Abstract
Purpose
Papillon-Lefèvre syndrome (PLS) is a rare autosomal recessive disorder that involves palmoplantar keratosis (PK) and severe aggressive periodontitis. Cathepsin C (CTSC) gene mutations are etiologic for PLS, with more than 60 different mutations reported in different ethnic groups worldwide. The purpose of this study was to report a novel cathepsin C mutation in a Brazilian patient.
Methods
A 4-year-old boy presented with aggressive periodontitis, recession, missing teeth, and hyperkeratosis of the palms of hands and soles. Peripheral blood samples were obtained from family members for genomic DNA isolation. The coding region and exon/intron boundaries of the CTSC gene were amplified and sequenced.
Results
The patient had a PLS phenotype, which included PK and early-onset severe periodontitis. Sequence analysis showed a novel CTSC mutation (c.267–268del) present in the homozygous state.
Conclusion
This report described a novel mutation in a family with Brazilian Papillon-Lefèvre syndrome and presented a review of all cathepsin C (65) mutations reported to date.
Keywords: Cathepsin C, Papillon-Lefèvre Syndrome, periodontitis, hyperkeratosis
Papillon-Lefèvre syndrome (PLS; OMIM 245000)1 is an uncommon disorder with a prevalence of 1 to 4 million individuals4; and it is also a type IV palmoplantar keratoderma characterized by the presence of palmoplantar keratosis (PK) and aggressive periodontitis (AP) that affects both the primary and permanent dentitions and leads to premature tooth loss.2,3 The condition is transmitted as an autosomal recessive trait. An increased prevalence of parental consanguinity, first noted by Gorlin et al. (1964)4 is also noted in the literature reports (OMIM 245000).1
Although PK and AP destruction of both dentitions are the main clinical features of PLS, increased susceptibility to infections, decreased polymorphonuclear (PMN) leukocyte function, calcifications of the dura mater, and onychogryphosis have also been reported.4–6
The skin lesions first appear between 1- and 4-years-old, where the palms and soles present a dry, red, and scaly appearance that may progress to thickening and cracking of the skin. The dermal involvement may present as a diffuse or demarcated distribution, with the demarcated form being the most common presentation. Hyperkeratotic plaques and transgressions have also been noted on the legs, knees, elbows, cheeks, and labial comissures.7 In some children, PK appears simultaneously with the periodontal disease and regresses once the periodontally involved teeth are lost. Skin lesions are reported to improve with age, but some degree of PK usually remains throughout life. The clinical features of severe prepubertal periodontitis and palmar-plantar hyperkeratosis is pathognomonic for PLS.
The genetic basis for PLS is due to mutations that affect both alleles of the cathepsin C gene (CTSC) which is located on chromosome 11q14.8–10 Currently, more than 60 CTSC mutations have been identified in individuals diagnosed with PLS (OMIM-245000),1 Haim-Munk syndrome (OMIM-245010),1 and some forms of AP (OMIM-170650).1
CTSC gene, also named dipeptidyl-peptidase I, is an olygomeric lysosomal cysteine proteinase, from the papain superfamily, capable of removing dipeptides from the amino terminus of proteins and involved in the zymogen activation of granule-associated serine proteases. Specifically, functional CTSC is required to activate the neutrophil serine proteases cathepsin G, neutrophil elastase, and proteinase 3.11,12 CSTC is expressed in several tissues, including lungs, kidney, placenta, dermal epithelia, gingiva, immune inflammatory cells, and their precursors. Among the fully differentiated immune cells, the polymorphonuclear cells and the alveolar macrophages showed the strongest hybridization signal.9,13
While CTSC mutations have been identified from families around the world, relatively few have been reported in South America. The purpose of this study was to report a mutation analysis of the cathepsin C gene in a Brazilian family with a member diagnosed with Papillon-Lefevre syndrome.
METHODS
CLINICAL AND RADIOGRAPHIC EVALUATIONS
Ten members of a consanguineous Brazilian family were recruited for the study. The clinical diagnosis of PLS was made on the presence of AP and clinical appearance of PH. Family members received medical and dental examinations, including clinical and radiographic evaluations. The family history was recorded, and the pedigree was constructed.
PATIENT SAMPLES, DNA ISOLATION, AND MUTATIONAL ANALYSIS
Samples were available from the proband, and both parents. All studies were conducted with approval from the institutional IRB. All subjects provided consent for the study. Genomic DNA samples were isolated from peripheral blood samples obtained by standard venepuncture using the QIAamp Blood Kit (Qiagen, Inc., Valencia, CA) in order to obtain permit mutational analysis. The coding region and exon/intron boundaries of the CTSC gene were amplified and sequenced, as previously described.14
RESULTS
The proband was a 4-year-old boy referred to the Dental Anomalies Clinic of the University Hospital of Brasilia, Brazil, for dental evaluation due to premature tooth loss. His parents reported that he began loosing his teeth before 4-years-old. The family history revealed that the parents were first cousins from the city of Paracatú, a region of the state of Minas Gerais, Brazil (Figure 1—pedigree). Neither parent demonstrated hyperkeratotic lesions of their hand, feet, or any other location. Neither parent had a history of significant periodontitis. The parents reported dermatological problems in a paternal grandmother and in the proband’s oldest brother who died of unknown causes at the age of 15 months. The medical history of the proband indicated the presence of PK since he was 4-months-old. The condition was reported to be more severe during cold weather. The proband was frequently hospitalized during infancy due to gastrointestinal symptoms, recurrent pneumonia, chronic malnutrition, and anemia. Physical examination revealed retardation of the somatic development, hyperkeratosis of the palms of the hands and soles of the feet with fissures of the soles making locomotion difficult. Dermal lesions on the knees and elbows were evident in the proband (Figure 2). Dermal biopsy from the hand demonstrated hyperkeratosis, hypergranulosis, acanthosis, and irregular epidermal ridges. In addition, psoriasiform dermatitis and perivascular lymphocitic infiltrate was observed histologically.
Figure 1.
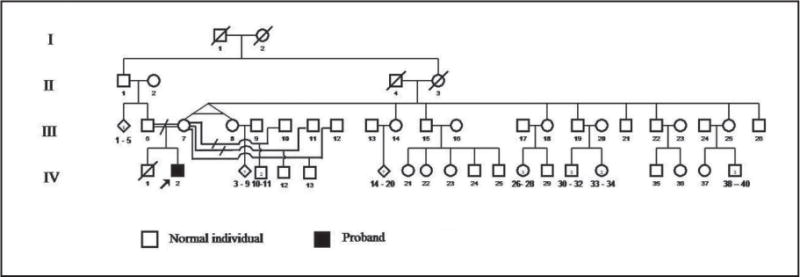
Pedigree diagram of the Brazilian family with Papillon-Lefevre syndrome.
Figure 2.
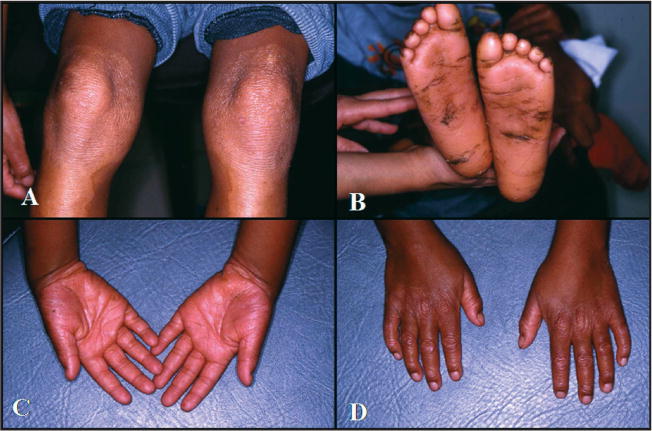
(A) Hyperkeratosis of transgressions on the knees. (B) Hyperkeratosis and fissures on the soles. (C–D) Hyperkeratosis of the palm.
Dental eruption in the proband began at 9-months-old and was uneventful. By 4 years, 10-months-old, however, all primary teeth demonstrated mobility and gingival inflammation that was followed by progressive tooth loss. The patient experienced great discomfort while eating and performing oral hygiene. An intraoral examination revealed an edentulous mandible and 3 maxillary teeth: the right canine and two second maxillary molars (Figure 3). Recession, evident around all 3 maxillary teeth, was particularly severe around the canine. The interproximal gingiva between the right canine and the right second molar was red and enlarged—consistent with significant inflammation. Suppuration was present around all 3 teeth.
Figure 3.
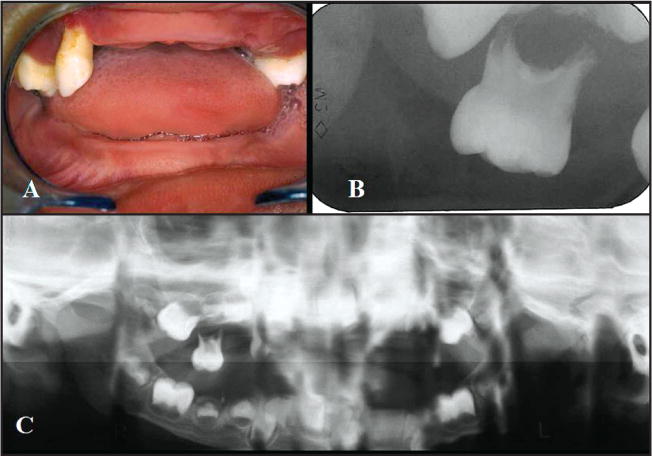
Clinical and radiographs findings of the Papillon-Lefevre syndrome patient. (A) Oral gingiva, showing aggressive periodontitis, missing teeth, and severe recession in the first dentition (4 years old). (B) Periapical radiograph-extensive loss of alveolar bone in the maxillary molar. (C) Panoramic showing generalized alveolar bone loss in mandibular and maxilla arch.
Although the mandible was clinically edentulous, radiographs revealed the presence of permanent teeth that were consistent with a normal developmental chronology. Radiographic examination of the maxillary arch revealed severe alveolar bone loss, and root resorption of the upper canine. Clinical treatment comprised the extraction of the remaining deciduous teeth due to severe periodontitis, mobility, and root resorption. Full mandibular and maxillary dentures were constructed. Physical and oral examinations of the other family members (III6; III7; III19; IV10; IV11; IV30; IV33 – Figure 1-pedigree) did not show any similar oral or cutaneous pathology.
The CTSC mutation analysis showed a (c.267–268del/ c.267–268del) mutation in exon 2, introducing a premature stop codon. The proband was homozygous for the mutation in exon 2 (c.267–268del/ c.267–268del). Both parents were heterozygous for the wild type and the mutant alleles (Wildtype/c.267_268del; Figure 4).
Figure 4.
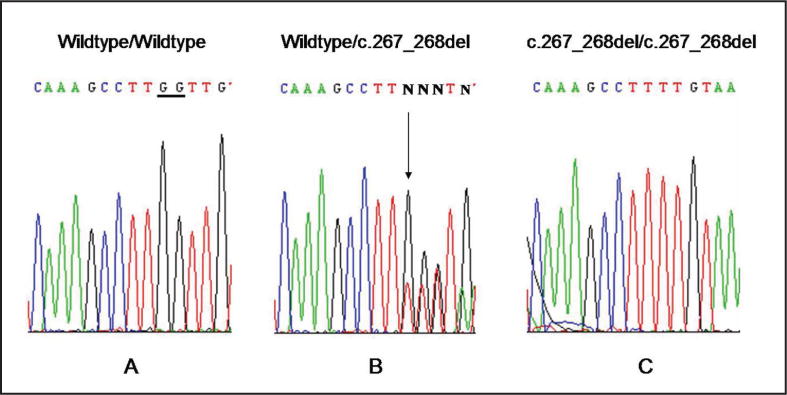
Sequence analysis of cathepsin C gene. (a) Wildtype/wildtype sequence. (b) Mother’s sequence Wildtype/c.267_268del – heterozygous. (c) Proband sequence c.267–268del/c.267–268del mutation.
DISCUSSION
Reports of Papillon-Lefèvre and related conditions have been noted in individuals from around the world, and more then 60 different CTSC mutations are reported in the literature. There have been relatively few reports, however, from South America (Table 1).
Table 1.
Table of All Mutations to Date, Including the Effect, Location, Origin of the Patients, and References
| Location | Mutation | Origin | References |
|---|---|---|---|
| Exon 1 | c.90C>A; c.90C>A; c.96T>G; c.112delCCTG; c.116G>C; c.145C>T | Europe and North Africa, Morocco, Thailand, North America, China, Puerto Rico, South India | 6, 19–25 |
| Exon 2 | c.199–222del; c.205C>T; c.267–268del | China, India, Brazil | 7, 24, this case report |
| Exon 3 | c.322A>T; c.380A>C; c.386T>A; c.415G>A; c.436delT; c.445–446insATGT; c.458C>T | Germany, France, Turkey, North America, China, Bangladesh, Scotland, Egypt | 7, 14, 18, 22, 23, 27, 28 |
| Intron 3 | IVS3-1G>A | Not specified | 10 |
| Exon 4 | c.555G>A; c.566–572Del; c.587T>C; c.622–623insC; c.628C>T; c.629–630 delGA | Brazil, Germany, Russia, Turkey, Algeria, India | 7, 10, 15, 16, 19, 27, 29 |
| Exon 5 | c.704G>A; c.706G>T; c.71 1del14; c.745G>T ; c.748C>T; c.755A>T; c.756 757ins130 | Iran, Spain, Europe, North Africa, Turkey, North America, China, Eritrea, Morocco | 7, 10, 19, 25, 28, 30–33 |
| Exon 6 | c.778T>C; c.815G>C; c.815G>A; c.854 C>T c.856C>T ; c.857A>G; c.872G>A; c.880T>C | China, Turkey, North America, France, Belgium, Morroco, India, Spain | 7, 9–11, 19, 20, 22, 23, 27, 30, 31, 34 |
| Exon 7 | c.898G>A; c.899G>A; c.901G>A; c.902G>T; c.910T>A ; c.912C>A; c.935A>G; c.947T>G; c.956A>G; c.984del7bp; c.1015C>T; c. 1019A>G ; c.1028–1029delCT c. 1040A>G, c.1047delA; c.1056delT; c.1141delC; c. 1156G>C; c.1213C>A; c. 1214A>G; c.1213–1215delCAT; c.1268G>C; c.1269G>A; c.1286G>A; c. 1287G>C; c.1360A>G | Vietnam, North America, Iran, Japan, Germany, Panama, South India, Egypt, Germany, France, Martinique, Turkey, North America, Pakistan, Sri Lanka | 6, 7, 9, 10, 11, 19, 24, 22, 27–29, 33, 35–37 |
It has been reported that PLS may actually be under-reported, in part due to incorrect diagnoses. While the initial clinical signs of skin involvement in PLS patients are usually evident during the first 4 years of life, the onset of oral findings are reported to be more variable. In this study, the child was initially treated for a dermatological condition, and a PLS diagnosis was only made after the premature loss of teeth had occurred. The differential diagnoses include Haim-Munk syndrome, which also exhibits additional findings on the hands, feet, and nails, including: arachnodactly; acroosteolysis; tapered and pointed distal phalanges; claw-like volar curve; pes planus; and onychogryposis.1 None of these features, however, were found in the present case.
By the time the patient was seen, the treatment plan decision was to extract the 3 remaining teeth—because they were mobile and the patient was in pain, and construct full dentures to improve mastication and esthetics. The permanent teeth will erupt and, unless antibiotic coverage is initiated, likely will repeat severe periodontal inflammation and lead to destruction, with premature exfoliation of the permanent dentition.5,18 Strategies to treat a PLS patient can be preplanned and can include: oral hygiene instruction; use of antimicrobial rinses; scaling and root planing (removal of sub/supra deposits, instrumentation of tooth and root surfaces); multiple antibiotic regimens; periodontal surgery; and extraction of hopeless teeth. Early diagnosis, treatment, and long-term maintenance are important to prevent or delay tooth loss and enhance early replacement of missing teeth for preservation of function and esthetics.
CONCLUSIONS
Sequence analysis of the CTSC gene in this family identified a novel CTSC gene mutation. The 2 base pair deletion resulted in a premature stop codon and premature termination of the CTSC gene in exon 2 (c.267_268del/p.N89fsX92). This mutation is expected to result in inactivation of the CTSC enzyme.10 Consequently, the proband would be expected to not have the ability to cleave the neutrophil serine proteases cathepsin G, neutrophil elastase, and proteinase 3.11,12 The inability to generate functional neutrophil serine proteases may also prevent proteolysis of macrophage inflammatory protein-1, which may be in part responsible for the severe periodontal inflammation associated with erupted teeth and associated alveolar bone loss. This novel mutation brings the total reported number of cathepsin C mutations to 65 (Table 1) and the number reported from South America to 3.15–17
Contributor Information
Debora Pallos, Department of Periodontics, University of Taubaté, São Paulo, Brazil.
Ana Carolina Acevedo, Department of Dentistry, School of Health Science, University of Brazilia – Oral Care Center for Inherited Diseases, University Hospital of Brasilia, Brasília, Brazil.
Heliana Dantas Mestrinho, Department of Dentistry, School of Health Science, University of Brazilia – Oral Care Center for Inherited Diseases, University Hospital of Brasilia, Brasília, Brazil.
Ilia Cordeiro, Department of Dentistry, School of Health Science, University of Brazilia – Oral Care Center for Inherited Diseases, University Hospital of Brasilia, Brasília, Brazil.
Thomas C. Hart, Human Craniofacial Genetics Section, National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Md.
P. Suzanne Hart, Office of the Clinical Director, National Human Genome Research Institute, National Institutes of Health, Bethesda, Md.
References
- 1.Online Mendelian Inheritance in Man, OMIM (TM) Johns Hopkins University; Baltimore, Md: Available at: www.ncbi.nlm.nih.gov/omim. Accessed March 6, 2009. [Google Scholar]
- 2.Papillon MN, Lefevre B. Two cases of familial symmetric palmoplantar keratosis (Maleda’s disease) in a brother and his sister. Alterations in both cases (in French) Bull Soc Fr Dermatol Syphiligr. 1924;31:81–4. [Google Scholar]
- 3.Gorlin RJ, Cohen MM, Hennekam RCM. Syndromes of the Head and Neck. 4. New York: Oxford University Press; 2001. pp. 1101–2. [Google Scholar]
- 4.Gorlin RJ, Sedano H, Anderson VE. The syndrome of palmar-plantar hyperkeratosis and premature periodontal destruction of the teeth: A clinical and genetic analysis of the Papillon-Lefevre syndrome. J Pediatr. 1964;65:895–908. doi: 10.1016/s0022-3476(64)80014-7. [DOI] [PubMed] [Google Scholar]
- 5.Haneke E. The Papillon-Lefevre syndrome: Keratosis palmoplantaris with periodontopathy. Report of a case and review of the cases in the literature. Hum Genet. 1979;51:1–35. doi: 10.1007/BF00278288. [DOI] [PubMed] [Google Scholar]
- 6.Nakano A, Nomura K, Nakano H, et al. Papillon-Lefevre syndrome: mutations and polymorphisms in the cathepsin C gene. J Invest Dermatol. 2001;116:339–43. doi: 10.1046/j.1523-1747.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 7.Hart PS, Zhang Y, Firatli E, et al. Identification of cathepsin C mutations in ethnically diverse Papillon-Lefevre syndrome patients. J Med Genet. 2000;37:927–32. doi: 10.1136/jmg.37.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart TC, Bowden DW, Ghaffar KA, et al. Sublocalization of the Papillon-Lefevre syndrome locus on 11q14-q21. Am J Med Genet. 1998;79:134–9. doi: 10.1002/(sici)1096-8628(19980901)79:2<134::aid-ajmg9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Hart TC, Hart PS, Bowden DW, et al. Mutations of the cathepsin C gene are responsible for Papillon-Lefevre syndrome. J Med Genet. 1999;36:881–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Toomes C, James J, Wood AJ, et al. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet. 1999;23:421–4. doi: 10.1038/70525. [DOI] [PubMed] [Google Scholar]
- 11.de Haar SF, Jansen DC, Schoenmaker T, De Vree H, Everts V, Beertsen W. Loss-of-function mutations in cathepsin C in two families with Papillon-Lefevre syndrome are associated with deficiency of serine proteinases in PMNs. Hum Mutat. 2004;23:524. doi: 10.1002/humu.9243. [DOI] [PubMed] [Google Scholar]
- 12.Pham CTN, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefevre syndrome: Correlating the molecular, cellular, and clinical consequences of the cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immun. 2004;173:7277–81. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 13.Rao NV, Rao GV, Hoidal JR. Human dipeptidyl-peptidase I. J Biol Chem. 1997;272:10260–5. doi: 10.1074/jbc.272.15.10260. [DOI] [PubMed] [Google Scholar]
- 14.Cagli NA, Hakki SS, Dursun R, et al. Clinical, genetic, and biochemical findings in two siblings with Papillon-Lefevre syndrome. J Periodontol. 2005;76:2322–9. doi: 10.1902/jop.2005.76.12.2322. [DOI] [PubMed] [Google Scholar]
- 15.Hart PS, Pallos D, Zhang Y, et al. Identification of a novel cathepsin C mutation (p.W185X) in a Brazilian kindred with Papillon-Lefevre syndrome. Mol Genet Metab. 2002;76:145–7. doi: 10.1016/s1096-7192(02)00031-8. [DOI] [PubMed] [Google Scholar]
- 16.Cury VF, Costa JE, Gomez RS, Boson WL, Loures CG, De ML. A novel mutation of the cathepsin C gene in Papillon-Lefevre syndrome. J Periodontol. 2002;73:307–12. doi: 10.1902/jop.2002.73.3.307. [DOI] [PubMed] [Google Scholar]
- 17.Cury VF, Gomez RS, Costa JE, Friedman E, Boson W, De Marco L. A homozygous cathepsin C mutation associated with Haim-Munk syndrome. Br J Dermatol. 2005;152:353–6. doi: 10.1111/j.1365-2133.2004.06278.x. [DOI] [PubMed] [Google Scholar]
- 18.Hart TC, Shapira L. Papillon-Lefèvre syndrome. Periodontol 2000. 1994;6:88–100. doi: 10.1111/j.1600-0757.1994.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 19.Lefevre C, Blanchet-Bardon C, Jobard F, et al. Novel point mutations, deletions, and polymorphisms in the cathepsin C gene in nine families from Europe and North Africa with Papillon-Lefevre syndrome. J Invest Dermatol. 2001;117:1657–61. doi: 10.1046/j.0022-202x.2001.01595.x. [DOI] [PubMed] [Google Scholar]
- 20.Allende LM, Moreno A, de Unamuno P. A genetic study of cathepsin C gene in two families with Papillon-Lefevre syndrome. Mol Genet Metab. 2003;79:146–8. doi: 10.1016/s1096-7192(03)00070-2. [DOI] [PubMed] [Google Scholar]
- 21.Nitta H, Wara-Aswapati N, Lertsirivorakul J, et al. A novel mutation of the cathepsin C gene in a Thai family with Papillon-Lefevre syndrome. J Periodontol. 2005;76:492–6. doi: 10.1902/jop.2005.76.3.492. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Hart PS, Moretti AJ, et al. Biochemical and mutational analyses of the cathepsin c gene (CTSC) in three North American families with Papillon-Lefevre syndrome. Hum Mutat. 2002;20:75. doi: 10.1002/humu.9040. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Bai X, Liu H, Li L, Cao C, Ge LJ. A novel mutation of cathepsin C gene in two Chinese patients with Papillon-Lefevre syndrome. Dent Res. 2007;86:735–8. doi: 10.1177/154405910708600809. [DOI] [PubMed] [Google Scholar]
- 24.Selvaraju V, Markandaya M, Prasad PV, et al. Mutation analysis of the cathepsin C gene in Indian families with Papillon-Lefevre syndrome. BMC Med Genet. 2003;12:4–5. doi: 10.1186/1471-2350-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Bai XW, Liu HS, Cao CF, Ge LH. Novel mu-tations of cathepsin C gene in two Chinese patients with Papillon-Lefevre syndrome. Zhonghua Kou Qiang Yi Xue Za Zhi. 2006;41:602–5. [PubMed] [Google Scholar]
- 26.Hewitt C, Wu CL, Hattab FN, et al. Coinheritance of two rare genodermatoses (Papillon-Lefevre syndrome and oculocutaneous albinism type 1) in two families: A genetic study. Br J Dermatol. 2004;151:1261–5. doi: 10.1111/j.1365-2133.2004.06237.x. [DOI] [PubMed] [Google Scholar]
- 27.Noack B, Görgens H, Schacher B, et al. Functional cathepsin C mutations cause different Papillon-Lefèvre syndrome phenotypes. J Clin Periodontol. 2008;35:311–6. doi: 10.1111/j.1600-051X.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 28.Hewitt C, McCormick D, Linden G, et al. The role of cathepsin C in Papillon-Lefevre syndrome, prepubertal periodontitis, and aggressive periodontitis. Hum Mutat. 2004;23:222–8. doi: 10.1002/humu.10314. [DOI] [PubMed] [Google Scholar]
- 29.Wani AA, Devkar N, Patole MS, Shouche YS. Description of two new cathepsin C gene mutations in patients with Papillon-Lefevre syndrome. J Periodontol. 2006;77:233–7. doi: 10.1902/jop.2006.050124. [DOI] [PubMed] [Google Scholar]
- 30.Allende LM, Garcia-Perez MA, Moreno A, et al. Cathepsin C gene: First compound heterozygous patient with Papillon-Lefevre syndrome and a novel symptom less mutation. Hum Mutat. 2001;17:152–3. doi: 10.1002/1098-1004(200102)17:2<152::AID-HUMU10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Pham CT, Ivanovich JL, Raptis SZ, Zehnbauer B, Ley TJ. Papillon-Lefèvre syndrome: Correlating the molecular, cellular, and clinical consequences of cathepsin C/dipeptidyl peptidase I deficiency in humans. J Immunol. 2004;173:7277–81. doi: 10.4049/jimmunol.173.12.7277. [DOI] [PubMed] [Google Scholar]
- 32.Schacher B, Baron F, Ludwig B, Valesky E, Noack B, Eickholz P. Periodontal therapy in siblings with Papillon-Lefèvre syndrome and tinea capitis: A report of two cases. J Clin Periodontol. 2006;33:829–36. doi: 10.1111/j.1600-051X.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- 33.Jouary T, Goizet C, Coupry I, et al. Detection of an intragenic deletion expands the spectrum of CTSC mutations in Papillon-Lefèvre syndrome. J Invest Dermatol. 2008;128:322–5. doi: 10.1038/sj.jid.5700987. [DOI] [PubMed] [Google Scholar]
- 34.Hart TC, Hart PS, Michalec Md, et al. Haim-Munk syndrome and Papillon-Lefevre syndrome are allelic mutations in cathepsin C. J Med Genet. 2000;37:88–94. doi: 10.1136/jmg.37.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Lundgren T, Renvert S, et al. Evidence of a founder effect for four cathepsin C gene mutations in Papillon-Lefevre syndrome patients. J Med Genet. 2001;38:96–101. doi: 10.1136/jmg.38.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noack B, Gorgens H, Hoffmann T, Fanghanel J, Kocher T, Eickholz P, Schackert HK. Novel mutations in the cathepsin C gene in patients with prepubertal aggressive periodontitis and Papillon-Lefevre syndrome. J Dent Res. 2004;83:368–70. doi: 10.1177/154405910408300503. [DOI] [PubMed] [Google Scholar]
- 37.Hart TC, Hart PS, Michalec Md, et al. Localisation of a gene for prepubertal periodontitis to chromosome 11q14 and identification of a cathepsin C gene mutation. J Med Genet. 2000;37:95–101. doi: 10.1136/jmg.37.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Haar SF, Mir M, Nguyen M, et al. Gene symbol: CTSC. Disease: Papillon-Lefevre syndrome. Hum Genet. 2005;116:545. [PubMed] [Google Scholar]


