Abstract
Background
Regular physical activity reduces falls, hip fractures, and all-cause mortality, but physical activity levels are low in older age groups.
Aim
To evaluate two exercise programmes promoting physical activity among older people.
Design and setting
Pragmatic three-arm, parallel-design cluster randomised controlled trial involving 1256 people aged ≥65 years (of 20 507 invited) recruited from 43 general practices in London, Nottingham, and Derby.
Method
Practices were randomised to the class-based Falls Management Exercise programme (FaME), the home-based Otago Exercise Program (OEP), or usual care. The primary outcome was the proportion reaching the recommended physical activity target 12 months post-intervention. Secondary outcomes included falls, quality of life, balance confidence, and costs.
Results
In total, 49% of FaME participants reached the physical activity target compared with 38% for usual care (adjusted odds ratio 1.78, 95% confidence interval [CI] =1.11 to 2.87, P = 0.02). Differences between FaME and usual care persisted 24 months after intervention. There was no significant difference comparing those in the OEP (43% reaching target at 12 months) and usual-care arms. Participants in the FaME arm added around 15 minutes of moderate-to-vigorous physical activity per day to their baseline level; this group also had a significantly lower rate of falls (incident rate ratio 0.74, 95% CI = 0.55 to 0.99, P = 0.042). Balance confidence was significantly improved in both intervention arms. The mean cost per extra person achieving the physical activity target was £1740. Attrition and rates of adverse reactions were similar.
Conclusion
The FaME programme increases self-reported physical activity for at least 12 months post-intervention and reduces falls in people aged ≥65 years, but uptake is low. There was no statistically significant difference in reaching the target, or in falls, between the OEP and usual-care arms.
Keywords: aged people, exercise promotion, falls, general practice, physical activity
INTRODUCTION
Physical activity reduces the risk of cardiovascular disease, type 2 diabetes, osteoporosis, falls, hip fractures, certain cancers, and all-cause mortality.1–3 Promoting physical activity in older people could prevent functional decline, frailty, falls, and fractures.4 Current physical activity recommendations are 150 minutes of moderate-to-vigorous physical activity (MVPA) per week,5 including activities that challenge balance. However, physical inactivity is widespread6 and the most effective ways of increasing and maintaining physical activity remain unclear. This article reports the findings from a trial of exercise programmes for older people on achievement and maintenance of recommended physical activity targets.
METHOD
The full trial protocol is reported elsewhere,7 as are changes to the protocol introduced during the trial.8 Full details of the trial design, recruitment, analysis, and reporting of findings (including a CONSORT statement) are available from the Health Technology Assessment (HTA) report of the study.9
A three-arm, parallel-design cluster controlled trial was used, with allocation at the level of general practice in two centres (London and Nottingham/Derby).
Eligibility
Practices were recruited with assistance from Primary Care Research Networks for London, Derby, and Nottingham. Patients aged ≥65 years were identified by searches of computerised medical records and invited to participate by letter from their GP.
Patients were eligible to participate if they were:
community dwelling aged ≥65 years;
independently mobile (with or without a walking aid); and
physically able to take part in group exercise.
Patients were excluded if they:
had experienced ≥3 falls in the previous year;
had unstable clinical conditions;
would be unable to follow instructions about exercise safely; or
were receiving palliative care.
Attempts were made to exclude those who were already exercising at, or above, the target level through a pre-assessment screening telephone call.
Interventions
The interventions were the home-based Otago Exercise Programme (OEP),10 and a group-based exercise programme called Falls Management Exercise (FaME).11 The FaME intervention took place in a group once a week and included exercises to be carried out at home, unsupervised, twice weekly. The OEP was undertaken at home, unsupervised, and comprised exercises to be done three times per week. The programmes included the following progression:
OEP — ankle weights and hand holds; and
FaME — resistance bands and hand holds, plus a move to more dynamic balance work and floor work with postural stability instructors.
How this fits in
Physical activity protects against disablement and a range of diseases in later life, but the older population is largely inactive. The most effective ways to increase and maintain physical activity levels in older people over prolonged periods are unclear. A class-based exercise programme added, on average, around 15 minutes of moderate-to-vigorous physical activity per day in older people and class participants experienced significantly fewer falls, but uptake of exercise promotion was low.
Volunteer peer mentors supported participants in the OEP arm, and weekly FaME classes were run in local venues by postural stability instructors trained to work with older people. Both interventions were delivered for 24 weeks. Participants in the usual-care arm were not offered either programme.
Outcomes and outcome measures
The primary outcome was the proportion of participants who reported reaching ≥150 minutes of MVPA per week. Physical activity was assessed using three validated questionnaires: Phone-FITT, the Physical Activity Scale for the Elderly (PASE), and the Community Healthy Activities Model Program for Seniors (CHAMPS). The primary outcome measure was the CHAMPS questionnaire.
Secondary outcomes are described in full in the trial protocol7 but included:
balance confidence (CONFbal scale);
falls efficacy (Falls Efficacy Scale-International [FES-I]);
positive and negative outcomes expectations for exercise (Outcome Expectations for Exercise scale [OEE]);
quality of life (Older People’s Quality of Life Questionnaire [OPQOL], EQ-5D, and 12-item Short Form Health Survey [SF-12]);
social network size and density (brief Lubben Social Network Scale);
perceived social support (Multidimensional Scale of Perceived Social Support [MSPSS]);
falls risk (Falls Risk Assessment Tool [FRAT]);
Timed Up and Go;
forward reach; and
the 30-second chair stand.
Demographic information, comorbidities, and medication were recorded at baseline; use of general practice and hospital and community social services was recorded at follow-up assessments. Falls were ascertained from diaries returned by participants every 4 weeks during the intervention and every 3 months in the subsequent year, and from reports given in follow-up telephone interviews using the Phone-FITT questionnaire. The NHS costs of delivering each exercise programme were captured from study records and included staff, facilities, equipment, and overheads.
Participants were followed up every 6 months after the end of the intervention period, until 24 months. The primary time point was chosen as 12 months post-intervention to match other trials.7
Sample size
Sample size estimates were based on detecting differences between each intervention and the control arm in proportions of participants reaching ≥150 minutes of MVPA per week using 90% power, a two-sided 5% significance level, an intra-cluster correlation coefficient of 0.01, 30% attrition, a mean cluster size post-attrition of 32, and the percentage achieving physical activity target of 14.6% in the intervention arms and 4.9% in the control arm.12
Randomisation
Minimisation using site, practice size, and deprivation was used to allocate practices to treatment arms,13 once all participants from a practice were recruited. Full details are available in the trial report.7
Blinding
General practices, participants, and researchers having contact with practices and participants were blind to the treatment arm until all participants within a practice were recruited. Analyses were undertaken without researchers being blind to the treatment arm allocation. To minimise bias an a priori statistical analysis plan with the trial steering committee was agreed.
Statistical methods
Participants were analysed in the groups to which they were randomised, regardless of whether they received the intervention or not, and multiple imputation was used to include all participants in the analyses. Baseline characteristics were compared informally between treatment arms. Comparisons between treatment arms were made using random-effects regression models to allow for clustering between practices. Linear models were used for continuous outcome variables, logistic models for binary outcome variables, and negative binomial models for data on rate of falls. The CHAMPS score measuring minutes of physical activity followed a lognormal distribution with many zeros, and was therefore transformed to log e(CHAMPSscore+1).
The proportions of participants whose weekly MVPA exceeded 150 minutes, and those who reported zero MVPA, were tabulated for all time points. All analyses were undertaken adjusted for minimisation variables and for baseline outcome measure values. Differential effects of the intervention by age (>/<75 years) and sex were assessed for the primary outcome measures by adding interaction terms to the model.
The primary analysis was carried out on 572 participants with complete data on CHAMPS score at baseline and 12 months follow-up. Also carried out were two multiple imputation analyses on participants who:
had Phone-FITT data at 0 and 12 months, even if CHAMPS data was missing (n = 07); and
had entered into the study (n = 1254).
All other variables in the substantive model (randomisation arm, practice size, location, and deprivation status) were used to impute 12-month CHAMPS scores for the second analysis, while Phone-FITT scores at 0 and 12 months were additionally used for the first analysis. Fifty imputations were carried out in each case; results were combined using Rubin’s rules.
Adherence
Minutes of exercise undertaken by participants using activity diaries and attendance registers from FaME class instructors were estimated by the researchers. Adherence was defined as (minutes spent exercising/intended minutes) 100%, where patients with values exceeding 75% were regarded as ‘adherent’.10
Economic analysis
An embedded economic evaluation adopted the NHS perspective. The resources involved in the delivery of each intervention were gathered from study records at each site (London and Nottingham). Resources fell into four categories:
hire of venues (FaME classes, OEP induction session);
procurement of exercise equipment;
reimbursement of postural stability instructors for FaME and peer mentors for OEP; and
intervention management costs (including training of postural stability instructors and peer mentors).
A full economic cost was calculated in pound sterling at 2011 prices. Actual expenditures were used for the cost of non-human resources. The cost of postural stability instructors was based on the unit costs of an equivalent NHS grade (community physiotherapist), inclusive of oncosts and overheads.14 The value of volunteer peer mentor time for the OEP was established by replacement cost methods using the unit cost to the NHS of community clinical support workers.14 The total cost of each intervention at each site was established, and the cost per participant was calculated.
Safety
GPs checked the medical records of recruited participants for eligibility prior to commencement of the interventions. Exercise safety guidelines15,16 were followed. Adverse events and serious adverse events were assessed for seriousness, expectedness, and causality.
Written, informed consent was obtained from all participants and NHS Research and Development approval was granted for all practices.
RESULTS
In total, 43 practices invited 20 507 eligible patients to participate in the study. A total of 1256 (6%) were recruited between July 2009 and September 2011; one dropped out between recruitment and allocation, and one withdrew all data from the trial, giving a study population of 1254. The average age of participants was 73 (65–94) years, with 84% of participants aged <80 years; 62% of participants were female. In total, 34 languages were spoken as a first language by participants (33 in London and 12 in Nottinghamshire/Derbyshire) and 14% of participants were non-white, with greater ethnic diversity among the London participants. All in all, 44% of participants had completed some form of further education. On average, each participant had 1.7 comorbidities (range 0–7, standard deviation [SD] 1.4) and was taking 3.7 medications on repeat prescription (range 0–18, SD 3.7), with no differences between study arms. Trial participants performed below normative levels on most scales, except for Phone-FITT, PASE, CONFbal, and OPQOL; more details can be found in the HTA report.9
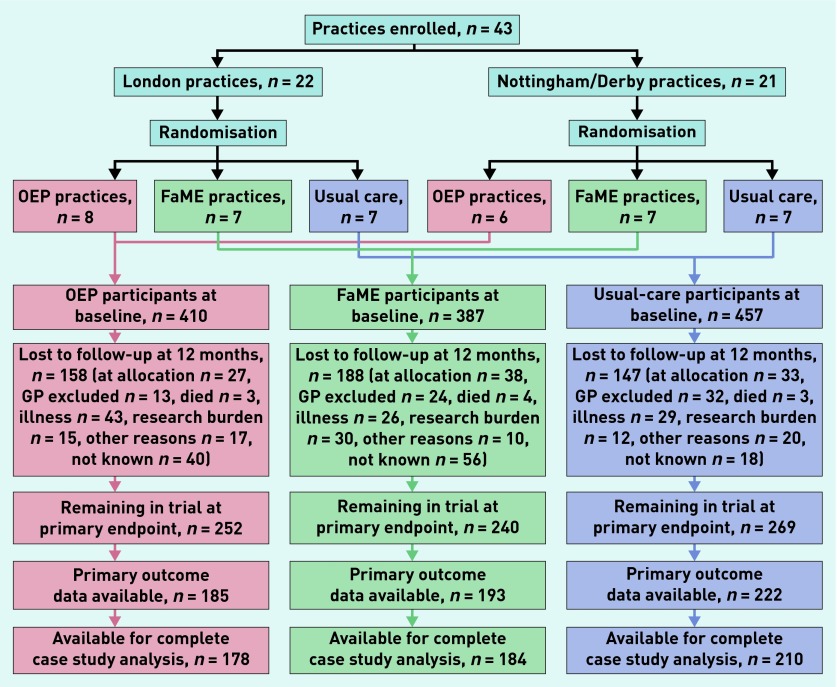
The progress of practices and participants through the trial is shown in Figure 1. A total of 761 (61%) participants remained in the trial at the primary end-point 12 months after the end of the intervention period. Of these, 600 (48%) had primary outcome data (CHAMPS score at 12 months) available and 572 (46%) had data available for complete case analysis at the primary endpoint (48%, 43%, and 46% for participants in the FaME, OEP, and usual-care arms respectively).
Figure 1.
Trial progress of practices and participants. FaME = Falls Management Exercise. OEP = Otago Exercise Programme. Research burden is the judgement by participants that research activities (completion of diaries and questionnaires) are burdensome to them.
All FaME classes were fully staffed but recruitment of peer mentors was difficult (as described elsewhere17) and not all participants in the OEP arm received per protocol peer mentor support. Baseline characteristics of practices and participants are shown by arm in Table 1; treatment arms were well balanced.
Table 1.
Baseline characteristics of practices and participants, by allocation arma
| Randomisation arm | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Usual care | Missing value | FaME | Missing value | OEP | Missing value | |
| Practice characteristics | ||||||
|
| ||||||
| Practices, n | 15 | – | 14 | – | 14 | – |
|
| ||||||
| Patients per practice, n (range) | 30.5 (5–49) | – | 27.6 (6–46) | – | 29.3 (15–43) | – |
|
| ||||||
| Practice size, n (range) | 6426 (2663–15 000) | – | 6883 (3000–11 200) | – | 7520 (2650–18 000) | – |
|
| ||||||
| Practice deprivation score (range)b | 24.9 (9.8–57) | – | 22.6 (3.1–57) | – | 29.9 (12.4–61.4) | – |
|
| ||||||
| Patient characteristics | ||||||
|
| ||||||
| Participants, nc | 457 | – | 387 | – | 410 | – |
| London | 204 | – | 168 | – | 231 | – |
| Nottingham | 253 | – | 219 | – | 179 | – |
|
| ||||||
| Sex, n (% female) | 283 (61.9) | – | 239 (61.8) | – | 260 (63.4) | – |
|
| ||||||
| Age, years (SD) [range] | 73.1 (6.2) [65–92] | – | 72.9 (6.1) [65–94] | – | 72.8 (5.8) [65–94] | – |
|
| ||||||
| BMI, kg/m2 (SD) [range] | 27.0 (5.1) [15.4–53.3] | 15 | 27.1 (5.3) [16.7–64.2] | 13 | 26.6 (4.9) [17.0–48.7] | 20 |
|
| ||||||
| Comorbidities, n (IQR) | 2 (1–3) | 0 | 2 (1–3) | 0 | 2 (1–3) | 0 |
|
| ||||||
| Current medications, n (IQR) | 4 (1–6) | 3 | 3 (2–6) | 1 | 4 (1–6) | 4 |
|
| ||||||
| English first language, n (%) | 411 (90.9) | 5 | 334 (87.7) | 6 | 358 (88.0) | 3 |
|
| ||||||
| Confidence in balance (10–30d) | 12.55 (3.93) | 68 | 12.63 (3.98) | 57 | 12.48 (3.76) | 57 |
|
| ||||||
| Falls efficacy (1–4d) (SD) | 9.36 (4.08) | 61 | 8.99 (3.56) | 54 | 8.89 (3.49) | 51 |
|
| ||||||
| EQ-5D (5–15d) (SD) | 0.68 (0.08) | 7 | 0.67 (0.09) | 7 | 0.68 (0.09) | 11 |
|
| ||||||
| OPQOL (35–175d) (SD) | 130.75 (13.53) | 115 | 129.36 (13.54) | 114 | 129.36 (12.69) | 98 |
|
| ||||||
| Lubben Social Network Scale (0–30d) (SD) | 15.93 (5.70) | 65 | 16.47 (5.76) | 57 | 15.44 (5.48) | 59 |
|
| ||||||
| MSPSS (12–84d) (SD) | 65.81 (17.96) | 82 | 65.93 (15.57) | 82 | 66.60 (15.49) | 80 |
|
| ||||||
| PASE (0–361d) (SD) | 119.19 (60.42) | 57 | 109.11 (52.21) | 45 | 119.85 (50.60) | 48 |
|
| ||||||
| Phone-FITT (frequency × durationd) (SD) | 36.80 (13.65) | 80 | 37.68 (13.67) | 71 | 41.18 (13.11) | 56 |
|
| ||||||
| CHAMPS MVPA (frequency × durationd) (SD) | 179.06 (239.38) | 57 | 171.14 (234.28) | 45 | 193.38 (244.96) | 48 |
|
| ||||||
| FRAT (1–5d) (SD) | 1.03 (0.96) | 4 | 0.89 (0.90) | 4 | 0.98 (0.90) | 8 |
|
| ||||||
| SF-12 physical (0–100d) (SD) | 38.74 (5.50) | 3 | 38.74 (5.64) | 1 | 38.78 (5.64) | 3 |
|
| ||||||
| SF-12 mental (0–100d) (SD) | 49.88 (6.09) | 3 | 49.60 (6.02) | 0 | 50.15 (5.86) | 3 |
|
| ||||||
| 30-second sit-to-stand, n (SD) | 10.49 (3.31) | 8 | 10.48 (3.64) | 10 | 10.27 (2.81) | 10 |
|
| ||||||
| Functional reach, cm (SD) | 24.68 (7.43) | 19 | 25.60 (6.98) | 16 | 25.57 (7.43) | 8 |
|
| ||||||
| Timed up and go, seconds (SD) | 11.11 (4.61) | 19 | 10.95 (4.94) | 50 | 11.18 (7.84) | 35 |
|
| ||||||
| OEE +ve (1–5d) (SD) | 3.84 (0.58) | 85 | 3.85 (0.62) | 78 | 3.85 (0.60) | 61 |
|
| ||||||
| OEE –ve (1–5d) (SD) | 3.85 (0.81) | 90 | 3.96 (0.75) | 67 | 3.90 (0.85) | 71 |
Mean figures are given unless otherwise stated.
Index of Multiple Deprivation, 2007.
One participant withdrew after assessment but before allocation, and another withdrew all data from the study.
Score. CHAMPS = Community Healthy Activities Model Program for Seniors. FaME = Falls Management Exercise. FRAT = Falls Risk Assessment Tool. IQR = interquartile range. MSPSS = Multidimensional Scale of Perceived Social Support. MVPA = moderate-to-vigorous physical activity. OEE = Outcome and Expectation for Exercise. OEP = Otago Exercise Programme. OPQOL = Older People’s Quality of Life. PASE = Physical Activity Scale for the Elderly. SD = standard deviation. SF-12 = 12-item Short Form Health Survey.
Primary outcome
The proportions reporting at least 150 minutes of MVPA per week rose from 40% (136/342) to 49% (95/193) in the FaME arm, 41% (150/362) to 43% (79/185) in the OEP arm, and 37.5% (150/400) to 38% (84/222) in the usual-care arm 12 months after the end of the intervention. Participants in the FaME arm, compared with usual care, reported more MVPA at 12 months, adding around 15 minutes of MVPA per day. The odds ratio (OR) for reaching or exceeding the target of 150 minutes of MVPA per weeks in the FaME arm was 1.78 (95% confidence interval [CI] =1.11 to 2.87, P = 0.02). There was no statistically significant difference in reaching the target between the OEP and usual-care arms (OR 1.17 (95% CI = 0.72 to 1.92), P = 0.52). Effect sizes did not vary significantly by age (P = 0.96) or sex (P = 0.63). Multiple imputation analysis indicated findings were robust to missing data.
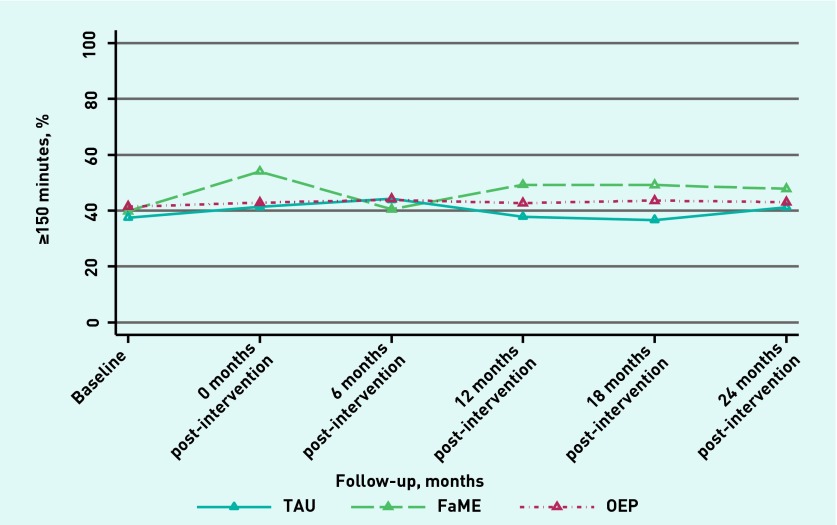
The increase in self-reported physical activity in participants in the FaME arm, compared with usual care, persisted at 24 months, as shown in Figure 2. Although the graph suggests a narrowing of the gap between the usual-care and FaME groups at 24 months, the test for a time × arm interaction was non-significant (P = 0.88).
Figure 2.
Trajectory over time of proportions of participants meeting the physical activity target. FaME = Falls Management Exercise. OEP = Otago Exercise Programme. TAU = treatment as usual.
Secondary outcomes
Table 2 shows secondary outcomes at 12 months post-intervention, and Table 3 shows falls rates by study arm. There were no statistically significant differences in secondary outcomes except for falls, balance confidence, and expectations of exercise (P = 0.05). There was a statistically significant reduction in the rate of falls in the FaME arm compared with the usual-care arm in the 12 months following the intervention (incidence rate ratio [IRR] 0.74, 95% CI = 0.55 to 0.99, P = 0.042), and a statistically non-significant reduction in the OEP arm (IRR 0.76, 95% CI = 0.53 to 1.09, P = 0.14) (Table 3). Balance confidence was significantly improved in both intervention arms (P = 0.03 for both) (Table 2). Those with negative expectations of exercise at baseline became significantly more positive in both arms (P = 0.001 for both); those already positive at baseline became significantly more so in the FaME arm (P = 0.003).
Table 2.
Secondary outcome variables at 12 months post intervention, with multi-level modelling results (group effects versus usual care)
| Scale | Arm, n at baseline | 12 months post-intervention | Multi-level modelling results | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| n | Mean (SD) | n | Estimate | 95% CI | P-value | ||
| PASE | Usual care, 400 | 222 | 122.5 (51.8) | 572 | n/a | n/a | n/a |
| FaME, 342 | 193 | 124.2 (53.3) | 11.19 | 0.194 to 22.191 | 0.05 | ||
| OEP, 362 | 185 | 126.8 (61.3) | 7.48 | −3.826 to 18.794 | 0.20 | ||
|
| |||||||
| Phone-FITT | Usual care, 377 | 225 | 47.71 (17.41) | 628 | n/a | n/a | n/a |
| FaME, 316 | 208 | 49.52 (15.95) | 2.303 | −0.531 to 5.137 | 0.11 | ||
| OEP, 354 | 237 | 49.38 (16.50) | 1.340 | −1.494 to 4.174 | 0.35 | ||
|
| |||||||
| OPQOL | Usual care, 342 | 185 | 134.80 (14.82) | 444 | n/a | n/a | n/a |
| FaME, 273 | 169 | 132.31 (15.98) | −0.794 | −2.848 to 1.260 | 0.45 | ||
| OEP, 312 | 156 | 133.72 (14.95) | 0.374 | −1.772 to 2.520 | 0.73 | ||
|
| |||||||
| SF-12 physical | Usual care, 454 | 217 | 39.11 (5.00) | 583 | n/a | n/a | n/a |
| FaME, 386 | 186 | 38.85 (4.92) | −0.211 | −1.125 to 0.703 | 0.65 | ||
| OEP, 407 | 183 | 39.30 (4.73) | 0.278 | −0.672 to 1.229 | 0.57 | ||
|
| |||||||
| SF-12 mental scale | Usual care, 454 | 217 | 49.16 (5.60) | 584 | n/a | n/a | n/a |
| FaME, 387 | 186 | 48.74 (5.81) | −0.430 | −1.506 to 0.646 | 0.43 | ||
| OEP, 407 | 183 | 49.05 (5.11) | −0.172 | −1.291 to 0.947 | 0.76 | ||
|
| |||||||
| Balance confidence (CONFbal) | Usual care, 389 | 218 | 12.38 (4.05) | 546 | n/a | n/a | n/a |
| FaME, 330 | 183 | 12.13 (3.65) | −0.529 | −0.998 to −0.061 | 0.03 | ||
| OEP, 353 | 179 | 12.23 (3.71) | −0.545 | −1.033 to −0.057 | 0.03 | ||
|
| |||||||
| Social network (MSPSS) | Usual care, 375 | 209 | 67.23(16.54) | 500 | n/a | n/a | n/a |
| FaME, 305 | 183 | 63.27 (17.69) | −2.480 | −5.637 to 0.677 | 0.12 | ||
| OEP, 330 | 171 | 63.46 (18.14) | −2.373 | −5.700 to 0.953 | 0.16 | ||
|
| |||||||
| Social network (Lubben Social Network Scale) | Usual care, 392 | 210 | 16.41 (5.79) | 533 | n/a | n/a | n/a |
| FaME, 330 | 181 | 15.68 (5.82) | −0.651 | −1.411 to 0.110 | 0.09 | ||
| OEP, 351 | 180 | 15.43 (5.35) | 0.176 | −0.624 to 0.976 | 0.67 | ||
|
| |||||||
| EQ-5D | Usual care, 450 | 212 | 0.68 (0.07) | 558 | n/a | n/a | n/a |
| FaME, 380 | 179 | 0.67 (0.07) | −0.009 | −0.022 to 0.005 | 0.23 | ||
| OEP, 399 | 176 | 0.68 (0.07) | 0.000 | −0.014 to 0.015 | 0.96 | ||
|
| |||||||
| FES-I | Usual care, 396 | 220 | 8.94 (3.66) | 561 | n/a | n/a | n/a |
| FaME, 333 | 188 | 9.20 (4.56) | 0.102 | −0.653 to 0.856 | 0.79 | ||
| OEP, 359 | 185 | 9.09 (4.19) | 0.045 | −0.740 to 0.831 | 0.91 | ||
|
| |||||||
| OEE +ive | Usual care, 372 | 252 | 3.85 (0.64) | 614 | n/a | n/a | n/a |
| FaME, 309 | 206 | 4.02 (0.55) | 0.130 | 0.043 to 0.216 | 0.003 | ||
| OEP, 349 | 211 | 3.93 (0.65) | 0.083 | −0.006 to0.171 | 0.066 | ||
|
| |||||||
| OEE –ive | Usual care, 367 | 248 | 3.96 (0.87) | 595 | n/a | n/a | n/a |
| FaME, 320 | 204 | 4.19 (0.75) | 0.200 | 0.077 to 0.323 | 0.001 | ||
| OEP, 339 | 203 | 4.20 (0.710 | 0.252 | 0.077 to 0.323 | 0.001 | ||
FaME = Falls Management Exercise. FES-I = Falls Efficacy Scale — International. MSPSS = Multidimensional Scale of Perceived Social Support. OEE = Outcome and Expectation for Exercise. OEP = Otago Exercise Programme. OPQOL = Older People’s Quality of Life. PASE = Physical Activity Scale for the Elderly. SD = standard deviation. SF-12 = 12-item Short Form Health Survey.
Table 3.
Comparisons of reported falls between intervention arms
| OEP | FaME | Usual care | |
|---|---|---|---|
| Falls during intervention | 108 | 96 | 118 |
| Person years at risk during intervention | 129.8 | 117.9 | 133.9 |
| Falls per person year during intervention | 0.80 | 0.81 | 0.87 |
| Rate ratio (95% CI) [P-value] during intervention compared with usual care | 0.93 (0.64 to 1.37) [0.72] | 0.91 (0.54 to 1.52) [0.72] | Reference |
| Falls in the first year post intervention | 98 | 100 | 153 |
| Person years at risk in the first year post intervention | 184 | 187.3 | 221.3 |
| Falls per person year in the first year post intervention | 0.54 | 0.57 | 0.71 |
| Rate ratio (95% CI) [P-value] in the first year post intervention (compared with usual care) | 0.76 (0.53 to 1.09) [0.14] | 0.74 (0.55 to 0.99) [0.04] | Reference |
FaME = Falls Management Exercise. OEP = Otago Exercise Programme.
FaME was more expensive than OEP (£269 versus £88 per participant in London; £218 versus £117 in Nottingham/Derby at 2011 prices). The cost per additional person achieving the target of ≥150 minutes of MVPA at the primary endpoint in the FaME arm was £1920 in London and £1560 in Nottingham (mean £1740).
Safety
Rates of adverse reactions were similar across arms, both during and after the intervention period (for further details see the trial report).7
Adherence
Two analyses were carried out on data from participants in the FaME arm:
assuming that those not returning relevant diaries had not undertaken any exercise during that 4-week period; and
omitting any participant who had not returned all six diaries.
There was no evidence of difference in primary outcome between adherers and non-adherers for either analysis (P = 0.67 and 0.95 respectively). Similar analyses were carried out on data from participants in the OEP arm. No evidence of difference in outcome was apparent between adherers and non-adherers in any analysis (data not shown).
DISCUSSION
Summary
Older people participating in FaME classes reported significantly increased physical activity at 12 months after cessation of the intervention. If 38% of patients in the usual-care arm met the target of ≥150 minutes of MVPA per week at 12 months post-intervention, an OR of 1.78 associated with FaME 12 months post-intervention would mean that 52% of participants would be meeting the guideline, giving an absolute increase of 14%. This equates to a number needed to treat of around 8, and an additional mean daily increase of 15 minutes of MVPA. There was no statistically significant increase in MVPA in the OEP arm, compared with usual care.
The difference due to FaME was noticeable 24 months post-intervention, with no significant evidence of benefit diminishing with time. The FaME programme also significantly reduced falls compared with the usual-care arm, while the OEP arm had a slightly lesser (non-significant) reduction. FaME was more expensive than OEP; the mean cost per participant achieving or exceeding the target level of physical activity was £1740.
Strengths and limitations
To the best of the researchers’ knowledge, this study is the largest general practice-based trial of exercise interventions for older people in the UK to date, and the first to deploy peer mentors to augment an exercise programme. Both exercise interventions were evidence based, but also feasible to use in general practice and tailored to individual participants’ capabilities by postural stability instructors and peer mentors.
This trial addressed many of the weaknesses inherent in previous general practice-based studies17 by including standard scales for assessing physical activity, long follow-up, an intention-to-treat analysis controlling for baseline values of outcome measures, use of multiple imputation techniques to assess robustness to missing data, and a comprehensive assessment of the safety of the interventions.18 In addition, the researchers attempted to maintain a balance between internal and external validity, and measure adherence as an outcome.19 Blinding of analysts proved difficult, although there is little evidence that this has an effect on outcomes;20 bias was minimised by agreeing an a priori statistical analysis plan with the trial steering committee.
Only 13% of those invited to take part in the trial expressed an interest in doing so and only 6% enrolled; almost two-thirds of participants were female. Although expectations of research burden may have inhibited some older people from participating, the findings suggest that the uptake of a service based on this study could be relatively small. The trial also experienced considerable attrition, but this was similar across treatment arms and multiple imputation analysis demonstrated that the findings were robust to missing data. Being adherent to the intervention did not appear to influence the treatment benefit observed, but the trial was not powered for this subgroup analysis so this finding should be treated with caution.
The trial estimated the costs of the two exercise interventions, but did not take account of savings attributable to the significant reduction in falls, which would need to be considered in decisions about commissioning exercise interventions.
As a result of the difficulties recruiting sufficient peer mentors, a consistent dose of peer mentoring could not be ensured, and so the full impact of the OEP intervention may not have been measured. However, this is very similar to the real-life situation where a supervised home-based intervention is unlikely to have the staffing required to provide home visits and telephone support.
This trial was reliant on self-reported physical activity. Although self-reported physical activity is appropriate for measuring change in behaviour,21 social desirability may influence self-reporting, resulting in an overestimation of the actual levels of activity.22,23 There is no reason to suspect differential reporting of physical activity between treatment arms in this trial. Characteristics known to be associated with reporting physical activity were similar across treatment arms, and attrition did not vary significantly between arms. Self-reported activity can be an appropriate outcome measure to use as it has predicted both self-reported and measured functional ability 3–5 years later24 and all-cause mortality in middle-aged males 21 years later.25 Recommendations for 150 minutes of MVPA are based on associations between self-reported physical activity and health outcomes.26,27
Comparison with existing literature
A systematic review of the effectiveness of physical activity interventions for adults aged ≥50 years delivered through general practice,28 identified six studies published between 1998 and 2011, involving a total of 1522 participants. Three studies showed no impact on physical activity levels after intervention, even in younger populations;29–31 one showed a statistically significant increase in physical activity, which was lost at 12 months after intervention,32 in a younger population. Two other studies in comparable populations of older people, from New Zealand and Australia, showed a significant increase in leisure activity (but not MVPA) at 12 months follow-up,33 and improvement in V02max (a test of aerobic capacity) at 12 months follow-up.34 None of these studies showed an improvement in MVPA at 12 months after cessation of the intervention, as found in this trial. As this trial was larger than previous trials, increased statistical power is a potential explanation for the findings.
Implications for practice
The FaME programme is feasible for primary care use, and has been shown to be effective. The estimates of effectiveness and costs presented here can be used to inform evidence-based physical activity and falls prevention policies and strategies. Further research is required to explore the reasons why community-dwelling older people decline participation in group exercise classes Factors associated with adherence should also be explored further.
Acknowledgments
We thank all those who participated in this study, including the research team, practice staff, postural stability instructors, peer mentors, and members of the trial steering committee.
Funding
The project described in this article was funded by the Health Technology Assessment stream of the National Institute for Health Research (NIHR) (project number 06/36/04). The views expressed here are those of the authors, not of the Department of Health or the NIHR. The funders proposed modifications to the original study design, which were incorporated, but had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
Ethical approval was obtained from Nottingham Research Ethics Committee 2 (application number 08/H0408/72).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
There were no financial relationships with any organisations that may have an interest in the submitted work other than Later Life Training Ltd for Dawn A Skelton and Susie Dinan-Young. The other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Department of Health . Choosing activity: a physical activity action plan. London: DH; 2005. [Google Scholar]
- 2.Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc. 2000;48(8):883–893. doi: 10.1111/j.1532-5415.2000.tb06884.x. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kampert JB, Kohl HW, 3rd, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 4.McClure R, Turner C, Peel N, et al. Population-based interventions for the prevention of fall-related injuries in older people. Cochrane Database Syst Rev. 2005;1:CD004441. doi: 10.1002/14651858.CD004441.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health . Start active, stay active: a report on physical activity for health from the four home countries’ Chief Medical Officers. London: DH; 2011. [Google Scholar]
- 6.Health & Social Care Information Centre . Health survey for England — 2012. Leeds: HSCIC; 2013. http://www.hscic.gov.uk/catalogue/PUB13218 (accessed 8 Sep 2015). [Google Scholar]
- 7.Iliffe S, Kendrink D, Morris R, et al. Multi-centre cluster randomised trial comparing a community group exercise programme with home based exercise with usual care for people ages 65 and over in primary care: protocol of the ProAct 65+ trial. Trials. 2010;11:6. doi: 10.1186/1745-6215-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens Z, Carpenter H, Gawler S, et al. Lessons learnt during a complex, multi-centre cluster randomised controlled trial: the ProAct65+ trial. Trials. 2013;14:192. doi: 10.1186/1745-6215-14-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iliffe S, Kendrick D, Morris R, et al. Multi-centre cluster randomised trial comparing a community group exercise programme with home based exercise with usual care for people aged 65 and over in primary care. Health Technol Assess. 2014. DOI: http://dx.doi.org/10.3310/hta18490. [DOI] [PMC free article] [PubMed]
- 10.Robertson MC, Devlin N, Gardener MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: randomised controlled trial. BMJ. 2001;322(7288):697–701. doi: 10.1136/bmj.322.7288.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skelton D, Dinan S, Campbell M, Rutherford O. Tailored group exercise (Falls Management Exercise — FaME) reduces falls in community-dwelling older frequent fallers (an RCT) Age Ageing. 2005;34(6):636–639. doi: 10.1093/ageing/afi174. [DOI] [PubMed] [Google Scholar]
- 12.Adams G, Gulliford MC, Obloha C, et al. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57(8):785–794. doi: 10.1016/j.jclinepi.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Pocock SJ. Clinical trials: a practical approach. Chichester: John Wiley & Sons; 1983. [Google Scholar]
- 14.Curtis L. Unit costs of health and social care 2011. Canterbury: Personal Social Services Research Unit; 2011. http://www.pssru.ac.uk (accessed 18 May 2015). [Google Scholar]
- 15.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 16.Dinan S. Delivering an exercise prescription for vulnerable older patients. In: Young A, Harries M, editors. Physical activity for patients: an exercise prescription. London: Royal College of Physicians; 2002. pp. 53–70. [Google Scholar]
- 17.Stevens Z, Barlow C, Iliffe S. Promoting physical activity among older people in primary care using peer mentors. Prim Health Care Res Dev. 2015;16(2):201–206. doi: 10.1017/S1463423613000510. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLOS Clin Trial. 2006;1(1):e9. doi: 10.1371/journal.pctr.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agency for Healthcare Research and Quality . The empirical evidence of bias in trials measuring treatment differences. Rockville, MD: Agency for Healthcare Research and Quality; 2012. [PubMed] [Google Scholar]
- 21.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37(3):197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc. 2014;46(1):99–106. doi: 10.1249/MSS.0b013e3182a0595f. [DOI] [PubMed] [Google Scholar]
- 23.Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005;161(4):389–398. doi: 10.1093/aje/kwi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young DR, Masaki KH, Curb JD. Associations of physical activity with performance-based and self-reported physical functioning in older men: the Honolulu Heart Program. J Am Geriatr Soc. 1995;43(8):845–854. doi: 10.1111/j.1532-5415.1995.tb05525.x. [DOI] [PubMed] [Google Scholar]
- 25.Eaton CB, Medalie JH, Flocke SA, et al. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities: twenty-one-year follow-up of the Israeli Ischaemic Heart Disease study. Arch Fam Med. 1995;4(4):323–329. doi: 10.1001/archfami.4.4.323. [DOI] [PubMed] [Google Scholar]
- 26.Gulsvik AK, Thelle DS, Samuelsen SO, et al. Ageing, physical activity and mortality — a 42-year follow-up study. Int J Epidemiol. 2012;41(2):521–530. doi: 10.1093/ije/dyr205. [DOI] [PubMed] [Google Scholar]
- 27.Celis-Morales CA, Perez-Bravo F, Ibañez L, et al. Objective vs self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PloS One. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens Z, Barlow C, Kendrick D, et al. Effectiveness of general practice-based exercise promotion for older adults: a systematic review. Prim Health Care Res & Develop. 2013 doi: 10.1017/S1463423613000017. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein MG, Pinto BM, Marcus BH, et al. Physician-based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med. 1999;21(1):40–47. doi: 10.1007/BF02895032. [DOI] [PubMed] [Google Scholar]
- 30.Halbert JA, Silagy CA, Finucane PM, et al. Physical activity and cardiovascular risk factors: effect of advice from an exercise specialist in Australian general practice. Med J Aust. 2000;173(2):84–87. doi: 10.5694/j.1326-5377.2000.tb139250.x. [DOI] [PubMed] [Google Scholar]
- 31.Kerse N, Elley CR, Robinson E, Arroll B. Is physical activity counseling effective for older people? A cluster randomized, controlled trial in primary care. J Am Geriatr Soc. 2005;53(11):1951–1956. doi: 10.1111/j.1532-5415.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 32.Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity one year later? A randomized controlled trial. J Public Health. 2005;27(1):25–32. doi: 10.1093/pubmed/fdh197. [DOI] [PubMed] [Google Scholar]
- 33.Kolt GS, Schofield GM, Kerse N, et al. Effect of telephone counseling on physical activity for low-active older people in primary care: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(7):986–992. doi: 10.1111/j.1532-5415.2007.01203.x. [DOI] [PubMed] [Google Scholar]
- 34.Petrella RJ, Koval JJ, Cunningham DA, Paterson DH. Can primary care doctors prescribe exercise to improve fitness? The Step Test Exercise Prescription (STEP) project. Am J Prev Med. 2003;24(4):316–322. doi: 10.1016/s0749-3797(03)00022-9. [DOI] [PubMed] [Google Scholar]