The prevalence of dementia is increasing worldwide, with a predicted rise from 35.6 million in 2010 to 115.4 million in 2050.1 The economic cost of medical, social, and informal care was estimated at £391 billion in 2010 (€533 billion; US$604 billion), exceeding the economies of many countries.1 The lack of treatment options also increases fear and uncertainty for individuals and their families, leading the English Department of Health to say that ‘Among the over-55s, dementia is feared more than any other illness.’ 2
The challenge of dementia has been recognised internationally. Many countries have now produced dementia strategies encompassing diagnosis, support for patients and carers, and increased research funding; however, improving diagnosis has been contentious. In England, one particular strategy was to improve the rate of diagnosis and appropriate post-diagnosis support by March 2015. To achieve this, dementia diagnosis was incentivised in general practice by implementing an optional enhanced service under the terms of the general medical services contract. Taking a case-finding approach, practices that joined the enhanced service received a financial payment for every patient assessed for the early signs of dementia. However, this has been controversial with critics highlighting the difficulty of accurate diagnosis, particularly in the early stages of dementia, as no more than half of those patients with mild cognitive impairment develop dementia.3 The lack of appropriate care in the community, fragmented health and social care, and the lack of effective treatment options have also been raised. Finally, this approach to dementia screening fails the UK’s own National Screening Committee criteria.4 Given these difficulties, perhaps the focus should shift from improving early diagnosis to improving mid-life prevention.
THE ROLE OF MODIFIABLE RISK FACTORS
While non-modifiable risk factors, in particular age and genetic factors, play a major role in the development of dementia, an increasing body of evidence has highlighted the role for modifiable risk factors that exacerbate, or reduce, one’s risk of developing dementia in later life.5,6 Good quality evidence exists identifying the following as exacerbating risk: depression; type 2 diabetes; smoking; mid-life hypertension; mid-life obesity; physical inactivity; and low educational attainment. Although the evidence is weaker, factors that may reduce the risk include vegetable intake, Mediterranean diet, and increased cognitive activity. Finally, there is weak evidence suggesting that early-life events, such as the death of a parent, and chronic sleep disturbances in mid-life may also exacerbate the risk of developing dementia.5
The importance of cardiometabolic risk factors that develop in mid-life, such as hypertension and obesity, as well as the contribution of smoking and physical inactivity, have led to the view that approaches are required that target populations well before they develop dementia, while still in their 40s and 50s.5,7 Several countries have now acknowledged the need for preventive strategies targeting dementia and the clear overlap with cardiovascular risk and the risk that diabetes poses suggests that dementia could be added to current chronic disease management programmes located in primary care5 and to wider programmes of public health. The recent Blackfriars Consensus statement on promoting brain health suggests strategies to bring dementia prevention into wider health policy and healthcare delivery,8 and these have now been incorporated into draft public health guidance from the National Institute for Health and Care Excellence.9 However, it is apparent that the public need to be more aware of the association of these modifiable risk factors with later dementia risk and that practitioners need to be more proactive in raising this with patients.
AWARENESS OF DEMENTIA RISK
Despite national campaigns, the public is not yet fully aware of the links between mid-life health-related behaviour and dementia.10 For example, Alzheimer’s Australia found that while 51% of Australians believed risk reduction was possible, 20% believed nothing could be done to reduce dementia risk (and a further 28% were unsure).10 There was greater awareness of a role for mental, social, and physical activity in reducing dementia risk, but few knew about the role of vascular risk factors, for example smoking, hypertension, and high cholesterol, in developing dementia. Thus, it is clear that there is still much work to be done in raising awareness and educating the general public about the links between risk factors associated with cardiovascular disease, linked to dementia, and the association between diabetes and later risk of dementia. There has been little or no research exploring the views of general GPs and other primary care staff, such as practice nurses, with respect to their knowledge of modifiable risk factors related to dementia, or to what extent they discuss such issues with patients in mid-life. Indeed, there is evidence that those working in the public health field have a low awareness of the role that modifiable risk factors have in dementia development,11 and, therefore, it is likely that a similar situation exists for general practice and primary care. Addressing these issues is part of the rationale for the In-MINDD (the Innovative Midlife Intervention for Dementia Deterrence) project.
In-MINDD
In-MINDD brings together these two important concepts: first, that there is a group of potentially modifiable and interlinked risk factors which, if addressed in mid-life, may reduce the risk of developing dementia in later life, or at least delay its onset; and second, that primary care, and in particular general practice, can play an important role in ensuring that patients are more aware of these links and can be supported to make the necessary lifestyle changes to address these risks. This work is being carried out in four European primary care systems: France, Ireland, the Netherlands, and Scotland, UK. An important distinction between In-MINDD and other dementia-related randomised controlled trials (RCTs) targeting cardiovascular and lifestyle-related risk factors, is that In-MINDD is targeting those in mid-life, namely 40–60-year-olds.
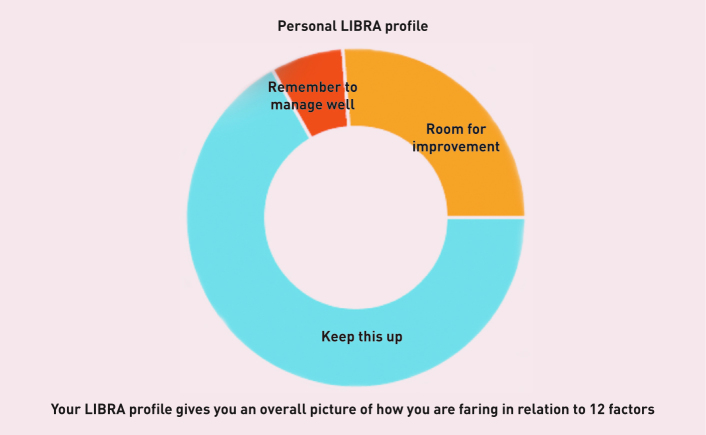
In-MINDD is tackling this in three stages. First, we have identified and ranked putative risk factors.6 Then, using these risk factors, we have developed an online profiler based on personalised demographic, lifestyle, and clinical information. This results in individuals receiving information in the form of a personalised Lifestyle for Brain Health (LIBRA) global score and profile (Figure 1).
Figure 1.
LIBRA profile. ‘Keep this up’ = risk factors that patients are currently managing well or conditions that they currently do not have. ‘Room for improvement’ = areas that could be targeted for behaviour change strategies, for example smoking or physical inactivity. ‘Remember to manage well’ = if patients already have a condition such as diabetes or coronary heart disease.
A linked online support environment gives individuals information on their identified risk factors, outlines the national recommendations in their relevant country and supports goal setting to change behaviour. The final stage of the work is testing the effectiveness of this approach through a feasibility RCT. As part of the RCT, we will conduct qualitative interviews with participants, GPs, and practice nurses to explore their use of the LIBRA score and profile and, importantly, their awareness and understanding of modifiable risk factors for dementia.
Conducting this work will offer important opportunities to better understand what both patients and practitioners in primary care know and understand about modifiable risk factors for dementia and how we can best design and implement interventions to support them to address and incorporate the necessary health-related behaviour change into everyday life. It will also raise awareness about the inter-connectedness of dementia prevention with that of better recognised diseases, such as diabetes and cardiovascular disease, and may provide opportunities for general practice, and primary care more widely, to address dementia risk in a more timely and appropriate way than some of the diagnosis strategies being proposed for general practice. Although still a major health problem, dementia incidence and prevalence are decreasing in some countries, possibly as a result of systematic approaches targeting cardiometabolic disease.5 Research is needed to help us understand which risk factors healthcare professionals should target, and to understand the comparative benefits of targeting non-metabolic risk factors, such as cognitive activity, alongside cardiometabolic risk factors such as hypertension control, lowering of cholesterol, and smoking cessation. This should help general practice identify and develop its role in the prevention or delay of dementia.
Acknowledgments
Thanks to Maria Pierce, Susan Browne, Kay Deckers, Martin PJ van Boxtel and Frans Verhey for their insightful contributions to this editorial. For more information about the In-MINDD project see: http://www.inmindd.eu/
Funding
In-MINDD is funded by the European Community’s Framework Programme Seven (FP7) under contract #304979. All materials developed as part of this study have been funded from this project.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
REFERENCES
- 1.Wimo A, Prince M. World Alzheimer Report 2010 The global economic impact of dementia. http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf (accessed 21 Sep 2015). [DOI] [PubMed]
- 2.Department of Health Prime Minister’s challenge on dementia Delivering major improvements in dementia care and research by 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215101/dh_133176.pdf (accessed 21 Sep 2015).
- 3.Brunet MD, McCartney M, Heath I, et al. There is no evidence base for proposed dementia screening. BMJ. 2012;345:e8588. doi: 10.1136/bmj.e8588. [DOI] [PubMed] [Google Scholar]
- 4.Fox C, Lafortune L, Boustani M, et al. The pros and cons of early diagnosis in dementia. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X669374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prince M, Albanese E, Guerchet M, et al. World Alzheimer Report 2014. Dementia and risk reduction: an analysis of protective and modifiable factors. http://www.alz.co.uk/research/WorldAlzheimerReport2014.pdf (accessed 21 Sep 2015).
- 6.Deckers K, van Boxtel MPJ, Schiepers OJG, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234–246. doi: 10.1002/gps.4245. [DOI] [PubMed] [Google Scholar]
- 7.Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 8.Public Health England, UK Health Forum Blackfriars Consensus on promoting brain health: reducing risks for dementia in the population. http://nhfshare.heartforum.org.uk/RMAssets/Reports/Blackfriars%20consensus%20%20_V18.pdf (accessed 21 Sep 2015).
- 9.National Institute for Health and Care Excellence Dementia, disability and frailty in later life — mid-life approaches to prevention. https://www.nice.org.uk/guidance/gid-phg64/resources/disability-dementia-and-frailty-in-later-life-midlife-approaches-to-prevention-draft-guidance2 (accessed 21 Sep 2015).
- 10.Smith B, Ali S, Quach H. Public knowledge and beliefs about dementia risk reduction: a national survey of Australians. BMC Public Health. 2014;14(1):661. doi: 10.1186/1471-2458-14-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Health Forum Knowledge and awareness among the public health workforce in the UK about the prevention of dementia. http://nhfshare.heartforum.org.uk/RMAssets/Reports/Dementia%20Workforce%20survey%20report_Final.pdf (accessed 21 Sep 2015).