Background and Purpose—
We sought to establish whether the presence (versus absence) of a lesion on magnetic resonance imaging (MRI) with diffusion weighting (DWI-MRI) at presentation with acute stroke is associated with worse clinical outcomes at 1 year.
Methods—
We recruited consecutive patients with a nondisabling ischemic stroke and performed DWI-MRI. Patients were followed up at 1 year to establish stroke recurrence (clinical or on MRI), cognitive impairment (Addenbrooke Cognitive Assessment Revised,<88) and modified Rankin Scale.
Results—
A median of 4 days post stroke, one third (76/264; 29%) of patients did not have a DWI lesion (95% confidence interval, 23%–35%). There was no statistically significant difference between those with and without a DWI lesion with respect to age or vascular risk factors. Patients without a lesion were more likely to be women or have previous stroke. At 1 year, 11 of 76 (14%) patients with a DWI-negative index stroke had a clinical diagnosis of recurrent stroke or transient ischemic attack, 33% had cognitive impairment (Addenbrooke Cognitive Assessment Revised <88), and 40% still had modified Rankin Scale >1, no different from DWI-positive patients; DWI-positive patients were more likely to have a new lesion on MRI (14%), symptomatic or asymptomatic, than DWI-negative patients (2%; P=0.02). Our data were consistent with 6 other studies (total n=976), pooled proportion of DWI-negative patients was 21% (95% confidence interval, 12%–32%).
Conclusions—
Nearly one third of patients with nondisabling stroke do not have a relevant lesion on acute DWI-MRI. Patients with negative DWI-MRI had no better prognosis than patients with a lesion. DWI-negative stroke patients should receive secondary prevention.
Keywords: cognition; diffusion; ischemic attack, transient; magnetic resonance imaging; stroke
Magnetic resonance imaging (MRI) with diffusion weighting (DWI-MRI) detects more ischemic stroke lesions than computed tomography1 and is recommended to diagnose stroke in national stroke guidelines.2 There is an increasing reluctance to diagnose stroke in patients who have clinical features of stroke and a negative DWI. Previous studies found that DWI-MRI did not identify a relevant ischemic lesion in one third of patients with a nondisabling stroke,3,4 but there is little information on these patients’ long-term prognosis. If patients without a DWI-MRI lesion (DWI-negative) have not had a stroke, then we would expect that they would have a better prognosis with a lower risk of long-term dependency, recurrent stroke, and cognitive impairment than patients with a DWI-visible ischemic lesion (DWI-positive).
Objectives
We aimed to determine (1) the proportion of patients with nondisabling stroke without an acute ischemic lesion on DWI-MRI, (2) whether these patients are clinically different at presentation from those with a lesion, and (3) whether they have a reduced risk of recurrent stroke, ongoing significant symptoms, dependency, or cognitive impairment at 1 year. We also updated a systematic review and meta-analysis of all recent studies.
Methods
Study Population and Recruitment
We performed a prospective observational study, recruiting consecutive inpatients and outpatients with mild (ie, nondisabling) ischemic stroke who presented to the Lothian regional stroke service from May 2010 to May 2012. We defined a nondisabling ischemic stroke as focal onset of neurological symptoms lasting >24 hours, with no other explanation, which did not cause significant impairment in basic activities of daily living at presentation.
We excluded patients who did not have a diagnosis of stroke, those whose symptoms resolved within 24 hours, those who were unable to consent (eg, because of dementia or aphasia), those who had a contraindication to MRI, those with clearly disabling stroke, and those with another medical condition, which meant that they were unlikely to survive for a year.
All participants involved in the study gave written informed consent, and the study was approved by the Lothian Research Ethics committee (ref 09/S1101/54).
Collection of Clinical Data
The clinical research fellow (S.D.J.M.) assessed all patients and recorded clinical features, baseline demographics, and risk factors (Table 1). We classified the stroke subtype using the Oxfordshire Community Stroke Project classification.5 We determined whether symptoms were likely to relate to anterior or posterior circulation and recorded whether the patient had previous stroke or transient ischemic attack (TIA), ischemic heart disease, atrial fibrillation, hypertension (defined as blood pressure of ≥140/90 mm Hg on presentation or a previous diagnosis), hyperlipidemia (cholesterol >5 mmol/L on presentation or a previous diagnosis), previous diagnosis or current symptoms of peripheral vascular disease, diabetes mellitus (previous diagnosis or diagnosed on admission in accordance with the World Health Organization criteria), family history of stroke, and smoking status. We recorded the worst National Institutes of Health Stroke Scale score; if symptoms had improved before the assessment, we estimated the score at the worst point from the history and referral information.
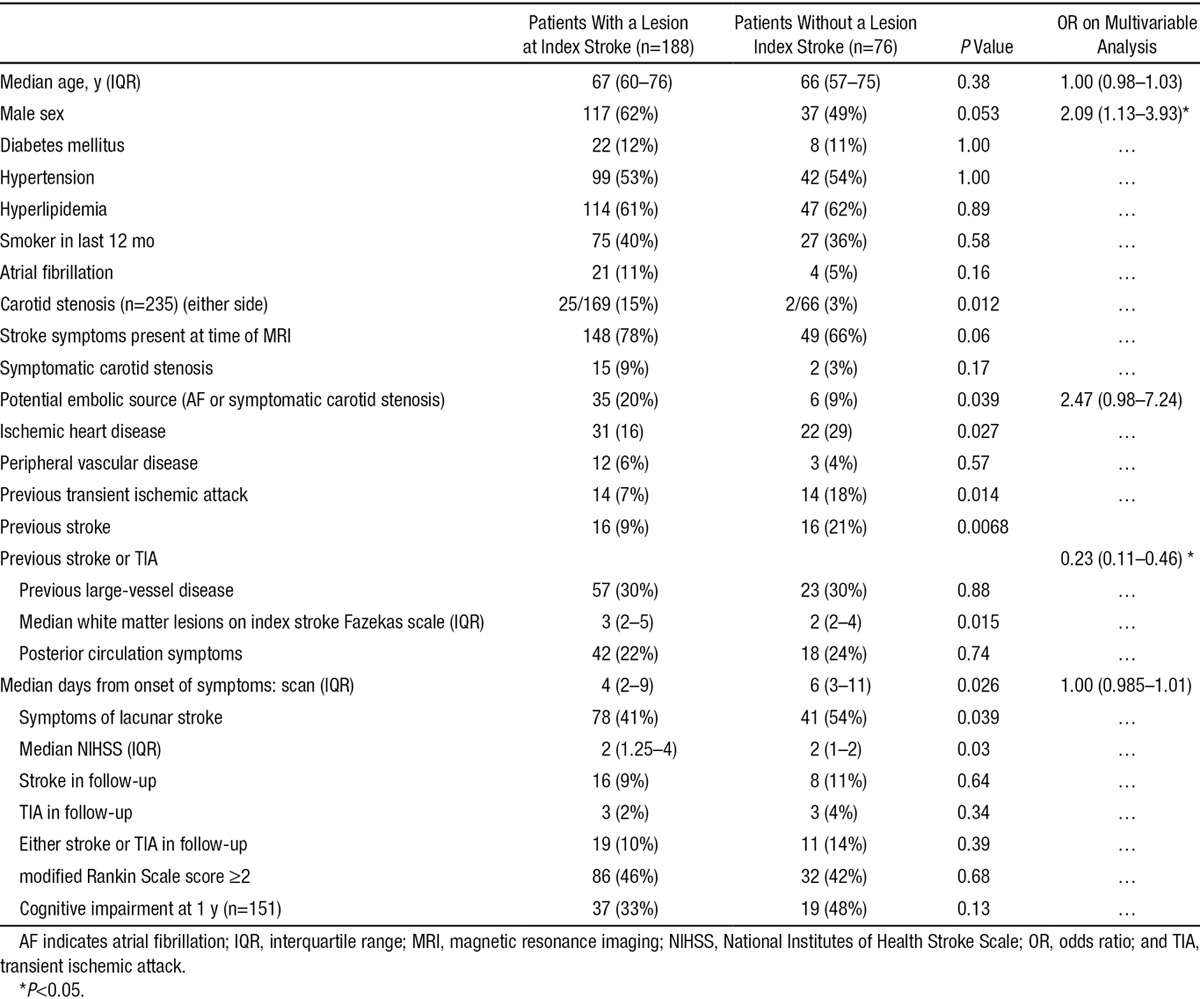
Table 1.
Comparison of Patients With and Without a Lesion on Magnetic Resonance Imaging
Imaging
All patients underwent a brain MRI scan on the same 1.5 Tesla MRI scanner (Signa LX; General Electric, Milwaukee, WI) operating in research mode, and using a self-shielding gradient set with maximum strength of 33 mT/m, and an 8-channel phased-array head coil. The scanner was operated within a tight quality assurance program to maintain uniform performance. Sequences included axial DWI (30-direction axial diffusion tensor imaging, b=1000 s/mm2 and 2×b0 acquisitions, repetition time/echo time [TR/TE]=7700/82 ms, 24×24 cm field of view [FoV], 128×128 acquisition matrix, 28×5-mm slices), T2-weighted (TR/TE=6000/90 ms, 24×24 cm FoV, 384×384 propeller acquisition, 1.5 averages), axial fluid-attenuated inversion recovery (TR/TE/inversion time =9000/153/2200, 24×24 cm FoV, 384 (anterior-posterior)×224 acquisition matrix), T2* (TR/TE=800/15 ms, 20° flip angle, 24 (anterior-posterior)×18 cm FoV, 384×168 acquisition matrix, 2 averages, all with 28×5-mm slices and 1-mm slice gap), and sagittal 3D T1-weighted (TR/TE/inversion time=7.3/2.9/500 ms, 8° flip angle, 330 (superior-inferior)×214.5 cm FoV, 256×146 acquisition matrix, 100×1.8-mm slices). An ECG and routine blood tests (including serum glucose, lipids, erythrocyte sedimentation rate, and renal and liver function tests) were performed. Patients had carotid Doppler ultrasound imaging to identify carotid stenosis, defining symptomatic carotid stenosis as >50% stenosis on the relevant side measured by the North American Symptomatic Carotid Endarterectomy Trial criteria.6 Echocardiography and other investigations were performed according to clinical practice guidelines.2
Diagnosis
A panel of neurologists, stroke physicians, and neuroradiologists met weekly to discuss the clinical findings, MRI images, all other investigations and to reach a final consensus diagnosis. We included a patient if the final diagnosis was of ischemic stroke defined as a sudden onset of neurological symptoms, lasting >24 hours, with no other more likely causes identified.
An experienced neuroradiologist (J.M.W.) assessed all scans for acute ischemic lesions, according to well-defined criteria for identifying ischemic lesions on neuroimaging.7 We classed a lesion as relevant to the presenting symptoms if it was of the appropriate size and shape for an infarct, in the appropriate brain location, and appeared of the right age to have caused the stroke symptoms. We defined lacunar infarct as a lesion <20-mm axial diameter in the deep gray or white matter of the cerebral hemispheres or brain stem. We defined cortical infarct as a lesion involving cortex or a large subcortical infarct >20-mm axial diameter (striatocapsular). We rated white matter hyperintensities (WMH) on the Fazekas scale8 and summed the peripheral and deep WMH scores to create a Total Fazekas score.
Follow-Up
We followed up all participants at 1 year after the stroke, with face-to-face assessment, review of hospital records, and a repeat MRI. If a participant could not attend a follow-up appointment, we performed a telephone interview or sent a postal questionnaire. If we could not contact patients or their carers, we obtained information from their general practitioners.
At 1 year, we recorded whether a recurrent stroke, TIA, or other vascular event had been diagnosed formally or whether the participant had any new symptoms suggestive of recurrent stroke or TIA. We also established whether there had been further clinical events that called the original diagnosis of stroke into question, eg, recurrent stereotyped events that may indicate migraine. We rated disability using the structured interview version of the modified Rankin Scale9 and assessed cognitive function using the Addenbrooke Cognitive Assessment Revised.10 We defined a participant as symptomatic of the index stroke if they were still experiencing symptoms, which had led to a change in their lifestyle (modified Rankin Scale score, >1). We defined cognitive impairment as Addenbrooke Cognitive Assessment Revised <88, which is recognized to be consistent with dementia.10
All 1-year MRI scans were read by an experienced neuroradiologist (J.M.W.) to identify any new lesions. The scan rating was performed blind to clinical follow-up details, first blind to and then comparing the follow-up with the presentation MRI and noting the location of any new ischemic or hemorrhagic lesions. The location of any new lesion was then compared with any symptoms of recurrent stroke to see whether the MRI lesion corresponded with the symptoms.
Statistical Analysis
We compared nonparametric continuous variables using the Mann–Whitney U test, and dichotomous variables using Fisher exact test. We created 2 further variables: any potential embolic source, meaning the presence of either atrial fibrillation or symptomatic carotid stenosis (>50%) and any large-vessel disease, meaning the presence of ischemic heart disease, peripheral vascular disease, or carotid stenosis on either side. We used binary logistic regression to perform a multivariate analysis, selecting variables based on our hypothesis and those that were statistically significant in the univariate analysis. We analyzed the data using R Version 2.15.2 (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0; http://www.R-project.org/).
Systematic Review
We referred to the recent systematic review of DWI in patients with both TIA and minor stroke11 and identified those studies that had provided data for nondisabling stroke separately from TIA. We updated the search that identified prospective studies of MRI in nondisabling minor stroke or TIA, to identify articles published up to October 2014. We included articles that provided data on mild stroke patients who underwent MRI and excluded retrospective case note reviews. We extracted data on study setting, inclusion/exclusion criteria, median National Institutes of Health Stroke Scale, delay between symptom onset and scan, imaging details, and number of participants with and without a lesion. We performed a meta-analysis of the proportion of patients with a DWI-negative stroke with a random-effects model using Stats Direct version 2.7.9 (StatsDirect statistical software; http://www.statsdirect.com; StatsDirect Ltd, England, 2013), both with and without the data from our study.
Results
Participant Recruitment
During the study period (May 2010 to April 2012), 471 patients were referred as potentially suitable for the study, of whom 264 were recruited (Figure 1), with final expert panel confirmed stroke. At 1-year post index stroke, 197 participants had in-person clinical and MRI follow-up, 151 had cognitive testing, and the remainder were followed up by post or by telephone. No patients were lost to follow-up.
Figure 1.
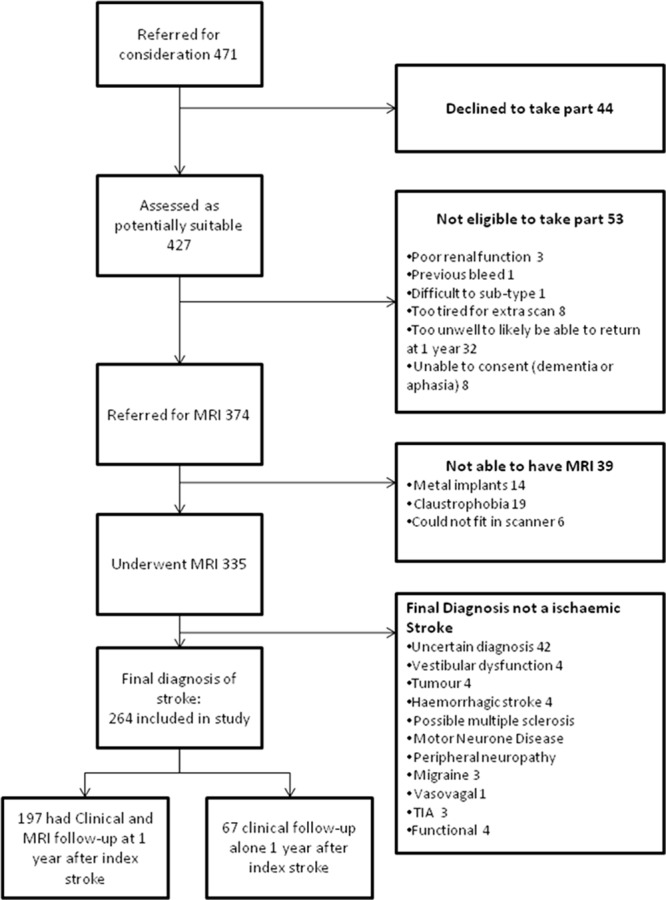
Recruitment into the study. MRI indicates magnetic resonance imaging; and TIA, transient ischemic attack.
Main Findings
Of the 264 who had a final diagnosis of index stroke, 76 (29% of patients; 95% confidence interval, 23%–34%) had no acute ischemic MRI lesion. Most of the 188 acute ischemic lesions were seen on DWI (178 patients) and 10 of 264 patients had relevant acute lesions on T2/fluid-attenuated inversion recovery only. Women and participants with a previous stroke or TIA were more likely to have no acute ischemic lesion on MRI (Table 1). Participants without an MRI lesion had a similar profile of vascular risk factors (diabetes mellitus, hypertension, high cholesterol, or smoking), noncarotid large-vessel artheromatous disease (peripheral vascular disease or ischemic heart disease), and were of a similar age to those with an acute ischemic lesion on MRI. DWI-positive patients were more likely to have carotid stenosis, atrial fibrillation, higher National Institutes of Health Stroke Scale, more WMH, and cortical stroke. DWI-negative patients were more likely to have ischemic heart disease or a previous stroke and be scanned later. After adjustment for age and sex, any embolic source and time to scanning were no longer associated with lesion presence.
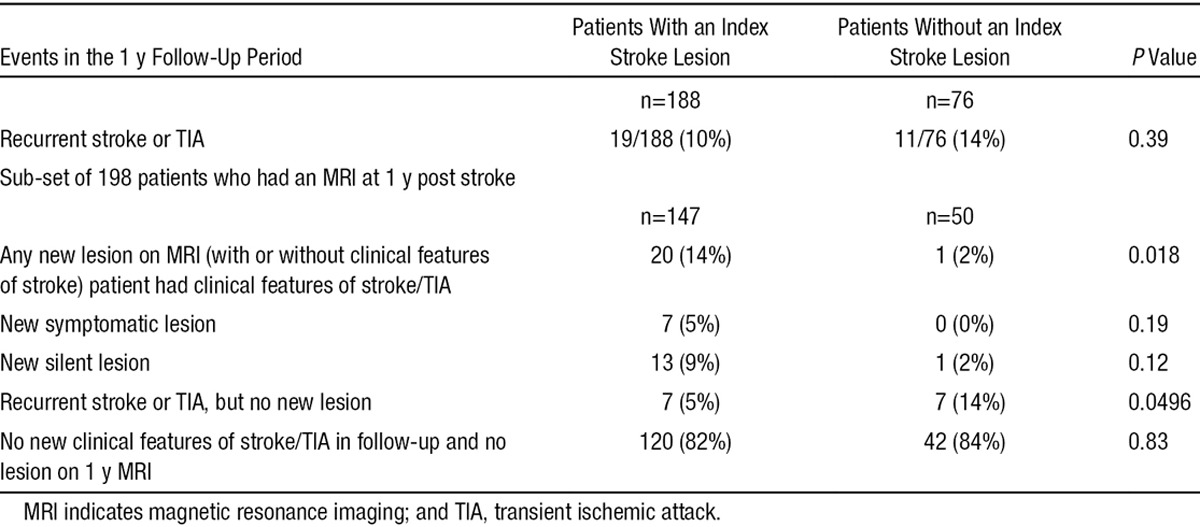
At 1 year, 198 were scanned: 25 were too unwell, 35 declined a further scan, and 3 were deceased. Of the 198 with a 1-year scan, the patients without an acute ischemic index MRI lesion were just as likely to have persistent symptoms, cognitive impairment, or recurrent stroke/TIA (Table 1) as those who had a DWI-positive index lesion. In addition, participants with an index lesion on DWI-MRI were more likely to have a new lesion (symptomatic or asymptomatic) on 1-year follow-up MRI (20/147; 14%) than patients who had not had an index ischemic MRI lesion (1/50 2%; P=0.02; Table 2). If silent and symptomatic lesions at 1-year follow-up are considered separately, both were more common in DWI-positive patients, but neither were statistically significant: 7 of 147, 5% of DWI-positive patients had a symptomatic lesion and 13/147, 9% had a silent lesion; by contrast, no DWI-negative patients had a symptomatic lesion on 1-year follow-up and just 1 of 50 (2%) had a silent lesion
Table 2.
Clinical and Imaging Recurrence of Stroke in Patients With and Without a Lesion Who Attended for 1-Year Follow-Up MRI
At 1 year post stroke, 4 patients with no index lesion had further events that had led to the original diagnosis of stroke being questioned: of these, 2 were thought to have epilepsy, 1 may have had functional symptoms, and 1 may have had dementia. One patient who had a DWI-MRI index lesion and clinical features of stroke contacted us at 3 years post stroke (ie, 2 years after the last follow-up) to inform us that that patient had since been diagnosed with multiple sclerosis.
Systematic Review
Identification of Articles
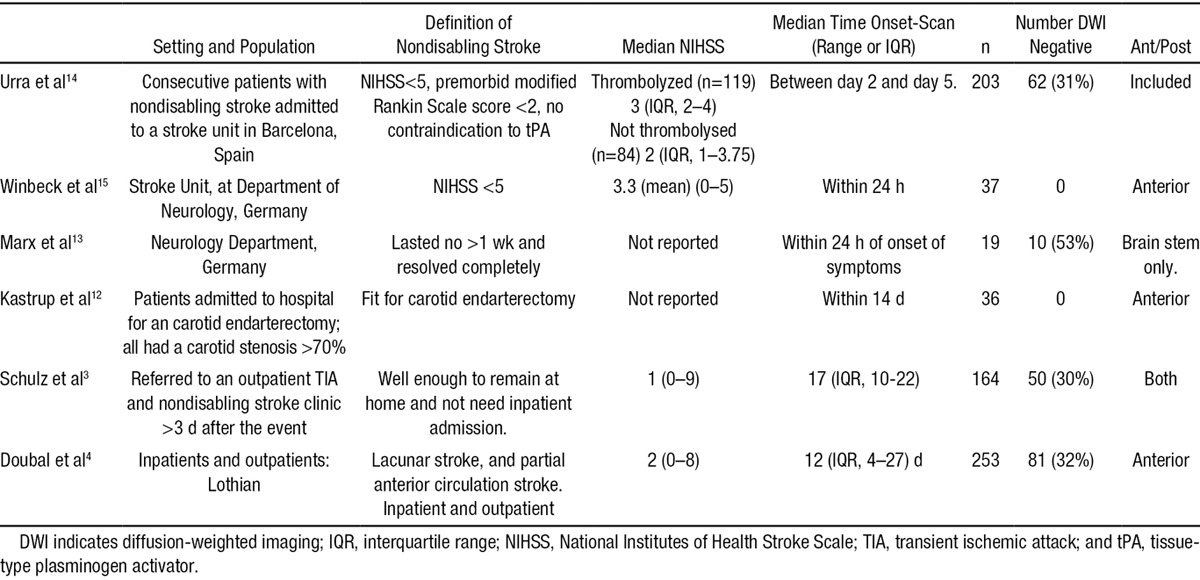
We included 6 previous studies3,4,12–15 of 712 participants plus the present study, total sample of 976 participants (Table 3).
Table 3.
Characteristics of Studies Included in Systematic Review
Study Characteristics
The studies used different definitions of nondisabling stroke: Schulz et al3 (n=164) included patients not requiring hospital admission; Winbeck et al15 (n=37) and Urra et al14 (n=208) included patients with National Institutes of Health Stroke Scale score of ≤5; Marx et al13 (n=19) only included patients with brain stem ischemia whose symptoms resolved completely within 1 week; Kastrup et al12 (n=37) only included patients referred for a carotid endarterectomy; and Doubal et al4 (n=253) (also performed in Edinburgh) used the same definition as the present study.
Meta-Analysis
Including the present study, the pooled estimate of the proportion of patients with DWI-negative nondisabling stroke was 21% (95% confidence interval, 12%–32%; Figure 2), with significant heterogeneity (I2; 93.3%). Excluding our study, the pooled proportion was 27% (95% confidence interval, 24%–30%), indicating that our findings are consistent with the other studies.
Figure 2.
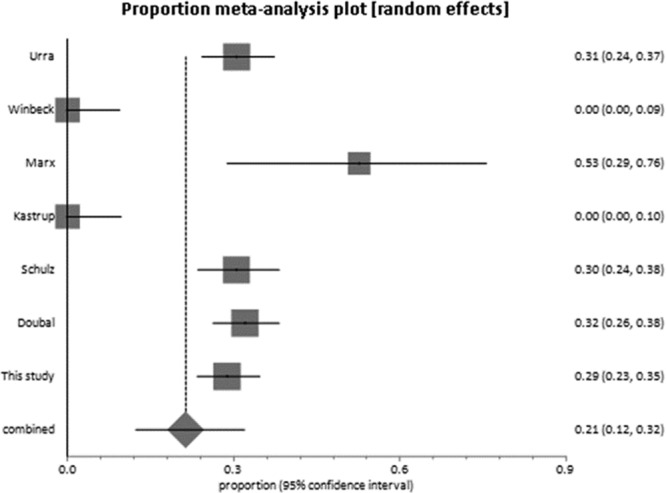
Forest plot of the pooled proportion of subjects with nondisabling stroke without a lesion on magnetic resonance imaging with diffusion weighting: median National Institutes of Health Stroke Scale <5 in all studies.
Discussion
We found that nearly one third of patients with a nondisabling stroke have no visible acute ischemic lesion on DWI-MRI. One year later, they were just as likely to have recurrent stroke, cognitive impairment (Addenbrooke Cognitive Assessment Revised <88), or stroke-related disability (modified Rankin Scale score ≥2) as the patients with an acute index lesion. The only difference was that DWI-positive index stroke patients were more likely to have a new lesion on MRI at 1 year (symptomatic or asymptomatic) than the DWI-negative index stroke patients.
Of the studies identified in our review, ours is the largest, and the only one to follow-up all patients at 1 year with repeat MRI for those able to attend. The patients’ diagnoses were all assessed by a panel of stroke experts with access to all clinical information and investigations, and we only included patients for whom the panel was unable to find a more likely explanation for the symptoms and signs than stroke. We followed up all patients at 1 year and carefully sought alternative diagnoses. It remains possible that some of the participants without an acute index lesion may have had a stroke mimic, such as migraine. But one might expect patients presenting with a stroke mimic to experience further events that would clarify the diagnosis, but in 95% of the DWI-negative patients, no other diagnosis has been made and several represented with recurrent stroke (including one who was given thrombolytic treatment).
Although it was the largest study on this topic to date, the sample is limited for a common disease like stroke. We scanned patients on the day of presentation to stroke specialists, but for some this may have been too late; our findings may not be applicable to a population where all are assessed immediately at symptom onset. However, our cohort represents a typical population of patients presenting with stroke and is consistent with other studies. Repeated scanning might identify some additional lesions, but this would be impractical and not representative of routine clinical practice. Although no patients were lost to follow-up, 25% were not able to have repeat MRI scanning at 1 year.
Our findings are consistent with the 6 other studies identified in our systematic review (n=718, n=978 including our study). Our finding that 11% of subjects without an index MRI lesion had a recurrent stroke in the first year is similar to the findings by Fujimoto et al16 who found that 7.8% of 102 subjects without a DWI lesion had a recurrent stroke in the first 3 months (only published in abstract at present so not in our review). A retrospective study of a stroke unit population with more severe stroke17 found a much smaller proportion of stroke patients without a lesion on DWI-MRI (16/701) although retrospective studies are prone to bias.
Why did some patients not have a DWI-MRI lesion? Perhaps the diagnosis was wrong; however, no DWI-negative patients were proven to have a nonvascular cause of their symptoms on either a subsequent scan or autopsy. DWI-MRI may also have false positives because DWI is not specific for ischemia.18 Four patients had some evidence that their index event could possibly have been a stroke mimic because they had further events that led to the original diagnosis being questioned although no alternative was proven. However, in general, patients with mimics such as migraine or seizures should not be disabled or cognitively impaired at 1 year, and 42% of DWI-negative patients had a modified Rankin Scale score ≥2 at 1 year, similar to those with a DWI-positive index lesion. The excess of previous vascular disease in patients without an index acute lesion on MRI suggests that these patients may be more aware of the need to seek medical attention for any new neurological symptoms.
DWI is sensitive to acute ischemia. The increased signal that makes the ischemic tissue visible is because of restricted water diffusion in the extracellular space as water shifts from the extra- to the intracellular space (intracellular edema) in early ischemia. However, consideration of the pathophysiological changes that lead to cellular edema shows that the reduction in cerebral blood flow required to initiate cell swelling is more severe than that required to produce acute neurological symptoms (Figure 3).19 Additionally, the chance of seeing an acute ischemic lesion on DWI-MRI increases as the severity of the stroke increases and with the duration of ischemia.20 Data from highly standardized experimental large artery stroke models demonstrate variability in infarct extent on DWI and a close relationship between depth and duration of the perfusion reduction and DWI visibility,21 but there are no data (to our knowledge) on DWI appearances in experimental minor stroke. Therefore, it is possible that some small (nondisabling) strokes reflect a reduction in blood flow severe enough to cause symptoms, but not severe enough to cause a DWI lesion, or that the DWI lesion was transient, was missed by scanning, and did not show up on structural MR sequences either (these are known to be less sensitive to small infarcts).22
Figure 3.
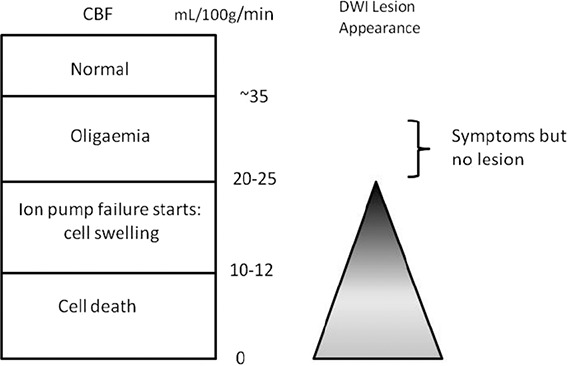
Relationship between symptoms, cerebral blood flow (CBF) and diffusion-weighted imaging (DWI) lesion.
It is intriguing that there were more recurrent infarcts, including silent infarcts, visible on the 1-year follow-up MRI in the patients with a visible index DWI-MRI lesion than in those without. This might suggest some differential vulnerability to developing infarction, which might be consistent with there being more WMH in the patients with DWI-positive index lesions than in those with DWI-negative lesions; WMH are associated with more infarct growth after hyperacute stroke,23 which has been interpreted as greater susceptibility to ischemia. Alternatively, the excess of previous vascular disease in patients without an index acute lesion on MRI suggests that these patients may be more aware of the need to seek medical attention for any new neurological symptoms.
Additional studies should include long-term follow-up to determine why some patients with no other explanation for their neurological symptoms apart from stroke do not to develop a visible lesion on DWI yet have similar cognitive and physical outcomes to those with a DWI lesion. Similar patients must have been included in secondary prevention studies, many of which were performed before widespread DWI availability or did not use DWI positivity as an entry requirement. Meantime patients with a clinical diagnosis of stroke who are DWI-negative have the same high risk of recurrent stroke and disability as DWI-positive patients and should receive secondary stroke prevention until further evidence on their specific management is available.
Acknowledgments
Dr Makin recruited and assessed the patients, performed statistical analysis, drafted and edited the article. Dr Wardlaw conceived and supervised the original project, provided input and direction, edited the articles, and prepared the final version. Dr Doubal provided guidance and edited the manuscript. Dr Dennis identified patients, participated in expert panel, read and critically appraised the article.
Sources of Funding
The study was funded by the Wellcome Trust ref. 088134/Z/09/A.
Disclosures
Drs Wardlaw and Dennis received funding from The Wellcome Trust to perform the study (funding for scanning, image analysis, SM salary support).
Footnotes
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
References
- 1.Brazzelli M, Sandercock PAG, Grazia Celani M, Righetti E, Chappell FM, Arestis N, et al. MRI versus CT for detection of acute vascular lesions in patients presenting with stroke symptoms. Stroke. 2010;41:e27–e28. doi: 10.1002/14651858.CD007424.pub2. [DOI] [PubMed] [Google Scholar]
- 2.National Collaborating Centre for Chronic Conditions (UK) National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA) Royal College of Physicians of London.; 2008. [PubMed] [Google Scholar]
- 3.Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke. 2004;35:2459–2465. doi: 10.1161/01.STR.0000143455.55877.b9. doi: 10.1161/01.STR.0000143455.55877.b9. [DOI] [PubMed] [Google Scholar]
- 4.Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross-sectional study. J Neurol Neurosurg Psychiatry. 2011;82:540–542. doi: 10.1136/jnnp.2009.190298. doi: 10.1136/jnnp.2009.190298. [DOI] [PubMed] [Google Scholar]
- 5.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–1758. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM. Stroke: A Practical Guide to Management. 2nd Ed. Oxford: Blackwell Scientific Ltd; 2001. What pathological type of stroke is it? pp. 151–222. [Google Scholar]
- 8.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 9.Quinn TJ, McArthur K, Dawson J, Walters MR, Lees KR. Reliability of structured modified rankin scale assessment. Stroke. 2010;41:e602. doi: 10.1161/STROKEAHA.110.590547. [DOI] [PubMed] [Google Scholar]
- 10.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 11.Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, Sandercock PA, et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol. 2013 doi: 10.1002/ana.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastrup A, Schulz JB, Mader I, Dichgans J, Küker W. Diffusion-weighted MRI in patients with symptomatic internal carotid artery disease. J Neurol. 2002;249:1168–1174. doi: 10.1007/s00415-002-0793-2. doi: 10.1007/s00415-002-0793-2. [DOI] [PubMed] [Google Scholar]
- 13.Marx JJ, Mika-Gruettner A, Thoemke F, Fitzek S, Fitzek C, Vucurevic G, et al. Diffusion weighted magnetic resonance imaging in the diagnosis of reversible ischaemic deficits of the brainstem. J Neurol Neurosurg Psychiatry. 2002;72:572–575. doi: 10.1136/jnnp.72.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urra X, Ariño H, Llull L, Amaro S, Obach V, Cervera Á, et al. The outcome of patients with mild stroke improves after treatment with systemic thrombolysis. PLoS One. 2013;8:e59420. doi: 10.1371/journal.pone.0059420. doi: 10.1371/journal.pone.0059420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winbeck K, Bruckmaier K, Etgen T, von Einsiedel HG, Röttinger M, Sander D. Transient ischemic attack and stroke can be differentiated by analyzing early diffusion-weighted imaging signal intensity changes. Stroke. 2004;35:1095–1099. doi: 10.1161/01.STR.0000125720.02983.fe. doi: 10.1161/01.STR.0000125720.02983.fe. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto S, Junnouch J, Matsuki T, Mezuki S, Ishitsucka T, Kitazono T. Stroke without Acute Ishchaemic Lesions on Diffusion Weighted Imaging - Fukuoka Stroke Registrary. Cerebrovasc Dis. 2012;34(suppl 1):95. [Google Scholar]
- 17.Watts J, Wood B, Kelly A, Alvaro A. Stroke syndromes associated with DWI-negative MRI include ataxic hemiparesis and isolated internuclear ophthalmoplegia. Neurol Clin Pract. 2013;3:186–191. doi: 10.1212/CPJ.0b013e318296f288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaarslan E, Arslan A. Diffusion weighted MR imaging in non-infarct lesions of the brain. Eur J Radiol. 2008;65:402–416. doi: 10.1016/j.ejrad.2007.04.023. doi: 10.1016/j.ejrad.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738 suppl):A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 20.Hand PJ, Wardlaw JM, Rivers CS, Armitage PA, Bastin ME, Lindley RI, et al. MR diffusion-weighted imaging and outcome prediction after ischemic stroke. Neurology. 2006;66:1159–1163. doi: 10.1212/01.wnl.0000202524.43850.81. doi: 10.1212/01.wnl.0000202524.43850.81. [DOI] [PubMed] [Google Scholar]
- 21.Rivers CS, Wardlaw JM. What has diffusion imaging in animals told us about diffusion imaging in patients with ischaemic stroke? Cerebrovasc Dis. 2005;19:328–336. doi: 10.1159/000084691. doi: 10.1159/000084691. [DOI] [PubMed] [Google Scholar]
- 22.Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the “small stuff”: the details of acute cerebral ischaemia. Lancet Neurol. 2004;3:39–45. doi: 10.1016/s1474-4422(03)00621-5. [DOI] [PubMed] [Google Scholar]
- 23.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]


