Supplemental Digital Content is available in the text.
Keywords: epidemiologic studies, genetics, genome-wide association study, sex, stroke
Background and Purpose—
Evidence from epidemiological studies points to differences in factors predisposing to stroke by age and sex. Whether these arise because of different genetic influences remained untested. Here, we use data from 4 genome-wide association data sets to study the relationship between genetic influence on stroke with both age and sex.
Methods—
Using genomic-relatedness-matrix restricted maximum likelihood methods, we performed 4 analyses: (1) we calculated the genetic correlation between groups divided by age and (2) by sex, (3) we calculated the heritability of age-at-stroke-onset, and (4) we evaluated the evidence that heritability of stroke is greater in women than in men.
Results—
We found that genetic factors influence age at stroke onset (h2 [SE]=18.0 [6.8]; P=0.0038), with a trend toward a stronger influence in women (women: h2 [SE]=21.6 [3.5]; Men: h2 [SE]=13.9 [2.8]). Although a moderate proportion of genetic factors was shared between sexes (rG [SE]=0.68 [0.16]) and between younger and older cases (rG [SE]=0.70 [0.17]), there was evidence to suggest that there are genetic susceptibility factors that are specific to sex (P=0.037) and to younger or older groups (P=0.056), particularly for women (P=0.0068). Finally, we found a trend toward higher heritability of stroke in women although this was not significantly greater than in men (P=0.084).
Conclusions—
Our results indicate that there are genetic factors that are either unique to or have a different effect between younger and older age groups and between women and men. Performing large, well-powered genome-wide association study analyses in these groups is likely to uncover further associations.
Considerable evidence from epidemiological studies points to differences between men and women in factors predisposing to stroke. Differences in lifetime stroke risk,1 poststroke quality-of-life,2 and clinical presentation between men and women are well established.3 Family history studies have shown stronger effects in women when compared to men,4,5 and a significant excess maternal risk in women.4,5 Different risk factors also appear to be more important in either sex. Women tend to have higher rates of hypertension and atrial fibrillation, whereas men are more likely to have a history of alcohol or tobacco use, or a history of heart disease and diabetes mellitus.6 Similarly, differences in clinical characteristics, prevalence of risk factors, and outcome by age-at-stroke onset are considerable.7 Older patients tend to have higher prevalence of hypertension and atrial fibrillation and are more likely to be women,8 whereas younger patients are more likely to have rarer stroke causes9 and in general have lower rates of cardiovascular risk factors.8
An important unanswered question is whether genetic susceptibility factors contribute to these differences. Over the past decade, genome-wide association studies have transformed our understanding of complex disease, identifying hundreds of common genetic associations.10–12 Many loci have been identified that contribute to risk of ischemic and hemorrhagic stroke.13–15 In addition to these principal findings, many genetic association studies have also reported sex-specific associations with complex traits, including type 2 diabetes mellitus,11 and numerous other traits.16–18 Similarly, several associations have been identified that are specific to early or late-onset groups.19–21 These findings show that some genetic susceptibility factors are specific to sex or age groups and emphasize the importance of considering heterogeneity in genetic association studies.
With this in mind, we determined whether such associations might exist for ischemic stroke by interrogating the genetic liability to stroke for age and sex groups, estimating the degree to which genetic liability is shared between groups. We used a recently developed approach (genomic-relatedness-matrix restricted maximum likelihood) that uses the distant relatedness between individuals based on common variation tagged on genotyping arrays to estimate the heritability and coheritability of traits explained by common genetic variation.22–24 We used this approach to interrogate differences between groups of stroke cases when divided by age and sex. In doing so, we show that there exist differences in the underlying common genetic contribution to stroke by age and sex.
Methods
Cohort Characteristics
The data set of 5076 ischemic stroke cases and 7623 controls was derived from 4 genome-wide association studies of stroke from United Kingdom, Germany, Italy, and Australia, all of which have been described previously.13,25,26 Stroke was defined as a typical clinical syndrome with radiological confirmation. Stroke subtyping was performed using the Trial of Organization 10172 in Acute Stroke Treatment (TOAST) classification system.27
Genotyping
All cohorts were genotyped on commercial arrays from Illumina, comprising the 610K, 660W, and 1M arrays. Quality control was performed on each data set separately, as previously described.13,25,26 A consensus set of 381 428 single-nucleotide polymorphisms was then identified, which was consistent across the 4 populations and the populations were merged for these single-nucleotide polymorphisms. We then performed principal components analysis using EIGENSTRAT software on an LD-pruned set of single-nucleotide polymorphisms from the combined data set removing any population outliers,28 defined as >6 SDs from the mean on the first 5 principal components. Thirty-seven individuals were removed in total (29 from ASGC and 8 from Milano; 31 cases and 6 controls). After removing these outliers, we repeated the principal components analysis, calculating the first 20 principal components, which we used in our analyses. No imputation of the data set was performed.
Genomic-Relatedness-Matrix Restricted Maximum Likelihood analyses
For each analysis, we removed distantly related individuals above a relatedness threshold (relatedness threshold kinship coefficient >0.05, at most 30 individuals) because related individuals can lead to overestimation of heritability. We performed each analysis using GCTA (version 1.24.2), including 20 principal components each time to rule out the potentially confounding influence of population stratification. The following analyses were performed:
Age-at-Onset as a Quantitative Trait
We first estimated the heritability of age-at-onset of stroke as a quantitative trait in stroke cases only. This value represents to the extent to which genetic factors influence the age at which stroke occurs. Age-at-onset was defined as age-at-first stroke where available; in the absence of this information, age at blood taken was used instead (<5% of cases). We then performed secondary analyses, stratified by sex.
Genetic Correlation Between Groups Stratified by Median Age
We next estimated the genetic correlation (rG) between the oldest half of cases (age, ≥72 years) and the youngest half of cases (age, ≤71 years). We tested whether the correlation was significantly different from zero (rG≠0), and whether it was significantly <1. We interpreted a significant test for rG≠0 as evidence that genetic liability was shared between the 2 traits and a significant test for rG<1 as evidence that not all genetic liability was shared between the traits. We performed secondary analyses stratified by sex, performing the same calculations each time.
Genetic Correlation Between Groups Stratified by Sex
Next, we estimated the genetic correlation between all ischemic stroke cases, divided by sex. We again tested whether the correlation was significantly different from zero (rG≠0), and whether it was significantly <1, interpreting the results as previously. We performed secondary analyses stratified by median age-at-onset, performing the same calculations each time.
In above 3 analyses, we used P<0.05 to evaluate evidence of significant differences between groups in the main analysis and then used a Bonferroni-corrected value (P=0.017) to assess significance in secondary analyses, correcting for the 3 overall analyses.
Test for Higher Heritability in Women than in Men
To evaluate whether the heritability of stroke was greater in women versus men, as previously reported,4,5 we estimated an empirical P value using permutations as follows. We first calculated the heritability of stroke in men and women separately, including all controls in both analyses. We then generated 1000 random permutations, splitting the cases into 2 groups of equivalent size to the number of men and women in the actual data set. We estimated the heritability 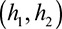 , with SEs
, with SEs 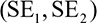 for the 2 groups in each permutation, including all controls in each analysis. For each permutation, we calculated
for the 2 groups in each permutation, including all controls in each analysis. For each permutation, we calculated 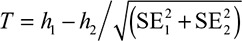 and estimated a P value for significance by dividing the number of permutations, where T exceeded that from our actual data set by the total number of permutations. We used P<0.05 to evaluate significance.
and estimated a P value for significance by dividing the number of permutations, where T exceeded that from our actual data set by the total number of permutations. We used P<0.05 to evaluate significance.
Results
Cohort Characteristics
A total of 5076 cases and 7623 controls were used in the analysis. Clinical characteristics for all cases are given in Table 1. Age at stroke onset was significantly higher in women than in men (Figure; P=9.9×10−37; mean [SD] in women=71.9 [14.5]; mean [SD] in men = 67.8 [12.9]).
Table 1.
Description of Ischemic Stroke Cases

Figure.
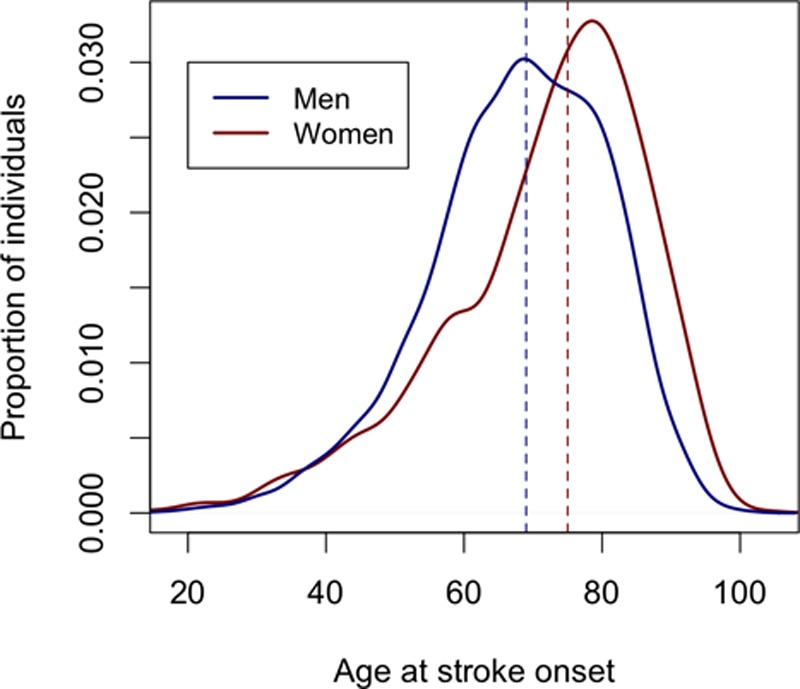
Age at stroke distributions by sex, with dotted lines indicating mean age at stroke onset for men and women.
Age-at-Onset as a Quantitative Trait
We first tested to see whether we could identify a genetic influence on age at stroke onset. The results showed that age at onset was significantly heritable (h2 [SE]=18.0 [6.8]; P=0.0038; n=5044). When stratifying on sex, we observed a trend toward a stronger effect in women (h2 [SE]=30.8 [16.3]; P=0.033; n=2145) than in men (h2=20.4 [11.8]; P=0.042; n=2907).
Genetic Correlation Between Groups Divided by Median Age
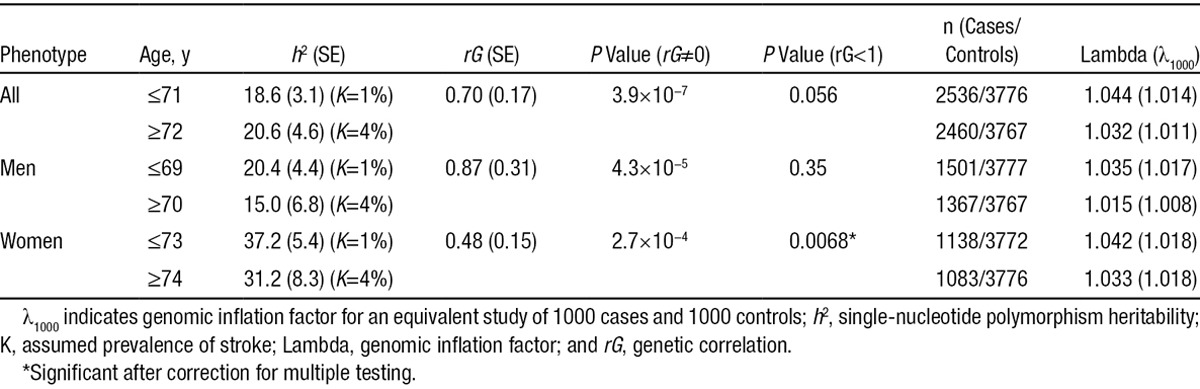
We next tested to see whether the genetic effects on younger onset cases were shared with later onset cases, estimating the genetic correlation (rG) between the 2 groups. The results showed moderate genetic correlation between younger and later onset cases (rG [SE]=0.70 [0.17]): a 2-tailed test for rG≠0 was highly significant (P=3.9×10−7), indicating significant sharing of genetic effects between the 2 groups (Table 2). A 1-tailed test for rG<1 showed a near-significant difference in genetic liability between groups when considering all stroke cases (P=0.056). However, when we repeated the analysis stratified on sex, we found that the genetic correlation between younger and later onset cases was significantly different to 1 in women (rG [SE]=0.48 [0.15]; P=0.0068), but not in men (rG [SE]=0.87 [0.31]; P=0.35). This result points to differences in genetic liability by age in women, thereby suggesting potential differences between younger and later onset female cases.
Table 2.
Heritability of Stroke Subtypes Divided by Median Age-at-Onset, and Genetic Correlation Between Groups
Genetic Correlation Between Groups Divided by Sex
We next tested to see whether the genetic effects on men with ischemic stroke were shared with women, estimating the genetic correlation (rG) between the 2 sexes. There was a significant genetic correlation (rG [SE]=0.68 [0.16]) between the sexes (P=2.7×10−7 for the test of rG≠0), indicating that a significant proportion of the genetic susceptibility factors are shared between men and women. However, we also found significant differences between men and women (P=0.037 for rG<1), suggesting the existence of some genetic susceptibility factors specific to either of the sexes. Genetic correlation values were similar when stratifying on median age-at-onset although a significant effect was not detected in either group (Table 3).
Table 3.
Heritability of Stroke Subtypes by Sex and Genetic Correlation Between Groups

Test for Higher Heritability in Women Than in Men
Finally, we tested whether heritability was higher in women than in men. Heritability was higher in women (h2 [SE]=21.6 [3.5]) than in men (h2 [SE]=13.9 [2.8]) although not significantly so (P=0.084).
Discussion
In this study, we performed analyses to investigate how differences in stroke characteristics by age and sex are affected by underlying genetic susceptibility. First, we investigated the relationship between age-at-stroke and genetic susceptibility. We showed that age-at-stroke onset is significantly heritable, suggesting that in addition to genetic effects influencing risk of stroke, there are also genetic effects that influence the age at which stroke occurs. When stratifying by sex, we found a trend toward this effect being stronger in women. Second, we investigated the genetic correlation between younger and later onset cases. We found evidence that a large proportion of genetic effects are shared between younger and later onset cases, and a suggestion that there are genetic susceptibility factors that are either specific to or stronger in either of the groups. When stratifying by sex, these age specific were significant in women, with lower correlation between younger and later-onset women. These results point to different genetic effects by age at stroke onset, and suggest that this effect may be stronger in women, although more evidence will be needed to confirm this hypothesis. Gene expression changes with age are well established;29,30 such changes might be hypothesized to lead to different processes being more important at different age ranges.
The evaluation of differences in genetic susceptibility to stroke between men and women demonstrated that there are genetic factors that are either specific to or have stronger effects in either of the sexes although a moderate proportion of genetic effects was found to be shared between the 2 groups. This result could arise because of 2 factors. First, it could imply that some genetic factors are specific to either of the 2 sexes as a result of sex-specific physiological features. An example of this might be the influence of age at menopause and age at menarche, which are thought to influence stroke risk in women.31 Second, it could imply that genetic factors exert different influences in the 2 sexes. This could arise because of their interaction with environmental exposures such as alcohol consumption or smoking, which are more common in men.8 Other possibilities include epigenetic mechanisms, or different prevalence of cardiovascular risk factors between sexes, although these would be expected to also have a genetic component.
Our results also showed a trend toward genetic influence on stroke being stronger in women. However, taken in the context of family history and heritability studies, which show stronger effects in women,4,5 these findings are consistent with the hypothesis that the underlying contribution from common genetic factors is stronger in women. One explanation for this difference might be that a larger proportion of disease risk is explained by environmental factors in men. For example, risk factors such as alcohol consumption and smoking are more prevalent in men.8 An alternative explanation might be that women have additional genetic factors influencing age at menarche, age at menopause and estrogen levels, all of which have been hypothesized to influence stroke risk.32 Finally, differences in other modifiable lifestyle factors such as stress,33 which is known to influence cardiovascular disease risk,34 may also play a part.
This study has limitations. Cases and controls were derived from 4 populations from Europe and Australia. Therefore, we cannot rule out the influence of differences in case ascertainment on the results. In addition, we were underpowered to perform analyses by TOAST subcategories. Results were based on analyses of common genetic variants (minor allele frequency >0.01), meaning we are unable to comment on differences in heritability arising from rare variation. Our estimates of heritability were in general lower than those for many other neurological disorders such as multiple sclerosis (30%) and Alzheimer disease (24%), which may indicate a comparatively lower genetic contribution to disease.35 The results may also be related to the fact that TOAST categories have different prevalences in different age groups (Figure I in the online-only Data Supplement). Although we were underpowered to perform the same analyses in TOAST categories, we did perform these as secondary analyses: results are provided in Tables I and II in the online-only Data Supplement. Similarly, this study has strengths. The sample size used was relatively large, and sex was confirmed genetically in all cases and matched to database records, reducing the possibility of incorrect assignment.
Our results show that genetic susceptibility to stroke differs by age and by sex. Many genetic association studies have reported sex-specific associations with complex traits.11,16 Our results suggest that sex and age-at-onset–specific associations are likely to exist with ischemic stroke and therefore motivate the implementation of methods that boost power by allowing heterogeneity of single-nucleotide polymorphism effects.36 Such approaches are likely to detect novel associations with ischemic stroke and provide insights into age- and sex-specific influences on ischemic stroke.
Acknowledgments
We thank the Wellcome Trust Case Control Consortium 2.
Sources of Funding
Funding for genotyping, and initial analysis of stroke samples was provided by Wellcome Trust Case Control Consortium-2 (TSA 2013/01). Funding information on the ASGC and Milano studies is available in their original articles.13,26 Dr Traylor was supported by a project grant from the Stroke Association (TSA 2013/01). Dr Rutten-Jacobs was supported by programme grant funding from the British Heart Foundation/Stroke Association (TSA/BHF 2010/01). H.S. Markus was supported by an National Institute for Health Research (NIHR) Senior Investigator award. H. Markus and Dr Bevan were supported by the NIHR Cambridge University Hospitals Comprehensive Biomedical Research Centre. P.M. Rothwell holds NIHR and Wellcome Trust Senior Investigator Awards. C. Sudlow was supported by UK Biobank and the Scottish Funding Council. Dr Lewis’s research was supported by the National Institute for Health Research Biomedical Research Centre (BRC) based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, and the BRC for Mental Health at South London and Maudsley NHS Foundation Trust and King’s College London.
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.115.009816/-/DC1.
References
- 1.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 2.Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu-Bettger J, Zimmer L, et al. Sex differences in quality of life after ischemic stroke. Neurology. 2014;82:922–931. doi: 10.1212/WNL.0000000000000208. doi: 10.1212/WNL.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. European BIOMED Study of Stroke Care Group. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 4.Touzé E, Rothwell PM. Heritability of ischaemic stroke in women compared with men: a genetic epidemiological study. Lancet Neurol. 2007;6:125–133. doi: 10.1016/S1474-4422(06)70683-4. doi: 10.1016/S1474-4422(06)70683-4. [DOI] [PubMed] [Google Scholar]
- 5.Touzé E, Rothwell PM. Sex differences in heritability of ischemic stroke: a systematic review and meta-analysis. Stroke. 2008;39:16–23. doi: 10.1161/STROKEAHA.107.484618. doi: 10.1161/STROKEAHA.107.484618. [DOI] [PubMed] [Google Scholar]
- 6.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, et al. Get With the Guidelines-Stroke Steering Committee and Investigators. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. doi: 10.1161/CIRCULATIONAHA.109.892497. doi: 10.1161/CIRCULATIONAHA.109.892497. [DOI] [PubMed] [Google Scholar]
- 8.Andersen KK, Andersen ZJ, Olsen TS. Age- and gender-specific prevalence of cardiovascular risk factors in 40,102 patients with first-ever ischemic stroke: a Nationwide Danish Study. Stroke. 2010;41:2768–2774. doi: 10.1161/STROKEAHA.110.595785. doi: 10.1161/STROKEAHA.110.595785. [DOI] [PubMed] [Google Scholar]
- 9.Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, van Dijk EJ, de Leeuw FE. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol. 2014;10:315–325. doi: 10.1038/nrneurol.2014.72. doi: 10.1038/nrneurol.2014.72. [DOI] [PubMed] [Google Scholar]
- 10.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, et al. Wellcome Trust Case Control Consortium; Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators; Genetic Investigation of ANthropometric Traits (GIANT) Consortium; Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Australian Stroke Genetics Collaborative, Wellcome Trust Case Control Consortium 2 (WTCCC2); International Stroke Genetics Consortium. Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilarski LL, Achterberg S, Devan WJ, Traylor M, Malik R, Lindgren A, et al. GARNET Collaborative Research Group, Wellcome Trust Case Control Consortium 2, Australian Stroke Genetic Collaborative, the METASTROKE Consortium, and the International Stroke Genetics Consortium. Meta-analysis in more than 17,900 cases of ischemic stroke reveals a novel association at 12q24.12. Neurology. 2014;83:678–685. doi: 10.1212/WNL.0000000000000707. doi: 10.1212/WNL.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo D, Falcone GJ, Devan WJ, Brown WM, Biffi A, Howard TD, et al. International Stroke Genetics Consortium. Meta-analysis of genome-wide association studies identifies 1q22 as a susceptibility locus for intracerebral hemorrhage. Am J Hum Genet. 2014;94:511–521. doi: 10.1016/j.ajhg.2014.02.012. doi: 10.1016/j.ajhg.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randall JC, Winkler TW, Kutalik Z, Berndt SI, Jackson AU, Monda KL, et al. DIAGRAM Consortium; MAGIC Investigators. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9:e1003500. doi: 10.1371/journal.pgen.1003500. doi: 10.1371/journal.pgen.1003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orozco G, Ioannidis JP, Morris A, Zeggini E DIAGRAM consortium. Sex-specific differences in effect size estimates at established complex trait loci. Int J Epidemiol. 2012;41:1376–1382. doi: 10.1093/ije/dys104. doi: 10.1093/ije/dys104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu LY, Schaub MA, Sirota M, Butte AJ. Sex differences in disease risk from reported genome-wide association study findings. Hum Genet. 2012;131:353–364. doi: 10.1007/s00439-011-1081-y. doi: 10.1007/s00439-011-1081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imielinski M, Baldassano RN, Griffiths A, Russell RK, Annese V, Dubinsky M, et al. Western Regional Alliance for Pediatric IBD; International IBD Genetics Consortium; NIDDK IBD Genetics Consortium; Belgian-French IBD Consortium; Wellcome Trust Case Control Consortium. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat Genet. 2009;41:1335–1340. doi: 10.1038/ng.489. doi: 10.1038/ng.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet. 2013;45:513–517. doi: 10.1038/ng.2607. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witt H, Beer S, Rosendahl J, Chen JM, Chandak GR, Masamune A, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet. 2013;45:1216–1220. doi: 10.1038/ng.2730. doi: 10.1038/ng.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Manolio TA, Pasquale LR, Boerwinkle E, Caporaso N, Cunningham JM, et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat Genet. 2011;43:519–525. doi: 10.1038/ng.823. doi: 10.1038/ng.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SH, Yang J, Goddard ME, Visscher PM, Wray NR. Estimation of pleiotropy between complex diseases using single-nucleotide polymorphism-derived genomic relationships and restricted maximum likelihood. Bioinformatics. 2012;28:2540–2542. doi: 10.1093/bioinformatics/bts474. doi: 10.1093/bioinformatics/bts474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holliday EG, Traylor M, Malik R, Bevan S, Falcone G, Hopewell JC, et al. Australian Stroke Genetics Collaborative; Wellcome Trust Case Control Consortium 2; International Stroke Genetics Consortium. Genetic overlap between diagnostic subtypes of ischemic stroke. Stroke. 2015;46:615–619. doi: 10.1161/STROKEAHA.114.007930. doi: 10.1161/STROKEAHA.114.007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellenguez C, Bevan S, Gschwendtner A, Spencer CC, Burgess AI, Pirinen M, et al. Genome-wide association study identifies a variant in hdac9 associated with large vessel ischemic stroke. Nat. Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holliday EG, Maguire JM, Evans TJ, Koblar SA, Jannes J, Sturm JW, et al. Australian Stroke Genetics Collaborative; International Stroke Genetics Consortium; Wellcome Trust Case Control Consortium 2. Common variants at 6p21.1 are associated with large artery atherosclerotic stroke. Nat Genet. 2012;44:1147–1151. doi: 10.1038/ng.2397. doi: 10.1038/ng.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 28.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 29.Glass D, Viñuela A, Davies MN, Ramasamy A, Parts L, Knowles D, et al. UK Brain Expression consortium; MuTHER consortium. Gene expression changes with age in skin, adipose tissue, blood and brain. Genome Biol. 2013;14:R75. doi: 10.1186/gb-2013-14-7-r75. doi: 10.1186/gb-2013-14-7-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Magalhães JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40:1044–1049. doi: 10.1161/STROKEAHA.108.542993. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol. 2012;11:82–91. doi: 10.1016/S1474-4422(11)70269-1. doi: 10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Everson-Rose SA, Roetker NS, Lutsey PL, Kershaw KN, Longstreth WT, Jr, Sacco RL, et al. Chronic stress, depressive symptoms, anger, hostility, and risk of stroke and transient ischemic attack in the multi-ethnic study of atherosclerosis. Stroke. 2014;45:2318–2323. doi: 10.1161/STROKEAHA.114.004815. doi: 10.1161/STROKEAHA.114.004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kivimäki M, Nyberg ST, Batty GD, Fransson EI, Heikkilä K, Alfredsson L, et al. IPD-Work Consortium. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380:1491–1497. doi: 10.1016/S0140-6736(12)60994-5. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SH, Harold D, Nyholt DR, Goddard ME, Zondervan KT, Williams J, et al. ANZGene Consortium; International Endogene Consortium; Genetic and Environmental Risk for Alzheimer’s disease Consortium. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum Mol Genet. 2013;22:832–841. doi: 10.1093/hmg/dds491. doi: 10.1093/hmg/dds491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magi R, Lindgren CM, Morris AP. Meta-analysis of sex-specific genome-wide association studies. Genet Epidemiol. 2010;34:846–853. doi: 10.1002/gepi.20540. doi: 10.1002/gepi.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]


