Abstract
Therapeutics based on transcription factors have the potential to revolutionize medicine but have had limited clinical success due to delivery problems1–4. The delivery of transcription factors is challenging because it requires developing a delivery vehicle that can complex transcription factors, target cells, and stimulate endosomal disruption, with minimal toxicity5,6. In this report we present a novel multifunctional oligonucleotide, termed DARTs (DNA Assembled Recombinant Transcription factors), which can deliver transcription factors with high efficiency in vivo. DARTs are composed of an oligonucleotide that contains a transcription factor binding sequence and hydrophobic membrane disruptive chains that are masked by acid cleavable galactose residues. DARTs have a unique molecular architecture, which allows them to bind transcription factors, trigger endocytosis in hepatocytes, and stimulate endosomal disruption. The DARTs target hepatocytes as a result of the galactose residues and can disrupt endosomes efficiently with minimal toxicity, because unmasking of their hydrophobic domains selectively occurs in the acidic environment of the endosome. We show here that DARTs can deliver the transcription factor Nuclear erythroid 2-related factor 2 (Nrf2) to the liver, catalyze the transcription of Nrf2 downstream genes, and rescue mice from acetaminophen induced liver injury.
The delivery of transcription factors is a central challenge in medicine. Transcription factors control every major physiological process within a cell, ranging from cell fate determination to inflammation resolution2,3,7, and have the ability to correct the fundamental causes of a wide range of diseases. For example, the transcription factor Nrf2 transcribes genes that resolve states of chronic oxidative stress and inflammation, and can protect against numerous incurable inflammatory diseases, such as atherosclerosis, Alzheimer’s, and drug induced liver failure4,8,9. Therapeutics based on transcription factors, such as Nrf2, have tremendous therapeutic potential, but have been challenging to develop as therapeutics because of delivery problems4,10,11, and there is a great need for new transcription factor delivery vehicles9,12.
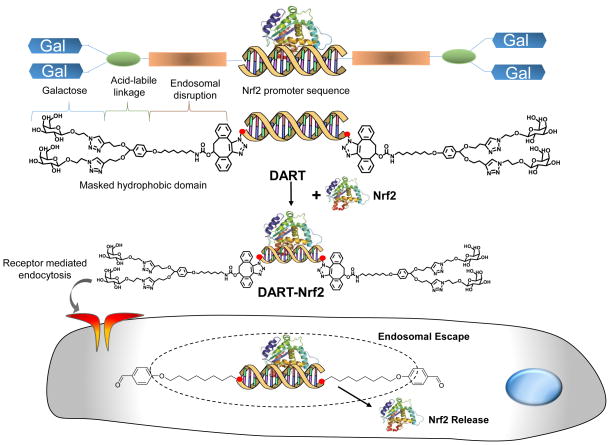
In this report we present a transcription factor delivery vehicle, termed DARTs, which can deliver Nrf2 to the liver and rescue mice from acute liver failure. The chemical structure of the DARTs and the mechanism by which they are designed to deliver transcription factors are shown in Figure 1. DARTs are composed of an oligonucleotide that contains an Nrf2 binding sequence and hydrophobic C32 alkyl chains that are “masked” by acid cleavable galactose residues, which are conjugated via acetal linkages13. The DARTs are able to complex Nrf2 because they contain an Nrf2 promoter sequence, which binds Nrf2 with high affinity, and can also target hepatocytes because they have galactose conjugated to their 3′ ends. In addition, the unique molecular architecture of the DARTs allows them to be pH sensitive membrane disruptive agents, and disrupt endosomes efficiently with minimal toxicity. The DARTs are designed to have minimal toxicity while they are circulating in the blood, because at pH 7.4, their hydrophobic domains are unable to interact with cell membranes due to the adjacent galactose groups. However, after endocytosis, the galactose groups of the DARTs are cleaved as a result of hydrolysis, and this activates the hydrophobic domains and allows them to disrupt the endosomal membrane.
Figure 1. DARTs are multifunctional oligonucleotides that can deliver the transcription factor Nrf2 in vivo.
DARTs are composed of an oligonucleotide that contains an Nrf2 promoter sequence and hydrophobic membrane disruptive domains that are masked by acid cleavable galactose residues. DARTs complex Nrf2 and deliver Nrf2 to the liver via galactose mediated endocytosis. After endocytosis, DARTs stimulate endosomal disruption and the release of Nrf2 into the cytoplasm, due to acid catalyzed unmasking of their hydrophobic domains.
The DARTs are designed to address several key challenges associated with delivering transcription factors in vivo. For example, transcription factors can be delivered via the DARTs by simply mixing them together. In contrast traditional protein delivery strategies require chemical modification of the protein or exposure to organic solvents, both of which frequently cause protein denaturation or the generation of antigenicity14. DARTs are well-defined molecules that can also be synthesized on a large scale, and avoid the characterization and manufacturing problems associated with self-assembled systems. In addition, DARTs are able to disrupt endosomes without using cationic peptides or polymers and thus overcome the toxicity problems associated with traditional endosomal disruptive strategies15. Finally, the DART delivery strategy is modular, and a variety of transcription factors can potentially be delivered via the DART strategy by simply changing their oligonucleotide sequence and targeting moieties.
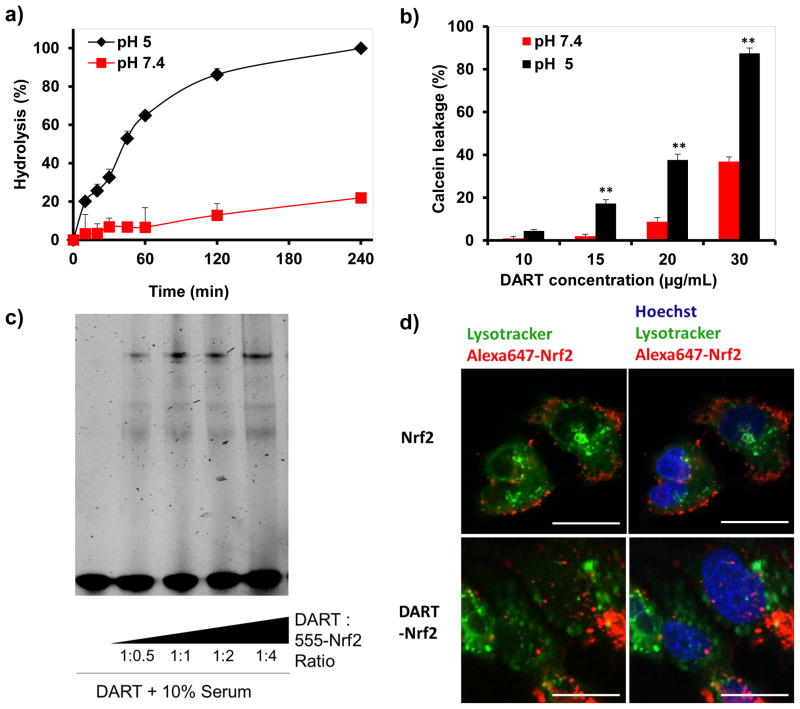
The pH sensitive hydrolysis of the acetal linkage in the DARTs is a critical feature of their molecular design. The DARTs need to be stable at pH 7.4 to allow for circulation in the blood with low toxicity, but hydrolyze rapidly at pH 5.0 and trigger endosomal disruption, before extensive degradation in the lysosome occurs. The hydrolysis kinetics of the DARTs was measured at pH 5.0 and pH 7.4, via gel electrophoresis (see Supplementary Information S3 for details). Figure 2a demonstrates that DARTs undergo pH sensitive hydrolysis with an estimated half-life of 41 minutes at pH 5.0, but have an estimated half-life of 12 hours at pH 7.4. Intravenously injected galactose containing molecules are internalized by the liver within 1 hour16, and the pH 7.4 hydrolysis kinetics of the DARTs should be suitable for delivering transcription factors to the liver. In addition, the rapid hydrolysis of the DARTs at pH 5.0, suggests that they will trigger endosomal escape before extensive degradation of Nrf2 in the lysosomes occurs.
Figure 2. The DARTs are acid sensitive endosomal disruptive agents and their hydrolysis is pH sensitive.
a, The hydrolysis of the DARTs is acid catalyzed. DART hydrolysis was conducted at pH 5 and pH 7.4 PBS, ± S.E, n=5.
b, DARTs disrupt calcein containing liposomes in a pH-dependent manner. Liposome disruption by the DARTs was assessed by calcein leakage, mean ± S.E, n=5. **, p < 0.01.
c, Nrf2 binds the DARTs in 10% serum. An electrophoretic mobility shift assay on Nrf2-DART complexes demonstrates that the migration of the DARTs is shifted after complexation with Nrf2 in serum.
d, DARTs alter the intracellular delivery of Nrf2 and enhance lysosomal release. Alexa555 labeled Nrf2 (red color) was complexed with the DARTs and incubated with HepG2 cells. Endosomes were counterstained with Lysotracker green (green color). Nrf2 complexed with the DARTs have a substantially different intracellular distribution than free Nrf2, with a large fraction existing outside of the lysosome, whereas free Nrf2 is predominantly on the cell membrane or in lysosomes. (Scale bar: 5 μm).
The acid catalyzed hydrolysis of the DARTs is designed to trigger endosomal disruption via unmasking of their hydrophobic domains. We therefore investigated if DARTs could disrupt liposomes in a pH sensitive manner using a calcein leakage assay (see Supplementary Information S4 for details). DARTs were incubated with calcein containing liposomes at either pH 5.0 or 7.4 for 30 minutes, at concentrations between 10 – 30 μg/mL, and the disruption of liposomes was measured via the increase in calcein fluorescence. Figure 2b demonstrates that DARTs have pH sensitive membrane disruptive properties, and can disrupt lipid bilayers in an efficient manner. For example, calcein containing liposomes incubated with DARTs at a 20 μg/mL (1 μM) concentration at pH 5.0 released approximately 40% of their encapsulated calcein in 30 minutes, whereas liposomes incubated at pH 7.4 released only 10% of their encapsulated calcein. DARTs have a membrane disruptive capacity similar to commonly used endosomal disruptive polymers, such as co-polymers of diethylaminoethyl methacrylate and butyl methacrylate, which also require μg/mL concentrations to disrupt membranes, and this suggests that the DARTs will be able to enhance the cytoplasmic delivery of transcription factors17.
The DARTs are designed to complex Nrf2 because they contain the antioxidant responsive element (ARE) sequence, which is a DNA sequence found in the promoter region of Nrf2 dependent genes. We performed experiments to determine if the DARTs could complex Nrf2. The binding of the DARTs with Nrf2 was determined via a competitive ELISA between DARTs and ARE DNA for binding to Nrf2. The results of this experiment are shown in the supporting information Figure S5 (see Supplementary Information S6 for details), and demonstrate that the DARTs bind Nrf2 with an affinity similar to unmodified ARE DNA. In addition, we performed experiments to determine if the DARTs could bind Nrf2 in serum. Alexa555-Nrf2 was complexed with the DARTs in 10% serum, and binding to the DARTs was analyzed by gel electrophoresis (see Supplementary Information S8 for details). Figure 2c demonstrates that Nrf2 can complex the DARTs in 10% serum, addition of Nrf2 to DARTs generated a high molecular weight band that was significantly larger than the free DARTs.
We performed fluorescent microscopy experiments to determine if DARTs could enhance the endosomal release of Nrf2. HepG2 cells were treated with DART-Alexa555-Nrf2 complexes and the intracellular localization of Nrf2 was investigated via confocal microscopy, using Lysotracker green to mark endosomes and lysosomes (see Supplementary Information S9.1 for details). Figure 2d demonstrates that DARTs can dramatically alter the intracellular distribution of Nrf2, and causes a significant fraction of the delivered Nrf2 to localize in compartments distinct from lysosomes. In contrast, free Nrf2 is mostly found on the cell membrane or in lysosomes.
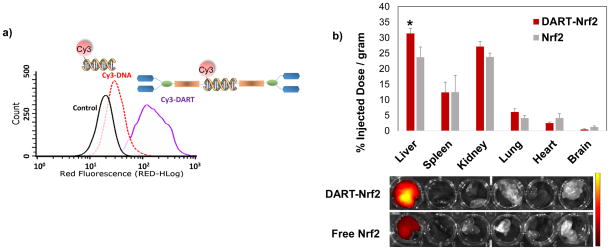
DARTs contain a tetravalent galactose group for hepatocyte targeting. We performed experiments to determine if the DARTs were efficiently internalized by hepatocytes. DARTs containing Cy3 were incubated with Hepa-1C1C7 and their uptake was compared against Cy3-labeled DNA via flow cytometry (see Supplementary Information S9 for details). Figure 3a demonstrates that the DARTs dramatically enhance the uptake of DNA, causing an 8 fold increase in mean red fluorescence versus free DNA. Based on these results we further performed in vivo biodistribution and pharmacokinetic experiments with Nrf2 complexed with DARTs, to determine if the DARTs could enhance the accumulation of Nrf2 in the liver (see Supplementary Information S11.1 for details). Figure 3b demonstrates that the DARTs can increase the delivery of Nrf2 to the liver, causing an approximately 1.4 fold increase in liver delivery compared to free Nrf2, suggesting that the galactose targeting via the DARTs increases the delivery of Nrf2 to the liver. An increase in serum half-life was also observed with DART-Nrf2, which had a 1.3 fold increase in serum half-life over free Nrf2 (104 mins versus 83 mins) (see Supplementary Information S11.2 for details). The 1.3 fold increase in serum half-life is consistent with the modest increase in molecular weight of Nrf2 caused by complexation with the DARTs. The molecular weight of the DART is approximately 20 kD and the DART-Nrf2 complex should therefore have a molecular weight that is only 35 % greater than free Nrf2. In addition, no evidence of systemic toxicity was observed in mice injected with Nrf2 complexed with the DARTs, as determined by blood TNF-α levels (Fig. S9).
Figure 3. The DARTs can target hepatocytes and enhance the delivery of Nrf2 to the liver.
a, Hepa-1C1C7 cells were treated with Cy3-DNA or Cy3-DART for 4 hours and uptake was measured via flow cytometry. Cy3 labeled DARTs have enhanced uptake in Hepa-1C1C7 cells and have 8 times higher mean red fluorescence than free DNA.
b, DARTs enhance the liver delivery of Nrf2. Nrf2 labeled with IRDye 800CW-NHS-ester was complexed with the DARTs, and injected into mice. The biodistribution of Nrf2 was quantified via fluorescence 4 hours after Nrf2 injection. DARTs enhance the liver delivery of Nrf2 by a factor of 1.4. mean ± S.E, n=6. *, p < 0.05.
DARTs are designed to deliver Nrf2 to hepatocytes, induce the expression of antioxidant genes, and protect hepatocytes from inflammatory diseases associated with oxidative stress18,19. We investigated if DARTs could deliver Nrf2 to HepG2 cells and upregulate the transcription of anti-oxidant genes that are regulated by Nrf2, in particular, Heme oxygenase-1 (HO-1), NAD(P)H dehydrogenase (quinone) (NQO1), and Glutamate-cysteine ligase catalytic subunit (GCLC) 4,20. We selected these three genes for analysis because of their central role in protecting against oxidative stress, and because they are signatures of Nrf2 activity. For example, HO-1 increases carbon monoxide production and is therapeutic against a wide variety of inflammatory diseases21. In addition, NQO1 up-regulates quinone anti-oxidant production and GCLC increases the production of glutathione, both of which protect against oxidative stress by remodeling metabolic and cell signaling pathways 4,22.
Nrf2 was bound to DARTs at a 1:1 molar ratio (DART-Nrf2) and was incubated with HepG2 cells at a 0.6 μM concentration overnight and then analyzed for gene expression via reverse transcription PCR (RT-PCR). As a control, HepG2 cells were treated with free Nrf2. Figure 4a demonstrates that DARTs can deliver functional Nrf2 into hepatocytes and induce the expression of Nrf2 target genes. For example, HepG2 cells treated with DART-Nrf2 had a significant increase in HO-1 expression in comparison to cells treated with Nrf2 or control cells. Similarly, DART-Nrf2 was able to increase the transcription of NQO1 and GCLC, whereas free Nrf2 had no effect on these genes.
Figure 4. DARTs are able to deliver Nrf2 to hepatocytes, up-regulate Nrf2 downstream genes, and can protect hepatocytes against reactive oxygen species (ROS).
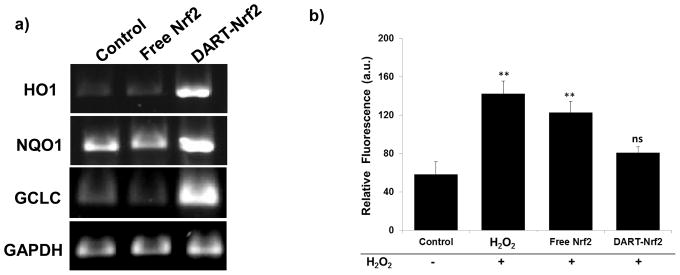
a, Nrf2 delivered by DARTs enhances the expression of HO1, NQO1, and GCLC. RT-PCR of HepG2 cells treated with DART-Nrf2 complexes up-regulate HO1, NQO1, and GCLC. Free Nrf2 had no effect on HO1, NQO1, and GCLC gene expression.
b, Nrf2 delivered by DARTs reduces ROS levels in HepG2 cells stressed with hydrogen peroxide. ROS levels in hydrogen peroxide stressed cells were not significantly reduced by free Nrf2, whereas DART-Nrf2 decreased ROS production down to levels comparable to control cells, mean ± S.E, n=9. **, p < 0.01, ns = statistically not significant.
We also investigated if the transcription of Nrf2 downstream genes by DART delivered Nrf2 could protect hepatocytes from oxidative stress. HepG2 cells were pretreated with a 200 μM concentration of hydrogen peroxide for 4 hours, to induce oxidative stress, and then treated with either free Nrf2 or DART-Nrf2 (0.6 μM) for 14 hours. Cellular oxidative stress was measured with CM-H2DCFDA using flow cytometry (see Supplementary Information S9.3 for details). Figure 4b demonstrates that DART delivered Nrf2 can protect hepatocytes from hydrogen peroxide induced oxidative stress. Cells treated with hydrogen peroxide had a 2.5 fold increase in CM-H2DCFDA oxidation, whereas this was reduced down to levels comparable to the control with DART-Nrf2. In contrast, Nrf2 by itself had a minimal effect on cellular oxidative stress levels. Thus, DARTs are able to deliver functional Nrf2, induce the transcription of antioxidant genes, and protect cells against oxidative stress.
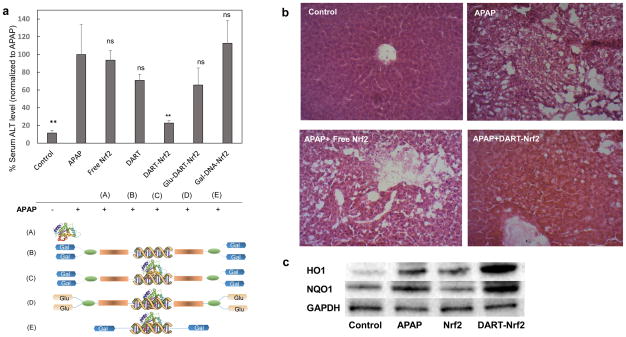
Nrf2 delivered by DARTs has great potential for treating liver associated inflammatory diseases, such as acute liver failure and liver fibrosis, which collectively effect millions of people each year4,10,11. We investigated if Nrf2 delivered by DARTs could protect mice against acetaminophen (APAP) induced liver injury. APAP induced liver injury was selected as an initial disease model for the DARTs because of the great need for new therapeutics against this disease23,24 and the well-established role of Nrf2 in protecting against APAP induced liver injury. APAP induced toxicity in mice was induced via the i.p. injection of APAP (500mg/kg), and 1 hour afterwards an intravenous injection of either free Nrf2 or DART-Nrf2 was given. Liver toxicity was determined 24 hours later by measuring ALT levels in the serum and also via histology. Additionally, liver tissue was isolated from these mice and analyzed by western blotting to determine if Nrf2 delivered by the DARTs could induce the expression of Nrf2 downstream genes (see Supplementary Information S11 for details).
Figures 5a–c demonstrate that DARTs can enhance the delivery of Nrf2 in vivo, induce the transcription of Nrf2 downstream genes, and protect mice from APAP induced liver injury. For example, Figure 5a demonstrates that mice receiving DART-Nrf2 and APAP had a four-fold decrease in ALT levels in comparison to mice that received APAP only, or mice that received APAP and free Nrf2. A similar therapeutic benefit was observed in the histology sections of the mice that received DART-Nrf2 and APAP, which had a histological profile similar to healthy mice, in contrast mice receiving free Nrf2 and APAP had significant signs of toxicity (Fig. 5b). Importantly, DART-Nrf2 was able to have a therapeutic effect on APAP treated mice, even though it was given an hour after APAP administration, suggesting that it can be effective in a clinical scenario where patients present hours after drug intake. In addition, we investigated if Nrf2 complexed with DARTs could stimulate the transcription of Nrf2 downstream genes in vivo, via western blotting for the Nrf2 downstream genes HO1 and NQO1. Figure 5c demonstrates that Nrf2 delivered by the DARTs can increase the expression of HO1 and NQO1 in the liver, and suggests that the therapeutic effects of DART delivered Nrf2 are due to the enhanced transcription of Nrf2 downstream genes.
Figure 5. The DARTs can deliver Nrf2 to the liver, up-regulate Nrf2 downstream genes, and rescue mice from acetaminophen induced liver injury.
a, Nrf2 delivered by DARTs rescue mice from acetaminophen (APAP) induced liver injury. DART-Nrf2 complexes reduce serum alanine transaminase (ALT) levels in APAP treated mice by a factor of 4. Free Nrf2 had no effect. Nrf2 complexed with either Glu-DARTs or Gal-DNA, and free DARTs, did not cause a statistically significant reduction in ALT levels. mean ± S.E, n=10. p < 0.01, ns = statistically not significant to APAP.
b, Nrf2 delivered by DARTs reduces liver damage in APAP treated mice. Histological sections of livers from APAP treated mice had significant inflammation. In contrast, histological sections of livers from APAP mice treated with DART-Nrf2 had reduced liver damage and resembled the histological sections of healthy mice. Free Nrf2 was unable to suppress liver damage. (Scale bar: 40 μm).
c, Nrf2 delivered by DARTs enhances the expression of the proteins HO1 and NQO1 in the liver. APAP treated mice were given either DART-Nrf2 or free Nrf2, and their livers were harvested and western blot analysis was conducted for the proteins HO1, NQO1, and GAPDH. Mice treated with DART-Nrf2 complexes have increased HO1 and NQO1 expression in comparison to free Nrf2 or APAP treated mice. Control = no treatment, APAP = APAP only, Nrf2 = APAP+Nrf2, DART = APAP+DART-Nrf2.
We performed additional experiments to determine if the endosomal disruptive or galactose targeting groups of the DARTs were essential for Nrf2 delivery in vivo. To determine the importance of the endosomal disruptive unit, we synthesized galactose conjugated DNA and investigated its ability to deliver Nrf2 and rescue mice from APAP induced liver injury. Figure 5a demonstrates that galactose conjugated DNA is unable to deliver Nrf2 in vivo, demonstrating that the endosomal disruptive unit of the DARTs is critical for efficacy. In addition, we performed experiments to determine if the galactose targeting groups of the DARTs were critical for Nrf2 delivery. DARTs containing glucose residues instead of galactose were synthesized and their ability to rescue mice from APAP induced liver injury was investigated. Figure 5a demonstrates that glucose containing DARTs were inefficient at delivering Nrf2, but were more effective at delivering Nrf2 than galactose conjugated DNA. Thus the galactose targeting plays a role in enhancing Nrf2 delivery in vivo, but is perhaps not as important as the endosomal disruption unit.
In this report we present a multifunctional oligonucleotide, termed DARTs, which can deliver transcription factors in vivo. The DART delivery strategy has numerous potential applications given the central role of transcription factors in liver biology. In addition, it should be possible to deliver transcription factors to cell types outside of the liver by using targeting groups other than galactose. Finally, the DART delivery strategy demonstrates that DNA based scaffolds can be used to deliver DNA binding proteins and provides a platform for the development of DNA origami-based delivery vehicles.
Materials and Methods
Synthesis of the DARTs
The synthesis of the DARTs is shown in detail in the supporting information and is described in Figures S1 and S2.
In vitro delivery of Nrf2 with DARTs
HepG2 cells were cultured in DMEM media containing 10% FBS and antibiotics (Pen Strep). Cells with a passage number less than 15 were used for experiments. HepG2 and Hepa-1C1C7 cells were seeded at 40% confluency the day before experiments. Nrf2 and DARTs were mixed at equal molar ratios and incubated for 15 min in binding buffer before the addition to cells. For all in vitro studies, 0.6 μM of Nrf2 or 0.6 μM of Nrf2 complexed with 0.6 μM of DARTs were used.
Reverse transcription PCR (RT-PCR) analysis of HepG2 cells treated with Nrf2 delivered by DARTs
HepG2 cells were treated with either free Nrf2 or DART-Nrf2 in culture media for 24 h and the RNA was extracted from the cells using the RNeasy® Mini Kit with additional DNase I digestion (Qiagen, Hilden, Germany), according to the manufacturer’s protocol. RNA purity and concentration were determined by measuring the optical density using an ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA). Purified mRNAs were normalized by total mRNA concentration, and Qiagen one-step RT-PCR was conducted with primers for HO1, NQO1, GCLC, and GAPDH, which were purchased from Origene. Amplified DNAs were visualized with a ChemiDoc XRS system, after gel electrophoresis in a 1% agarose gel and staining with SYBR safe.
Intracellular localization of Nrf2 delivered by DARTs
The intracellular localization of Nrf2 delivered by the DARTs was observed by confocal microscopy (Swept Field Confocal microscope-Prairie Technology). Nrf2 was labeled with Alexa555 to visualize its intracellular localization. 500 μg of Nrf2 (500 μg/mL) was reacted with 9 times molar excess of Alexa555-NHS-ester (Life Technologies) at 4°C overnight in 0.1 M phosphate buffer (pH 8.5). A PD-10 column was used to remove unreacted Alexa555. The concentration of Nrf2 and conjugated Alexa555 were measured with Nanodrop 2000 (Thermo). Alexa555-Nrf2 and DART were mixed at equal molar ratios and incubated for 15 min. HepG2 cells in 35 cm culture dishes were treated with 0.6 μM of Alexa555-Nrf2 prepared with or without DARTs for 30 min. After PBS wash, cells were incubated for additional 4 hr in culture medium. 100nM of Lysotracker Green DND-26 (Life Technologies) was added to the cells to visualize endosomes and lysosomes. HepG2 cells were washed with PBS and images were taken using confocal microscopy.
Intracellular uptake analysis of Cy3-DART in HepG2 cells
3′-azide functionalized oligonucleotides containing the Nrf2 consensus sequence and Cy3, (forward strand 5′-/Cy3/GTC ACA GTG ACT CAG CAG AAT CTG TTT T-N3-3′) and (reverse strand 5′-CAG ATT CTG CTG AGT CAC TGT GAC TTT TT-N3-3′) were purchased from IDT. Cy3 labeled DARTs were synthesized following procedures described in the Supplementary Information (see Supplementary Information S2.2 for details). HepG2 cells were treated with 0.6 μM of either Cy3-DNA or Cy3-DARTs in OptiMEM medium with 1% FBS for 24hr. HepG2 cells were washed 3 times with PBS and analyzed with flow cytometry (Guava easyCyte™).
Treatment of acetaminophen (APAP) induced liver injury in mice with DART-Nrf2
After overnight fasting, mice were administered an intraperitoneal injection of APAP (500 mg/kg) dissolved in PBS (5% DMSO). One group of mice received free Nrf2 at the dose of 25 μg per mouse (1.25 mg/kg of Nrf2), and the other groups of mice were administered DARTs, DART-Nrf2, glucose-DART-Nrf2, and Gal-DNA-Nrf2 in 100 μL of PBS by tail-vein injection. 24 h after the injection of APAP, the mice were euthanized by CO2 asphyxiation. The blood was collected by cardiac puncture and the livers were isolated. APAP-induced liver injury was determined by measuring the alanine aminotransferase (ALT) activity in the serum using a commercial ALT assay kit (Biovision). Liver samples were used for RNA isolation and histology to assess the liver damage. For histological analysis livers sections were stained by Hematoxylin and eosin, observed under light microscopy (Zeiss AxioCam ERc 5s), and the necrosis was compared between the groups of control, Nrf2 and DART-Nrf2 treated mice. Western blotting was performed with the liver samples to analyze the expression of HO1, NQO1, and GAPDH, using standard procedures. Transfer of proteins from liver homogenates to the nitrocellulose membrane (Bio-rad) was performed at 65V for 60 min. Primary antibodies (1:500) were incubated overnight at 4C and the secondary antibody, bovine anti-rabbit-HRP, (1:2000) was incubated for 2 hr at RT. Images were taken using a ChemiDoc XRS system (Bio-Rad Laboratories). HO1 antibody (sc-10789), NQO1 antibody (sc-25591), and bovine anti-rabbit IgG-HRP (sc-2370) were purchased from Santa Cruz Biotech.
Detailed experimental procedures regarding the DART synthesis, characterization, and in vitro and in vivo testing are described in the Supplementary Information.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH, U01 268201000043C-0-0-1, RO1 AI107116-01, and RO1 AI088023-03. In addition, this work was supported by the WM Keck Foundation, based in Los Angeles, USA. We thank S. Maity and M. Webb for chemistry advice and M. West in the Berkeley Stem Cell Center for technical assistance.
References
- 1.Zhou HY, et al. Generation of Induced Pluripotent Stem Cells Using Recombinant Proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Huang P, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. doi: 10.1038/nature10116. [DOI] [PubMed] [Google Scholar]
- 4.Bataille AM, Manautou JE. Nrf2: a potential target for new therapeutics in liver disease. Clinical pharmacology and therapeutics. 2012;92:340–348. doi: 10.1038/clpt.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, et al. Delivery of Intact Transcription Factor by Using Self-Assembled Supramolecular Nanoparticles. Angew Chem Int Edit. 2011;50:3058–3062. doi: 10.1002/anie.201005740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan M, et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5:48–53. doi: 10.1038/Nnano.2009.341. [DOI] [PubMed] [Google Scholar]
- 7.Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 8.Giudice A, Arra C, Turco MC. Review of molecular mechanisms involved in the activation of the Nrf2-ARE signaling pathway by chemopreventive agents. Methods Mol Biol. 2010;647:37–74. doi: 10.1007/978-1-60761-738-9_3. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuishi Y, et al. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Cuadrado A, Moreno-Murciano P, Pedraza-Chaverri J. The transcription factor Nrf2 as a new therapeutic target in Parkinson’s disease. Expert opinion on therapeutic targets. 2009;13:319–329. doi: 10.1517/13543780802716501. [DOI] [PubMed] [Google Scholar]
- 11.Crawford R, et al. Non-covalent single transcription factor encapsulation inside a DNA cage. Angew Chem Int Ed Engl. 2013;52:2284–2288. doi: 10.1002/anie.201207914. [DOI] [PubMed] [Google Scholar]
- 12.Biswas A, Liu Y, Liu T, Fan G, Tang Y. Polyethylene glycol-based protein nanocapsules for functional delivery of a differentiation transcription factor. Biomaterials. 2012;33:5459–5467. doi: 10.1016/j.biomaterials.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Murthy N, Campbell J, Fausto N, Hoffman AS, Stayton PS. Bioinspired pH-responsive polymers for the intracellular delivery of biomolecular drugs. Bioconjugate chemistry. 2003;14:412–419. doi: 10.1021/Bc020056d. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, et al. Delivery of intact transcription factor by using self-assembled supramolecular nanoparticles. Angew Chem Int Ed Engl. 2011;50:3058–3062. doi: 10.1002/anie.201005740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K, Lee H, Lee KW, Park TG. Optical imaging of intracellular reactive oxygen species for the assessment of the cytotoxicity of nanoparticles. Biomaterials. 2011;32:2556–2565. doi: 10.1016/j.biomaterials.2010.11.072. [DOI] [PubMed] [Google Scholar]
- 16.Dube D, Khatri K, Goyal AK, Mishra N, Vyas SP. Preparation and evaluation of galactosylated vesicular carrier for hepatic targeting of silibinin. Drug development and industrial pharmacy. 2010;36:547–555. doi: 10.3109/03639040903325560. [DOI] [PubMed] [Google Scholar]
- 17.Cheng C, Convertine AJ, Stayton PS, Bryers JD. Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials. 2012;33:6868–6876. doi: 10.1016/j.biomaterials.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Molecular aspects of medicine. 2011;32:234–246. doi: 10.1016/j.mam.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. The Journal of pharmacology and experimental therapeutics. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 20.Sekhar KR, et al. NADPH oxidase activity is essential for Keap1/Nrf2-mediated induction of GCLC in response to 2-indol-3-yl-methylenequinuclidin-3-ols. Cancer research. 2003;63:5636–5645. [PubMed] [Google Scholar]
- 21.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. Journal of biochemistry and molecular biology. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 22.Suh JH, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holubek WJ, Kalman S, Hoffman RS. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2006;43:880. doi: 10.1002/hep.21106. author reply 882. [DOI] [PubMed] [Google Scholar]
- 24.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.