Abstract
The informed consent process for genetic testing does not commonly address preferences regarding disclosure of results in the event of the patient’s death. Adults being tested for familial colorectal cancer were asked whether they want their exome sequencing results disclosed to another person in the event of their death prior to receiving the results. Of 78 participants, 92% designated an individual and 8% declined to. Further research will help refine practices for informed consent.
Introduction
The incorporation of exome and genome sequencing into research and clinical practice raises the possibility of providing a range of genomic results to relatives in the event of the death of the research participant or patient.1 Genomic data can be of direct relevance to the medical care of relatives. However, some test subjects (e.g., cancer patients) are at higher risk of dying before they receive their test results and thus may not be able to share useful information with family members. We created an Institutional Review Board (IRB)-approved document with talking points on the possibility of disclosure of results to family members after an individual’s death to discuss during the informed consent process for genomic testing with participants in a study of exome sequencing in the context of familial colorectal cancer/polyposis.
There is general agreement that patients having a clinical exome or genome sequencing test should have the option of receiving clinically important, medically actionable findings that are unrelated to their diagnosis in addition to their diagnostic findings.2 Providers customarily recommend that patients disclose these findings to their relatives who are at risk for sharing the same genetic variants; however, disclosure is generally left to the discretion of the patient. If the patient elects not to share results, clinicians and researchers generally do not overrule this decision. The dissemination of research results to the family members of research participants may also be encouraged. However, death or loss of capacity of the patient or participant prior to receiving these results, including results that may be generated based on new knowledge at a future time, may prevent test subjects from sharing results with relatives. A plan for disclosure to an individual designated by the patient or participant prior to their death can facilitate return of relevant genomic information to surviving family members.3 A passive approach of researchers and/or clinicians disclosing results only to relatives who seek out this information has been advocated by some4; however, others support active and direct disclosure,5 and even an ethical “duty to warn” if a finding implies a high risk for the development of a severe medically actionable disease in a relative.6 While there is not a professional consensus on this point, it has been proposed that health care professionals have, at a minimum, a responsibility to encourage patients to share genetic information with family members who may also have an inherited risk.7 Such encouragement of sharing is part of usual care in the medical genetics clinical setting. However, in both clinical and research settings, disclosure of results after a patient or participant’s death has received little attention to date.8
This article reports empirical data concerning the decisions of adult participants in cancer-related genomic medicine research regarding disclosure of their exome sequencing results to family members after the participant’s death. We present our approach to discussing the potential of return of results in the event of death, report preliminary trends in participant choices, and discuss related legal and ethical considerations.
Methods
A. Participants
Adult patients being evaluated for hereditary colorectal cancer and/or polyps at either of two Seattle-area participating genetic medicine clinics are offered enrollment into the University of Washington, New Exome Technology in (NEXT) Medicine study. The NEXT Medicine study is a National Human Genome Research Institute (NHGRI) and National Cancer Institute (NCI) funded randomized control trial that began enrollment in early 2012. The project is part of the Clinical Sequencing Exploratory Research (CSER) consortium,9 which is investigating the incorporation of sequencing technology into clinical medicine. NEXT Medicine participants are randomized to receive standard clinical genetic testing or exome sequencing in addition to clinical genetic testing. Exome sequencing takes place in a clinical laboratory with Clinical Laboratory Improvement Amendments (CLIA) certification. The results of exome sequencing are entered into the electronic health records of participants as portable document format (PDF) reports. All enrolled study participants are expected to survive for the duration of their enrollment in the trial (approximately 12–14 months) and have indicated interest in receiving medically actionable incidental findings in addition to diagnostic genetic results.
B. Consent
Either a genetic counselor or a research coordinator conducts informed consent conversations with patients for enrollment in the trial. In planning the study, we realized that a participant might die before receiving exome results, precluding their sharing of research findings with family members. Thus, we sought approval from the University of Washington IRB to ask about participants’ wishes regarding disclosure of genetic information to another person in the event of their death, after they consented to enrollment in the study. The content of this discussion is based on the points included in a supplemental written consent document which describes the protocol (Supplemental Figure 1). That consent document was developed based on medical power of attorney and record release documents identified by an Internet search of the terms “Power of Attorney, Washington.” The format was loosely based on the University of Washington Medical Center authorization form for disclosure or release of protected health information. The document states, “Genetic results information is important for my blood relatives to learn as we share similar genetic material.” However, the document does not require that the participant’s designee be a blood relative or other family member. Authorization for disclosure is valid until a date decided by the participant or until the end of the NEXT Medicine study. Participants are advised that results disclosed may include those generated by both clinical and research testing, and that the form will be destroyed when all study-related genetic results from the NEXT Medicine study have been provided to that participant. The designated individual’s name and contact information (mailing address, phone number and/or email address), as well as their relationship to the research participant, are recorded. Participants are advised that we would contact their designee using the contact information they provide, and are not obliged to share more than one form of contact information. We chose to request only a single designee for logistical reasons. Participants who decline to authorize return of genetic results to another individual in the event of their death are still eligible to participate in the trial.
Results
To date, 78 participants (33 female and 45 male) enrolled in the study have been asked about their preference for disclosure of genetic information in the event of their death. Forty of these 78 participants (51%) were then randomized to exome sequencing and 38 (49%) to usual care. The mean age of these 78 participants was 52 years old (range 19–80 years) at the time of enrollment, the majority self-reported their ethnicity as Caucasian (80%). The remaining participants self-reported their ethnicity as Asian (9%), Pacific Islander (1%), or mixed (10%). Eighty-six percent had a post-high-school education, and most had a spouse (including partners; 70.5%). All participants had a clinical colorectal cancer genetic test ordered due to a personal and/or family history of colorectal cancer and/or polyps. Current clinical testing for study participants typically includes ordering either colorectal tumor tissue testing to inform targeted gene sequencing or one of several gene sequencing panels that test for pathogenic variants in genes associated with a risk for colorectal cancer and/or polyps. Of these 78 participants, 28 had a diagnosis of colorectal cancer with or without colon polyps, 42 had colon polyps and not colorectal cancer, and 8 had a suggestive family history without a personal history of colorectal cancer or polyps.
A. Participant choices
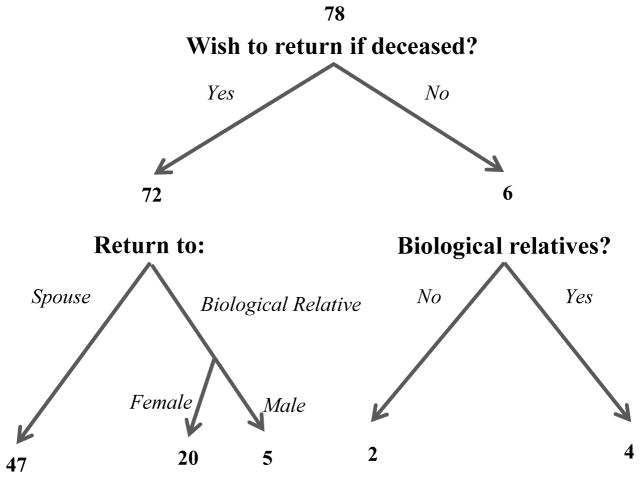
Of these 78 participants, 72 (92%) chose to designate an individual to receive their genomic results in the case of their death (Figure 1). The decisions of male and female participants are presented in Table 1. While many of the participants made their decision without noticeable hesitation, a few participants deliberated about whether to make a designation and whom to designate. In the latter scenario, some participants indicated they were considering whether they should designate an individual who would be at risk to share their genetic findings, as well as whether the designated individual would likely disseminate results to relevant family members who are also at risk.
Figure 1.
Participant decisions on return of genetic information after participant death.
Table 1.
Participant designees for return of genetic information after participant death, by participant sex.
| Participant sex | Return to spouse N (%) |
Return to male biological relative N (%) |
Return to female biological relative N (%) |
Declined to state a designee | Total N |
|---|---|---|---|---|---|
| Female | 17 (52%) | 2 (6%) | 9 (27%) | 5 (15%) | 33 |
| Male | 30 (67%) | 3 (7%) | 11 (24%) | 1 (2%) | 45 |
| Total | 47 (60%) | 5 (6%) | 20 (16%) | 6 (8%) | 78 |
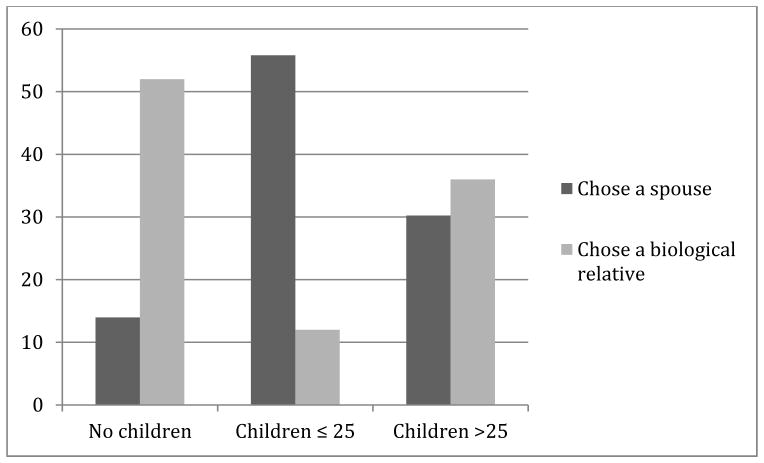
Of those selecting a designee, 47/72 (65%) opted for results to be returned to a spouse and 25 (35%) designated a blood relative. No one designated anyone outside of their family. Family history information was available for 39 of the 43 participants who designated their spouse. Of these 43 participants, 24 had children aged 25 years or less, 6 had no children, and 13 had at least one child over the age of 25 years. Of the 25 participants designating a blood relative, 3 had children aged 25 years or less, 13 had no children, and 9 had at least one child over the age of 25 years. The data suggest that participants with young children preferentially choose their spouse (Fisher’s exact p-value = 0.00025). The ages of the children of participants who designated a spouse or a blood relative are presented in Figure 2.
Figure 2.
Percent of participants who chose a spouse or chose a biological relative, comparing participants without children, with children ≤25years old, and with children >25 years old.
B. Participants who selected a blood relative
Of the 25 participants who designated a blood relative, 9 were currently married or living with a partner, 8 were never married, and 8 were divorced or widowed. More of those blood relatives designated were female than is predicted by chance (20/25 or 80% two-tailed binomial test p=0.002); 2 designated their mother, 0 their father, 10 their sister, 2 their brother, 6 their daughter, 3 their son, 1 their aunt, and 1 their grandmother. All 9 participants (6 female, 3 male) who were married or living with a partner but selected relatives and not their spouse, selected female relatives. Two of the 5 participants who designated male relatives also had a female first-degree relative. One of the 3 participants who designated a son also had a daughter, and 1 of the 2 participants who designated a brother also had a sister.
C. Participants who declined
Of the 6 participants who declined to authorize return of results to a specific individual, 1 had no known biological relatives (as the person was an adoptee) and 1 had no relationship with living biological family members. Neither of these participants had a spouse. The remaining 4 participants (5%) had a relationship with biological relatives but chose not to have the results shared. Two of these participants had a spouse. One participant did not feel comfortable making the decision to share unknown results and wanted to make decisions about sharing results after seeing them. Another participant felt that his results were private information and did not want to burden another individual with them. Two participants cited not having biological children as their rationale for declining, and one of these participants did not want results disclosed to her siblings.
Discussion
Genetic information differs from other health information by providing specific risk information that may indicate the need for genetic testing or clinical evaluation of biological relatives. While an individual’s diagnosis of hypertension, for example, might indicate the need for blood pressure screening in a relative, genetic test results in an affected family member provide more specific indication of risk and are essential to determine the most cost-effective and informative testing strategy in an unaffected relative. In particular, a negative result in an unaffected family member cannot be conclusively interpreted unless that individual has been tested for a known pathogenic variant found in an affected relative. If an unaffected relative tests negative for a known familial pathogenic variant, he or she would have the general population risk for the disease, rather than the higher risk associated with the familial variant. Without knowledge of the genetic basis for disease in the affected relative, it would be impossible to determine whether an unaffected relative with a negative test result would be at increased risk. Additionally, knowledge of a specific pathogenic variant in an affected individual allows direct testing for that particular variant in the family, which is faster and much less costly than sequencing one or more related genes.
In the context of participants in a cancer genetics study, who are also patients having a clinical genetic test or that clinical test plus research exome sequencing, we found that the vast majority of participants with a spouse or who had contact with biological relatives (74/78, 95%) chose to designate an individual to receive results in the case of their death prior to receiving the results. The decisions made by female and male participants were almost identical. While thus far only 4 (5%) participants with a spouse and/or in touch with biological relatives declined to designate a recipient, it is important to note that this is a choice some participants made. In a larger population, 5% would constitute a significant number of individuals.
Most participants choosing a designee selected their spouse (65%) and only 8/55 participants (14.5%) with a spouse selected a biological relative instead; spouses were thus preferred over biological relatives by most married or partnered participants. This suggests that the spouses, while generally sharing no genetic relationship, were trusted to share the information appropriately with those at risk. This designation is different than the recommendation by geneticists to discuss results with relatives who are at risk to share the same genetic findings. Most participants (86%) who selected a spouse had children and generally these children were aged 25 or younger; participants may have felt that these children were too young to be burdened with the designation to receive the genetic results directly. Approximately half of those designating a blood relative did not have children and only 12% had children less than 25 years old. Thus, participants with young children were more likely to select their spouse, suggesting that they prioritized the careful dissemination of risk information to their children when making this decision. Both male and female participants disproportionately selected female family members when a spouse was not the designee. This likely reflects the previously documented role of women as more often being the keepers and sharers of family medical information,10 including information related to genetic disorders11 and specifically familial colorectal cancer.12
Limitations of this work include the small sample size and that the participants were all adults from a specific patient population (having clinical genetic testing for hereditary colorectal cancer and/or polyps). The majority of participants was also of Caucasian ancestry and had post-secondary education.
Two questions surround our choice of a medical power of attorney as the vehicle for designating a representative to receive genetic test results following a research participant’s incapacity or death. The first question is whether this level of documentation is necessary: do other legal mechanisms already provide adequate access to genetic test results by family members? The second question is whether this approach is effective: will a medical power of attorney, signed while the participant is alive, effectuate the participant’s wishes to share genetic test results with a designated family member after death? We examined these questions under the assumption that the research institution is a HIPAA-covered entity, to which the HIPAA Privacy Rule applies.
As amended in 2013,13 the HIPAA Privacy Rule treats an individual’s health data as protected health information (PHI) for a period of 50 years after death, and genetic information is expressly recognized as being health information.14 The Privacy Rule recognizes that under applicable law (usually, state law), more than one person may have been appointed to perform various functions on behalf of a deceased person or the deceased person’s estate, and not all of these appointed roles warrant access to the decedent’s PHI. The Privacy Rule’s section 164.502(g)(4) requires HIPAA-covered entities to treat a person as a “personal representative” of the deceased person only “with respect to PHI relevant to such personal representation.”15 After the 2013 amendments, persons who qualify as a personal representative “continue to have a right to access the decedent’s protected health information relevant to such personal representation, and have authority to authorize uses and disclosures of the decedent’s protected health information that are not otherwise permitted or required by the Privacy Rule.”16 The amendments recognized, however, that:
family members, relatives, and others, many of whom may have had access to the health information of the deceased individual prior to death, have had difficulty obtaining access to such information after the death of the individual, because many do not qualify as a “personal representative” of the decedent under the Privacy Rule at § 164.502(g)(4).17
Accordingly, the amendments created a new access pathway under section 164.510(b)(5), which permits covered entities “to disclose a decedent’s information to family members and others who were involved in the care or payment for care of the decedent prior to death, unless doing so is inconsistent with any prior expressed preference of the individual that is known to the covered entity.” 18
If an individual dies without having designated a personal representative with broad powers to access all of his or her medical information after death, most states’ laws grant broad powers to a default personal representative—often, an executor or administrator or close relative of the deceased. The default provisions of state law often are adequate to ensure access to genetic test results by biologically related family members. This is not always the case, however. For example, the default personal representative under state law might be the surviving spouse, who may be estranged from the deceased’s children from a previous marriage, who are biological relatives of the deceased and have a strong interest in the test results. In genomic research, with its potential to generate medically significant diagnostic results and incidental findings, it is prudent to give participants—who know their family dynamics better than the state does—an opportunity to designate who should receive that genomic information. Our empirical results support the notion that one-size-fits-all default provisions of state law may not meet the needs of all research participants: while most participants with a spouse chose the spouse, 14.5% did not do so. This suggests that research participants may value the opportunity to tailor plans for return of their results after death.
A second question concerns our choice of medical powers of attorney for this purpose. Under general principles of agency law, powers of attorney are extinguished upon death of the principal (the person who granted the power of attorney) unless special circumstances apply.19 The Department of Health and Human Services (HHS) does not automatically recognize persons who held medical powers of attorney during a deceased person’s lifetime as personal representatives for purposes of accessing information after death.20 Whether such people have continued access to data after death would be a question of state law. Family members granted a power of attorney to receive genomic results thus may or may not qualify as a personal representative for purposes of disclosures after death after under section 164.502(g)(4). Such people can, however, use the medical power of attorney to demonstrate that they were involved in the participant’s healthcare prior to death, thus allowing access under the Privacy Rule’s new section 164.510(b)(5) access pathway created in 2013. We note, however, that access under section 164.510(b)(5) is subject to certain limitations: First, family members only can gain access to data relevant to the role they were playing in the deceased person’s healthcare while the deceased was alive (e.g., if they only were involved with decisions about cancer care, their scope of access would be limited to data relevant to that). Second, covered entities are permitted, but not required, to disclose results to the family members. Finally, access may be subject to confidentiality restrictions that the participant requested prior to death (such as excluding return to a specific family member).21 Despite these limitations, this pathway has an offsetting advantage: It avoids any interference with the power of the personal representative empowered, under other applicable law, to access all of the decedent’s medical information. HHS has emphasized that the new section164.510(b)(5) pathway of access for family members “would not change the authority of a decedent’s personal representative.”22 Also, the use of medical powers of attorney offers a fairly strong way to ensure focused access to incidental findings by the participant’s designee, without opening up broad access to the whole of the decedent’s medical record by the designee. In devising an approach to eliciting participant preferences on return of results after death or loss of capacity, investigators should consider federal, state, and relevant case law applicable to their own research sites.23
In difficult situations where a participant has asked that genetic results not be disclosed to a family member, the Privacy Rule provides an additional pathway for communicating medically important information to the surviving relatives.24 The HIPAA Privacy Rule allows disclosure of health information without an individual’s authorization if the disclosure is for “treatment” purposes, and this can include treatment of a third party, such as a relative. The Privacy Rule “does permit a doctor to disclose protected health information about a patient to another health care provider for the purpose of treating another patient (e.g., to assist the other health care provider with treating a family member of the doctor’s patient).”25 This creates a pathway that allows the information to be disclosed to the family member’s health care providers, who can then use it in deciding appropriate follow-up care.
Although we collected these data in the context of a research study, all participants were clinic patients who had a genetic test as part of their clinical care. Our long-term goal is to identify the best and most useful processes for managing clinical genomic information. In a clinical context, a family member who requests the deceased patient’s medical results may not realize there could be genetic information present that is also relevant to their own health. While a provider aware of such relevant information could in theory initiate the offer of disclosure to family members after the death of a patient, in practice there is not a process for this within usual medical care. Our study was carried out in a HIPAA-covered research institution, and we note that options for sharing a decedent’s test results with family members may be even more limited in non-HIPAA-covered research setting. In non-HIPAA research settings, IRBs may seek to prevent researchers from sharing these data, even if they so desire. Thus, documenting a research participant’s desire to share genetic information is particularly important. The discussion with a patient or participant should also allow them to identify with whom they prefer the information to be shared. The patient or participant is best positioned to know which designee is optimal to ensure the dissemination of results to at-risk family members. Although our data are limited, the variation in the relative designated by the participant and the apparent preference for a female relative suggest that the person designated may not always be the individuals to whom investigators or clinicians might turn without participant guidance, the legal next of kin or executor.
Eliciting preferences for return of results in the event of a patient or participant’s death during the informed consent process for genomic sequencing tests can generate ethical concerns, including concern over imposing genetic information on a designee who may be a relative at risk of sharing the variant. The designee or other persons they share information with are thus potentially learning of a personal genetic risk, just as they would have if the patient had shared the results. While a relative learning of a genetic risk has the autonomy to decide whether to pursue clinical follow-up after receiving such information, they cannot unlearn the information that they may be at increased risk. This information may be unexpected and unwanted.26 Challenges also arise if the designee who receives the results refuses to pass on information to at-risk family members.
A separate issue is whether it is ethically permissible to give research participants the option to prohibit researchers from sharing potentially valuable genomic findings with their family members after their death. We allow this and, at HIPAA-covered institutions, individuals have a right to request privacy protections that may include restrictions on disclosures to family members.27 However, some observers may object to this option as it withholds potentially important medical information, while others may uphold individual choice based on personal privacy rights. As noted above, when HIPAA applies (that is, when research is conducted at an institution that is a HIPAA-covered entity), confidentiality restrictions requested by a research participant may be overcome if the information is needed for purposes of treating the family member.28 However, the responsibility of health care providers to reach out to family members who do not seek this information is debatable.29 Under our model, we would contact the designated relative, rather than waiting to be asked by any family member.
Conclusion
Addressing consent for sharing genomic information after death, and with whom the research participant wants information shared, should be considered part of the consent process for a genomic test. The vast majority of participants in our study elected to designate an individual for return, but participant preferences varied, highlighting the importance of asking this question prior to the initiation of genomic sequencing tests. This is especially important in projects addressing cancer and other life-limiting conditions; allowing participants to express their preferences about the future dissemination of their genomic information after death is an important dimension of respect for autonomy and privacy. Similar considerations militate in favor of eliciting these preferences from patients in clinical genomic testing. Further research with different study populations and in a clinical setting will help refine practices for addressing this topic with both patients and research participants being consented for genomic sequencing tests.
Supplementary Material
Acknowledgments
This work was funded by the National Institutes of Health (NIH), including the National Human Genome Research Institute (NHGRI) and the National Cancer Institute (NCI) Clinical Sequencing Exploratory Research (CSER) Consortium, U01HG006507 and U01HG007307 (Jarvik, PI). Additional support came from NHGRI U01HG006375 (Jarvik, PI) and NHGRI P50 HG003374 (Burke, PI). Editorial review of this article was supported by NIH/NCI/NHGRI R01CA154517 (Petersen, Koenig, Wolf, PIs). All views expressed are those of the authors and do not necessarily reflect the views of NIH, NCI, or NHGRI.
Biographies
Laura M. Amendola, M.S., is a clinical and research genetic counselor in the University of Washington, Division of Medical Genetics. Ms. Amendola received her MS in Genetic Counseling in Houston, Texas at the University of Texas, Graduate School of Biomedical Sciences.
Martha Horike-Pyne, M.P.H., C.I.P., is a Research Coordinator in the Division of Medical Genetics at the University of Washington, Seattle. She received her M.P.H. at the University of Massachusetts, Amherst, and is scientist board member for two Institutional Review Boards (IRBs) in Seattle.
Susan B. Trinidad, M.A., is a research scientist in the Department of Bioethics and Humanities at the University of Washington. She holds a master’s degree from the Interdisciplinary Program in Health and Humanities at Michigan State University.
Stephanie M. Fullerton, D.Phil., is an Associate Professor of Bioethics and Humanities at the University of Washington and Adjunct Associate Professor of Epidemiology and Genome Sciences. She completed a Ph.D. in Human Population Genetics at the University of Oxford, U.K., and a postdoctoral fellowship in the Ethical, Legal, and Social Implications (ELSI) of genetic research at the Pennsylvania State University.
Barbara J. Evans, Ph.D., J.D., is Professor of Law and holds the George Butler Research professorship at University of Houston Law Center, where she is Director of the Center for Biotechnology & Law. She earned her Ph.D. at Stanford University and J.D. at Yale Law School.
Wylie Burke, M.D., Ph.D., is a Professor of Bioethics and Humanities at the University of Washington and Adjunct Professor of Medicine (Medical Genetics). She completed a Ph.D. in Genetics and an M.D. at the University of Washington where she also trained in Internal Medicine.
Gail P. Jarvik, M.D., Ph.D., is the Head of the Division of Medical Genetics and holds the Arno G. Motulsky Chair of Medicine at the University of Washington, Seattle, where she is Professor of Medicine (Medical Genetics) and Genome Sciences. She received her Ph.D. in Human Genetics at the University of Michigan and her M.D. at the University of Iowa in the Medical Scientists Training Program. She completed her residency in Internal Medicine at the Hospital of the University of Pennsylvania and her fellowship in Medical Genetics at the University of Washington.
References
- 1.Chan B, et al. Genomic Inheritances: Disclosing Individual Research Results from Whole-Exome Sequencing to Deceased Participants’ Relatives. AJOB. 2012;12(10):1–8. doi: 10.1080/15265161.2012.699138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf SM. Return of Individual Research Results and Incidental Findings: Facing the Challenges of Translational Science. Annual Review of Genomics and Human Genetics. 2013;14:557–577. doi: 10.1146/annurev-genom-091212-153506. [DOI] [PMC free article] [PubMed] [Google Scholar]; Green RC, et al. ACMG Recommendations for Reporting of Incidental Findings in Clinical Exome and Genome Sequencing. Genetics in Medicine. 2013;15(7):565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]; American College of Medical Genetics. ACMG Updates Recommendation on “Opt Out” for Genome Sequencing Return of Results. 2014 available at < https://www.acmg.net/docs/Release_ACMGUpdatesRecommendations_final.pdf> (last visited January 15, 2015)
- 3.Chan, supra note 1.; Fullerton SM, et al. Beneficence, Clinical Urgency, and the Return of Individual Research Results to relatives. AJOB. 2012;12(10):9–10. doi: 10.1080/15265161.2012.699153.Bredenoord AL, van Delden JJ. Disclosing Individual Genetic Research Results to Deceased Participants’ Relatives by Means of a Qualified Disclosure Policy. AJOB. 2012;12(10):10–12. doi: 10.1080/15265161.2012.699145.
- 4.Chan, supra note 1.
- 5.Fullerton, supra note 3.
- 6.Bombard Y, et al. Risks to Relatives in Genomic Research: A Duty to Warn? AJOB. 2012;12(10):12–14. doi: 10.1080/15265161.2012.699157. [DOI] [PubMed] [Google Scholar]
- 7.Offit K, et al. The ‘Duty to Warn’ a Patient’s Family Members about Hereditary Disease Risks. JAMA. 2004;292(12):1469–1473. doi: 10.1001/jama.292.12.1469. [DOI] [PubMed] [Google Scholar]
- 8.Forest LE, et al. Communicating Genetic Information in Families--A Review of Guidelines and Position Papers. European Journal of Human Genetics. 2007;15(6):612–618. doi: 10.1038/sj.ejhg.5201822. [DOI] [PubMed] [Google Scholar]
- 9.CSER: Clinical Sequencing Exploratory Research. at < https://cser-consortium.org/> (last visited April 2, 2015); National Human Genome Research Institute (NHGRI), Clinical Sequencing Exploratory Research (CSER), . at < http://www.genome.gov/27546194> (last visited April 2, 2015)
- 10.Dodd-McCue D, et al. The Role of Women in the Donation Consent Decision: Building on Previous Research. Progress in Transplantation. 2007;17(3):209–214. doi: 10.1177/152692480701700308. [DOI] [PubMed] [Google Scholar]
- 11.Batte B, et al. Family Communication in a Population at Risk for Hypertrophic Cardiomyopathy. Journal of Genetic Counseling. 2014;24(2):336–348. doi: 10.1007/s10897-014-9774-8. [DOI] [PubMed] [Google Scholar]; Green J, et al. Family Communication and Genetic Counseling: The Case of Hereditary Breast and Ovarian Cancer. Journal of Genetic Counseling. 1997;6(1):45–60. doi: 10.1023/A:1025611818643. [DOI] [PubMed] [Google Scholar]
- 12.Palmquist AE, et al. ‘The Cancer Bond’: Exploring the Formation of Cancer Risk Perception in Families with Lynch Syndrome. Journal of Genetic Counseling. 2010;19(5):473–486. doi: 10.1007/s10897-010-9299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services, Office for Civil Rights, Modifications to the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules Under the Health Information Technology for Economic and Clinical Health Act and the Genetic Information Nondiscrimination Act; Other Modifications to the HIPAA Rules, 78 . Federal Register. 2013:5566–5702. [PubMed] [Google Scholar]
- 14.45 C.F.R. § 160.103 (2014).
- 15.45 C.F.R. § 164.502(g)(4) (2014); U.S. Department of Health and Human Services, supra note 12, at 5614–15.
- 16.U.S. Department of Health and Human Services, supra note 12, at 5616.
- 17.Id., at 5614.
- 18.Id., at 5615; see also 45 C.F.R. § 164.510(b)(5) (Providing, “Uses and disclosures when the individual is deceased. If the individual is deceased, a covered entity may disclose to a family member, or other persons identified in paragraph (b)(1) of this section who were involved in the individual’s care or payment for health care prior to the individual’s death, protected health information of the individual that is relevant to such person’s involvement, unless doing so is inconsistent with any prior expressed preference of the individual that is known to the covered entity.”).
- 19.Brunner MT. What constitutes power coupled with interest within the rule as to termination of agency. 1953;1:11. 28 A.L.R.2d 1243, §§. updated to 2015. [Google Scholar]
- 20.U.S. Department of Health and Human Services, supra note 12, at 5616.
- 21.45 C.F.R. § 164.522; U.S. Department of Health & Human Services, Office for Civil Rights, Health Information of Deceased Individuals (2013), available at <http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/decedents.html> (last visited January 15, 2015).
- 22.U.S. Department of Health and Human Services, supra note 12, at 5615.
- 23.Bombard et al., supra note 5; Offit et al., supra note 6.
- 24.U.S. Department of Health & Human Services, Office for Civil Rights. How Can Family Members of a Deceased Individual Obtain the Deceased Individual’s Protected Health Information that is Relevant to their Own Care? 2013 available at < http://www.hhs.gov/ocr/privacy/hipaa/faq/personal_representatives_and_minors/222.html> (last visited April 5, 2015)
- 25.U.S. Department of Health & Human Services, Office for Civil Rights. Under the HIPAA Privacy Rule, May a Health Care Provider Disclose Protected Health Information About an Individual to Another Provider, when Such Information Is Requested for the Treatment of a Family Member of the Individual? 2009 available at < http://www.hhs.gov/ocr/privacy/hipaa/faq/disclosures_to_friends_and_family/512.html> (last visited January 15, 2015)
- 26.Galvin K, Clayman ML. Disclosure/Disruption: Considering Why Not to Disclose Genetic Information after Death. AJOB. 2012;12(10):14–16. doi: 10.1080/15265161.2012.699148. [DOI] [PubMed] [Google Scholar]
- 27.45 C.F.R. § 164.522.
- 28.U.S. Department of Health & Human Services, supra note 24.
- 29.Bredenoord and van Delden, supra note 3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.