Summary
Introduction
Platelet Rich Plasma (PRP) contains numerous growth factors; Platelet poor plasma (PPP) is plasma proteins without platelets, containing growth factors other than platelet derived. We planned to evaluate the effect of both autologous PRP & PPP on human ACL cell growth characteristics in culture conditions to see if one was better than the other.
Methods
ACL remnants were collected from eleven patients during ACL reconstruction surgery; PPP and PRP were prepared from blood of these patients. Cells were isolated, identified and cultured and were then divided into six groups. Groups A–D had Fetal Bovine Serum (FBS) added to them along with different concentrations of PRP and PPP. Groups E and F had 5% and 10% PRP respectively but lacked FBS. Cell viability was assayed by MTT and Annexin V assay, and DNA content was evaluated by propidium iodide staining and flow cytometry.
Results
analysis of cultured cells showed that addition of PRP (5 or 10%) increased the viability of ACL cells in 4 out of 11 and promoted cell proliferation in 8 of 11 donor samples; 10% PRP was more effective than 5% PRP. However, the difference in effectiveness of 10% PRP was not significantly better than 5% PRP. 5% PPP had no significant effect on cell viability, but it led to an increase in DNA content in 5 of 11. There was no statistically significant effect of either PRP or PPP in preventing cell death (depicted by apoptosis rate).
Conclusion
PRP may have an enhancing effect on ACL cell viability and promotion of cell proliferation but the ideal concentration of PRP for these positive effects needs to be determined before it could be used in clinical settings for enhancing primary repair of torn ACL. Also larger, more controlled and better studies are needed to confirm its clinical utility.
Keywords: Platelet rich plasma, platelet poor plasma, ACL injury, cell culture study
Introduction
Anterior cruciate ligament (ACL) is the most commonly injured knee ligament and its injury predisposes to knee instability and decreased function1–3. Treatment of ACL injuries has evolved significantly, with expectation of returning to sports/high demand physical activities being the principal driving factor4. ACL not only has a mechanical role but it also possesses mechanoreceptors necessary for proprioception5. Hence, repair of the torn ACL would be the best management option for regaining its function. While there have been attempts at primary repair of the torn ligament6–8, results have been less encouraging and ligament reconstruction with an autograft or allograft is considered a better alternative9, 10. The very fact that ACL is an intra articular structure which is bathed with synovial fluid, makes chances of its healing remote, and hence the need for using a ligament/tendon for the reconstruction11. Although there are several methods of ACL reconstruction used today, no single current surgical procedure has been proven to reliably restore complete long-term knee function after an ACL tear9, 10. Because of the limitations associated with available treatment modalities and poor healing ability of the ACL, ligament tissue engineering with orthobiologics is being explored as an alternative approach.
Investigators have used PRP injected into graft material or as a gel scaffold along with the graft with variable results4,12–14. While some of the investigators have reported good ligamentization of graft and good clinical results4, 13 others have not had much improvement in results14. None of the investigators have looked into the effect that PRP has on the native ACL cells, although Yoshida et al.14 and Cheng et al.15 have done work related to growth of porcine ACL tissue in vitro.
We conceptualized this study in an attempt to evaluate the effect of both PRP and PPP on ACL tissue harvested during arthroscopic ACL reconstruction surgery. We attempted to obtain growth of cells from ACL tissue from the ACL remnants obtained from the stump during surgery. Following which we tried to examine the effect of addition of PRP and PPP in various concentrations on the ACL cell cultures. The results obtained are presented here.
Materials and methods
The study was conducted at the Post Graduate Institute of Medical Education and Research (PGIMER) Chandigarh after obtaining clearance from the Institutional Ethics Committee and the Departmental Review Board. The study was performed as a controlled laboratory study. The study meets the ethical standards of this journal16.
We included patients with ACL deficient knees between 18 to 40 years of age with an injury period of more than three months who underwent ACL reconstruction surgery. An informed consent was obtained from all patients undergoing ACL reconstruction surgery, explaining the use of tissues obtained during surgery for experimental purposes, along with consent for withdrawing blood for PRP preparation. ACL remnant tissue was removed from 28 patients and was taken as the source of ACL cells.
Collection of ACL tissue
The cells were obtained during arthroscopic ACL reconstruction surgery done by the senior surgeon. After the diagnostic arthroscopy, ACL remnant tissue was obtained with an arthroscopic punch from the tibial stump of torn ACL. This was then kept in a sterile test tube containing normal saline and was transported to the laboratory in an ice box within 10 minutes of harvesting.
Cell isolation and culture
Cells were isolated from the remnant tissue by means of sequential enzymatic digestion with trypsin and stored for one hour at 37° C; the cells were identified morphologically under inverted microscope. After several washes in RPMI 1640 media, the isolated cells were cultured in a monolayer in complete media containing 10% fetal bovine serum, 25 mcg ascorbic acid, 360 mg/ml L-glutamine, and 50 mg/ml gentamicin at 37° C and 5% CO2 in air atmosphere.
Collection of blood for PRP preparation
Once a successful primary culture of ACL cells was obtained, blood was collected from the patients for preparation of PRP. Preparation of PRP was done in a sterile manner by the Department of Transfusion Medicine of our institution, following a pre defined protocol which has been previously used in other studies by us17–19.
The whole blood obtained from the patient’s antecubital vein was transferred from the blood bag into two different sterile tubes of 50 ml each using a blood transfusion set, inside the bio safety cabinet (BIOAIR-Safe flow 1.2, type 2). One of the tubes was then centrifuged with a table top centrifuge. The blood was separated into Platelet rich plasma (PRP) and residual RBC’s with the buffy coat. Hereafter the procedure was completely done inside the bio safety cabinet. The PRP was then extracted through a pipette and transferred to a separate test tube. The second tube containing the remaining 50 ml of whole blood was given a hard spin (3000 rpm) on a table top centrifuge. Subsequently, the Platelet poor plasma (PPP) was then separated and the RBCs were left in the tube along with the buffy coat; leukocytes were not filtered from the blood. The platelet count was measured in the patient’s peripheral blood as well as in the final PRP and PPP product. The prepared PRP and PPP were stored at a temperature of −80° C.
Culture protocol for the studies
The cells from the initial culture were checked for cell viability and growth. After 24 to 48 hours of pre-culture in complete medium, the cells were placed in the serum-free medium for 12 hours, which consisted of RPMI 1640 media with supplements as described above. For incubation with fibroblasts derived from the ACLs we prepared 5% concentration (475 microliter of Culture medium + 25 microliter of PRP) and 10% concentration (450 microliter of culture medium + 50 microliter of PRP) of PRP and 5% PPP (475 microliter of Culture medium + 25 microliter of PPP). Whenever PRP was added, it was activated by addition of calcium chloride (M/40) in a ratio of 4:1 (PRP: calcium chloride). Activation is required for the release of growth factors from the platelets. The cultured cells were grouped into six sub groups as detailed below and were then incubated for 24 hours (day 1) and 48 hours (day 2).
Group A – 10% fetal bovine serum (FBS) + cells + media (taken as control)
Group B – Control + 5% PRP
Group C – Control + 5% PPP
Group D – Control + 10% PRP
Group E – Cells + media + 5% PRP (no FBS)
Group F – Cells + media + 10% PRP (no FBS).
Analysis of the cultured cells
The cell cultures were analyzed for cell viability and DNA content to gauge the effect which varying concentration of PRP and PPP have on the cultured cells. For the MTT assay (cell viability) 50,000 cells were seeded in 96 well plate; for the apoptotic assay (Annexin V) 100,000 cells were seeded in 6-well plate and for the SPF (DNA content analysis) 100,000 cells were seeded in a 6-well plate. At least 20,000 events were analyzed by flow cytometry.
Cell viability analysis
Cell viability: it was assessed by using MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] dye assay. The cells were incubated with 5 mg/ml of MTT for 3.5 hours at 37° C in culture hood. The media was removed and 150 micro liter of MTT solvent was added. The absorbance was then read at 590 nm in a spectrophotometer.
Annexin V assay for cell apoptosis: the percentage of apoptotic cells was checked by incubating the cells with PRP and PPP using Annexin V fluos and PI staining. For staining, the cells were washed with PBS and incubated with Annexin-V-Fluoresce in Hepes buffer containing PI for 10–15 min at 15–25° C. Analysis was carried out by flow cytometry.
DNA content analysis/‘S’ phase fraction: the cells were analyzed for their DNA content by propidium iodide staining and flow cytometry. For analysis the cells were fixed and permeabilized with 70% ethanol and stained with PI (200 microliter of 1mg/ml stock) in triton X-100 (0.1%v/v) and RNAse A solution and then analyzed. The analysis of all these sets was done separately in terms of the cell viability and its DNA content. These results were further compared accordingly to evaluate the efficacy of different concentrations of PRP and PPP in enhancing the growth of ACL cells in vitro.
The data so obtained was entered in a spread sheet in SPSS 20 (IBM Inc, Illinois). This was used to calculate the mean values, plot histograms and bar diagrams. The data from the different groups was analyzed using the Kruskall Wallis test, which is anon-parametric test. A p<0.05 was considered as significant through the study.
Results
Patients undergoing the ACL reconstruction surgery were between 18–40 years (mean 22 years) of age and the duration between injury and surgery was between 4 to 7 months.
A successful primary culture was obtained in 11 out of 28 samples of ACL remnant tissue. The cells started appearing at 3 days of incubation in culture flasks. Initially they were rounded in shape; after 4–5 days they started adhering to the surface and assumed a spindle shaped morphology of fibroblast. Thereafter, the cells increased in number and formed colonies, initially at the edges of the flask and then towards the center. A confluent growth of ACL cells was obtained by 10 to 11 days which were passed through culture media.
Further studies were carried out on ACL cells from the 11 successful primary cultures. PRP and PPP which were stored at −80° C were thawed and used for preparing the previously defined groups using cells from the primary culture. Group A was taken as the control group. Among the other groups, Groups B, C and D showed significant demonstrable results. However, Groups E and F (both lacked FBS) demonstrated insignificant cell growth and were excluded from further analysis. On day 1 of incubation with respective additives (PRP or PPP) growth was seen in all the 11 samples and the results were analyzed for all. On day 2, cells in 4 samples became differentiated and hence could not be analyzed. Thus, the day 2 analysis was of 7 samples only.
Cell viability
On day 1 of incubation of the cells with respective additives, cell viability was found to be more than control in only 1 of the 11 samples in Group B (5% PRP). Viability was increased in 1 and was same as controls in 2 samples in Group D (10% PRP). There was no increase in cell viability in the samples of Group C (5% PPP).
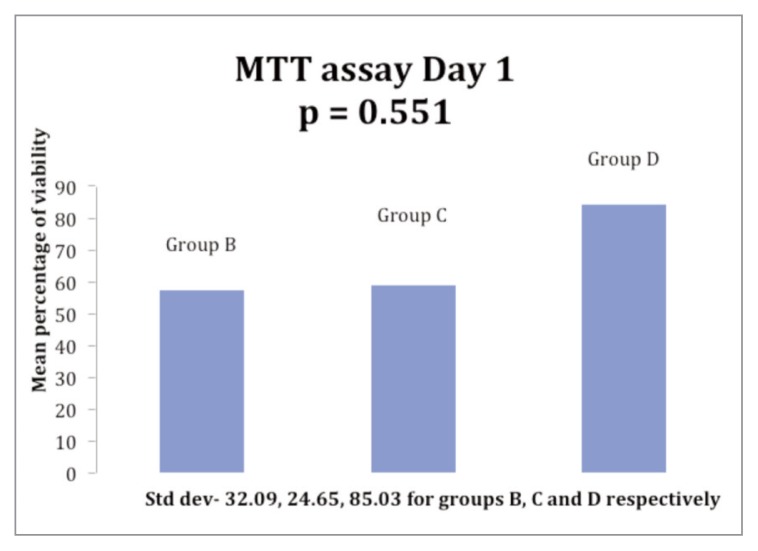
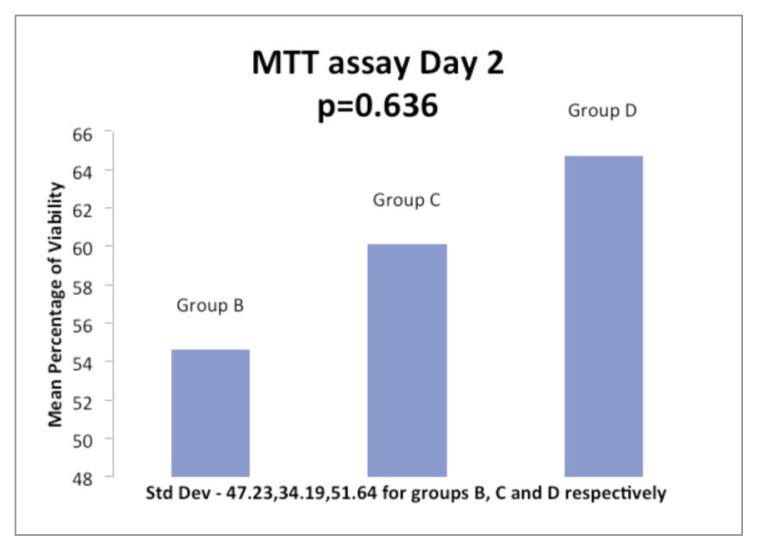
On day 2 of incubation with additives, the cell viability was more than controls in one sample each among the three Groups B, C and D. The mean cell viability on day 1 for the Groups B, C and D was 56.84%, 58.85% and 84.20% respectively while the cell viability on day 2 for the Groups was 54.61%, 60.09% and 64.74% respectively (Figs. 1, 2). The cell viability among the Groups was not found to be statistically significant.
Figure 1.
Histogram showing the comparison of mean ACL cell viability at day 1 of incubation with 5% PRP (Group B), 5% PPP (Group C) & 10% PRP (Group D).
Figure 2.
Histogram showing the comparison of mean ACL cell viability at day 2 of incubation with 5% PRP (Group B), 5% PPP (Group C) & 10% PRP (Group D).
Annexin V assay for cell apoptosis
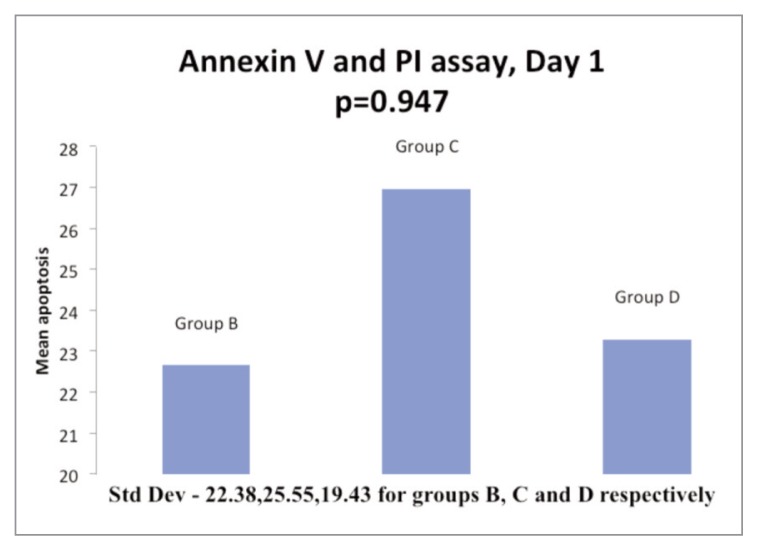
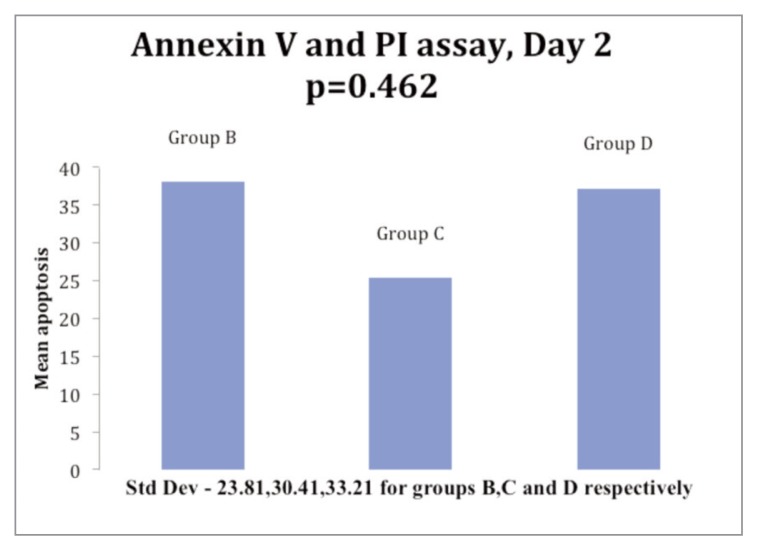
On day 1, cell apoptosis was more than controls in 8 samples and less than controls in 3 samples in Group B (5% PRP); it was more than controls in 9 samples and less than controls in 2 samples in Group C. In Group D, 7 samples had cell apoptosis more than control while it was less than controls in 4 samples. The mean cell apoptosis rate on day 1 was 22.65, 23.29 and 26.96 for the Groups B, C and D respectively (Fig. 3). On day 2, 7 samples were analyzed and cell apoptosis rate was more than controls in 6 and less than controls in 1 sample in Group B; it was more than controls 5 and less than controls in 2 samples in Group C. While in Group D, 5 samples had cell apoptosis rate more than controls and 2 samples had a rate less than controls. The mean cell apoptosis rate on day 2 for 7 samples was 38.05, 25.37 and 37.2 for the Groups B, C and D respectively (Fig. 4).
Figure 3.
Histogram in showing comparison of mean apoptosis of ACL cells at day 1 of incubation with 5% PRP (Group B), 5% PPP (Group C) & 10% PRP (Group D) respectively.
Figure 4.
Histogram in showing comparison of mean apoptosis of ACL cells at day 2 of incubation with 5% PRP (Group B), 5% PPP (Group C) & 10% PRP (Group D).
Thus, the rate of apoptosis did not show any consistent trend over the period of the study in any of the three Groups (B, C and D) but overall, the rate of apoptosis of cells was more in all the three Groups than in control Group A.
S phase fraction (SPF) by cell cycle analysis
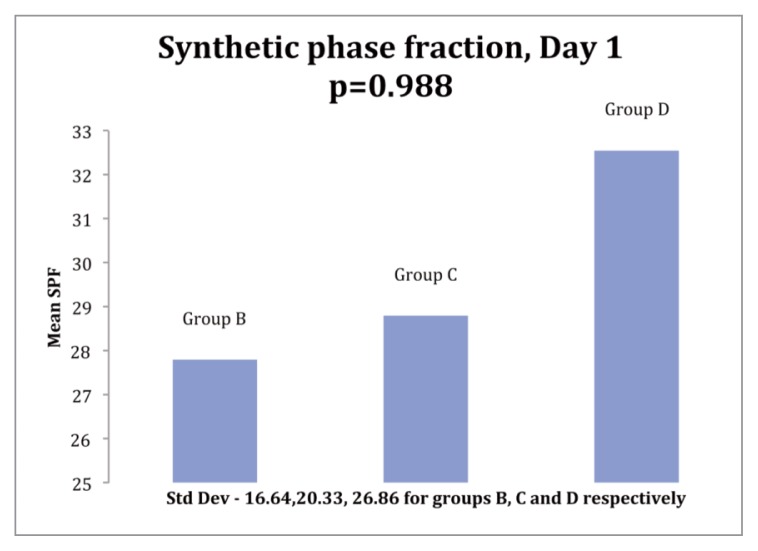
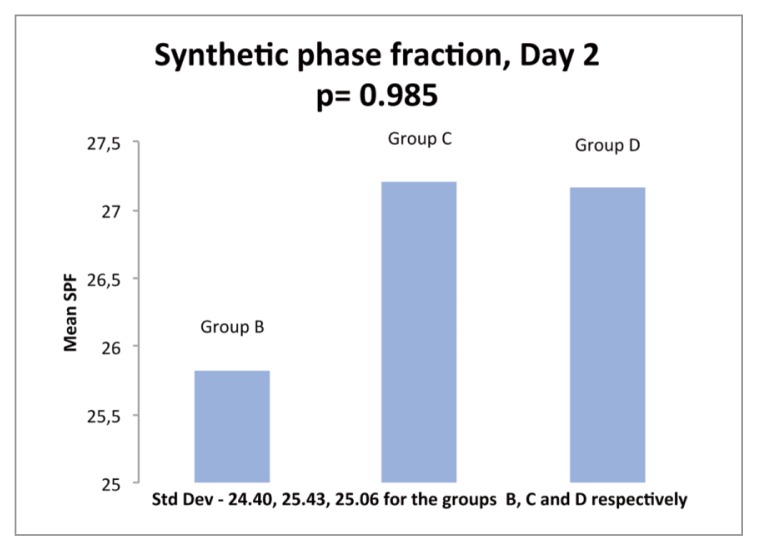
On day 1 of incubation with PRP or PPP, an increase in the SPF over control was seen in 6 samples and a decrease in 5 samples of Group B. 5 samples showed increase while 6 showed decrease in SPF over controls in Group C, and 7 showed an increase while 4 showed a decrease in Group D. The mean SPF values were 27.78, 28.78 and 32.54 for Groups B, C and D respectively (Fig. 5). On day 2 of incubation, an increase of SPF over controls was seen in 3 out of 7 samples in Group B while a decrease was seen in 4 samples. 4 samples in Group C showed an increase while 3 samples showed a decrease. In Group D, 5 samples showed an increase and 2 showed a decrease. The mean SPF was 25.82, 27.2 and 27.15 for Groups B, C and D respectively (Fig. 6). In summary, while an increase in SPF over controls was seen with the addition of growth factors, the increase seen among the three Groups B, C and D was statistically not significant.
Figure 5.
Histogram showing comparison of mean SPF (synthetic phase fraction) of ACL cells at day 1 of incubation as determined by cell cycle analysis with 5% PRP (Group B), 5% PPP (Group C) & 10% PRP (Group D).
Figure 6.
Histogram showing comparison of mean SPF (synthetic phase fraction) of ACL cells at day 2 of incubation as determined by cell cycle analysis with 5% PRP (Group B), 5% PPP (Group C) & 10% PRP (Group D).
Discussion
Orthobiologic substances especially platelet rich plasma has been used by researchers for various conditions of musculoskeletal system18, 20 in the recent past with conflicting results. ACL injury is a common injury and investigators have been trying to use bone marrow and PRP among others for improving the outcomes of ACL injuries. Many of such studies are experimental involving animal experiments21, 22. PRP has been used in clinical studies of ACL reconstruction4, 13, 23 attempting to enhance the graft-to-bone healing, graft maturation, and/or improvement in the clinical outcomes with variable results. A recent systematic review of the literature on PRP use in ACL surgery12 concluded that there was promising evidence that addition of PRP could be a synergic factor in acquiring maturitymore quickly than in grafts with no PRP; however, it was also noted that there was no proof of clinical outcomes being enhanced by the use of PRP in ACL surgery.
PRP has been used in animal models (porcine and canine) in attempts to improve the healing of primary ACL repair11, 24. The results of such animal studies have been encouraging, and actually suggest that PRP can enhance the growth of primary ACL cells. The clinical implication of such a scenario could be enormous and merits further investigations. Previously, a study of human ACL cell culture with autologous PRP was done by Fallouh et al.25; they found that in vitro treatment of ACL cells with platelet-rich clot releasate resulted in a significant increase in cell numbers compared with platelet-poor clot releasate. They noted that the collagen production by the platelet rich clot releasate-treated cells was significantly higher than that of the platelet-poor clot releasate-treated cells, probably due to enhanced cell proliferation, and that there was no significant effect of platelet-rich clot releasate treatment on gene expression for type-I collagen. Their study was on the ACL cells from four patients and was the first of its kind.
In the present study we attempted to include a relatively larger sample size than Fallouh et al.25 and tried to evaluate the effect that autologous PRP might have on cell viability, apoptosis and cell proliferation. Perhaps the biggest problem in today’s scenario is adequate growth ACL cells on being stimulated by growth factors, as we believe that it is necessary to be able to culture cells in vitro in sufficient numbers, and with relative ease, before any clinical use of such a technique could be conceptualized.
We encountered difficulties in cell culture as we were able to obtain an initial culture in only 11 out of 28 samples emphasizing the fact that this process remains inherently difficult. This low turnover might result from issues in any of the critical steps involved in the process, including harvesting, transportation, enzymatic degradation, cell separation and initial culture with stringent culture conditions. These difficulties have been noted in other studies.
The sub cultures which contained different concentrations of PRP or PPP were examined after day 1 and day 2 of culture. While four of the groups contained fetal bovine serum (FBS) and showed growth of cells, two groups which did not have any FBS added to them showed no significant growth and were excluded from further analysis.
We observed that the addition of PRP or PPP did not lead to cell viability more than the controls in most of the samples, although few specimens did show some increased cell viability with 10% PRP. When compared among the groups, 10% PRP showed greater cell viability than 5% PRP or 5% PPP but the difference was not statistically significant.
When the rate of apoptosis was evaluated, the addition of PRP and PPP to the ACL cell cultures was found to have increased the rate of apoptosis as compared to the controls. The rate of apoptosis was different in the PRP/PPP containing groups on both the days of the study. Nevertheless, we noted that the mean rate of overall apoptosis for the two days taken together was the highest in group D (10% PRP). Thus, it could be inferred that the addition of PRP in a greater amount may actually promote apoptosis; this assumes significance as a high concentration of growth factors applied to the cells may have deleterious effects instead of an enhancing effect.
Evaluation of the ‘S’ phase fraction (SPF), which reflects the proliferative status of the cells in culture showed that addition of PRP/PPP led to an increase in the SPF over controls. Though the increase was maximum in the 10% PRP group (Group D), the improvement was not statistically significant over Groups B and C. Interestingly, a good improvement in the SPF was noted in PPP treated ACL specimens also. This in turn reflects that the cell cycle phases concerned with preparation for further division of the cells is actually stimulated by growth factors but we are not clear which ones are responsible.
Our study has its fair share of limitations and short comings. Though we obtained 28 samples for the study, results could be obtained in only 11 samples. This could be due to a small amount of ACL tissue harvested in the remaining 17 cases, as the harvesting was done from patients and the yield of tissue might not have been adequate in all cases. Further subcultures led to evaluation of 11 samples on day 1 and 7 on day 2. Thus, effectively seven samples were evaluated on both the days. Thus, the sample size was somewhat small. We were unable to culture cells in absence of fetal bovine serum, which we believe is a major roadblock before clinical efficacy of PRP for ACL repairs can be proven beyond doubt. Additionally, one of the major limitation was that while we activated the platelets to release growth factors by adding calcium chloride, we could not quantify the amount of the individual growth factors in PRP and PPP groups and this might actually be a contributing reason that no particular trend was noticeable in the study samples. Individual growth factor identification and their specific role in cell proliferation is needed to be evaluated in future research. One may also argue that 48 hours could be a relatively short duration and evaluation after 72 hours would have been better. We acknowledge this as a limitation of the study.
In summary, even though the study design and execution was not completely flawless, our study can be taken as a work in continuation of the work done by Fallouh et al.25. Both suggest that PRP in correct amount could have a proliferative effect on ACL cells in vitro and this could be potentially harnessed for preparing a scaffold with cells and PRP as ACL ligaments, thus changing reconstruction techniques from allograft/autograft tendons to real ACL reconstructions. Moreover, partial ACL tears which are often managed conservatively may be more satisfactorily treated with the addition of PRP to stimulate repairs. It is important to note that, although growth factors stimulate cell proliferation, a fine interplay of growth factors decides the cell viability and apoptosis; hence further studies are needed to evaluate the actual growth potential of the ACL remnant cells and to devise a way to apply the growth factors in PRP at these sites in a cost effective, yet clinically efficient manner.
References
- 1.Rudolph KS, Axel MJ, Buchanan TS, et al. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9:62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Papannagari R, Defrate LE, et al. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. ActaOrthop. 2007;78:355–360. doi: 10.1080/17453670710013924. [DOI] [PubMed] [Google Scholar]
- 3.Daniel DM, Stone ML, Dobson BE, et al. Fate of the ACL injured patient: A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 4.Nin JR, Gasque GM, Azcárate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25(11):1206–1213. doi: 10.1016/j.arthro.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon MS, Bali K, Prabhakar S. Differences among mechanoreceptors in healthy and injured anterior cruciate ligaments and their clinical importance. Muscles Ligaments Tendons J. 2012;2(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 6.O’Donoghue DH, Rockwood CA, Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48:503–519. [PubMed] [Google Scholar]
- 7.Feagin JA, Jr, Curl WW. Isolated tear of the anterior cruciate ligament: 5-Year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 8.Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10:103–107. doi: 10.1177/036354658201000208. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2014 doi: 10.1016/j.knee.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Qu J, Xu D, Zhou J, Lu H. Allograft versus autograft for anterior cruciate ligament reconstruction: an up-to-date meta-analysis of prospective studies. Int Orthop. 2013;37(2):311–320. doi: 10.1007/s00264-012-1720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 12.Figueroa D, Figueroa F, Calvo R, Vaisman A, Ahumada X, Arellano S. Platelet-Rich Plasma Use in Anterior Cruciate Ligament Surgery: Systematic Review of the Literature. Arthroscopy. 2015 doi: 10.1016/j.arthro.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez M, Anitua E, Azofra J, Prado R, Muruzabal F, Andia I. Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy. 2010;26(4):470–480. doi: 10.1016/j.arthro.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida R, Cheng M, Murray MM. Increasing platelet concentration in platelet-rich plasma inhibits anterior cruciate ligament cell function in three-dimensional culture. J Orthop Res. 2014;32(2):291–295. doi: 10.1002/jor.22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng M, Wang H, Yoshida R, Murray MM. Platelets and plasma proteins are both required to stimulate collagen gene expression by anterior cruciate ligament cells in three-dimensional culture. Tissue Eng Part A. 2010;16(5):1479–1489. doi: 10.1089/ten.tea.2009.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padulo J, Oliva F, Frizziero A, Maffulli N. Muscles, Ligaments and Tendons Journal. Basic principles and recommendations in clinical and field science research. MLTJ. 2013;4:250–252. [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon MS, Behera P, Patel S, Shetty V. Orthobiologics and platelet rich plasma. Indian J Orthop. 2014;48(1):1–9. doi: 10.4103/0019-5413.125477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong) 2015;23(1):6–10. doi: 10.1177/230949901502300102. [DOI] [PubMed] [Google Scholar]
- 20.Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 21.Kanazawa T, Soejima T, Noguchi K, Tabuchi K, Noyama M, Nakamura K, Shiba N. Tendon-to-bone healing using autologous bone marrow-derived mesenchymal stem cells in ACL reconstruction without a tibial bone tunnel-A histological study. Muscles Ligaments Tendons J. 2014;4(2):201–206. [PMC free article] [PubMed] [Google Scholar]
- 22.Lovric V, Kanazawa T, Nakamura Y, Oliver RA, Yu Y, Walsh WR. Effects of Gaps Induced Into the ACL Tendon Graft on Tendon-Bone Healing in a Rodent ACL Reconstruction Model. Muscles Ligaments Tendons J. 2012;1(3):91–99. [PMC free article] [PubMed] [Google Scholar]
- 23.Figueroa D, Melean P, Calvo R, et al. Magnetic resonance imaging evaluation of the integration and maturation of semi-tendinosus-gracilis graft in anterior cruciate ligament reconstruction using autologous platelet concentrate. Arthroscopy. 2010;26:1318–1325. doi: 10.1016/j.arthro.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 25.Fallouh L, Nakagawa K, Sasho T, et al. Effects of autologous platelet-rich plasma on cell viability and collagen synthesis in injured human anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(18):2909–2916. doi: 10.2106/JBJS.I.01158. [DOI] [PubMed] [Google Scholar]