Abstract
Aging is associated with gradual deterioration of adaptive immune function, a hallmark of which is the profound loss of naïve T cells (TN) associated with decline in thymic output and export of new cells into the peripheral T cell pool. Since the lymphotropic cytokine IL-7 plays crucial roles in both development of TN in the thymus and TN homeostasis in the periphery, we sought to determine the extent to which therapeutic administration of IL-7 could reverse TN deficiency in aging rhesus macaques (RM), either by enhancement of the demonstrably reduced thymopoiesis or by peripheral TN expansion. Our results indicate that treatment of both adult (8–15 years) and old (>20 years) RM with recombinant simian IL-7 (rsIL-7) results in only transient increases in peripheral CD4+ and CD8+ TN numbers with no long-term benefit, even with repeated therapy. This transient effect was due to peripheral TN expansion and not enhanced thymic function, and appeared to be limited by induction of IL-7 non-responsiveness. However, rsIL-7 therapy had a more promising effect on the central memory T cell (TCM) population (both CD4+ and CD8+) in adult and old RM, doubling the numbers of these cells in circulation and maintaining this larger population long-term. IL-7 therapy didn't reduce TCR diversity of the memory T cell compartment, suggesting that rsIL-7-induced expansion was symmetrical. Thus, although rsIL-7 failed to counter age-associated TN loss, the ability of this therapy to expand clonotypically diverse CD4+ and CD8+ TCM populations might potentially improve adaptive immune responsiveness in the elderly.
INTRODUCTION
Immunological aging is characterized by a gradual decline in production, emigration and maintenance of TN in peripheral circulation. At the heart of TN decline is the involution of the thymus, which starts soon after birth and continues well into adulthood. It is still largely unclear precisely how the thymus involutes with age; however, a number of factors including a depletion or loss of T cell progenitors from the bone marrow, decline in thymic epithelial integrity and function, reduced efficacy of thymic positive and negative selection, altered cytokine production in the thymic microenvironment and an increase in sex steroid hormone levels after puberty have all been implicated in driving this process (1–5). This defect in TN homeostasis has profound effects on peripheral T cell subset distribution. In addition to the decline in thymic output, continuous life-long exposure to antigenic stimulation continuously drives TN differentiation into the circulating TM pool (6–10). The benefit of this process would be the retention in advanced age of immunologic memory to a diverse range of antigenic specificities previously encountered over the lifetime of the host. However, loss of TN populations would be disadvantageous to the extent that it limits the ability of the immune system to protect the aging host against new or evolving pathogenic challenges, or to reconstitute effectively after conditions of lymphopenia. Indeed, rejuvenation of TN production and maintenance of peripheral TCR repertoire diversity is widely considered to be a crucial goal in overcoming the deleterious effects of immunological aging (10–14).
After maturation in the thymus, newly produced TN emigrate to secondary lymphoid tissues (e.g., lymph nodes, Peyer’s patches, and the spleen) and join the pool of circulating peripheral lymphocytes, where they preserve TCR diversity and mediate immune surveillance. In the periphery, TN are predominantly maintained by trophic signals received by interaction of their TCR with self peptide-MHC complexes and IL-7, a member of the common γ-chain family of cytokines. IL-7 serves as a key pro-survival factor for TN and also plays a major, albeit non-exclusive, role in TM homeostasis (15,16). IL-7 is secreted by stromal and epithelial cells of the thymus and is important for the development of immature thymocytes, particularly those in the double negative (DN) 2 to DN3 transition stages. Thymic cellularity and thymocyte expansion are significantly reduced in IL-7−/− and IL-7R−/− mice, and expression of IL-7 transcripts by thymic stromal cells has been shown to decline with age (17–20). IL-7 signals via the receptor IL-7R, a heterodimer consisting of the α-chain (CD127) and the γ-chain (CD132), and its effects are predominantly mediated through the Jak/STAT signaling pathway. Activation of the Jak/STAT pathway leads to the downstream recruitment and phosphorylation of the transcription factor STAT5, which in turn translocates to the nucleus and modulates the expression of genes associated with T cell proliferation and survival. IL-7 signaling also activates the PI3K signaling pathway leading to the activation of AKT and the regulation of genes associated with T cell survival such as Bad and p27, and is important in metabolic integrity of TN (21). The T cell response to IL-7 is modulated by CD127 expression, and IL-7 signaling drives antigen-independent homeostatic proliferation of the responding cells and the up-regulation of anti-apoptotic genes such as Bcl-2. The lack of IL-7 signaling prevents the inhibition of pro-apoptotic signals resulting in cell death. Defects in IL-7 signaling have been reported in patients with SCID, a condition characterized by a profound absence of T cells, highlighting the importance of IL-7 in human T cell development.
With its roles in T cell development and survival, IL-7 has been used as an immunotherapeutic agent to support immune reconstitution in conditions of T cell deficiency (22–24). Patients with metastatic cancer treated with 8 doses of IL-7 every 3 days for 21 days saw rapid, but transient, increases in the absolute numbers of CD4+ and CD8+ T cells and a decrease in fraction of CD4+ regulatory T cells (25). In a separate trial, patients with refractory malignancy received multiple subcutaneous doses (ranging from 3–60 µg/Kg) of recombinant IL-7 over a period of 14 days. This treatment induced a dose-dependent, but age-independent, increase in the number of circulating CD4+ and CD8+ T cells, which remained elevated for several weeks after the start of therapy (26). IL-7 dosing was further linked with increasing recent thymic emigrants, circulating TN and TCR repertoire diversity (27). HIV-infected patients with low CD4 counts (between 101–400 cells/µl) demonstrated sustained increases in CD4+ TN and CD4+ TCM populations in response to IL-7 treatment (28). Similarly in healthy and SIV-infected macaques, IL-7 treatment significantly increased the proliferation and absolute numbers of peripheral TN and memory (TM) populations (29–35).
Taken together, these studies indicate that IL-7 can dramatically affect the numbers and turnover of peripheral T cells. However, it remains unclear whether IL-7 has the potential to stimulate thymopoiesis and initiate T cell reconstitution in old age. In this study, we assessed the effect of rsIL-7 administration on TN and TM homeostasis in RM for its ability to regenerate T cell compartments in old RM. While rsIL-7 administration acutely increased the proliferative fraction and absolute numbers of TN and TM in the peripheral blood, the kinetics of this expansion were indistinguishable between surgically thymectomized RM and sham treated controls. In old RM, the response to rsIL-7 was transient and there was no long-term benefit to TN population stability with repeat dosing. In contrast, rsIL-7 treatment had a prolonged effect on CD4+ and CD8+ TCM expansion regardless of age, which was sustained with repeated therapy. Moreover, TCR diversity in TM, as judged by CDR3 length polymorphism analysis (spectratyping), was not adversely affected by this treatment, and in fact, showed signs of improvement.
MATERIALS AND METHODS
Animals
Colony-bred male and female RM of Indian origin were maintained according to the federal, state, and local guidelines. For CD8 depletion studies, 16 male juvenile RM (average = 3 years, range 1–6 years) were subjected to surgical thymectomy or sham surgery (n = 8 per group) prior to receiving the CD8+ T cell-depleting monoclonal antibody (mAb), cM-T807, with the first dose given s.c. at 20mg/kg body weight followed by 3 subsequent doses administered i.v. at 10mg/kg body weight on days 3, 7 and 10 (36). In addition, 4 old (average = 23.7 years, range 23–25 years), 4 adult (average = 11.5 years, range 8–15 years) and 2 juvenile (4 years each) RM were also administered cM-T807 as described above. For IL-7 studies, two cohorts consisting of adult and old RM received either 7 single doses (on days 0, 7, 108, 185, 304, 423 and 542) or 9 clustered doses (on days 0, 7, 14, 35, 42, 49, 70, 77 and 84) of rsIL-7 given s.c. at 30µg/kg body weight (31). Another cohort consisting of 12 juvenile male RM were subjected to surgical thymectomy or sham surgery prior to receiving rsIL-7 (30µg/kg) on days 0 and 7. The characteristics of all IL-7-treated RMs are described in Supplementary Table 1. BrdU (Sigma-Aldrich) was prepared as described previously (37) and administered i.v. in 4 separate doses of 30mg/kg body weight on days 10, 11, 12 and 13, after the first rsIL-7 dose. All RMs were housed at the Oregon National Primate Research Center in accordance with standards of the Center’s Institutional Animal Care and Use Committee and the “NIH Guide for the Care and Use of Laboratory Animals”. RM that developed disease states that were not clinically manageable were euthanized in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Measurement of plasma IL-7 concentration
The concentration of IL-7 in the plasma was measured by ELISA using the IL-7 Quantikine HS kit according to the manufacturer’s instructions (R&D Systems).
Cytokines and antibodies
Glycosylated rsIL-7 was obtained from Cytheris SA (Issy les Moulineaux Cedex, France). Francois Villinger (Emory University) kindly provided the recombinant simian IL-15 (rail-15). MAbs L200 (CD4; AmCyan), SP34-2 (CD3; Alexa 700, Pacific Blue), SK1 (CD8α; PerCP-Cy5.5, AmCyan, PE-Cy7, FITC), B56 (Ki-67; FITC), DX2 (CD95; PE, PE-Cy7, FITC), 3A9 (CCR5; APC), B44 (BrdU; FITC, APC) and pY694 (Phospho-STAT5; PE) were obtained from BD Biosciences. CD28.2 (PE-Texas Red) was obtained from Beckman Coulter. DK25 (CD8α; PE and APC), which is non–cross-reactive with cM-T807, was obtained from Dako. SK1 (CD8; PerCP-eFluro 710) was obtained from eBiosciences. The 150503 mAb (anti-CCR7) was purchased as purified immunoglobulin from R&D Systems, conjugated to biotin using the EZ-Link Maleimide-PEO solid phase biotinylation kit (Fisher), and visualized with streptavidin–Pacific Blue (Invitrogen). The anti-CD8 cM-T807 depleting mAb was provided through the National Institutes of Health’s Nonhuman Primate Reagent Resource Program.
Immunofluorescent staining and flow cytometric analysis
Whole blood was obtained and stained for flow cytometric analysis as previously described (37,38). Polychromatic (8–12 parameter) flow cytometric analysis was performed on an LSR II BD instrument using Pacific Blue, AmCyan, FITC, PE, PE-Texas Red, PE-Cy7, PerCP-Cy5.5, APC, APC-Cy7 and Alexa 700 as the available fluorescent parameters. Instrument set-up and data acquisition procedures were performed as previously described (39,40). List mode multi-parameter data files were analyzed using the FlowJo software program (Tree Star). Criteria for delineating naïve and memory T cell subsets and for setting + vs. − markers for CCR5 and Ki-67 expression have been previously described in detail (37,38,40,41). In brief, TN constitute a uniform cluster of cells with a CD28moderate, CCR7+, CCR5−, CD95low phenotype, which is clearly distinguishable from the phenotypically diverse memory population that is CD95high or displays one or more of the following “non-naïve” phenotypic features: CD28−, CCR7−, CCR5+. The TCM, transitional memory (TTrM) and effector memory (TEM) cell components of the memory subset in the blood were further delineated based on the following phenotypic criteria: TCM (CD28+, CCR7+, CCR5−), TTrM (CD28+, CCR7+/−, CCR5+) and TEM (CD28−, CCR7−, CCR5dim). For each subset to be quantified, the percentages of the subset within the overall small lymphocyte and/or small T cell (CD3+ small lymphocyte) populations were determined. For quantification of peripheral blood subsets, absolute small lymphocyte counts were obtained using an AcT5diff cell counter (Beckman Coulter) and, from these values, absolute counts for the relevant subset were calculated based on the subset percentages within the light scatter–defined small lymphocyte population on the flow cytometer. Baseline values were determined as the average of values at day -14, -7 and 0. Absolute counts are indicated as % change from baseline with baseline shown as 100%. Changes in proliferative fraction are indicated as the difference in the %Ki-67+ (Δ%Ki-67+) measured at the designated time points from baseline (0% = no change).
For analysis of phosphorylated STAT5 (pSTAT5) expression, whole blood (200µl) was added to polystyrene flow tubes and stained with the following fluorochrome-conjugated mAbs: anti-CD3, anti-CD4 and anti-CD8, at room temperature (RT) for 15 min. Phosflow was then performed on the whole blood, which was either left unstimulated or stimulated with increasing concentrations of rsIL-7 or rsIL-15 (ranging from 0.125 – 128ng/ml) for 15 min at 37°C/5% CO2. Detection of pSTAT5 was assessed with the BD Phosflow staining protocol according to the manufactures instructions. Briefly, cells were fixed with the BD Phosflow Lyse/Fix buffer for 5 min at RT and permeabilized in BD PhosFlow perm buffer IV for 5min at RT. After washing, cells were stained for intracellular markers with fluorochrome-conjugated anti-pSTAT5 and anti-Ki-67 for 45 min at RT. Cells were washed and flow cytometric analysis was performed on LSR II.
RNA extraction, cDNA synthesis and TCRBV CDR3 length polymorphism analysis (spectratyping)
The RNA extraction for TCRBV spectratype analysis was performed on a total of 176 samples (naïve and total memory CD4+ and CD8+ T cells) from 2 cohorts of rsIL-7-treated RM. In cohort 1, 4 adults and 7 old RM were treated with repeated single doses of rsIL-7; cohort 2 comprised of 5 adult and 6 old RM treated with 9 cluster doses of rsIL-7. A minimum of 5 × 104 naïve (CD28+ CD95−) or memory (CD28+/− CD95+) CD4+ and CD8+ T cells per animal were sort purified using a FACS Aria II (BD Biosciences) directly in Tri reagent (Molecular Research Center, Inc.) and stored at −80oC for RNA isolation. 5µl of polyacryl carrier (Molecular Research Center, Inc.) was added in each Tri reagent sample prior to starting the RNA isolation procedure. Total RNA was extracted using the standard Tri reagent RNA isolation protocol (Molecular Research Center, Inc.). cDNA was synthesized by using RT-PCR kits (Invitrogen) and subjected to a set of 24 separate TCR Vβ specific PCR reactions using previously published primers (Supplementary Table 2) (42). The forward primer used to detect Vβ18 and Cβ-FAM, was modified as follows: Vβ18 (5’-GAGTCAGGA ATG CCA AAGGA-3’) and Cβ-FAM (5’-CACGTGGTCGGGGT AGAAGCC-3’). The primer sequences of Vβ and Cβ segments were synthesized at Invitrogen and the spectratype analysis was conducted with modifications. In brief, 30ng of cDNA was added to a final 20µl mixture containing 2µl of PCR buffer (Invitrogen), 0.2mM dNTP mix, 1.5mM MgCl2, 10pmol of 5’ Vβ and Cβ primers, and 1.05U of Platinum Taq (Invitrogen). PCR conditions were: 40 cycles of 95°C for 30s, 55°C for 30s, and 72°C for 1 min, with a final extension at 72°C for 10 min. One microliter of the PCR product was then used as a template for a nested PCR (runoff reaction) using only a Fam-labeled 3’ Cβ primer in a 10µl reaction. PCR was conducted as follows: 10 cycles of 95°C for 30s, 60°C for 30s, and 72°C for 1 min, with a final extension at 72°C for 10 min. Runoff elongation products (2µl) were mixed with 8µl of loading buffer (1ml of Hi-Di formamide + 30µl of Genescan 500LIZ size standard of ABI) and denatured at 95°C for 2 min. Samples were then loaded to an Applied Biosystems 3130 Genetic Analyzer (ABI). GeneMapper software version 4.0 (ABI) was used to analyze the spectratype data.
Statistical analysis
For single timepoint cross-sectional outcomes (absolute counts and IL-7 plasma concentrations), the Kruskal-Wallis test was used to compare difference between age groups. Non-parametric Kruskal-Wallis test was applied over parametric statistical methods due to the unequal variation between groups and/or skewed distribution of outcomes. Repeated measures ANOVA was used to evaluate the effect of cM-T807 on CD8+ TN counts as a response variable and age group as between group factor, and days post-CD8+ T cell depletion as quantitative within group factors. Multivariate regression modeling was applied with days post-CD8 depletion and age group as independent variables and log transformed CD8+ TN counts as dependent variable to fit the regression line for each age group as well as to perform statistical hypothesis tests for equal regression slope between groups. Multiple regression model was used to evaluate association between % max BrdU+ and days post-treatment. To assess differences in the decay rates between sham and thymectomized animals, thymectomy status (thymectomy/sham) was added to regression model as a form of indicator variable so that interaction between days post-treatment and thymectomy status serves to test equal rates of decay between thymectomy and sham group. To evaluate the effect of rsIL-7 therapy on T cell population dynamics (absolute counts and proliferative fraction), repeated measures ANOVA was used with thymectomy status or age group as between group factor and days post-first rsIL-7 treatment as within group factor. Since in a typical experiment using repeated measures, two measurements taken at adjacent times are more highly correlated than two measurements taken several time points apart (also due to the limited sample size), first order auto-regressive (AR1) was used as correlation within each animal. False Discovery Rate adjustment was used to control for multiple comparisons. The p values < 0.05 were considered significant.
Spectratyping data were analyzed using a diversity complexity index (DCI) as previously described (43). If two distributions are identical, then DCI value will be zero. Positive DCI value indicates potential improvement on spectratype profile; in other words, administration of IL-7 increases TCR repertoire diversity, while negative DCI value indicates potential loss of spectratype profile diversity. Calculated overall DCI value between age group and dose regimens were compared using permutation tests due to the small sample size and unknown distributional assumption for the DCI. A permutation test gives a simple way to compute the sampling distribution for any test statistic by randomly shuffling (permutations) the exposures. In other words, the method by which group assignments are allocated to subjects in an experimental design is mirrored in the analysis of that design. The p-value is determined by sample distribution of test statistics; that is, the ranking of the test statistic among the shuffled test statistics gives a p-value.
RESULTS
The thymus is required for de novo TN reconstitution after efficient peripheral TN cell depletion
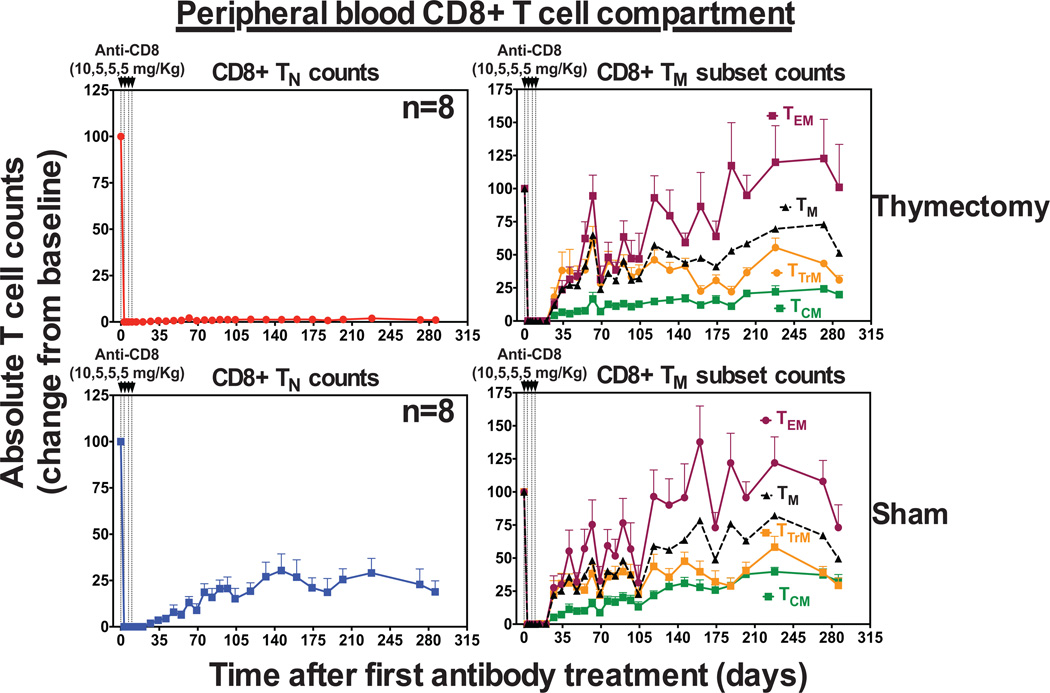
The goals of this study were to characterize the effects of rsIL-7 on peripheral T cell population dynamics in RM and determine whether rsIL-7 can stimulate thymopoiesis and/or immune reconstitution in old age. To systematically approach this question, we first examined the role of the thymus in de novo CD8+ TN production in RM. We know from previous studies that TN populations in juvenile RM with intact thymi reconstitute after efficient depletion with T cell- depleting mAbs (44–46). In particular, we have shown that antibody-mediated CD4+ T cell depletion of thymectomized RM results in profound deficiency of CD4+ TN in blood and peripheral lymph nodes (46). Using a similar approach, we subjected 16 juvenile RM between 1–4 years of age to surgical thymectomy (n=8) or sham surgery (n=8) followed by total CD8+ T cell depletion. As shown in Fig. 1, administration of the CD8+ T cell-depleting mAb cM-T807 to these RM led to the profound depletion of circulating CD8+ T cells in the blood through day 35. In RM, the peripheral blood TM compartment can be sub-classified based on the surface expression of CD28, CCR5 and CCR7 into TCM (CD28+CCR5−CCR7+), TTrM (CD28+CCR5+CCR7−/+), and TEM (CD28−CCR7−) (38,41,46,47). Note that while absolute numbers of CD8+ TM (including TCM, TTrM, and TEM subsets) in blood rebounded in both the thymectomized (athymic) and sham-treated (euthymic) RM, TN numbers rebound only in euthymic RM. Indeed, even after 280 days following CD8+ T cell depletion, athymic RM manifest almost a complete absence of circulating CD8+ TN in the blood. This data, together with our previous findings, demonstrates that if the peripheral CD4+ or CD8+ T cell compartment is efficiently depleted, neither CD4+ nor CD8+ TN regenerate in the absence of a functioning thymus, consistent with the idea that the thymus is the only significant source of de novo TN production in RM.
Figure 1. Thymectomy abrogates CD8+ TN recovery after antibody-mediated CD8+ T cell depletion.
Analysis of CD8+ TN and TM population dynamics, including TCM, TTrM and TEM subsets after mAb cM-T807 treatment in RM with complete initial CD8+ T cell depletion in blood that were previously subjected to thymectomy (n=8) or sham surgery (n=8). Results (mean + SEM) are shown as change from baseline value, with baseline represented as 100% and subsequent changes are shown relative to this baseline value determined as the average of absolute counts (see materials and methods) at days -14, -7 and 0 relative to the first dose of cM-T807.
Thymus function persists but significantly declines with age
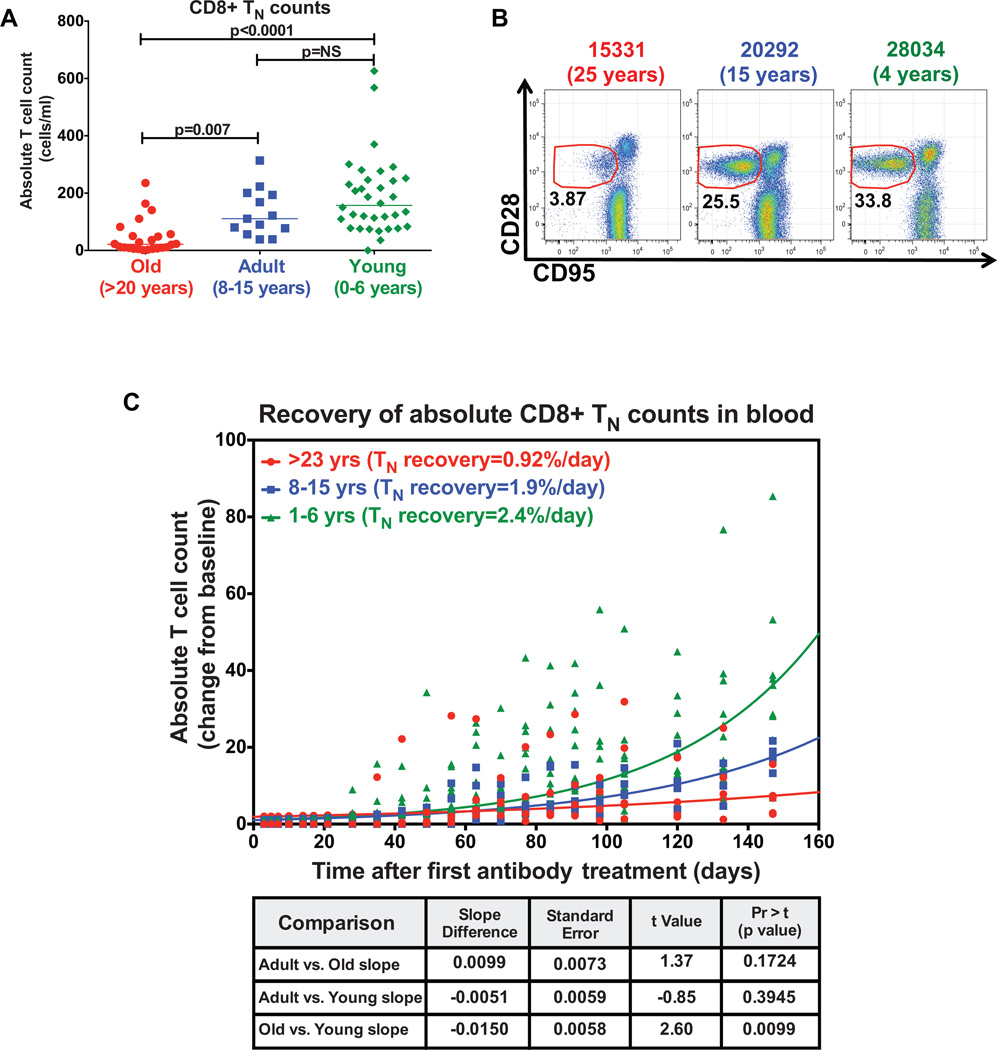
Having established that the thymus is indispensable for CD8+ TN production after the existing compartment has been efficiently depleted, we next sought to determine the impact of age on the ability of the thymus to reconstitute CD8+ TN after depletion. Similar to observations in humans (48,49), the absolute numbers and fractional representation of CD8+ TN in the peripheral blood of RM dramatically decline with age (Fig. 2A–B). Despite this, we examined the rate of CD8+ TN recovery after total CD8+ T cell depletion in a cohort of old (>20 years, n=4), adult (8–15 years, n=4) and young (0–6 years, n=10) RM. Administration of cM-T807 induced a profound depletion of CD8+ T cells in the blood through day 35 in all age groups. As expected, CD8+ TN regeneration was robust in the young RM with CD8+ TN counts increasing an average of 2.4% per day. The rate of recovery was slightly slower at an average of 1.92% per day in the adult RM. Interestingly, CD8+ TN numbers rebounded in the old RM, but at a significantly reduced rate of just 0.92% per day (p=0.0099) compared to the young RM (Fig. 2C). As TN reconstitution after total CD8+ T cell depletion only occurs in the presence of a functioning thymus (Fig. 1A), this data would suggest that the rebound in CD8+ TN numbers in old RM can only be associated with the production and export of new cells from the thymus. Thus, although aging is associated with an unequivocal decline in thymic output in RM, some new TN production must be present even in very old animals.
Figure 2. Thymic function persists in old RM.
(a) Comparison of the CD8+ TN absolute counts in peripheral blood of old (>20 years, n=20), adult (8–15 years, n=20) and young (0–6 years, n=20) RM. The p value was obtained using Kruskal-Wallis test to compare difference between groups (significant p values are shown; NS, non-significant). (b) Representative flow cytometric profiles showing decline in the fractional representation of CD8+ TN in blood of RM with age. The CD28 vs. CD95 profiles shown were gated on CD3+, CD8+, small lymphocytes. The red lines delineate the border between CD95low TN and CD95high TM with % of gated events in the naïve cluster shown in black. (c) Comparison of the recovery of CD8+ TN absolute counts (shown as percentage of baseline) in blood of old (n=4) vs. adult (n=4) vs. young (n=10) RM after cM-T807 treatment. Significance of difference in the rates of CD8+ TN recovery between age groups was assessed as described in the materials and methods.
IL-7 production and signaling are maintained in old RM
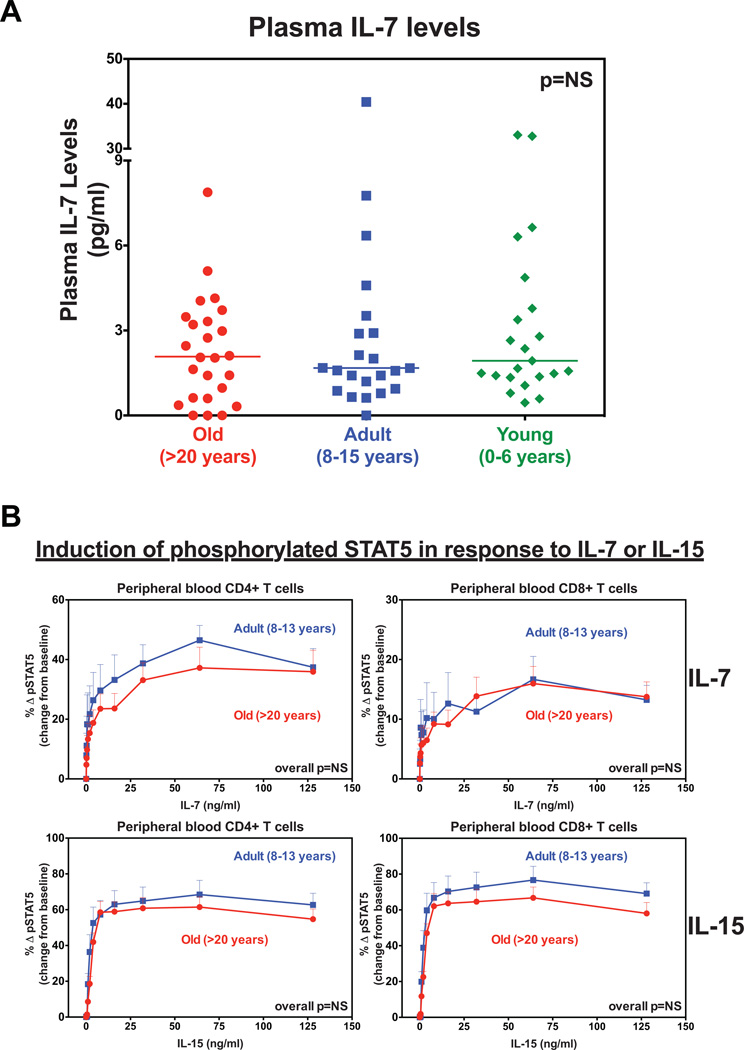
Prior to testing IL-7’s therapeutic effects in old RM, we examined whether there were defects in IL-7 production or signaling with age. In assessing this we first measured the levels of IL-7 in the plasma of young (1–6 years), adult (8–15 years) and old (>20 years) RM to determine whether IL-7 levels decline with age. We observed that the concentrations of plasma IL-7 were statistically similar between the three age groups, with median values of 1.93 pg/ml (IQR: 1.3 – 4.8), 1.67 pg/ml (IQR: 1 – 4.3) and 2.07 pg/ml (IQR: 0.6 – 3.5), for young, adult and old RM, respectively (Fig. 3A). This suggests that the production of the cytokine is not significantly altered with age. We then examined whether there were defects in the ability of T cells from old RM to respond to IL-7 stimulation and subsequent activation of the JAK-STAT signaling pathway. We stimulated whole blood ex vivo from a cohort of old (>20 years, n=7) and adult (8–15 years, n=4) RM with increasing concentrations of rsIL-7, and for comparison IL-15, and measured increases in pSTAT5 expression. As shown in Fig. 3B, both cytokines induced a dose-dependent increase (relative to baseline) in the fraction of CD4+ and CD8+ T cells with pSTAT5. Importantly, we found that the rate of induction of pSTAT5 expression was statistically identical between old and adult RM. We would note that the small sample sizes and the lack of comparison between memory and naïve T cells limit drawing definitive conclusions from these results. In addition, these results do not indicate whether down-stream gene expression (i.e., proliferation, survival, metabolic activity) is defective in T cells of old RM. However, these results do suggest that activation of the JAK-STAT pathway by IL-7 is not significantly compromised in old RM.
Figure 3. The production and T cell responsiveness to IL-7 are maintained in old RM.
(a) Comparison of plasma IL-7 concentrations in old (n=25) vs. adult (n=22) vs. young (n=25) RM. The p value was obtained using Kruskal-Wallis test to compare difference in concentration between groups (NS = non-significant). (b) Comparison of the dose response curves of pSTAT5 expression in peripheral blood CD4+ and CD8+ T cells between old (n=7) vs. adult (n=4) RM after ex vivo stimulation with 0, 0.5, 1, 2, 4, 8, 16, 32, 64, 128 ng/mL of rsIL-7 or IL-15. Results (mean + SEM) are shown as change from baseline (Δ) and significance of difference was assessed as described in the materials and methods (NS, non-significant).
Clustered rsIL-7 dosing transiently increases T cell counts but does not sustain TN reconstitution in old RM
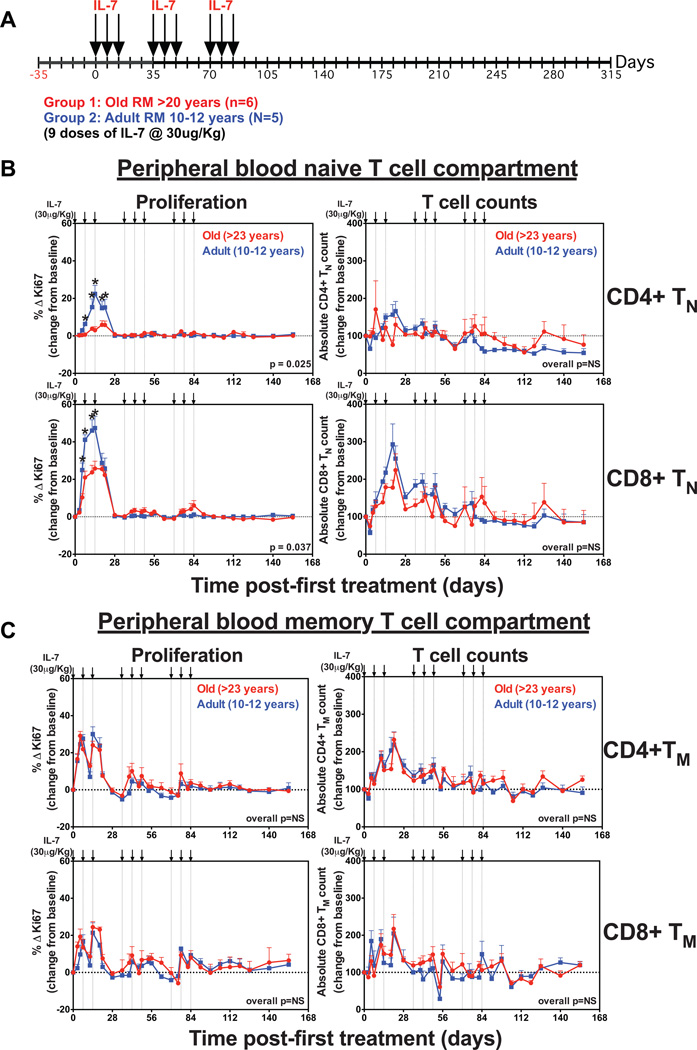
In a previous study, we reported that a clustered dosing regimen of 3 weekly doses of rsIL-7 at 30µg/kg followed by 2 weeks of no therapy and then 2 repeat cycles of therapy induced a rebound in the numbers of circulating TN in SIV-infected RM on antiretroviral therapy (ART) (31). This recovery occurred despite low levels of an IL-7-induced proliferative response and suggested that increased thymic output may have contributed to the rebound. Using this rsIL-7 dosing regimen, we compared the ability of rsIL-7 therapy to modify peripheral TN homeostasis in old (>20 years; n=6) vs. adult (10–12 years; n=5) RM (Fig. 4A). As shown in Fig. 4B, the first round of rsIL-7 induced a significant increase in the fraction of proliferating CD4+ and CD8+ TN, which was substantially higher in adult compared to old RM (baseline to peak increase [Δ %Ki-67+] for CD4+ and CD8+ TN = 22 vs. 6%, p = 0.025, and 47% vs, 25%, p = 0.037, respectively). Proliferation returned to pre-treatment levels in both groups by day 28, and subsequent administration of rsIL-7 resulted in little, if any, increase in the proliferating fraction of either CD4+ or CD8+ TN, similar to previous observations (31).
Figure 4. Clustered rsIL-7 dosing does not enhance TN reconstitution.
(a) A schematic representation of the clustered rsIL-7 dosing regimen in which 3 weekly s.c. injections (30µg/kg) followed by 2 weeks of no therapy and then 2 repeat cycles of therapy were administered (on days 0, 7, 14, 35, 42, 49, 70, 77 and 84) to 5 adult (10–12 years) and 6 old (>20 years) RM. (b) Analysis of the proliferative fraction and absolute counts of CD4+ TN and CD8+ TN, and (c) CD4+ TM and CD8+ TM in blood after clustered rsIL-7 treatment. Results (mean + SEM) are shown as percentage of Ki-67+, change (Δ) from baseline or absolute counts percentage of baseline. Significance of differences in these parameters between age groups was assessed as described in the materials and methods (*p < 0.05 at each time point).
Changes in absolute CD4+ or CD8+ TN counts in blood post-IL-7 therapy were more variable, but generally followed changes in proliferative fraction (Fig. 4B). After an acute drop, CD8+ TN counts in blood began to increase 7–12 days following the first rsIL-7 dose, with peak increases from baseline of ~3-fold in adult RM and ~2-fold in old RM noted 19–21 days following the initial dose, prior to falling back towards baseline. Subsequent rounds of IL-7 dosing had only a limited effect on CD8+ TN numbers, especially in old RM, and CD8+ TN numbers returned to baseline in all RM within 2 weeks of the last IL-7 dose. In adult RM, mean CD4+ TN numbers increased ~65% but returned to baseline midway through the second round of rsIL-7 dosing, and fell below baseline following the third round of dosing. In old RM, mean CD4+ TN numbers appear to fluctuate only modestly with rsIL-7 treatment, with no consistent increase. Taken together, these data demonstrate that the initial increase in bioavailable IL-7 afforded by rsIL-7 therapy induces an abrupt TN expansion in adult RM, particularly in the CD8+ lineage, but TN sensitivity to exogenous IL-7 falls off quickly and this expansion is not sustained despite continued rsIL-7 therapy. Importantly, the TN response was significantly blunted in old RM, suggesting that TN in aged animals are deficient in their ability to respond to IL-7 in vivo or that lymphoid tissues in old RM are defective in their ability to maintain viability of expanded TN populations. In this regard, we would note that both CD4+ and CD8+ memory T cells in these same animals, particularly the TCM and TTrM subsets, responded to rsIL-7 therapy with an abrupt increase in proliferative fraction and a 50–100% increase in the absolute numbers of these cells in blood (Fig. 4C; Supplementary Figure 1 and Supplementary Figure 2). Subsequent rounds of rsIL-7 dosing showed lower magnitude, but discernable, increases in TM proliferative fraction, and the expansion of TM numbers was more sustained than observed for TN (see below). Importantly, the response of TM to rsIL-7 was similar in adult vs. old RM, suggesting that the reduced response of TN to rsIL-7 therapy in old RM was due to specific age-related deficit in either TN themselves or TN-specific lymphoid microenvironment.
Intermittent IL-7 dosing induces sustained TCM expansion in adult and old RM
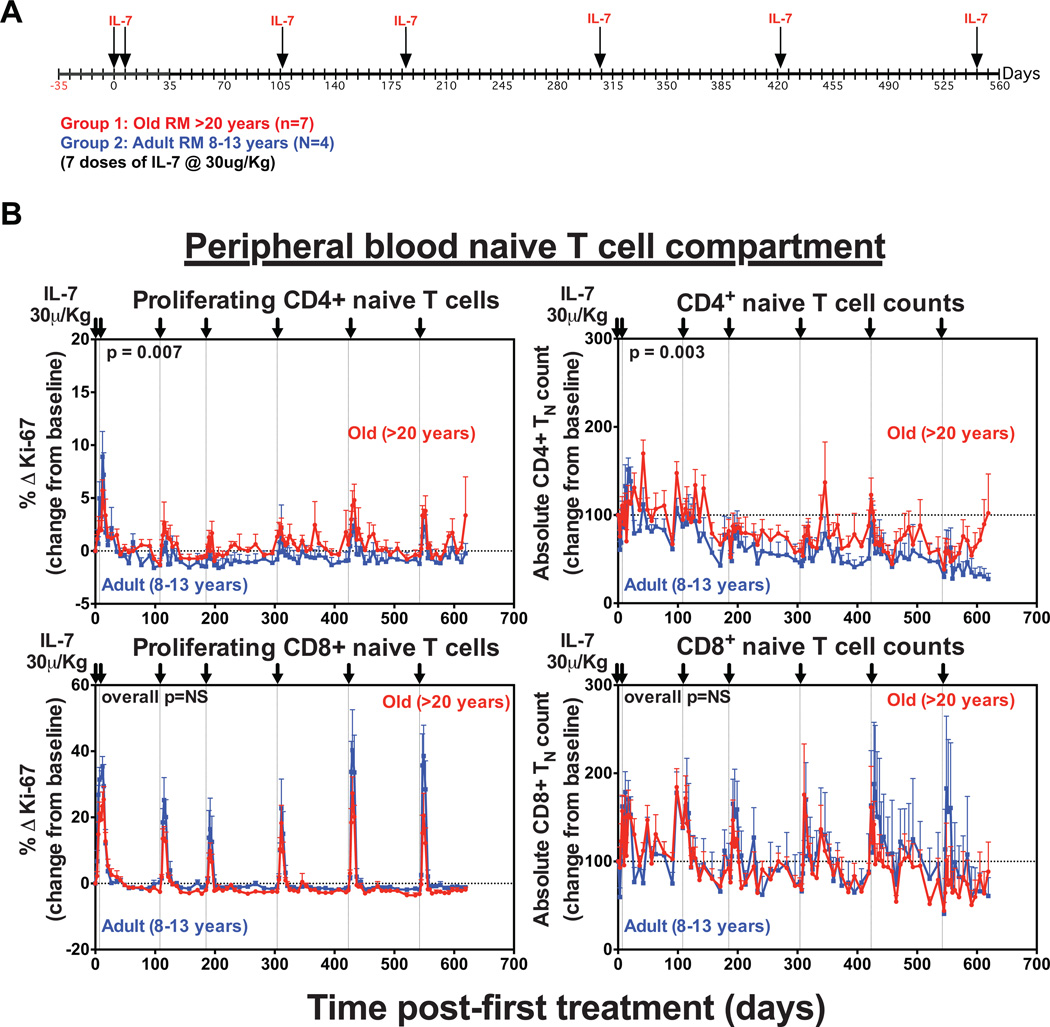
The short interval, clustered rsIL-7 dosing used in the initial trial of rsIL-7 therapy resulted in unambiguous tachyphylaxis, as the IL-7-mediated proliferative response in both the TN and TM subsets and the peripheral expansion in the TN population was markedly diminished or absent in the second and third rounds of rsIL-7 dosing. Indeed, the decline in CD4+ TN absolute counts below baseline after the third round of dosing in this experiment (Fig. 4B) raise the possibility that this rsIL-7 regimen reduced long-term sensitivity to endogenous IL-7. To determine whether reducing the frequency of rsIL-7 dosing would improve therapeutic outcome, we studied a second cohort of adult (10–12 years; n=4) vs. old (>20 years; n=7) RM that were given repeated single doses of rsIL-7 at longer dosing intervals over a period of 560 days (Fig. 5A). This change in dosing strategy also allowed more accurate analysis of the T cell response to each individual dose and how these responses were sustained over time. With longer rest periods between doses, rsIL-7 induced a transient baseline to peak increase in the proliferative fraction of circulating CD4+ and CD8+ TN in the blood after each dose, which were generally similar in the adult vs. old RM (Fig. 5B). This increase in Ki-67 expression was clearly apparent 5 days after the first rsIL-7 dose with levels peaking by day 7–10 before returning to baseline by day 14–17. Similar to previous observations, a higher fraction of CD8+ TN expressed Ki-67 (10–40%) than CD4+ TN (1–10%) in response to rsIL-7 (27). These proliferative responses were associated with transient increases in the absolute numbers of circulating CD4+ and CD8+ TN in the blood. Absolute CD8+ TN counts increased ~2-fold after each dose, but returned to pre-dose levels prior to administration of the next dose and were largely unchanged after the full course of therapy. Although CD4+ TN counts showed a similar pattern of transient post-dose expansion, the CD4+ TN baseline drifted down after the third rsIL-7 dose such that in most treated RM the absolute CD4+ TN counts were reduced at the end of the dosing regimen relative to pretreatment.
Figure 5. Long-term repeated single dosing with rsIL-7 has transient effects on CD4+ and CD8+ TN counts.
(a) A schematic representation of the dosing protocol in which 4 adult (8–13 years) and 7 old (>20 years) RM received 7 s.c. injections (30µg/kg) of rsIL-7 on days 0, 7, 108, 304, 423 and 542. (b) Analysis of the absolute counts and proliferative fraction of CD4+ and CD8+ TN in the blood after each dose of rsIL-7. Results (mean + SEM) are shown as percentage of Ki-67+, change (Δ) from baseline or absolute counts percentage of baseline. Significance of differences between age groups was assessed as described in the materials and methods (significant p values are shown; NS, non-significant).
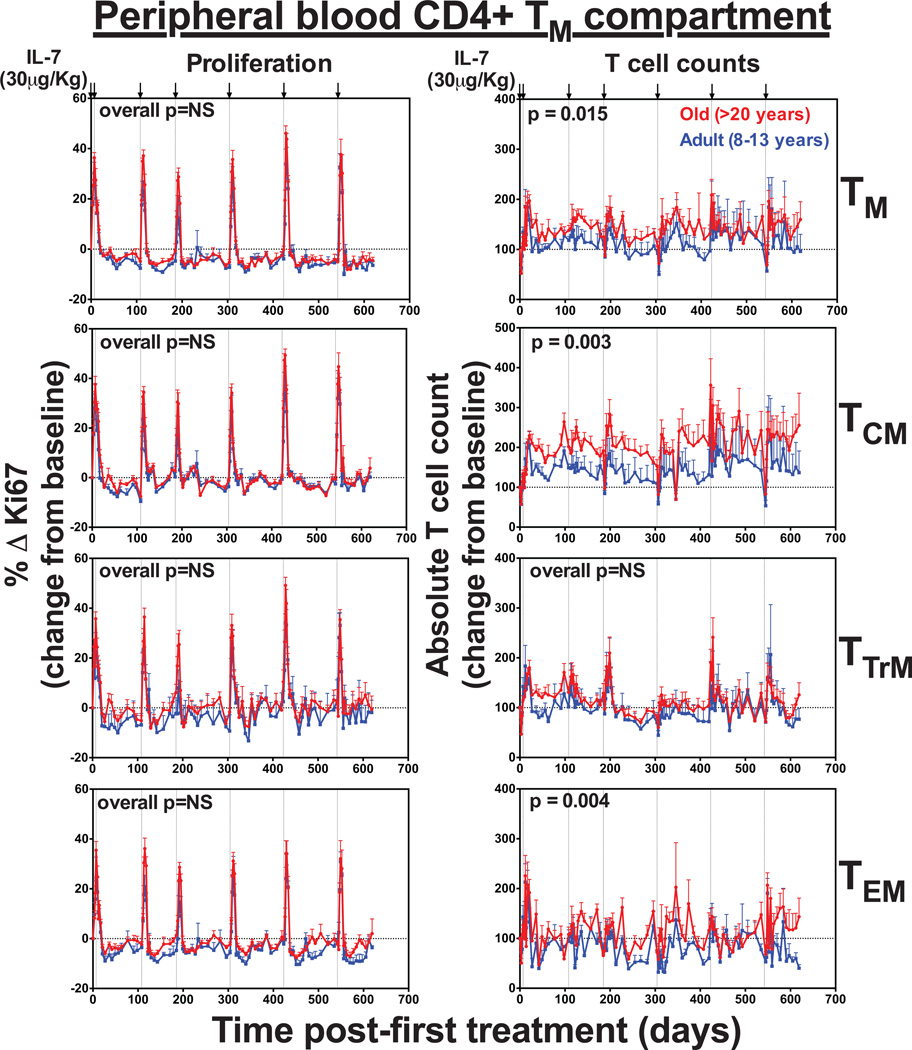
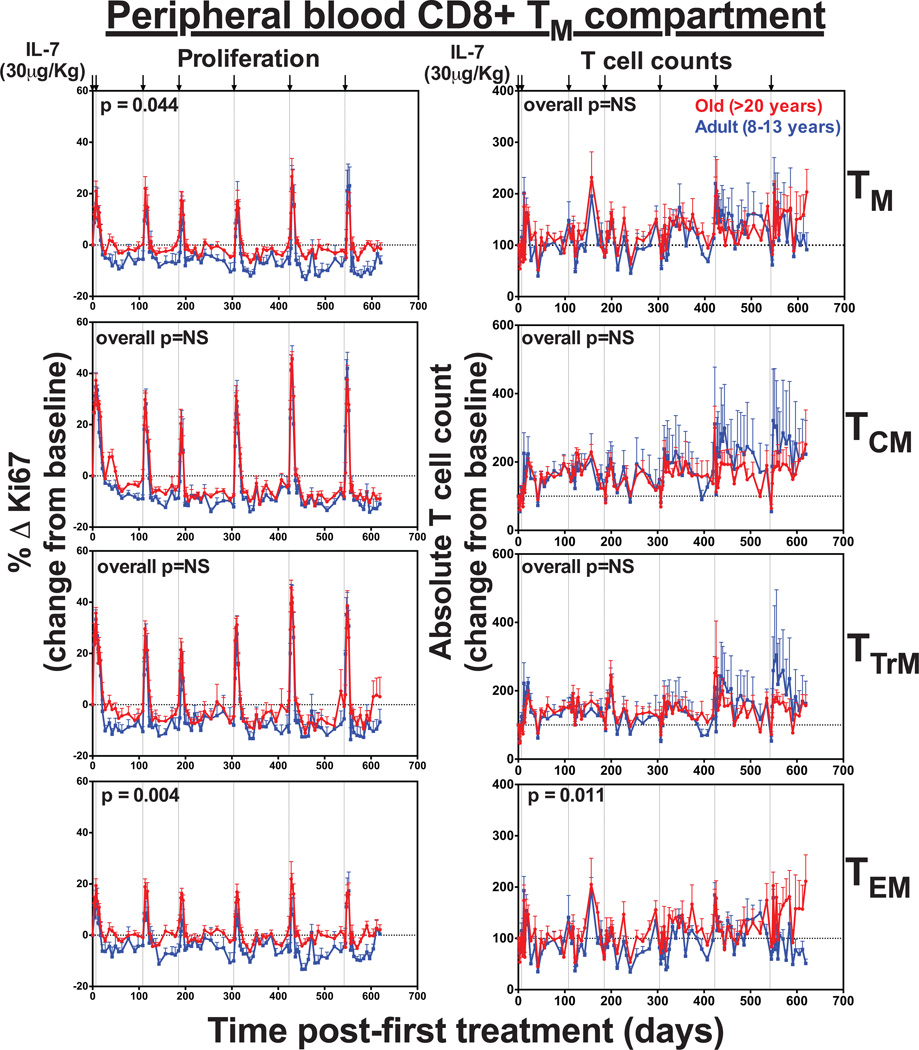
Each dose of rsIL-7 also induced a transient baseline to peak increase in the proliferative fractions of all CD4+ and CD8+ TM subsets (Fig. 6 and Fig. 7). Interestingly, the terminally differentiated TEM subset, which has low expression of IL-7R (CD127), showed robust proliferation in response to rsIL-7. However, it’s possible this does not reflect IL-7-mediated induction of proliferation in the TEM themselves, but rather, reflects the differentiation of IL-7-induced proliferating TCM and/or TTrM into the TEM pool (31). There was also a transient, 2–3-fold increase in absolute numbers of CD4+ and CD8+ TTrM and TEM in the blood after each dose. Strikingly, however, while the increase in TTrM and TEM counts were mainly transient, we observed a sustained increase in CD4+ and CD8+ TCM counts, which remained elevated with repeated therapy. CD8+ TCM expansion was similar between both adult and old animals, but CD4+ TCM expansion was significantly higher in the old RM (p=0.003). Overall, this data demonstrates that exogenous rsIL-7 administration does not improve TN reconstitution or long-term population stability in old RMs, but does have a sustained impact on TCM, both CD4+ and CD8+.
Figure 6. Long-term rsIL-7 dosing has a sustained effect on CD4+ TCM counts.
Figure shows the comparison of the absolute counts and proliferative fraction of CD4+ TM subsets in the blood. Results (mean + SEM) are shown as percentage of Ki-67+, change (Δ) from baseline or absolute counts percentage of baseline. Significance of differences in these parameters between age groups was assessed as described in the materials and methods (significant p values are shown; NS, non-significant).
Figure 7. The effect of rsIL-7 dosing on CD8+ TCM counts was similar between adult and old RM.
Figure shows the comparison of the absolute counts and proliferative fraction of CD8+ TM subsets in the blood. Results (mean + SEM) are shown as percentage of Ki-67+, change (Δ) from baseline or absolute counts percentage of baseline. Significance of differences in these parameters between age groups was assessed as described in the materials and methods (significant p values are shown; NS, non-significant).
TCR diversity is not adversely affected by repeated IL-7 administration in old TN and TM cells
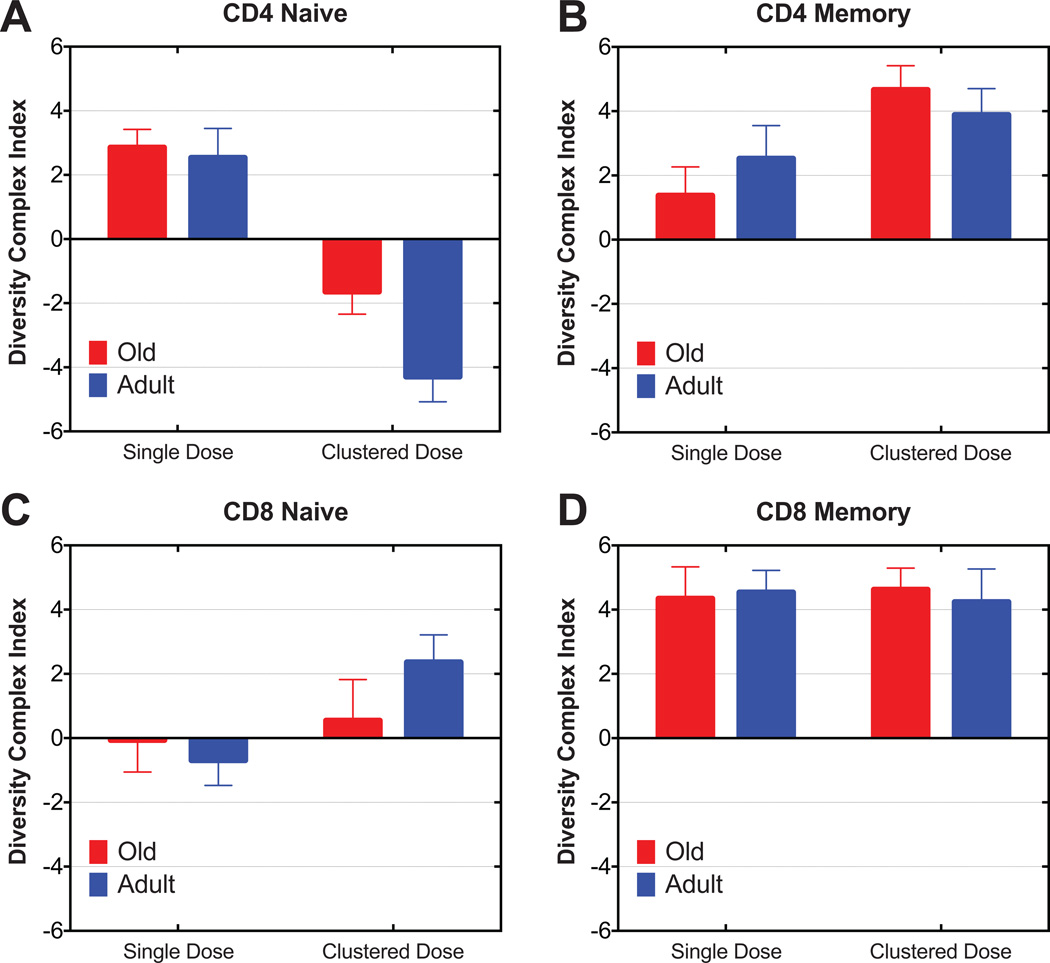
One concern with IL-7 cytokine therapy is that cytokine-driven homeostatic proliferation might expand only certain TN or TCM clones or could push only a limited number of TN clones into division and differentiation into TCM, which would have the effect of propagating only a few clones and thereby further reduce the diversity of the already eroded peripheral TCR repertoire in aged RM. To examine this possibility, we followed TCR diversity by TCRBV spectratyping of sorted peripheral TN and TM (total memory, CD95hi CD4+ or CD8+ T cells) before and on day 84 after cluster dose or day 91 after single dose rsIL-7 administration regimens, as previously described (7). Our data clearly showed that the composite TCRBV diversity index was not statistically reduced under either treatment condition in old animals (Fig. 8, A–D). In fact, this index showed a statistically significant improvement of CD8+ TM in both adult (single: p=0.007; clustered: p=0.014) and old (single: p=0.004 and clustered: p=0.001) RM under both treatments (Fig. 8D) and for CD4+ TM in both adult (p=0.008) and old (p=0.001) RM under cluster treatment (Fig. 8B). CD4+ TN under cluster, but not under single dose treatment, exhibited reduced TCRBV diversity, which was significant for the adult group (p=0.005) but not for the old group (Fig. 8A). In fact, CD4+ TN exhibited an improvement in TCRBV diversity in response to single dose rsIL-7 (p=0.002). CD8+ TN did not show significant effects of either treatment regimens, regardless of age (Fig. 8C). Overall, rsIL-7 treatment was compatible with improved TCR diversity particularly in CD4+ and CD8+ TM, which was similar in both old and adult RM. The impact on TN TCR diversity was less apparent. The improvement in CD4+ and CD8+ TM TCR diversity appeared to correspond with the highly proliferative effect of IL-7 on TM (Fig. 6 and Fig. 7), suggesting a symmetrical expansion of existing clones by IL-7 treatment, perhaps by also expanding clones that are present at lower copy number.
Figure 8. The comparison of the effect of rsIL-7 therapy on TCRBV repertoire diversity between adult and old RM.
CDR3 length polymorphism analysis (spectratyping) was conducted for FACS-sorted total naïve (CD95low) and memory (CD95hi) CD4+ and CD8+ T cells for 24 BV families. Diversity complex index (DCI) statistical analysis profile was created from sorted CD4+ naïve and memory (a, b) and CD8+ naïve and memory (c, d) T cells, respectively. Positive DCI value indicates potential improvement of spectratype profile, while negative DCI value indicates potential loss of spectratype profile diversity. DCI value is zero when two distributions are identical.
Treatment with rsIL-7 transiently increases TN and TM counts independent of thymus function
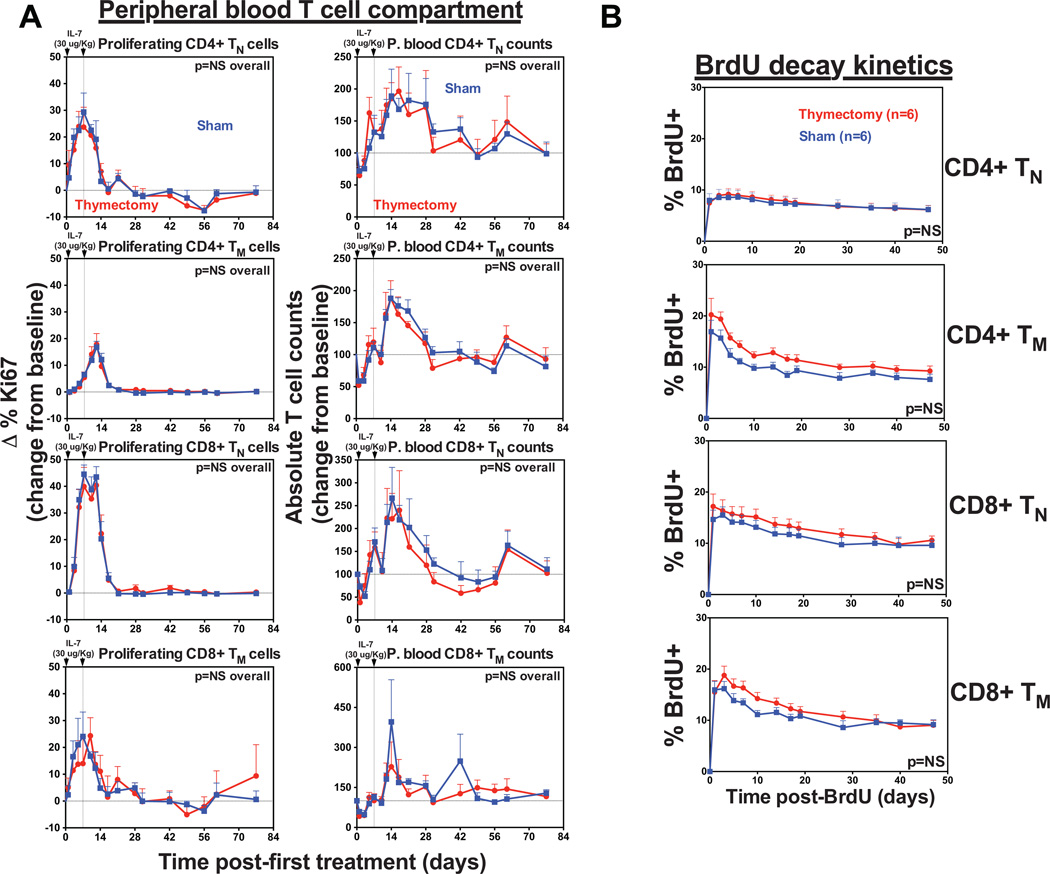
Finally, while murine models have shown that IL-7 therapy can increase thymopoiesis and T cell reconstitution after transplantation, and even restore thymopoiesis in IL-7-deficient mice, studies in humans and nonhuman primates (including our observations here) have failed to confirm whether thymic output contributes to peripheral T cell expansion after IL-7 treatment. To assess this we chose juvenile RM (1–4 years), as they possess fully functional thymi, thereby allowing optimal visualization of the thymic component of any effect after treatment. We subjected 12 juvenile RM to either surgical thymectomy (n=6) or sham surgery (n=6) prior to administering two doses of rsIL-7, one week apart and followed for changes in T cell dynamics in both groups. As shown in Fig. 9A, rsIL-7 induced a baseline to peak increase in the proliferative fraction of circulating CD4+ and CD8+ TN in the blood. This increase in proliferation peaked 7–12 days after the first dose before returning to pretreatment levels by day 21. The proliferative response induced on average a 2-fold increase in the absolute number of TN (both CD4+ and CD8+). Strikingly, the kinetics and magnitude of both the proliferative response and the change in absolute numbers of TN were statistically equivalent between athymic and euthymic RM. Moreover, determination of the turnover dynamics of CD4+ and CD8+ TN induced to proliferate after IL-7 administration by BrdU decay [(50,51); with BrdU administered 3–6 days after the last dose of rsIL-7, a time period that “captures” the peak of IL-7-induced T cell proliferation] revealed no differences between athymic and euthymic RM, similar to the turnover of thymic-independent TM populations in these animals (Fig. 9B). Taken together these data strongly suggest that the TN expansion in response to rsIL-7 occurs in the periphery in parallel with a similar TM response and is predominantly, if not exclusively, independent of thymic function.
Figure 9. IL-7-induced T cell expansion is independent of a thymus.
(a) Comparison of CD4+ and CD8+ TN and TM dynamics (absolute counts and proliferative fraction) in the blood of juvenile thymectomized (n=6) vs. sham (n=6)-treated RM after s.c. injections (30µg/kg) of rsIL-7 on days 0 and 7. Results (mean + SEM) are shown as percentage of Ki-67+, change (Δ) from baseline or absolute counts percentage of baseline. Significance of differences in these parameters between groups was assessed as described in the materials and methods (NS, non-significant) (b) Both groups of thymectomized and sham-treated RM were administered four daily i.v. doses (30 mg/kg) of BrdU beginning day 10 after the first rsIL-7 injection, and samples were obtained before BrdU administration (day 0) and at subsequent intervals for flow cytometric analysis of BrdU incorporation in CD4+ and CD8+ TN and TM. The first post-BrdU sampling was 24 h after the last BrdU dose and is designated day 1. The percentage of total BrdU+ cells was determined at each time point. Results (mean + SEM) are shown as percentage of BrdU+ change (Δ) from baseline. The significance of differences in BrdU decay kinetics between thymectomized and sham-treated RM was statistically analyzed as described in the materials and methods (NS, non-significant).
DISCUSSION
Aging-related immune dysregulation, also referred to as “immune senescence”, is associated with a dramatic decline in TN representation in the peripheral T cell pool (10,52–54). This loss in TN repertoire diversity is associated with an increased risk of infection, autoimmunity and cancer in old age. In this study we initially addressed the role the thymus plays in maintaining peripheral TN compartments in RM. Surgical thymectomy prior to CD8+ T cell depletion severely abrogated TN recovery in juvenile RM, resulting in profound TN deficiency, indicating that de novo CD8+ TN production is dependent on thymus output. We then examined the impact of age on thymic function by monitoring the rate of CD8+ TN reconstitution after total CD8+ T cell depletion. Interestingly, although CD8+ TN recovery was observed in all age groups, the rate of TN recovery was significantly lower in old RM, indicating that while thymic function can persist late in life, this function is considerably reduced. This result is consistent with data in humans showing residual thymocyte differentiation, active TCR gene rearrangement and measurable single joint TCR excision circles within the thymi of older adults (3,55). The presence of recent thymic emigrants as a marker of thymic output has also been reported in old mice (56). The question remains however as to whether this low-level thymic function can be therapeutically regenerated in old RM.
As IL-7 plays critical roles in both early thymocyte development and peripheral T cell homeostasis, we examined its potential as an immunotherapeutic for rejuvenating T cell compartments in old RM, which previously has mostly been assessed in murine models (57,58). Examining the impact of IL-7 treatment on TN population dynamics, we found that the increase in T cell proliferation, absolute counts and turnover after IL-7 treatment was similar between athymic and euthymic RM, suggesting IL-7 mainly stimulates thymus-independent T cell expansion even in juvenile RM with highly functional thymi. These observations are in contrast to studies in mice in which IL-7 has been shown to induce both thymic-dependent and thymic-independent T cell regeneration (59,60). However, while peripheral TN populations are predominantly maintained by thymic production throughout life in mice, proliferative expansion (and not thymic output) appears to play a more central role in maintaining TN homeostasis and TCR diversity in adult humans (61–63). Our data suggests that IL-7 may not improve thymic activity, but instead enhance the expansion of cells already in the peripheral T cell pool. While IL-7 levels remain stable over time and the intrinsic capacity of T cells to respond to IL-7 signaling in vitro does not appear to diminish with age (48,64,65), IL-7 may have an increasingly greater role in regulating TN homeostasis later in life. As TN numbers decline, the presence of excess IL-7 would induce the responding cells, predominantly TN and TCM, to fill the immunological space by mediating antigen-independent proliferative expansion. However, it is clear that this mechanism alone is insufficient to maintain TN population stability and TCR repertoire diversity long-term, and might have the detrimental effect of expanding persistent T cell clones.
In examining the therapeutic effects of exogenous IL-7 in old RM, we initially tested a short-interval clustered dosing regimen. This strategy was based on previous observations in SIV-infected RM in which absolute TN counts increased in response to IL-7 despite an attenuated proliferative response (31). Although this dosing regimen initially induced TN proliferation and resulted in a modest expansion of circulating TN populations in both old and adult RMs, this increase was transient (limited to the initial dose), and did not result in larger peripheral TN populations. In particular we saw no evidence of enhanced thymopoiesis or sustained TN reconstitution. It is also noteworthy that the response of TN in old RM to even the first dose of IL-7 was significantly reduced compared to adult RM, suggesting either reduced in vivo responsiveness of TN in old RM to IL-7 or to limitations in the lymphoid microenvironments supporting TN proliferation in old RM.
Although it is unclear why this dosing strategy had little impact on TN counts in this study of SIV-uninfected RM, it is possible that in the context of SIV infection, IL-7 countered infection-associated changes in TN survival or homing, rather than inducing an increase in thymic or peripheral TN production (66). With short-interval clustered dosing regimen, TN cells from SIV-uninfected RM rapidly lost sensitivity to therapeutic IL-7 administration, and given the long-term drop of CD4+ TN below baseline after cessation of rsIL-7 therapy, adult RM probably also lost sensitivity to endogenous IL-7. It is noteworthy that this long-term decline in TN numbers below baseline was less apparent in the old RM, likely reflecting the observations that the TN in old RM were considerably less responsive to the therapeutic IL-7 in the first place. This tachyphylaxis is similar to previous observations in RM treated therapeutically with IL-15 after short dosing intervals (32), and is thought to be secondary to feedback inhibition of the JAK/STAT signaling pathway through induction of members of the SOCS gene family (67–69). The binding of IL-7 has also been shown to down-regulate IL-7Rα by modulating its gene expression (69). A similar tachyphylaxis has recently been described with Interferon (IFN) treatment to RM during acute SIV infection. The expression of IFN-stimulated genes increased after the first dose of IFN-2α but decreased with repeated administrations resulting in no differences between treatment and placebo groups. Strikingly, it was shown that FOXO3a, a central regulator of IFN feedback was significantly upregulated after the first IFN dose, suggesting an intrinsic regulatory mechanism that leads to IFN desensitization (70). This would suggest that regulatory mechanisms exist that “turn off” or “attenuate” T cell responsiveness to cytokines when levels are in excess.
To determine whether the failure of clustered dose rsIL-7 therapy to affect a measureable expansion of peripheral TN populations was due to this multi-dose-associated induction of IL-7 response refractoriness, we treated a second cohort of RM with repeated single doses of IL-7 with longer intervals in between to allow sufficient time for T cells to regain IL-7 sensitivity. In this experiment, each dose of IL-7 induced both TN proliferation and proportional increases in TN absolute counts in blood. These responses were greater for CD8+ TN compared to CD4+ TN, in keeping with the higher proportion of IL-7-responsive cells in CD8+ TN vs. CD4+ TN, and in contrast to the more intense clustered dosing rsIL-7 regimen discussed above, were similar in magnitude in adult and old RM. However, the effect of therapy on TN populations was again transient with no apparent long-term benefit with repeated dosing. In fact, we saw a gradual decline in absolute CD4+ TN counts over time in both adult and old RM. As we did not maintain a cohort of untreated age-matched controls, we cannot rule out the possibility that this decline in TN counts was a consequence of reduced thymus output over time or to long-term changes in patterns of TN recirculation. It is also possible that rsIL-7 therapy reduced the size of TN populations by inducing the permanent phenotypic conversion of TN to TCM, although changes in TM population dynamics (abrupt and sustained increase in population size) do not correspond to the kinetics of TN population decline. Indeed, given the results of the cluster dosing experiment, it is possible that even with less frequent dosing of therapeutic IL-7, the abrupt exposure of TN to high levels of bioactive IL-7 induces refractoriness to endogenous IL-7 that results in loss of TN cells over time.
IL-7 therapy had a different effect on the TM compartment, in particular the highly IL-7 sensitive TCM population. Although similar to the TN response to IL-7, TM responded to the initial rsIL-7 dose with a dramatic increase in proliferative fraction and absolute counts, the loss of IL-7 sensitivity with subsequent clustered doses was less apparent than for TN, and the increase in absolute counts was sustained. This was true for both adult and old RM. Indeed, in the non-clustered rsIL-7 dosing regimen, both CD4+ and CD8+ TCM absolute counts in adult and old RM nearly doubled after the first rsIL-7 dose, and were maintained at this level for the duration of the experiment (with IL-7 administration every ~100 days). The numbers of circulating CD4+ and CD8+ TCM in both adult and old RM were also increased in the clustered dosing experiment, and only very slowly returned to baseline after the last rsIL-7 dose. These data indicate that IL-7 is a major regulator of TCM homeostasis in both adult and old RM, and suggest that levels of bioavailable IL-7 play a major role in determining the size of the recirculating TCM population. Importantly, in old RM, neither of the two dosage regimens had an adverse impact upon the TCRBV diversity as measured by spectratyping, and in total memory cells, the treatment actually increased diversity. This result is encouraging relative to the therapeutic use of IL-7, suggesting that the drug can be administered without fears of repertoire constriction.
In summary, this report documents the effects of IL-7 on peripheral T cell compartments in young adult and old RM. IL-7 showed no efficacy in stimulating thymopoiesis in old RM, and although peripheral TN responsiveness to IL-7 was largely preserved with age, rsIL-7 therapy had no long-term benefit in increasing TN population size in adult or old animals. In contrast, rsIL-7 therapy appeared to have salutary effects on TCM homeostasis, nearly doubling the size of this population in blood, and maintaining this enhanced population with only very infrequent repeat rsIL-7 dosing. Although IL-7 has shown great promise in increasing peripheral TCR repertoire diversity in clinical studies, more work is needed to fully define whether IL-7 therapy alone or in combination with other immune stimulatory factors can reverse some of the adverse consequences of immune senescence in old age.
Supplementary Material
ACKNOWLEDGMENTS
We thank Cytheris SA for providing rsIL-7, as well as Francois Villinger and the Resource for Nonhuman Primate Immune Reagents for providing rhesus IL-15. We also acknowledge the NIH Nonhuman Primate Reagent Resource supported by HHSN27220090037C and OD010976, from whom we obtained the CD8 T cell-depleting monoclonal antibody, cMT807. We thank J. Turner, P. Jewett, T. Swanson J. Dewane, and S. Planer for expert animal husbandry. We also thank E. McDonald, A. Kiddle, N. Hamilton, N. Currier, Q. DeGottardi, C. Pexton, I. Axthelm, S. Hagen, Y. Fukazawa, R. Lum, H. Park, A. Townsend and L. Boshears for technical or administrative assistance.
Grant Support: This work was supported by the US National Institutes of Health (including grants 5R01AI082529, 5R37AI054292, 5PO1AG023664, and 8P51OD01109255).
Abbreviations used in this article
- RM
rhesus macaques
- TN
naïve T cell
- TM
memory T cell
- TCM
central memory T cell
- TTrM
transitional memory T cell
- TEM
effector memory T cell
- pSTAT5
Phosphorylated STAT5
- rsIL-7
recombinant simian IL-7
- mAb
monoclonal antibody
Footnotes
Disclosures: All authors have no financial conflicts of interest.
REFERENCES
- 1.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin. Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berent-Maoz B, Montecino-Rodriguez E, Dorshkind K. Genetic regulation of thymocyte progenitor aging. Semin. Immunol. 2012;24:303–308. doi: 10.1016/j.smim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, Patel DD, Haynes BF. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J. Immunol. 2000;164:2180–2187. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- 4.Chen J. Senescence and functional failure in hematopoietic stem cells. Exp. Hematol. 2004;32:1025–1032. doi: 10.1016/j.exphem.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. The role of sex steroids and gonadectomy in the control of thymic involution. Cell. Immunol. 2008;252:122–138. doi: 10.1016/j.cellimm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc. Natl. Acad. Sc.i U. S. A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Lewinsohn DA, Nikolich-Zugich J. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J. Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicin-Sain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, Fischer MB, Koudelka CW, Axthelm MK, Nikolich-Zugich J, Picker LJ. Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J. Immunol. 2011;187:1722–1732. doi: 10.4049/jimmunol.1100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrando-Martinez S, Ruiz-Mateos E, Hernandez A, Gutierrez E, Rodriguez-Mendez Mdel M, Ordonez A, Leal M. Age-related deregulation of naive T cell homeostasis in elderly humans. Age. 2011;33:197–207. doi: 10.1007/s11357-010-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikolich-Zugich J. Non-human primate models of T-cell reconstitution. Semin. Immunol. 2007;19:310–317. doi: 10.1016/j.smim.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heng TS, Chidgey AP, Boyd RL. Getting back at nature: understanding thymic development and overcoming its atrophy. Curr. Opin. Pharmacol. 2010;10:425–433. doi: 10.1016/j.coph.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Hollander GA, Krenger W, Blazar BR. Emerging strategies to boost thymic function. Curr. Opin. Pharmacol. 2010;10:443–453. doi: 10.1016/j.coph.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ventevogel MS, Sempowski GD. Thymic rejuvenation and aging. Curr. Opin. Immunol. 2013;25:516–522. doi: 10.1016/j.coi.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chetoui N, Boisvert M, Gendron S, Aoudjit F. Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signalling pathway. Immunol. 2010;130:418–426. doi: 10.1111/j.1365-2567.2009.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaunders JJ, Levy Y, Seddiki N. Exploiting differential expression of the IL-7 receptor on memory T cells to modulate immune responses. Cytokine Growth Factor Rev. 2014;25:391–401. doi: 10.1016/j.cytogfr.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Andrew D, Aspinall R. Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp. Gerontol. 2002;37:455–463. doi: 10.1016/s0531-5565(01)00213-3. [DOI] [PubMed] [Google Scholar]
- 18.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 −/− mice. J. Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 19.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J. Immunol. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Capitini CM, Chisti AA, Mackall CL. Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. J. Intern. Med. 2009;266:141–153. doi: 10.1111/j.1365-2796.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ElKassar N, Gress RE. An overview of IL-7 biology and its use in immunotherapy. J. Immunotoxicol. 2010;7:1–7. doi: 10.3109/15476910903453296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stevenson Stetler-M, Morton KE, Mavroukakis SA, Morre M, Buffet R, Mackall CL, Gress RE. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sportes C, Babb RR, Krumlauf MC, Hakim FT, Steinberg SM, Chow CK, Brown MR, Fleisher TA, Noel P, Maric I, Stetler-Stevenson M, Engel J, Buffet R, Morre M, Amato RJ, Pecora A, Mackall CL, Gress RE. Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin. Cancer. Res. 2010;16:727–735. doi: 10.1158/1078-0432.CCR-09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, Fry TJ, Engels J, Buffet R, Morre M, Amato RJ, Venzon DJ, Korngold R, Pecora A, Gress RE, Mackall CL. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B, Rouzioux C, Avettand-Fenoel V, Croughs T, Beq S, Morre M, Poulin JF, Sekaly RP, Thiebaut R, Lederman MM. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 2012;55:291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aspinall R, Pido-Lopez J, Imami N, Henson SM, Ngom PT, Morre M, Niphuis H, Remarque E, Rosenwirth B, Heeney JL. Old rhesus macaques treated with interleukin-7 show increased TREC levels and respond well to influenza vaccination. Rejuvenation Res. 2007;10:5–17. doi: 10.1089/rej.2006.9098. [DOI] [PubMed] [Google Scholar]
- 30.Beq S, Rozlan S, Gautier D, Parker R, Mersseman V, Schilte C, Assouline B, Rance I, Lavedan P, Morre M, Cheynier R. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009;114:816–825. doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
- 31.Leone A, Rohankhedkar M, Okoye A, Legasse A, Axthelm MK, Villinger F, Piatak M, Jr, Lifson JD, Assouline B, Morre M, Picker LJ, Sodora DL. Increased CD4+ T cell levels during IL-7 administration of antiretroviral therapy-treated simian immunodeficiency virus-positive macaques are not dependent on strong proliferative responses. J. Immunol. 2010;185:1650–1659. doi: 10.4049/jimmunol.0902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picker LJ, Reed-Inderbitzin EF, Hagen SI, Edgar JB, Hansen SG, Legasse A, Planer S, Piatak M, Jr, Lifson JD, Maino VC, Axthelm MK, Villinger F. IL-15 induces CD4 effector memory T cell production and tissue emigration in nonhuman primates. J. Clin. Invest. 2006;116:1514–1524. doi: 10.1172/JCI27564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J, Franchini G, Mackall CL. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood. 2003;101:2294–2299. doi: 10.1182/blood-2002-07-2297. [DOI] [PubMed] [Google Scholar]
- 34.Moniuszko M, Fry T, Tsai WP, Morre M, Assouline B, Cortez P, Lewis MG, Cairns S, Mackall C, Franchini G. Recombinant interleukin-7 induces proliferation of naive macaque CD4+ and CD8+ T cells in vivo. J. Virol. 2004;78:9740–9749. doi: 10.1128/JVI.78.18.9740-9749.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nugeyre MT, Monceaux V, Beq S, Cumont MC, Ho Tsong Fang R, Chene L, Morre M, Barre-Sinoussi F, Hurtrel B, Israel N. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J. Immunol. 2003;171:4447–4453. doi: 10.4049/jimmunol.171.8.4447. [DOI] [PubMed] [Google Scholar]
- 36.Okoye A, Park H, Rohankhedkar M, Coyne-Johnson L, Lum R, Walker JM, Planer SL, Legasse AW, Sylwester AW, Piatak M, Jr, Lifson JD, Sodora DL, Villinger F, Axthelm MK, Schmitz JE, Picker LJ. Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J. Exp. Med. 2009;206:1575–1588. doi: 10.1084/jem.20090356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Picker LJ, Hagen SI, Lum R, Reed-Inderbitzin EF, Daly LM, Sylwester AW, Walker JM, Siess DC, Piatak M, Jr, Wang C, Allison DB, Maino VC, Lifson JD, Kodama T, Axthelm MK. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 2004;200:1299–1314. doi: 10.1084/jem.20041049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 39.Terstappen LW, Huang S, Picker LJ. Flow cytometric assessment of human T-cell differentiation in thymus and bone marrow. Blood. 1992;79:666–677. [PubMed] [Google Scholar]
- 40.Walker JM, Maecker HT, Maino VC, Picker LJ. Multicolor flow cytometric analysis in SIV-infected rhesus macaque. Methods Cell. Biol. 2004;75:535–557. doi: 10.1016/s0091-679x(04)75022-0. [DOI] [PubMed] [Google Scholar]
- 41.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol. Rev. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen ZW, Kou ZC, Shen L, Reimann KA, Letvin NL. Conserved T-cell receptor repertoire in simian immunodeficiency virus-infected rhesus monkeys. J. Immunol. 1993;151:2177–2187. [PubMed] [Google Scholar]
- 43.Park B, Samadder P, Okoye A, Mori M, Nikolich-Zugich J. JSM Proceedings, Biometrics Section. Alexandria, VA: American Statistical Association; 2014. Diversity Complexity Index (DCI) for Spectratype/Immunoscope Analysis of the Expressed TCR Repertoire; pp. 2814–2823. [Google Scholar]
- 44.Arron ST, Ribeiro RM, Gettie A, Bohm R, Blanchard J, Yu J, Perelson AS, Ho DD, Zhang L. Impact of thymectomy on the peripheral T cell pool in rhesus macaques before and after infection with simian immunodeficiency virus. Eur. J. Immunol. 2005;35:46–55. doi: 10.1002/eji.200424996. [DOI] [PubMed] [Google Scholar]
- 45.Engram JC, Cervasi B, Borghans JA, Klatt NR, Gordon SN, Chahroudi A, Else JG, Mittler RS, Sodora DL, de Boer RJ, Brenchley JM, Silvestri G, Paiardini M. Lineage-specific T-cell reconstitution following in vivo CD4+ and CD8+ lymphocyte depletion in nonhuman primates. Blood. 2010;116:748–758. doi: 10.1182/blood-2010-01-263814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okoye AA, Rohankhedkar M, Abana C, Pattenn A, Reyes M, Pexton C, Lum R, Sylwester A, Planer SL, Legasse A, Park BS, Piatak M, Jr, Lifson JD, Axthelm MK, Picker LJ. Naive T cells are dispensable for memory CD4+ T cell homeostasis in progressive simian immunodeficiency virus infection. J. Exp. Med. 2012;209:641–651. doi: 10.1084/jem.20112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okoye A, Meier-Schellersheim M, Brenchley JM, Hagen SI, Walker JM, Rohankhedkar M, Lum R, Edgar JB, Planer SL, Legasse A, Sylwester AW, Piatak M, Jr, Lifson JD, Maino VC, Sodora DL, Douek DC, Axthelm MK, Grossman Z, Picker LJ. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasi M, Troiano L, Lugli E, Pinti M, Ferraresi R, Monterastelli E, Mussi C, Salvioli G, Franceschi C, Cossarizza A. Thymic output and functionality of the IL-7/IL-7 receptor system in centenarians: implications for the neolymphogenesis at the limit of human life. Aging Cell. 2006;5:167–175. doi: 10.1111/j.1474-9726.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 49.Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur. J. Clin. Invest. 2005;35:380–387. doi: 10.1111/j.1365-2362.2005.01499.x. [DOI] [PubMed] [Google Scholar]
- 50.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vrisekoop N, den Braber I, de Boer AB, Ruiter AF, Ackermans MT, van der Crabben SN, Schrijver EH, Spierenburg G, Sauerwein HP, Hazenberg MD, de Boer RJ, Miedema F, Borghans JA, Tesselaar K. Sparse production but preferential incorporation of recently produced naive T cells in the human peripheral pool. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6115–6120. doi: 10.1073/pnas.0709713105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haberthur K, Engelman F, Barron A, Messaoudi I. Immune senescence in aged nonhuman primates. Exp. Gerontol. 2010;45:655–661. doi: 10.1016/j.exger.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moro-Garcia MA, Alonso-Arias R, Lopez-Larrea C C. When Aging Reaches CD4+ T-Cells: Phenotypic and Functional Changes. Front. Immunol. 2013;4:107. doi: 10.3389/fimmu.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid. Redox. Signal. 2011;14:1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 56.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrew D, Aspinall R. Il-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J. Immunol. 2001;166:1524–1530. doi: 10.4049/jimmunol.166.3.1524. [DOI] [PubMed] [Google Scholar]
- 58.Pido-Lopez J, Imami N, Andrew D, Aspinall R. Molecular quantitation of thymic output in mice and the effect of IL-7. Eur. J. Immunol. 2002;32:2827–2836. doi: 10.1002/1521-4141(2002010)32:10<2827::AID-IMMU2827>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 59.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001;97:1525–1533. doi: 10.1182/blood.v97.6.1525. [DOI] [PubMed] [Google Scholar]
- 60.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 61.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 62.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, Coutinho RA, Lange JM, Rinke de Wit TF, Tsegaye A, van Dongen JJ, Hamann D, de Boer RJ, Miedema F. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat. Med. 2000;6:1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 63.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 64.Marttila S, Jylhava J, Pesu M, Hamalainen S, Jylha M, Hervonen A, Hurme M. IL-7 concentration is increased in nonagenarians but is not associated with markers of T cell immunosenescence. Exp. Gerontol. 2011;46:1000–1002. doi: 10.1016/j.exger.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Sauce D, Larsen M, Fastenackels S, Roux A, Gorochov G, Katlama C, Sidi D, Sibony-Prat J, Appay V. Lymphopenia-driven homeostatic regulation of naive T cells in elderly and thymectomized young adults. J. Immunol. 2012;189:5541–5548. doi: 10.4049/jimmunol.1201235. [DOI] [PubMed] [Google Scholar]
- 66.Vassena L, Miao H, Cimbro R, Malnati MS, Cassina G, Proschan MA, Hirsch VM, Lafont BA, Morre M, Fauci AS, Lusso P. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS Pathog. 2012;8:e1002636. doi: 10.1371/journal.ppat.1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kimura MY, Pobezinsky LA, Guinter TI, Thomas J, Adams A, Park JH, Tai X, Singer A. IL-7 signaling must be intermittent, not continuous, during CD8(+) T cell homeostasis to promote cell survival instead of cell death. Nat. Immunol. 2013;14:143–151. doi: 10.1038/ni.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 69.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 70.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB, Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.