Abstract
Myeloid cell activation by lipopolysaccharides (LPS) involves two proteins, plasma LPS-binding protein (LBP) and cell-membrane CD14. Cell membrane CD14, anchored by a glycerophosphatidylinositol tail, is the cellular receptor for LPS-LBP complexes. Another form of CD14, without the lipid tail, circulates as a soluble plasma protein. In this work we show that soluble CD14 (sCD14) is required for activation of endothelial and epithelial cells by LPS. We propose that LPS-LBP complexes transfer LPS to sCD14, and the LPS-sCD14 complexes then bind to a cellular receptor. Support for this pathway comes from experiments in which LBP and CD14 in normal human serum are blocked by specific antibodies, experiments in which serum is replaced by purified LBP and sCD14, and experiments in which specific binding of [3H]LPS to epithelial cells is quantitated.
Full text
PDF

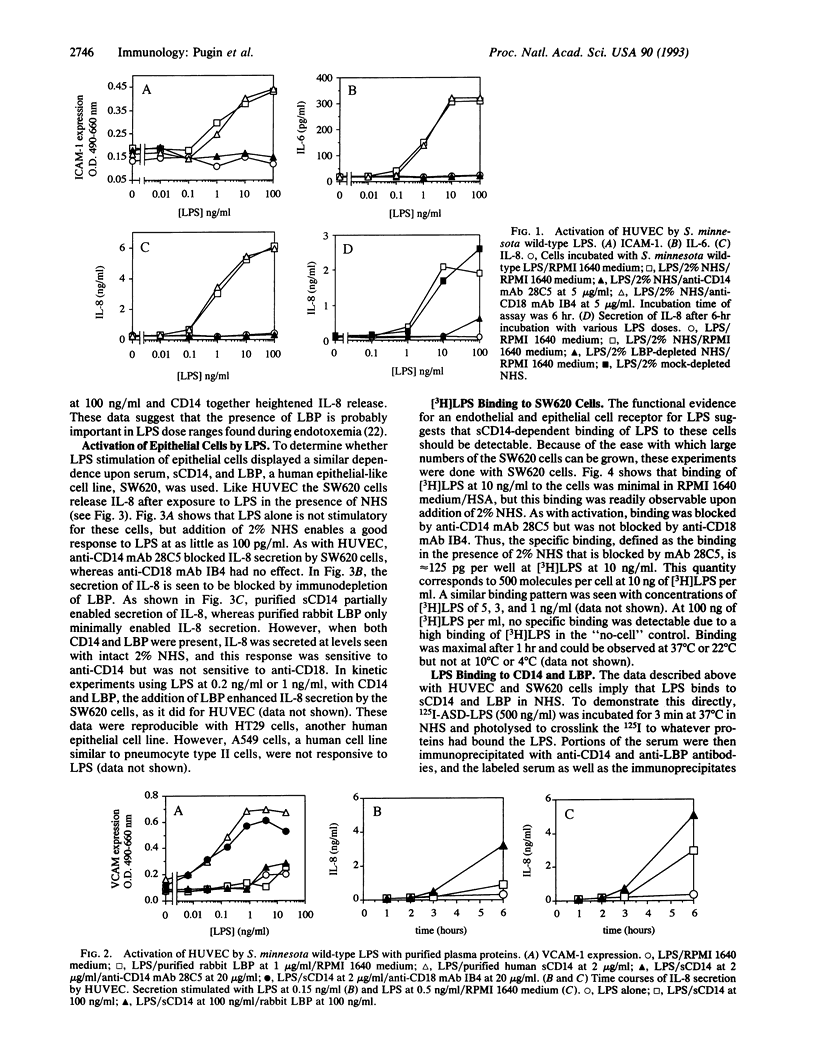
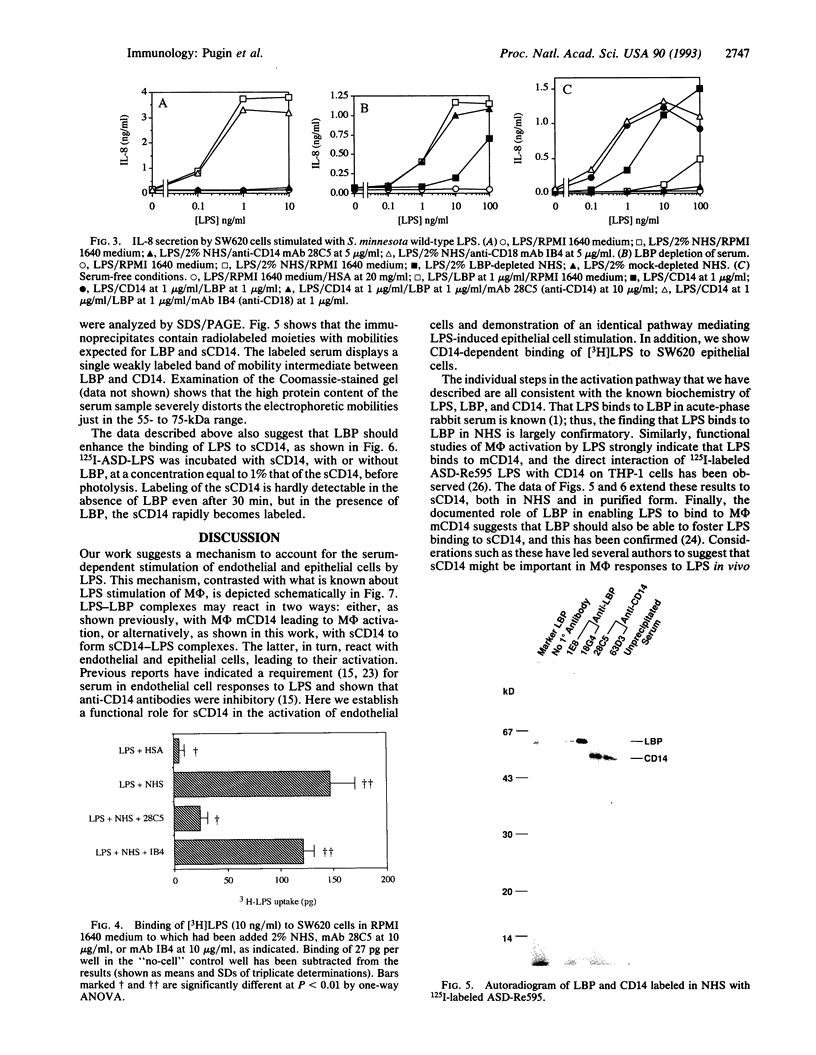
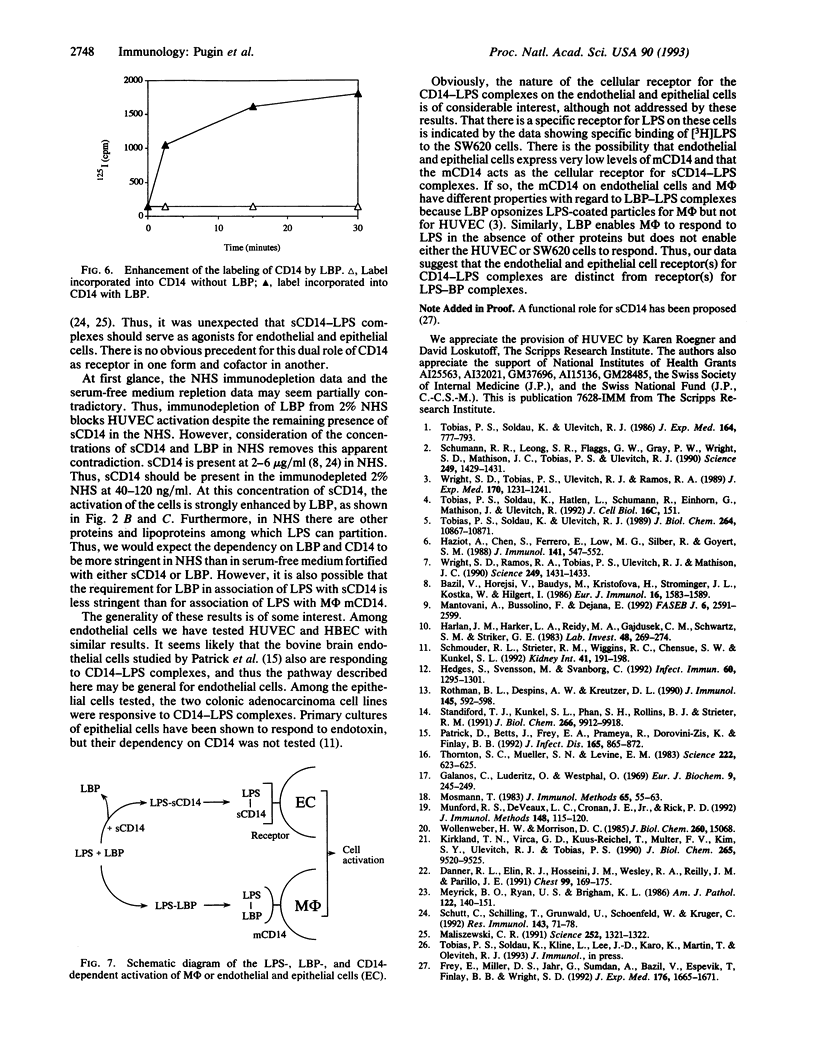
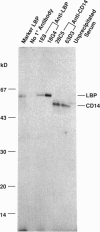
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazil V., Horejsí V., Baudys M., Kristofová H., Strominger J. L., Kostka W., Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986 Dec;16(12):1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- Danner R. L., Elin R. J., Hosseini J. M., Wesley R. A., Reilly J. M., Parillo J. E. Endotoxemia in human septic shock. Chest. 1991 Jan;99(1):169–175. doi: 10.1378/chest.99.1.169. [DOI] [PubMed] [Google Scholar]
- Frey E. A., Miller D. S., Jahr T. G., Sundan A., Bazil V., Espevik T., Finlay B. B., Wright S. D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992 Dec 1;176(6):1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Harker L. A., Reidy M. A., Gajdusek C. M., Schwartz S. M., Striker G. E. Lipopolysaccharide-mediated bovine endothelial cell injury in vitro. Lab Invest. 1983 Mar;48(3):269–274. [PubMed] [Google Scholar]
- Haziot A., Chen S., Ferrero E., Low M. G., Silber R., Goyert S. M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988 Jul 15;141(2):547–552. [PubMed] [Google Scholar]
- Hedges S., Svensson M., Svanborg C. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect Immun. 1992 Apr;60(4):1295–1301. doi: 10.1128/iai.60.4.1295-1301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Virca G. D., Kuus-Reichel T., Multer F. K., Kim S. Y., Ulevitch R. J., Tobias P. S. Identification of lipopolysaccharide-binding proteins in 70Z/3 cells by photoaffinity cross-linking. J Biol Chem. 1990 Jun 5;265(16):9520–9525. [PubMed] [Google Scholar]
- Maliszewski C. R. CD14 and immune response to lipopolysaccharide. Science. 1991 May 31;252(5010):1321–1322. doi: 10.1126/science.1718034. [DOI] [PubMed] [Google Scholar]
- Mantovani A., Bussolino F., Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992 May;6(8):2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Meyrick B. O., Ryan U. S., Brigham K. L. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986 Jan;122(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munford R. S., DeVeaux L. C., Cronan J. E., Jr, Rick P. D. Biosynthetic radiolabeling of bacterial lipopolysaccharide to high specific activity. J Immunol Methods. 1992 Apr 8;148(1-2):115–120. doi: 10.1016/0022-1759(92)90164-o. [DOI] [PubMed] [Google Scholar]
- Patrick D., Betts J., Frey E. A., Prameya R., Dorovini-Zis K., Finlay B. B. Haemophilus influenzae lipopolysaccharide disrupts confluent monolayers of bovine brain endothelial cells via a serum-dependent cytotoxic pathway. J Infect Dis. 1992 May;165(5):865–872. doi: 10.1093/infdis/165.5.865. [DOI] [PubMed] [Google Scholar]
- Rothman B. L., Despins A. W., Kreutzer D. L. Cytokine regulation of C3 and C5 production by the human type II pneumocyte cell line, A549. J Immunol. 1990 Jul 15;145(2):592–598. [PubMed] [Google Scholar]
- Schmouder R. L., Strieter R. M., Wiggins R. C., Chensue S. W., Kunkel S. L. In vitro and in vivo interleukin-8 production in human renal cortical epithelia. Kidney Int. 1992 Jan;41(1):191–198. doi: 10.1038/ki.1992.26. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Schütt C., Schilling T., Grunwald U., Schönfeld W., Krüger C. Endotoxin-neutralizing capacity of soluble CD14. Res Immunol. 1992 Jan;143(1):71–78. doi: 10.1016/0923-2494(92)80082-v. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Phan S. H., Rollins B. J., Strieter R. M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991 May 25;266(15):9912–9918. [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989 Jun 25;264(18):10867–10871. [PubMed] [Google Scholar]
- Tobias P. S., Soldau K., Ulevitch R. J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986 Sep 1;164(3):777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber H. W., Morrison D. C. Synthesis and biochemical characterization of a photoactivatable, iodinatable, cleavable bacterial lipopolysaccharide derivative. J Biol Chem. 1985 Dec 5;260(28):15068–15074. [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Tobias P. S., Ulevitch R. J., Ramos R. A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989 Oct 1;170(4):1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]