ABSTRACT
Extremity injury is a significant burden to those injured in explosive incidents and local ischaemia can result in poor functionality in salvaged limbs. This study examined whether blast injury to a limb resulted in a change in endothelial phenotype leading to changes to the surrounding tissue.
The hind limbs of terminally anaesthetized rabbits were subjected to one of four blast exposures (high, medium, low, or no blast). Blood samples were analyzed for circulating endothelial cells pre-injury and at 1, 6, and 11 h postinjury as well as analysis for endothelial activation pre-injury and at 1, 6, and 12 h postinjury. Post-mortem tissue (12 h post-injury) was analysed for both protein and mRNA expression and also for histopathology. The high blast group had significantly elevated levels of circulating endothelial cells 6 h postinjury. This group also had significantly elevated tissue mRNA expression of IL-6, E-selectin, TNF-α, HIF-1, thrombomodulin, and PDGF. There was a significant correlation between blast dose and the degree of tissue pathology (hemorrhage, neutrophil infiltrate, and oedema) with the worst scores in the high blast group. This study has demonstrated that blast injury can activate the endothelium and in some cases cause damage that in turn leads to pathological changes in the surrounding tissue. For the casualty injured by an explosion the damaging effects of hemorrhage and shock could be exacerbated by blast injury and vice versa so that even low levels of blast become damaging, all of which could affect tissue functionality and long-term outcomes.
Keywords: Blast, endothelial dysfunction, endothelium, inflammation, trauma
INTRODUCTION
Musculoskeletal trauma in combat casualties is often devastating and can involve soft tissue (including vascular tissue), and bone. Reports from recent conflicts suggest that extremity injury occurs in 54% of the severely battle injured (1) and additionally the rate of vascular injury is higher now than in previous conflicts with about 12% of combat wounded from Iraq and Afghanistan having a vascular injury (2). Muscle tissue is the most vascular structure in the extremities and during periods of activity has a high metabolic demand therefore it is very sensitive to ischaemic injury. Ischaemic muscle injury may result from a disruption of blood supply because of traumatic injury as well as a result of life-saving measures. These life-saving measures include tourniquet use and hypotensive resuscitation with the overall effect being reduced local blood flow. Indeed the treatment of combat casualties can require prioritising life saving measures over impact on tissue viability and long-term outcomes. The long-term impact of military extremity trauma is currently under investigation and recent reports suggest that functional outcomes after amputation are better than after limb salvage (3), and that more than half of 214 casualties with extremity vascular injury had unfavourable outcomes (4). One possible cause of poor outcome in limb salvage patients could be as a result of tissue ischaemia. Recent studies in pigs have demonstrated that the ischaemic threshold for neuromuscular recovery is significantly reduced with concomitant haemorrhagic shock (5), which is highly likely in severely injured military casualties.
Blast injury, as a result of the release of energy from an explosive, is an important injury mechanism for those injured on the battlefield with 70% of extremity injuries resulting from explosions (1). There has been little research to date on the effect of blast on the soft tissues of the extremities despite evidence of the debilitating nature of blast extremity injury (3). The aim of this study was to investigate the effect of a blast wave on the extremity with particular focus on blood vessels (endothelium), microcirculation, and surrounding tissue (muscle). The endothelium plays a vital role in tissue homeostasis and endothelial cells are sensitive to the effects of ischaemia (6). The microvasculature and endothelial dysfunction is becoming a focus for investigation following trauma (7) with treatment strategies looking to protect endothelial structure and function (8). A study that examines the effects of blast on the endothelium is required to help elucidate the effects of blast injury on the extremity and determine whether this mechanism of injury will impact on casualty outcomes and additionally indicate potential treatment strategies. This study examined whether blast injury to a limb resulted in damage to the endothelium as evidenced by the presence of endothelial cells in the circulation (CECs). Secondary outcome measures were changes in the vasculature to pro-inflammatory and procoagulant state over the first 12 h postinjury resulting in inflammatory changes in the surrounding tissue.
MATERIAL AND METHODS
Animals
This study was conducted following local ethical approval and was performed under the authority of the Animals (Scientific Procedures) Act 1986.
Thirty-eight New Zealand White rabbits 2.52 (2.00–3.52) kg (mean (range)) were sourced from a UK commercial supplier. Animals were housed in a conventional unit with 12 h light/dark (with 30 min dawn and dusk) and at a temperature of 16 to 20°C. Animals were kept in pairs in floor pens with sawdust and hay bedding mix with free access to water. Animals were fed Harlan Teklad 2,030 irradiated global rabbit diet ad libitum with a tropical forage mix scattered through the bedding for enrichment. Animals were acclimatized for a minimum of 7 days before use.
On the day of the experiment animals were premedicated with intramuscular midazolam (1–2 mg/kg). Following a period of pre-oxygenation via facemask animals were anaesthetized with intravenous alphaxalone (1–2 mg/kg) via a 24G over the needle catheter placed in the lateral ear vein. A surgical plane of anaesthesia was maintained via endotracheal tube with isoflurane in oxygen and nitrous oxide (50:50) throughout the study. Animals were culled with an overdose of pentobarbitone at the end of the study without regaining consciousness. Body temperature was monitored and maintained using homoeothermic blanket system (Harvard Apparatus Ltd, Kent, UK). Invasive blood pressure, heart rate, and end-tidal CO2 were monitored using a PropaqCS system (WelchAllyn, Buckinghamshire, UK). An infusion of 0.9% sodium chloride was infused at a rate of 6 mL/kg/h to replace insensible losses. Using aseptic technique the left carotid artery was surgically cannulated to allow blood pressure monitoring and blood sampling.
Following a period of 30 min post-surgery the baseline pre-injury blood samples were taken.
Blood samples were taken at predetermined time points for the analysis of markers of endothelial damage. Blood samples for CECs were taken at baseline, 1, 6, and 11 h postinjury. Blood samples for all other analyses were taken at baseline, 1 h postinjury, 6 h postinjury, and 12 h postinjury.
At the end of the study post-mortem tissue samples were collected and stored as appropriate for ELISA, RNA extraction, light microscopy, and electron microscopy.
The personnel undertaking blood and tissue sample analysis were blinded to blast loading.
Blast injury
Animals were randomized (via Excel randomization table) to receive one of four blast loads to both hind limbs (sham, low, medium, or high).
Shortly after baseline measurements both hind limbs (centered over gastrocnemius) were exposed to a blast wave via a compressed air device or no blast wave for the sham exposure group. Each limb received five blasts to expose the whole caudal aspect of the limb between the stifle (knee) and hock (ankle) to a blast injury. The blast apparatus is described in detail elsewhere (9) therefore in summary a 20,216 kPa compressed air cylinder was used to charge an air storage chamber (150 mL). Using a solenoid control system the stored air is discharged to the blast nozzle, and an aluminium disc at the base of the blast nozzle ruptures. This results in the formation of a shock wave which is emitted from the base of the nozzle. The distance between the nozzle and the target was changed to deliver different blast loads (9). The output from the compressed air device was checked each experimental day by exposing a piezoelectronic pressure sensor (MQ20), amplifier, and data capture system (9) to the randomized blast distance (minimum n=3 exposures) to ensure consistency of loading.
Circulating endothelial cells enumeration
One milliliter blood was taken to determine levels of CECs, using a modified CD146-based immunomagnetic separation method as described previously (10). Blood (1 mL in EDTA) was diluted with the same volume of buffer A (0.1% (v/v) BSA, 0.1% (v/v) sodium azide, 0.6% sodium citrate (v/v) in PBS) and incubated with a mouse antirabbit CD146 antibody (Millipore, Watford, UK) for 20 min at 4°C with gentle end-over-end rotation. The cells were then centrifuged (300 × g, 8 min) and resuspended in 2 mL buffer B (0.1% (v/v) BSA in PBS) and incubated for 20 min at 4°C with 25 μL pan mouse-IgG Dynabeads (Invitrogen, Paisley, UK) with rotation as before. The cells were then washed four times in buffer B and flushed through a 100 μL pipette tip 10 times during the final wash. The cells were then fixed in 500 μL ice-cold methanol at 4°C for 10 min, washed three times as before, resuspended in 1 mL buffer B, and stored overnight at 4°C for staining and visualisation. For staining and enumeration cells were placed in a magnet for 1 min, the supernatant discarded and they were resuspended in 100 μL 2 mg/mL FITC-coupled UEA-1 solution (Sigma-Aldrich, Gillingham, UK) and incubated for 1 h at 4°C with gentle mixing as before (in the dark). The sample was then washed twice in buffer B and the cell-bead suspension finally dissolved in 200 μL buffer B before counting. CECs were counted with fluorescence microscopy at 553 nm using a haemocytometer (Nexcelom Biosciences, Manchester, UK) by two researchers blinded to the groups. Statistical analysis was carried out on pilot studies to assess how many fields of view to include. CECs were defined as cells 10–50 μM in size with five or more magnetic beads attached and stained with UEA-1-FITC. CECs were expressed in numbers milliliter blood.
ELISA
Blood (2 mL) was collected into Lithium Heparin tubes and the plasma was then aliquoted into tubes and stored at −80°C until use. Plasma samples for von Willebrand Factor (vWF) and vascular endothelial growth factor (VEGF) as well as homogenized muscle tissue for stromal-cell derived factor 1 (SDF-1) and endothelin 1 (ET-1) were assayed in duplicate using ELISA kits from Cusabio Ltd (Wuhan, China) as per manufacturer's instructions.
qRT-PCR
The left gastronemius was excised post-mortem and cut in half along its length. One half was divided into three 0.25 cm3 sections and placed into RNA later and stored as per manufacturer's instructions until required. RNA was extracted from muscle using a hybrid Trizol/RNeasy kit (Qiagen, Manchester, UK) protocol. Following extraction RNA quantity and quality was assessed using NanoDrop 1,000 and Bioanalyser (BioRad, Hemel Hempstead, UK). All RIN numbers were >8.8. Any contaminating DNA was removed using Turbo DNase as per manufacturer's instructions (Applied Biosystems, Thermo Fisher Scientific, Loughborough, UK) and RNA re-assessed after DNase treatment. RNA was used in a reverse transcription reaction using SuperScript III (Invitrogen) to manufacturer's instructions with random hexamer primers. cDNA was diluted 1:5 with nuclease-free water for PCR. Real-time PCR was carried out using Taqman-style probe technology with primers and probes designed, synthesized and tested by PrimerDesign Ltd (Southampton, UK). For each PCR reaction 5 μL cDNA was used with 15 μL Precision R mastermix in duplicate with the following cycling conditions: 95°C 12 min, 40 cycles of 95°C 15 s, 60°C 1 min followed by a hold at 4°C. Calibrated normalized relative quantities (CNRQ) were calculated from Cq values using qBasePlus (Biogazelle Ltd., Gent, Belgium). Samples were normalized to the three most stable reference genes as determined by a GeNorm experiment and calibrated to the average of all samples. The efficiency of the PCR reaction for each gene was determined using a standard curve comprising pooled cDNA from at least 15 animals. The panel of markers assayed were as follows: tumor necrosis factor α (TNF-α), e-selectin, interleukin-6 (IL-6), thrombomodulin (THMB), hypoxia inducible factor-1 (HIF-1), peroxisome proliferator-activated receptor-γ 1-α (PGC-1α), VEGF, platelet-derived growth factor (PDGF), vascular cell adhesion molecule (VCAM), ET-1, and erythropoeitin.
Histology
The right gastrocnemius muscle was removed post-mortem for histological analysis. The muscle was divided into two equal sections and representative samples of tissue placed in gluteraldehyde for analysis using transition electron microscopy (EM) or in 10% neutral buffered formalin for analysis using light microscopy (LM). Saphenous blood vessels from both hind limbs were dissected away from the surrounding tissue and samples taken for LM and EM histological processing and analysis. Sections were stained with haematoxylin and eosin and examined using an Axioscop microscope for pathology scoring. Representative images were captured using an Axiocam MC5 digital camera and Axiovision software. Incidence of hemorrhage, inflammatory cell infiltration, and oedema were subjectively recorded as either not present (score = 0), mild (score = 1), moderate (score = 2), or severe (score = 4). The maximum percentage of muscle fiber bundles displaying evidence of necrosis was subjectively estimated in each section.
Statistical analysis
Differences in numbers CECs counted between groups were assessed with two-way ANOVA test (P < 0.05 and power = 0.8). Incidence of hemorrhage, oedema, and inflammatory cell infiltration was subjectively recorded as detailed above. Maximum percentage of muscle fiber necrosis was estimated as detailed above. These scores were assessed using a Cuzicks trend test (P < 0.05). Gene expression data (CNRQs) were log transformed before being assessed with one-way ANOVA (P < 0.05) with Dunnett's post-test compared with Sham.
RESULTS
Animals
Eleven animals were required to develop the CEC enumeration technique in the rabbit, therefore only data from 27 animals (sham n = 6, low n = 6, medium n = 6, high n = 9) were used for the CEC, ELISA, and qRT-PCR analysis. Histology, however, was performed on the tissue from all 38 animals.
Blast exposure
In this study three blast exposure levels were evaluated. It is not possible to record the blast wave during the actual tissue exposure, but the blast wave output from the apparatus was determined on the morning of the experiment. The blast apparatus produced three distinct and consistent blast waves as shown in Table 1.
Table 1.
Measurement of the blast overpressure and impulse output from the compressed air blast wave generator
| Group | Blast nozzle distance (cm) | Mean peak overpressure (95% CI) (kPa) | Mean impulse at 1.5 mS duration (95% CI) (sPa) |
| High | 1.5 | 1,665 (1,604–1,725) | 420.5 (412.8–428.2) |
| Medium | 3.0 | 962.6 (897.2–1,028) | 256.4 (241.7–271.2) |
| Low | 5.0 | 398.1 (356.4–439.8) | 119.7 (110.3–129.2) |
| Sham | N/A | 0 | 0 |
The impulse was calculated at a representative time 1.5 ms from the onset of shock wave initiation. CI, confidence interval.
Exposure to the blast wave resulted in localized bruising to the skin and underlying tissue only.
Outcome measures
The primary outcome of this study was the effect of the blast wave on the endothelium. In addition the surrounding tissue was assessed to determine both direct blast effects and indirect effects resulting from endothelial actication and/or damage.
Exposure to blast causes damage to the endothelium
Endothelial cells have been shown to be present at very low numbers in the circulating blood of healthy individuals and their appearance is associated with damage to the lining of blood vessels and so CECs are a specific marker of endothelial damage.
In the sham, low, and medium blast groups we saw no increase in CEC numbers over time compared with the baseline sample. In the high blast group, however, there were significantly higher numbers of CECs in the blood at 6 h compared with at 1 h postinjury and at this time point the levels were also significantly higher than in the sham group (Fig. 1).
Fig. 1.
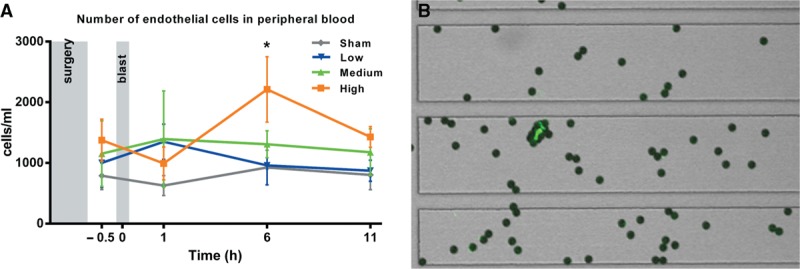
Number of endothelial cells in peripheral blood pre- and post-blast exposure (A). Sham, low, and medium groups n = 6, High group n = 9. Number of CECs was significantly higher at 6 h in high group compared with at 1 h and compared with sham at 6 h (P < 0.05, two-way ANOVA). Error bars show ± SEM. Example of a CEC stained with FITC-UEA-1 with 4 μm magnetic beads attached (B).
Exposure to blast results in local pathological changes in tissue
Histology
No adverse pathology was observed in hind limb muscles from the sham group or in the fore limb in any of the groups. Cell infiltrate, oedema, and hemorrhage, to varying extents, however, were observed in all three blast groups (Fig. 2) with a significant trend of increasing scores from sham to high (P < 0.0001). In addition, necrosis of the muscle tissue was observed. This was subjectively estimated and expressed as maximum percentage of muscle fiber bundles displaying evidence of necrosis in each section and when plotted by blast loading it also shows a significant increase with blast level (Fig. 3).
Fig. 2.
Pathology in H&E stained muscle tissue sections.
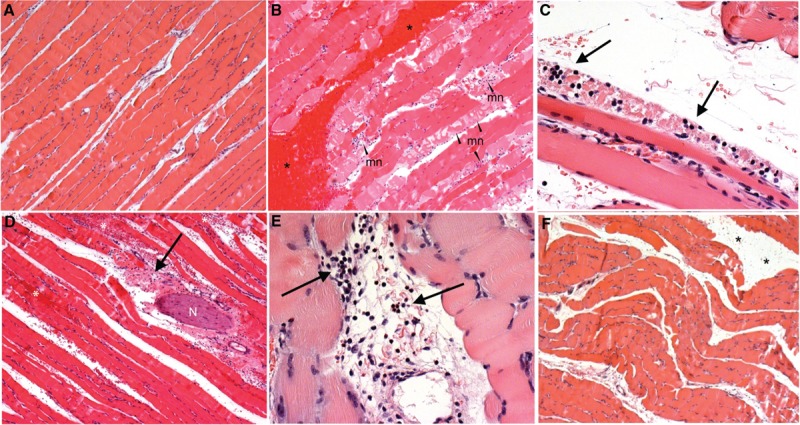
A, Muscle tissue from non-blasted (control) animals displayed normal morphology with no evidence of background pathology. B, Muscle tissues from many of the blast exposed animals displayed varying degrees of hemorrhage (∗) and muscle fiber necrosis (mn). C, Necrosis was frequently associated with concentrations of polymorphonuclear cells (heterophil) infiltrating the muscle tissues (↑). D, Examples of inflammatory cell infiltration (↑) and hemorrhage (∗) were also observed in adipose and connective tissues surrounding some nerve fiber bundles (N) within the area of injured muscle tissue. E, Some areas of connective and adipose tissue within the muscle were also seen to contain mononuclear lymphocytes (↑). F, Oedema was observed between muscle fiber bundles in some animals exposed to blast injury (∗). Images A, B, D and F scale = 400 μm, C scale = 50 μm, E scale = 100 μm.
Fig. 3.
Pathology scores of muscle tissue following blast exposure.
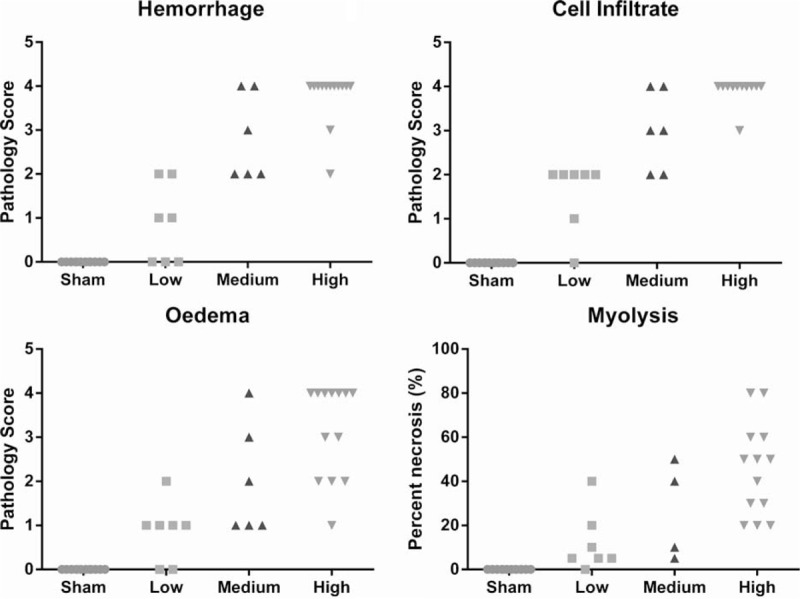
hemorrhage, cell infiltrate, and oedema were scored as not present (score = 0), mild (score = 1), moderate (score = 2), or severe (score = 4) (A–C), and the maximum percentage of muscle fibers showing signs of necrosis subjectively estimated (D). All showed a statistically significant increasing trend correlating with blast level (P < 0.0001 Cuzick's trend test).
Markers of immune response and endothelial activation
Circulating levels of inflammatory cells e.g. neutrophils showed some changes over the time course of the study but levels were almost identical between groups (data not shown).
Endothelial and immune cell activation was assessed in the muscle tissue excised at the end of the study.
Levels of both TNFα and E-selectin expression were significantly higher in the high blast group compared with sham (P < 0.01, one-way ANOVA with Dunnett's post-test compared with sham (Fig. 4).
Fig. 4.
Expression levels of TNFα (A) and E-selectin (B) within muscle tissue indicate activation of the endothelium after blast.
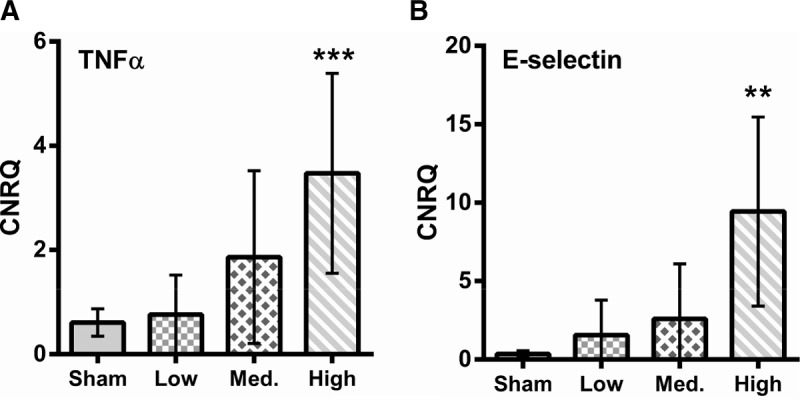
CNRQ, calibrated normalized relative quantities as calculated from Cq values using the qBasePlus program. ∗∗P < 0.01, one-way ANOVA with Dunett's post-test compared with sham. Error bars show 95% CI.
Levels of both IL-6 and THMB expression were significantly higher in the high blast group compared with sham (P < 0.01, one-way ANOVA with Dunnett's post-test compared with sham, Fig. 5).
Fig. 5.
Expression levels of Il-6 (A) and thrombomodulin (B) within muscle tissue indicate activation of the endothelium as well as immune activation after blast.
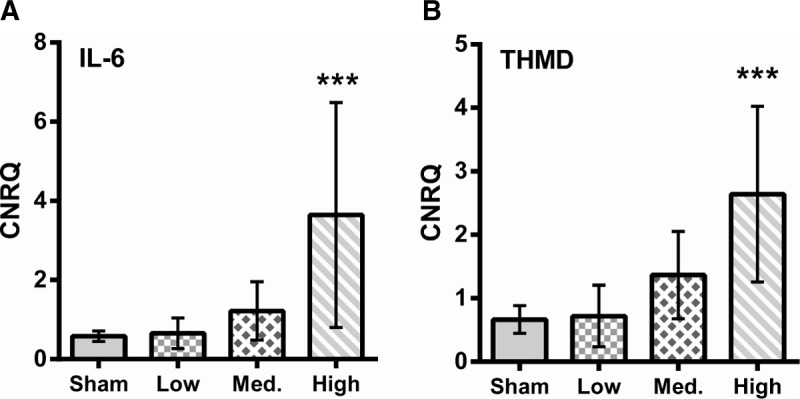
CNRQ, calibrated normalized relative quantities as calculated from Cq values using the qBasePlus program. ∗∗∗P < 0.01, one-way ANOVA with Dunett's post-test compared with sham. Error bars show 95% CI.
Local oxygen delivery
Blast injury may change local blood flow via the release of vasoactive substances and this in turn may affect local oxygen delivery. To investigate this tissue RNA expression levels of the classical hypoxic response regulator HIF-1α and the alternative transcription factor PGC-1α were investigated.
Increased blast loading caused a significant increase in the expression of HIF-1α in a highly correlative manner (Pearson rank correlation = 0.99 (P = 0.0135)). In contrast there was no significant increase in the expression of the transcription factor PGC-1α (Fig. 6).
Fig. 6.
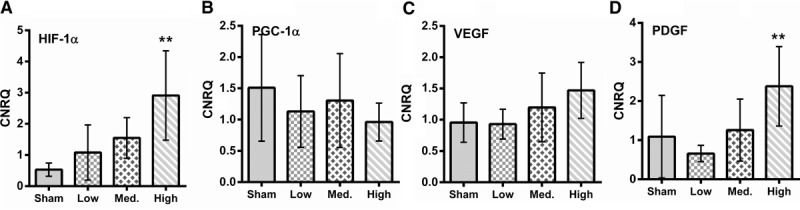
Expression levels of HIF-1α (A), PGC-1α (B), VEGF (C), and PDGA (D) in tissue. ∗∗Indicates significant difference compared with control.
Stimulation of angiogenesis
When the level of VEGF mRNA was assessed in muscle tissue no statistically significant increase in expression was observed between the different blast loadings. There was, however, a statistically significant increase in expression of PDGF in the high blast group (Fig. 6).
There were no statistically significant differences in plasma levels of vWF or VEGF between groups. There was no statistically significant difference in muscle levels of ET-1 or SDF-1 between groups. Nor were there statistically significant differences in muscle tissue gene expression of VCAM, ET-1 or erythropoeitin between groups, data not shown.
DISCUSSION
The endothelium is a diverse structure whose characteristics are dependent on the location and tissue type. The endothelium plays a vital role in maintenance and regulation of blood flow to tissues and in preventing thrombus formation. Endothelial cells secrete and express a number of products involved in coagulation/anticoagulation, inflammation, lipid metabolism, vasomotor control, and growth. Under normal circumstances in healthy individuals the endothelium has an anti-inflammatory and antithrombotic phenotype. When activated by injury for example, endothelial cells change functionality and become pro-inflammatory, prothrombotic, proproliferative, and vasoconstricting. Damage to endothelium from an injurious mechanism such as blast may be assessed by a number of circulating markers as wells as local gene and protein expression.
The present study has demonstrated that blast injury to the hind limbs of anaesthetized rabbits causes activation and damage to the endothelium. The magnitude of the effect is related to the level of blast loading with low blast exposures resulting in endothelial activation and with endothelial damage (evidenced by CECs) only evident after high blast exposure. Changes to the endothelium ultimately resulted in changes in the histological appearance of muscle tissue at and around the site of injury with oedema, cell infiltrate, and also necrosis.
The tissue changes associated with blast damage occurred locally, and over the 12-h postinjury period did not result in detectable systemic markers of endothelial damage with one exception. In the limbs exposed to the high blast loading there was a significant increase in CECs at 6-h postinjury compared with sham exposure. This is in contrast to the study by Ning et al. (11) in which the hind limbs of rats exposed to a blast injury caused a systemic inflammatory response and remote (lung) organ damage. The most likely cause of these different results is that the injury in their rats resulted in hemorrhage, fracture, soft tissue injury, and burns and so a much more severe insult than the current rabbit study. The “inflammatory spill-over” effect has also been observed after whole body blast exposure in the mouse with and without regional blast protection (12) and it is possible that given a longer post-blast exposure period remote organ effects may become apparent in the current study. After whole-body blast exposure it is highly likely that effects such as bradycardia, hypotension and apnoea would be seen. No physiological effects were observed post-blast in the current study demonstrating that the exposure was confined to the hind limbs and explains the more local effects in this study.
CECs are usually absent in healthy individuals but have been identified in diseases known to have vascular damage such as cancers, infectious diseases, and myocardial infarction (13) and are increased after stress and exercise (14). Following an insult it is thought that endothelial cells slough off the vessel wall (and become CECs). This insult may be mechanical injury, there may be changed or defective adhesion properties, or there may be necrosis or apoptosis. The time course associated with the appearance of increased levels of CECs in the current study is indicative of activation of biochemical pathways causing detachment rather than because of the initial mechanical force from the blast wave itself. The histological evidence of extra-vascular blood in the muscle tissue demonstrates that physical damage to vessel walls had occurred and therefore the expected finding if mechanical forces were the cause of endothelial cell detachment would have been a more immediate rise with a peak at 1 h rather than the actual “peak” at 6-h postinjury. Additionally, only the high blast group had significantly elevated CECs despite evidence of hemorrhage in the low blast group and a similar distribution of hemorrhage scores in the medium blast group. The apoptotic state of cells within the vessel wall was assessed on histological sections of the saphenous blood vessel. Unfortunately because of methodological problems it was not possible to complete this analysis and so it is not possible to conclusively determine the mechanism of endothelial cell detachment.
The presence of CECs demonstrates the severity of endothelial injury likely to have clinical consequences. It is possible that these CECs contributed to a pro-inflammatory state and this may be particularly true if the cells were necrotic releasing cellular substances such as HMGB-1 and DNA. A pro-inflammatory state may have local (cellular), regional (tissue), and systemic effects.
Local inflammatory responses were detected by the increased expression of the pro-inflammatory markers E-selectin, TNF-α, and IL-6. There were significant increases in IL-6, E-selectin, and TNFα gene expression in the high blast group compared with the sham group. The high blast group also demonstrated the most significant inflammatory cell infiltrate demonstrating a clinically significant effect of the increased pro-inflammatory gene expression. Hypoxia can induce endothelial expression of IL-6 (15) and that group have hypothesised that this increase is protective. In addition, E-selectin has been shown to be essential for homing of EPCs to ischaemic muscle (16) and so increased levels of E-selectin are essential for repair mechanisms following injury. The current study was not of sufficient duration to determine the long-term consequences of the inflammatory response and problems only arise when the inflammatory responses become exaggerated. A lack of increased expression of anti-inflammatory molecules however could be suggestive of an inappropriate response that may have a negative impact on outcomes. Tissue oedema was greatest in the high blast group indicating the greatest endothelial dysfunction and permeability.
Oxygen is essential for aerobic respiration and to maintain life, therefore mechanisms exist to ensure oxygen homeostasis and prevent the damaging effects of prolonged ischaemia. HIF-1α is a transcriptional regulator that mediates adaptive responses to reduced oxygen tension (17) via increased expression of a variety of growth factors that promote angiogenesis, including VEGF and PDGF (17). The transcriptional growth factor PGC-1α is a HIF-1α independent regulator of angiogenesis and this mechanism appears to be of particular relevance in limb ischaemia. There was no evidence of upregulated VEGF or PGC-1α expression in any of the blast injured groups in the current study despite clear evidence of tissue hypoxia in the high blast group (increased HIF-1 expression). It is possible that the time point at which the tissue samples were taken (12 h postinjury) was too short and that the degree of hypoxia/ischaemia was insufficient for the induction of increased PGC-1α expression and too early postinduction of HIF-1α expression for increased VEGF expression.
HIF-1α is known to change metabolism from oxidative to glycolytic and to stimulate the expression of glycolytic genes (17). The clinical significance of this switch in the context of the current study is the possibility that blast injured tissue is more likely to rely on anaerobic respiration and thus develop a metabolic acidosis, and in the context of a blast injured casualty blast could exacerbate the local effects of tissue hypoperfusion because of hemorrhage (common after explosive injury) which in turn would have implications for the initial resuscitation of injured personnel.
Thrombomodulin is the main orchestrator for antithrombotic nature of the endothelium in normal individuals. Thrombomodulin binds to thrombin to form a thrombin-thrombomudulin (TM-THMB) complex. This complex is directly anticoagulant and also has activity via the accelerated production of activated protein C (aPC). In the context of trauma and hypoperfusion aPC has additionally been implicated as a fibrinolytic (18). TM-THMB is also anti-inflammatory via activation of TAFI (thrombin-activatable fibrinolysis inhibitor) (19) and via degradation of HMGB-1 (20). In the current study there was the greatest thrombomodulin expression in the group with the greatest vascular damage, the high blast group. Inflammatory cytokines, such as TNF-α, are generally considered as downregulators of thrombomodulin (21) but in the current study the group with the greatest TNF-α expression also had the highest thrombomdulin expression. There are conflicted reports of the effect of shear stress (22, 23) and thrombin (24) on thrombomodulin expression in cell culture depending on the cell type under examination thus highlighting the complex nature of responses seen in vivo compared with in vitro. The presence of Heat Shock Proteins known to be released after trauma may have caused the increased thrombomodulin expression (25). PDGF in the high blast group was also significantly increased and this may have resulted in the increased thrombomodulin expression in the homogenized muscle tissue in this group (26).
Regardless of the cause of the increased thrombomodulin expression the clinical significance of the increase is of greater interest. On the one hand it can be considered as a therapeutic with recombinant soluble thrombomodulin showing potential (27–29). On the other hand in the context of trauma, overexpression of thrombomodulin may result in increased aPC production which may contribute to or exacerbate acute trauma coagulopathy. This would clearly have significant implications for the treatment of blast injured casualties. Unfortunately markers of fibrinolysis such as tPA were not assessed.
The current study does have limitations mainly because of the fact that the reagents available to examine markers of endothelial damage and activation were limited in the rabbit compared with either a rat or mouse. A time course of CEC measurements in addition to blood cytokine assays would not have been possible in either the rat or mouse without a large increase in the numbers of animals used. Studies to examine the time course of events would have been beneficial to elucidate the progression of the effects, as well as potentially determining whether blast had any effect on repair mechanisms, but again would have unnecessarily increased the number of animals used. The current study only examined a very focal blast exposure such that only a limited tissue bed was affected. Blast exposure to a greater area may have resulted in more systemic effects potentially leading to increased morbidity for the injured casualty.
It may be helpful to place the experimental blast data into an easily translatable clinical context but there are many caveats that need to be made to prevent inappropriate interpretation. The measured pressures given in Table 1 of this paper can be considered within the context of the output of real explosives, such as trinitrotoluene in a similar way to that published by Lashoff–Sullivan et al. (30) in their mouse model. The distance at which a specified reflected pressure is generated by an explosive increases with increasing charge size. The positive phase duration, however, does not scale in the same way so the combinations of the measured pressures with the positive phase duration of 1.5 ms (the duration of the measured blast wave from the blast apparatus) are only produced for certain charge sizes and distances. For spherical free air burst charges of TNT these charge sizes and distances can be estimated from the equations of Kingary and Bulmash (31) as implemented within the ConWep tool (32). Representative charge sizes and distances are 0.71 kg at 1.3 m, 0.67 kg at 1.5 m, and 0.3 kg at 1.6 m for the largest to smallest of the three pressures respectively. These should be considered as indicative predictions only, to put the pressure loadings into the context of explosive charges.
The work published by Bowen et al. (33) relates overpressure to an index of lethality and extrapolation of overpressure data in the current study indicates that (when modified to account for the differences in mass and response to blast between a rabbit and a person) exposing an unprotected person to these pressure loadings may be expected to produce survival outcomes from certain lethality at 24 h post-blast (probability > 0.99) for the highest pressure to a probability of lethality of about 0.10 for the lowest. These predictions, however, need to be put in context and it is very important to note that the lethality is based on a number of assumptions that need to be acknowledged to prevent misrepresentation of the data in the current study. The first assumption is that the whole body is exposed to the blast wave and all areas of the body receive the same loading. The reality is that different scenarios are going to produce highly variable pressure loadings to different parts of the body, for example an antipersonnel mine will produce a very localized injury to the leg with the rest of the body relatively uninjured. The second assumption is that no personal protective equipment is in place. The reality for military personnel is that there could well be areas of the body relatively exposed to the effects of blast whereas other areas are relatively protected. A third assumption is that no medical attention/treatment is given to the casualty in the first 24 h. The reality is that a person is likely to receive some form of medical care postinjury. Taken as a whole it is highly likely that the probability of lethality would be reduced, but it is not possible to determine the actual reduction, as that data is simply not available.
Lashof-Sullivan et al. (30) suggested that the degree of blast lung injury in their model affords a greater understanding of the blast overpressures, therefore data have been extrapolated from that presented by Jaffin et al. (9) to help with interpretation of the overpressure data in the current study. At 2.0 cm (overpressure equivalent to 1.5 cm in the current study) above the umbilicus in the rat there were multifocal hemorrhages or hemorrhages >0.5 cm3 in the intestines (9). Studies by Tatic et al. and Cripps and Cooper (34, 35) in rats and pigs respectively demonstrate that intestinal contusions from blast can lead to secondary perforations. Thus although not immediately fatal such lesions would require surgical intervention to reduce both morbidity and mortality. Therefore the blast exposures in the current study have been chosen to represent casualties with the whole spectrum of blast trauma ranging from those with minor injuries through to those with serious trauma.
Much of the clinical significance of the findings is speculative and further work is required to determine whether the “physical” changes observed in this study correspond to a functional change that would either affect functional outcomes or significantly influence casualty management. Future work should address this via the evaluation of critical oxygen delivery to the limb, for example, as well as the responsiveness to vasoactive substances.
CONCLUSION
This study has demonstrated that blast injury can activate the endothelium and in some cases cause damage which then in turn leads to effects in the surrounding tissue such as oedema and inflammatory cell infiltrate. For the casualty injured by an explosion the damaging effects of hemorrhage and resulting shock could be exacerbated by blast injury and vice versa so that even low levels of blast become damaging, impacting on overall recovery and functionality.
Footnotes
A.M.S. and E.M.D. are joint first authors.
This study was funded by Ministry of Defence. The authors report no conflicts of interest.
REFERENCES
- 1.Owens BD, Kragh JF, Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma 2008; 64:295–299. [DOI] [PubMed] [Google Scholar]
- 2.White JM, Stannard A, Burkhardt GE, Eastridge BJ, Blackbourne LH, Rasmussen TE. The epidemiology of vascular injury in the wars in Iraq and Afghanistan. Ann Surg 2011; 253:1184–1189. [DOI] [PubMed] [Google Scholar]
- 3.Doukas WC, Hayda RA, Frisch HM, Andersen RC, Mazurek MT, Ficke JR, Keeling JJ, Pasquina PF, Wain HJ, Carlini AR, et al. The Military Extremity Trauma Amputation/Limb Salvage (METALS) study: outcomes of amputation versus limb salvage following major lower-extremity trauma. J Bone Joint Surg Am 2013; 95:138–145. [DOI] [PubMed] [Google Scholar]
- 4.Scott DJ, Arthurs ZM, Stannard A, Monroe HM, Clouse WD, Rasmussen TE. Patient-based outcomes and quality of life after salvageable wartime extremity vascular injury. J Vasc Surg 2014; 59:173. [DOI] [PubMed] [Google Scholar]
- 5.Hancock HM, Stannard A, Burkhardt GE, Williams K, Dixon P, Cowart J, et al. Hemorrhagic shock worsens neuromuscular recovery in a porcine model of hind limb vascular injury and ischemia-reperfusion. J Vasc Surg 2011; 53:1052–1062. [DOI] [PubMed] [Google Scholar]
- 6.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002; 10:620–630. [DOI] [PubMed] [Google Scholar]
- 7.Giannoudis PV, Tosounidis TI, Kanakaris NK, Kontakis G. Quantification and characterisation of endothelial injury after trauma. Injury 2007; 38:1373–1381. [DOI] [PubMed] [Google Scholar]
- 8.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Hue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock 2013; 40:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffin JH, McKinney L, Kinney RC, Cunningham JA, Moritz DM, Kraimer JM, Graeber GM, Moe JB, Salander JM, Harmon JW. A laboratory model for studying blast overpressure injury. J Trauma 1987; 27:349–356. [DOI] [PubMed] [Google Scholar]
- 10.Woywodt A, Blann AD, Kirsch T, Erdbruegger U, Banzet N, Haubitz M, Dignat-George F. Isolation and enumeration of circulating endothelial cells by immunomagnetic isolation: proposal of a definition and a consensus protocol. J Thromb Haemost 2006; 4:671–677. [DOI] [PubMed] [Google Scholar]
- 11.Ning JL, Mo LW, Lu KZ, Lai XN, Wang ZG, Ma D. Lung injury following lower extremity blast trauma in rats. J Trauma Acute Care Surg 2012; 73:1537–1544. [DOI] [PubMed] [Google Scholar]
- 12.Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front Neurol 2010; 1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goon PK, Boos CJ, Lip GY. Circulating endothelial cells: markers of vascular dysfunction. Clin Lab 2005; 51:531–538. [PubMed] [Google Scholar]
- 14.Boos CJ, Balakrishnan B, Lip GY. The effects of exercise stress testing on soluble E-selectin, von Willebrand factor, and circulating endothelial cells as indices of endothelial damage/dysfunction. Ann Med 2008; 40:66–73. [DOI] [PubMed] [Google Scholar]
- 15.Yan SF, Ogawa S, Stern DM, Pinsky DJ. Hypoxia-induced modulation of endothelial cell properties: regulation of barrier function and expression of interleukin-6. Kidney Int 1997; 51:419–425. [DOI] [PubMed] [Google Scholar]
- 16.Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB, et al. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 2007; 110:3891–3899. [DOI] [PubMed] [Google Scholar]
- 17.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009; 24:97–106. [DOI] [PubMed] [Google Scholar]
- 18.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care 2007; 13:680–685. [DOI] [PubMed] [Google Scholar]
- 19.Myles T, Nishimura T, Yun TH, Nagashima M, Morser J, Patterson AJ, Pearl RG, Leung LL. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem 2003; 278:51059–51067. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shresthra B, Hashiguchi T, et al. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol 2008; 28:1825–1830. [DOI] [PubMed] [Google Scholar]
- 21.Seguin C, Abid MR, Spokes KC, Aird WC. Thrombin downregulates thrombomodulin expression and activity in primary human endothelial cells. Endothelium 2008; 15:143–148. [DOI] [PubMed] [Google Scholar]
- 22.Ishibazawa A, Nagaoka T, Takahashi T, Yamamoto K, Kamiya A, Ando J, Yoshida A. Effects of shear stress on the gene expressions of endothelial nitric oxide synthase, endothelin-1, and thrombomodulin in human retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci 2011; 52:8496–8504. [DOI] [PubMed] [Google Scholar]
- 23.Malek AM, Jackman R, Rosenberg RD, Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res 1994; 74:852–860. [DOI] [PubMed] [Google Scholar]
- 24.Bartha K, Brisson C, Archipoff G, de la Salle C, Lanza F, Cazenave JP, Beretz A. Thrombin regulates tissue factor and thrombomodulin mRNA levels and activities in human saphenous vein endothelial cells by distinct mechanisms. J Biol Chem 1993; 268:421–429. [PubMed] [Google Scholar]
- 25.Conway EM, Liu L, Nowakowski B, Steiner-Mosonyi M, Jackman RW. Heat shock of vascular endothelial cells induces an up-regulatory transcriptional response of the thrombomodulin gene that is delayed in onset and does not attenuate. J Biol Chem 1994; 269:22804–22810. [PubMed] [Google Scholar]
- 26.Lo IC, Lin TM, Chou LH, Liu SL, Wu LW, Shi GY, et al. Ets-1 mediates platelet-derived growth factor-BB-induced thrombomodulin expression in human vascular smooth muscle cells. Cardiovasc Res 2009; 81:771–779. [DOI] [PubMed] [Google Scholar]
- 27.Li JM, Singh MJ, Itani M, Vasiliu C, Hendricks G, Baker SP, et al. Recombinant human thrombomodulin inhibits arterial neointimal hyperplasia after balloon injury. J Vasc Surg. 2004; 39:1074–1083. [DOI] [PubMed] [Google Scholar]
- 28.Aikawa N, Shimazaki S, Yamamoto Y, Saito H, Maruyama I, Ohno R, et al. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock 2011; 35:349–354. [DOI] [PubMed] [Google Scholar]
- 29.Kearon C, Comp P, Douketis J, Royds R, Yamada K, Gent M. Dose-response study of recombinant human soluble thrombomodulin (ART-123) in the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 2005; 3:962–968. [DOI] [PubMed] [Google Scholar]
- 30.Lashof-Sullivan MM, Shoffstall E, Atkins KT, Keane N, Bir C, Vandevord P, Lavik EB. Intravenously administered nanoparticles increase survival following blast trauma. Proc Natl Acad Sci 2014; 111:10293–10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kingery CN, Bulmash G: Airblast Parameters from TNT Spherical Air Burst and Hemispherical Surface Burst, Defence Technical Information Center, Ballistic Research Laboratory, Aberdeen Proving Ground, Maryland, ARBRL-TR-02555, 1984. [Google Scholar]
- 32.ConWep 2.1.0.8, USAE Engineer Research and Development Center, Geotechnical/Structure Laboratory, Vicksberg, Mississippi. [Google Scholar]
- 33.Bowen IG, Fletcher ER, Richmond DR: Estimates of man's tolerance to the direct effects of air blast. DASA-2113, Washington DC, Defence Atomic Support Agency, 1968. [Google Scholar]
- 34.Tatic V, Ignjatovic D, Jevtic M, Jonanovic M, Draskovic M, Durdevic D. Morphological characteristics of primary nonperforated intestinal blast injuries in rats and their evolution to secondary perforations. J Trauma 1996; 40:S94–S99. [DOI] [PubMed] [Google Scholar]
- 35.Cripps NP, Cooper GJ. Risk of late perforation in intestinal contusions caused by explosive blast. Bri J Surg 1997; 84:1298–1303. [PubMed] [Google Scholar]


