SUMMARY
Porphyromonas gingivalis (Pg) expresses the enzyme peptidylarginine deiminase (PPAD), which has a strong preference for C-terminal arginines. Due to the combined activity of PPAD and Arg-specific gingipains, Pg on the cell surface is highly citrullinated. To investigate the contribution of PPAD to the interaction of Pg with primary human gingival fibroblasts (PHGF) and Pg-induced synthesis of prostaglandin E2 (PGE2), PHGF were infected with wild-type Pg ATCC 33277, an isogenic PPAD-knockout strain (Δppad) or a mutated strain (C351A) expressing an inactive enzyme in which the catalytic cysteine has been mutated to alanine (PPADC351A). Cells were infected in medium containing the mutants alone or in medium supplemented with purified, active PPAD. PHGF infection was assessed by colony-forming assay, microscopic analysis and flow cytometry. Expression of COX-2 and mPGES-1, key factors in the prostaglandin synthesis pathway, was examined by qRT-PCR, while PGE2 synthesis was evaluated by EIA. PHGF were infected more efficiently by wt-Pg than the Δppad strain, which correlated with strong induction of COX-2 and mPGES-1 expression by wt-Pg, but not by the PPAD activity-null mutant strains (ΔPPAD and C351A). The impaired ability of the ΔPPAD strain to adhere to and/or invade PHGF and both ΔPPAD and C351A to stimulate the PGE2-synthesis pathway was fully restored by the addition of purified PPAD. The latter effect was strongly inhibited by aspirin. Collectively, our results implicate PPAD activity, but not PPAD itself, as an important factor for gingival fibroblast infection and activation of PGE2 synthesis, the latter of which may strongly contribute to bone resorption and eventual tooth loss.
Keywords: P. gingivalis, peptidylarginine deiminase, citrullination, prostaglandin E2, gingival fibroblasts
INTRODUCTION
Periodontal disease (PD) is a major public health problem affecting over 30% of the adult population worldwide. The disease is clinically characterized by chronic inflammation of tooth-supporting structures, destruction of the periodontal ligament, the resorption of alveolar bone and formation of periodontal pockets. Untreated periodontitis may lead to tooth loss (Eke et al., 2012). One pathogen recognized as a principal causative agent of PD is the anaerobic, non-motile, asaccharolytic, Gram-negative bacterium, Porphyromonas gingivalis (Pg). The presence of Pg in subgingival plaque correlates with disease severity, as assessed by attachment loss, periodontal pocket depth and bleeding on probing (Bostanci & Belibasakis, 2012).
A wide variety of virulence factors, including lipopolysaccharides (LPS), fimbriae, proteinases (gingipains), haemagglutinins and haemolysins contribute to the pathogenicity of Pg (Bostanci & Belibasakis, 2012). Recently, considerable interest has been focused on peptidylarginine deiminase expressed by Pg (PPAD) (Wegner et al., 2010; Maresz et al., 2013). PPAD is able to modify proteins by deimination of peptidylarginine residues to produce peptidylcitrulline and ammonia (McGraw et al., 1999; Rodriguez et al., 2009). Unlike mammalian PADs, which act only on Arg within the polypeptide chain in a calcium-dependent manner, PPAD primarily citrullinates C-terminal residues and can also deiminate free L-arginine in the absence of calcium (Rodriguez et al., 2009; Bicker & Thompson, 2013).
The conversion of positively charged arginine into neutral citrulline may affect the folding and stability of proteins and peptides, alter their susceptibility to proteolysis and abrogate their biological activity. For example, citrullination of CXCL8 results in a considerable reduction in binding to glycosaminoglycans and prevents proteolytic truncation of the chemokine by plasmin or thrombin, thus precluding the ability of CXCL8 to recruit neutrophils (Loos et al., 2008; Proost et al., 2008; Loos et al., 2009). In turn, citrullination of the antibacterial peptide LL-37 by human PAD2 and PAD4 compromises its ability to neutralize lipopolysaccharides and makes the peptide more prone to degradation by proteases. Additionally, citrullination alters the immunomodulatory functions of LL-37 that are essential for the prevention of endotoxin-induced sepsis (Kilsgard et al., 2012, Koziel et al., 2014) and may upset other regulatory activities of this and other host defense peptides (Choi et al., 2012; Nijnik et al., 2012; Pulido et al., 2012; Semple & Dorin, 2012; Sall et al., 2013). Several recent reports indicate that protein citrullination catalyzed by PPAD may contribute to the pathogenesis of periodontitis and rheumatoid arthritis (RA). To the latter, citrullination of bacterial and host proteins by PPAD in inflamed gingival tissues is considered a molecular mechanism for generating antigens that initiate and/or enhance the autoimmune response in rheumatoid arthritis (Wegner et al., 2010; Nesse et al., 2012; Maresz et al., 2013). Moreover, it was recently reported that PPAD efficiently citrullinates the C-terminal arginine of epidermal growth factor (EGF), which subsequently impairs its biological activity (Pyrc et al., 2012). Decreased activity of EGF in gingival pockets may at least partially contribute to the tissue damage and delayed healing of the periodontium observed during Pg infection.
Chronic periodontitis, which is now recognized as a pathogen-driven dysbiotic disease (Hajishengallis & Lamont, 2012; Wright et al., 2013), entails multiple cycles of progression and remission mediated by the modulation of pro-inflammatory signaling networks (Demmer & Papapanou, 2010). One inflammatory mediator at the interface between bacterial infection and periodontal tissue damage is prostaglandin E2 (PGE2) (Offenbacher et al., 1986, 1993; Noguchi & Ishikawa, 2007; Taxman et al., 2012). PGE2 has several biological functions, including vasodilation and enhanced vascular permeability; however, in the context of the pathology of periodontitis, the induction of osteoclastogenesis is its most important function (Lerner, 1991, Brechter & Lerner, 2007). To this end, the correlation between PGE2 levels in the gingival crevicular fluid (GCF) and clinical parameters of periodontitis, such as periodontal attachment loss and bleeding on probing, is clearly documented (Preshaw & Heasman, 2002; Noguchi & Ishikawa, 2007; Zhong et al., 2007; Zhang et al., 2011; Taxman et al., 2012). However, the impact of PPAD on PGE2 signaling has not yet been elucidated.
The present study was undertaken to evaluate whether PPAD modulates prostaglandin signaling. To this end, we showed that PPAD activity, but not the protein alone, contributed to the infection of PHGF by Pg and activation of the PGE2 synthesis pathway, which is manifested by increased levels of COX-2 and mPGES-1 expression as well as significantly enhanced secretion of PGE2. The effect was linked at least partially to the citrullination of bacterial/host cell surface proteins and may contribute to alveolar bone loss at infected periodontitis sites.
MATERIALS AND METHODS
Construction of the P. gingivalis ΔPPAD mutant (ATCC 33277)
The mutant of P. gingivalis ATCC harboring a ppad gene deletion (Genbank accession number 188594442; locus tag PGN_0898) was obtained as described previously for the W83 strain (Wegner et al., 2010). Erythromycin-resistant clones were subcultured on selective plates and genomic integration was confirmed by PCR using primers flanking the integration site.
Plasmid construction for PPAD C351A (ATCC 33277) active site-directed inactivation
Three DNA fragments were amplified by PCR: 1.3 kb from the 5′ end of the ppad gene (TIGR accession no. PG1424; primers used were pUPPADa_F and pUPPADa_R), the tetracycline cassette (tetQ; primers used were tetQ_inf F and tetQ_PPAD_inf R), and a 960 bp fragment flanking the 3′ end of the ppad gene (primers used were pUPPADb_F and pUPPADb_R). The DNA sequences were cloned into the pUC19 plasmid using InFusion Cloning HD Kit (Clontech) in the E. coli DH5α strain. In the obtained plasmid, the PPAD-encoding sequence was further modified by substitution of the catalytic residue Cys351 with alanine to create the final plasmid PPAD_C351A. This was accomplished using the SLIM method (primers used were C351AFs, C351ARs, C351AFl and C351ARl) (Chiu et al., 2004). The correctness of the obtained constructs was confirmed by sequencing. The PPAD C351A plasmid was used for electroporation of P. gingivalis ATCC 33277 (Smith et al., 1990). The correct placement and orientation of the DNA segments were confirmed by sequencing and PPAD activity assay (Boyde & Rahmatullah, 1980).
Expression and purification of P. gingivalis PPAD
The Pg W83 strain was engineered to secrete PPAD in a soluble form using the same molecular strategy reported previously for the RgpB protease (Zhou et al., 2013). Subsequently, PPAD was purified from the culture medium via ion-exchange and gel filtration chromatography, as described previously (J. Potempa – unpublished data). The purity of PPAD was evaluated by SDS-PAGE followed by silver staining. PPAD activity was tested using a colorimetric assay, as described previously (Liao et al., 2005) with Nα-benzyloarginine ethyl ester (BAEE) as a substrate. Activity was expressed as mU/μL (1 mU = 1 nmol of citrulline produced within 1 h of the reaction).
Bacterial culture
P. gingivalis strains (wt-Pg ATCC 33277 and its isogenic mutant Δppad and C351A expressing catalytically inactive PPAD (PPADC351A) were grown on blood agar plates (BHI, brain heart infusion medium supplemented with 5% sheep blood, 5 μg mL−1 hemin and 0.5 μg mL−1 vitamin K in an anaerobic chamber (90% N2, 10% CO2, and 5% H2). The media were additionally supplemented with erythromycin (5 μg mL−1) or tetracycline (1 μg mL−1) for the growth of ΔPPAD or C351A mutants, respectively. After cultivation at 37°C for 7 days, bacterial cells were inoculated into enriched BHI broth (Becton Dickinson) for overnight culture. Prior to inoculation, bacteria were washed with phosphate-buffered saline (PBS) and resuspended in a fresh culture medium. Bacterial cell counts were determined using a spectrophotometer; an optical density of 1.0 at 600 nm corresponded to a concentration of 1 × 109 colony-forming units (CFU) per mL. Bacterial suspensions were used for further experiments.
Gingival fibroblast isolation and culture
Gingival biopsies were collected from healthy subjects presenting for orthodontic treatment at the Department of Periodontology and Oral Medicine, Jagiellonian University, Medical College, Institute of Dentistry in Krakow, Poland. The study was approved by the Bioethical Committee of the Jagiellonian University in Krakow, Poland (KBET/310/B/2012). Prior to the study written informed consent was obtained from all donors. Briefly, following enzymatic separation of the epithelial layer, the remaining connective tissue was digested with 0.1% collagenase I (Invitrogen). After digestion, the cell pellet was plated in a T25 flask in Dulbecco’s Modified Eagle Medium (DMEM, PAA GmbH) supplemented with 10% heat-inactivated foetal bovine serum (FBS, PAA), penicillin/streptomycin (50 U mL−1), gentamicin (50 U mL−1), and nystatin (10 μg mL−1, PAA), at 37°C in a humidified atmosphere containing 5% CO2. Immunofluorescent staining with anti-vimentin and anti-cytokeratin antibodies was conducted on samples from cultures at the second passage to evaluate the homogeneity of fibroblast cultures. Cell viability was confirmed by Trypan blue staining to be 95–98%. Tests for Mycoplasma spp. were negative. Fibroblast cultures at passages 3–6 were used for experiments.
Colony-forming assay
Gingival fibroblasts were grown until confluence in a T75 flask in DMEM supplemented with 10% FBS, penicillin/streptomycin (50 U mL−1), and gentamicin (50 U mL−1) at 37°C in a humidified atmosphere containing 5% CO2. Twenty hours before the experiment, cells were trypsinized and plated in a fresh culture media (DMEM, 2% FBS) in a 24-well plate at 9.5 × 105 cells per well. The next day, cells were infected with bacterial suspensions prepared from overnight cultures. In brief, overnight bacterial cultures were centrifuged, rinsed with PBS and adjusted to the appropriate MOI in fresh culture media. Cells were infected with wt-Pg ATCC 33277 and its isogenic mutant Δppad at an MOI of 100 for 1.5 h, 3 h, and 6 h, respectively.. To assess adhesion and invasion, infected cells were rinsed three times with PBS and lysed. Cell lysates were serially diluted and seeded on blood agar plates. After 10 days of growth in an anaerobic atmosphere at 37°C, bacterial colonies were counted and the results were expressed as CFU per mL. To evaluate bacterial invasion into fibroblasts, an antibiotic protection assay was performed. Immediately after infection, the cells were rinsed with PBS followed by 1.5 h incubation with gentamicin (100 μg mL−1). Subsequent steps were performed as described above (adhesion and invasion assay).
Supplementation of PPAD mutants with purified, active enzyme
Gingival fibroblasts were infected for 3 h at an MOI of 100 with the PPAD mutants (ΔPPAD and C351A) in medium supplemented with purified PPAD at 0.066 mU μL−1 (for a total of 66 mU), which corresponds to the mean PPAD activity of 20 h cultures of wt-Pg adjusted to Pg cell numbers used at an MOI of 100.
FACS and microscopic analysis of P. gingivalis adhesion to and invasion of PHGF
Overnight cultures of wt-Pg and the Δppad strain (ATCC 33277) were centrifuged, rinsed in PBS and resuspended in PBS to an OD of 1.5 in a total volume of 2 × 10 mL each. Next, bacterial cultures were labeled with fluorescent dye (Cell Trace CFSE Cell Proliferation Kit for Flow Cytometry, Life Technologies). In brief, CFSE dye dissolved in DMSO was diluted in PBS to a final concentration of 10 μM, mixed with bacterial pellets and incubated for 15 min at 37°C (in the dark). After incubation, bacterial pellets were rinsed several times with fresh PBS. Bacterial cell numbers were adjusted to 1 × 109 CFU per mL (OD = 1.0 at 600 nm). Primary human gingival fibroblasts (PHGF) were plated in fresh culture medium (DMEM, 2% FBS) at a density of 4 × 105 cells per well in a 24-well plate. The next day, cells were infected for 3 h at an MOI of 100 with bacterial suspensions prepared, as described earlier. Infection was conducted in three different ways. In the first set of experiments, infected cells were trypsinized, resuspended in fresh RPMI-1640 (Euroclone) supplemented with 0.5% FBS and analyzed using a FACScan flow cytometer (Becton Dickinson). Analysis was performed using CellQuest software to determine the percentage and mean fluorescence intensity of positive, infected cells. Two remaining sets of experiments were performed to assess adhesion and invasion or invasion alone using a fluorescent microscope. Bacterial invasion was assessed by employing an antibiotic protection assay, as described above. Infected cells were fixed in 4% formaldehyde, rinsed with PBS and analyzed with a fluorescent microscope (Eclipse E600, Nikon Inc.). Photographic documentation was obtained with a digital camera controlled by NIS-Elements software (Nikon Inc.). Analysis of adhesion and invasion or invasion only by fluorescently labeled bacteria was performed by quantification of the mean intensity of fluorescence within a defined area of interest (AOI) and expressed as arbitrary units [I/pix]. Evaluation of 12 AOIs was performed per experimental condition. The analysis was conducted with Imaging Software NIS Elements BR3.2.
Evaluation of PGE2 signaling
Gingival fibroblasts were cultured in DMEM, 10% FBS, penicillin/streptomycin and gentamicin until confluence. The day before the experiment, cells were trypsinized and plated in fresh DMEM supplemented with 2% FBS in a 6-well plate at a density of 9.5 × 105 cells per well. On the following day, cells were infected at an MOI of 100 for 3 h with wt-Pg, the Δppad and C351A mutant strains, and PPAD mutants were supplemented with purified PPAD. In parallel experiments, cells were infected for 3 h in the same way but in the presence of aspirin (50 μg mL−1), an inhibitor of cyclooxygenases. At specific times after infection, culture medium was collected, centrifuged and used for further analysis. Cells were rinsed three times with PBS and lysed using TriReagent solution. Collected lysates were used for the purification of total RNA and analysis of COX-2 and mPGES-1 gene expression by quantitative-RT PCR (qRT-PCR), as described below.
PGE2 synthesis
PGE2 synthesis was evaluated in culture media using a commercially available EIA kit (Cayman Chemical) following the manufacturer’s instructions.
Gene expression analysis
Total cellular RNA was extracted using TriReagent solution (Life Technologies), according to the manufacturer’s protocol. Isolated RNA was reverse transcribed with a high-capacity cDNA reverse transcription kit (Life Technologies) according to the manufacturer’s protocol, using 400 ng of previously extracted RNA in a total volume of 20 μL. Quantitative real-time PCR amplification was conducted with 2 × SYBR green mix (Sigma Aldrich, Poland). The reaction was carried out in a total volume of 10 μL in the presence of forward and reverse primers and template DNA (2 μL). The following primers were used: for the amplification of β-actin, β-act5 (5′-CCACACTGTGCCCATCTACG-3′), and β-act3 (5′-AGGATCTTCATGAGGTAGTCAGTCAG-3′); for the amplification of COX-2, 5_COX-2 (5′-AGCCCTTCCTCCTGTGCCT-3′) and 3_COX-2 (5′-TCCATTTTTCGTCGAAGGACTAA-3′); for the amplification of mPGES-1, 5_mPGES-1 (5′-CACGCTGCTGGTCATCAAGAT-3′) and 3_mPGES-1 (5′-TCCTACGGGACTCTGTGCC-3′). Each primer was used at 500 nM. ROX was used as a reference dye. The PCR cycling conditions were an initial denaturation for 5 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 55°C and 45 s at 72°C. Real-time amplification was followed by melting-curve assessment to confirm product identity.
RESULTS
PPAD is involved in efficient interaction with, adhesion to and invasion of gingival fibroblasts by P. gingivalis
The absence of Pg surface protein citrullination in the Δppad strain and/or inability to citrullinate host cell surface proteins may affect its interaction with cells. Therefore, we first compared the efficiency of infection of gingival fibroblasts by wt-Pg and the ΔPPAD mutant strain. In all tested conditions at an MOI of 100, wt-Pg adhered to and invaded gingival fibroblasts more efficiently than the Δppad strain (Table 1). Of note, regardless of the level of PPAD activity, infection at an MOI of 500 for 3 h resulted in progressive cell death, i.e., apoptosis of fibroblasts (data not shown).
Table 1. Comparison of adhesion/invasion or invasion alone of primary human gingival fibroblasts (PHGF) by P. gingivalis (ATCC 33277).
Cells were infected with 108 CFU (MOI = 100) of wt-Pg and the Δppad strain for 1.5 h, 3 h and 6 h. Infected cell monolayers were washed and cells were lysed. Lysates were serially diluted and plated on blood agar, and CFU were counted after 10 days of cultivation. Invasion was assessed using an antibiotic protection assay. Results are expressed as means ± SD and represent three independent experiments.
| Strain | Inoculum [CFU] | Infection time | Adhering and invading bacteria | Invading bacteria only | ||
|---|---|---|---|---|---|---|
| [CFU] | Student t test | [CFU] | Student t test | |||
| wt-Pg | 1x108 ± 3.3×106 | 1.5 h | 1.8×107 ± 2.2×106 | p = 0.005 | 1.4×107 ± 2.1×106 | p = 0.0001 |
| PgΔPPAD | 1×108 ± 4.3×106 | 1.4×107 ± 2.1×106 | 0.9×107 ± 1.4×106 | |||
| wt-Pg | 1×108 ± 5.0×106 | 3 h | 2.6×107 ± 2.2×106 | p = 0.0004 | 2.1×107 ± 1.5×106 | p = 6.3 × 10−07 |
| PgΔPPAD | 1×108 ± 6.3×106 | 1.8×107 ± 2.2×106 | 1.2×107 ± 0.7×106 | |||
| wt-Pg | 1×108 ± 6.6×106 | 6 h | 2.1×107 ± 1.8×106 | p = 0.0005 | 1.7×107 ± 2.0×106 | p = 0.003 |
| PgΔPPAD | 1×108 ± 6.6×106 | 1.4×107 ± 2.1×106 | 1.1×107 ± 1.2×106 | |||
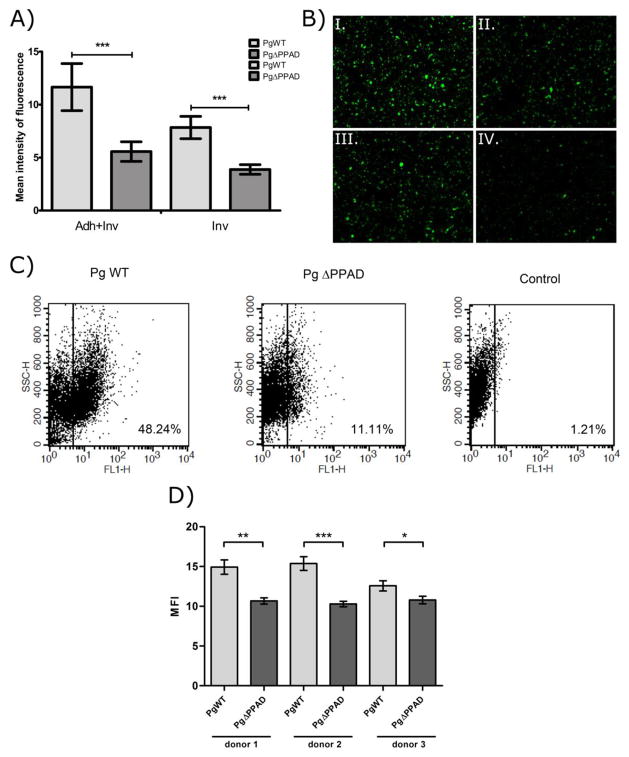
The ability of wt-Pg and the Δppad strain to infect fibroblasts was further investigated using fluorescently labeled bacteria (Fig. 1). Fluorescent microscopy (Fig. 1A and B) and flow cytometry (Fig. 1C and D) analysis revealed that the ΔPPAD mutant adhered to and invaded gingival cells to a considerably lower degree than the parental strain. This impaired ability of the ΔPPAD mutant to infect cells was independent of fibroblast donor (Fig. 1D). These data indicate that PPAD is involved in the interaction of P. gingivalis with gingival fibroblasts and contributes to the effective adhesion to and invasion of gingival fibroblasts by this periodontopathogen.
Figure 1. P. gingivalis adheres to and invades primary human gingival fibroblasts (PHGF) more efficiently than P. gingivalis-ΔPPAD mutants.
Human fibroblasts were infected with CFSE-labeled wt-Pg or the ΔPPAD mutant. (A) Mean intensity of fluorescence for adhesion/invasion (Adh+Inv) or invasion only (Inv) within a defined area of interest (AOI) was determined and expressed as arbitrary units [I/pix]. (B) Representative images from microscopic analysis of adhesion/invasion (I. and II.) and invasion only (III. and IV.) by wt-Pg (I. and III.) and the Δppad strain (II. and IV.) are shown. Magnification = 200×. (C) Dot plots of the percentage of infected cells and (D) the mean fluorescence intensity (MFI) of infection (adhesion and invasion) determined by flow cytometry analysis of human fibroblasts infected with CFSE-labeled wt-Pg and Δppad strains. In all experiments, PHGF were infected for 3 h at an MOI of 100, and all experiments were performed three times. Results are expressed as means ± SD (***, p < 0.001; **, p < 0.01; and *, p < 0.05).
External addition of active PPAD restored the ability of ΔPPAD to adhere to/invade fibroblasts
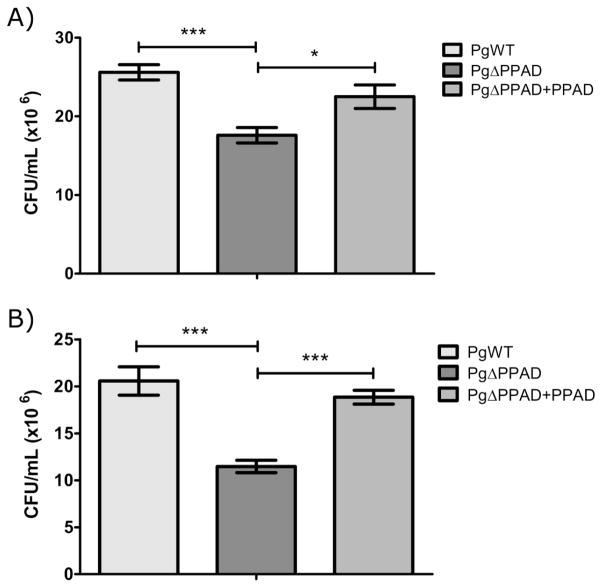
To verify the role of PPAD in the adhesion to and invasion of gingival fibroblasts, purified enzyme in an amount equivalent to that of the infection dose of wt-Pg was added to the medium together with the ΔPPAD mutant. As shown in Figure 2, the addition of exogenous PPAD restored the ability of the Pg mutant to adhere to and invade fibroblasts. This result excluded a possible polar effect of ppad gene deletion in the mutant strain phenotype and fully confirmed the role of PPAD in the interaction of Pg with fibroblasts.
Figure 2. PPAD supplementation restores the ability of P. gingivalis ΔPPAD to adhere to and invade primary human gingival fibroblasts (PHGF).
Purified PPAD (0.066 mU/μL, total 66 mU) was added to a culture medium of PHGF cells during infection with the ΔPPAD mutant, and adherence/invasion (A) and invasion alone (B) were determined. In all experiments, PHGF were infected for 3 h at an MOI of 100. Data represent three independent experiments and are expressed as means ± SD (***, p < 0.001; **, p < 0.01; and *, p < 0.05).
PGE2 signaling is affected by PPAD upon infection of gingival fibroblasts with P. gingivalis
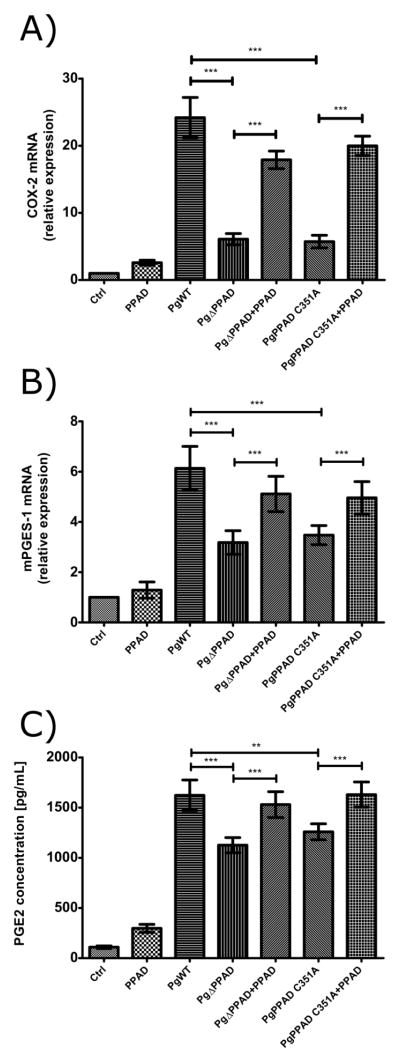
Next, we attempted to verify whether PPAD can affect an immune response through the modulation of the prostaglandin-dependent pathway. As depicted in Figure 3, the infection of fibroblasts with the wt-Pg strain upregulated the expression of two key enzymes that participate in PGE2 synthesis, cyclooxygenase-2 (COX-2; Fig. 3A) and microsomal PGE synthase-1 (mPGES-1; Fig. 3B), and increased the synthesis of PGE2 (Fig. 3C). Remarkably, upregulation of both enzymes was strongly reduced in cells infected with the Δppad strain and the strain C351A, which expresses a catalytically inactive form of the enzyme (Fig. 3A and B). In agreement with this, the level of synthesized PGE2 was significantly lower in the conditioned media of cells infected with the mutant strains compared to the media of cells infected with wt-Pg (Fig. 3C). Significantly, the addition of purified PPAD into the culture media during incubation of the mutant strain with fibroblasts restored the expression of COX-2 and mPGES-1 as well as the synthesis of PGE2 to levels comparable to those observed during infection with wt-Pg. These results clearly argue that PPAD activity is important for the stimulation of the prostaglandin synthesis pathway in Pg-infected fibroblasts.
Figure 3. PPAD induces prostaglandin E2 (PGE2) signaling in primary human gingival fibroblasts (PHGF).
Relative expression levels of cyclooxygenase-2 (COX-2) (A) and microsomal PGE synthase-1 (mPGES-1) (B), and the concentration of PGE2 (C) in PHGF infected with wt-Pg, ΔPPAD and C351A mutant strains for 3 h at an MOI of 100. Infection with mutant strains was also performed in the presence of purified PPAD (0.066 mU/μL, total 66 mU). Quantitative real-time PCR was performed using β-actin as a reference gene. PGE2 concentrations were evaluated by EIA test. Data represent three independent experiments and are expressed as means ± SD (***, p < 0.001; **, p < 0.01; and *, p < 0.05).
Activation of the prostaglandin synthesis pathway in infected fibroblasts is inhibited by aspirin
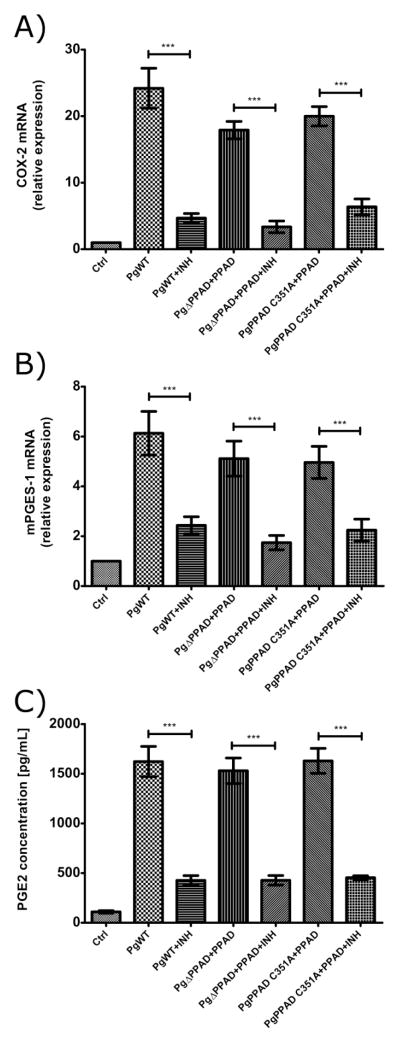
Aspirin is an effective anti-inflammatory drug which affects prostaglandin pathway through non-selective, irreversible inhibition of cyclooxygenases (Fuster & Sweeny, 2011; Funosas et al., 2012; Schmidt et al., 2014; Shiloah et al., 2014; Therefore, to validate PPAD activity-dependent stimulation of the prostaglandin synthesis pathway by P. gingivalis, fibroblasts were infected with Pg strains in combination with externally added PPAD and treated with aspirin. In all cases, aspirin reduced the expression of COX-2 (Fig. 4A) and mPGES-1 (Fig. 4B) and inhibited PGE2 synthesis (Fig. 4C). Together, these results fully confirm upregulation of the PGE2 synthesis pathway in infected fibroblasts and the importance of PPAD activity in this process.
Figure 4. PPAD-induced prostaglandin E2 (PGE2) synthesis in primary human gingival fibroblasts (PHGF) is inhibited by aspirin.
PHGF infection for 3 h at an MOI of 100 with wt-Pg or PPAD mutants (ΔPPAD and C351A) supplemented with purified PPAD (0.066 mU/μL, total 66 mU) resulted in an increased expression of cyclooxygenase-2 (COX-2) (A), microsomal PGE synthase-1 (mPGES-1) (B) and upregulated synthesis of PGE2 (C). The addition of aspirin (INH, 50 μg mL−1) to infected cells caused inhibition of PPAD-induced PGE2 signaling. Experiments were performed three times and data are expressed as means ± SD (***, p < 0.001; **, p < 0.01; and *, p < 0.05).
DISCUSSION
In the oral cavity, the gingival epithelium acts as a barrier that prevents the intrusion of oral bacteria into subepithelial tissues (Amano, 2007). A number of reports show that P. gingivalis, a primary aetiological agent of PD, is able to enter gingival epithelial cells. More importantly, it has been shown that this periodontopathogen spreads through the upper layers of the gingival epithelial barrier, penetrates the basement membrane and invades deeper into the connective tissue, where fibroblasts are the most prevalent cell type (Yilmaz et al., 2006; Amano, 2007). To date, the impact of PPAD on bacterial adhesion to and invasion of gingival cells, particularly gingival fibroblasts, has not been elucidated.
In this report, we show unambiguously that PPAD significantly contributes to the interaction of Pg with gingival fibroblasts. In comparison to the parental ATCC 33277 strain, the isogenic Δppad strain showed a two-fold decrease in the ability to adhere to and/or invade fibroblasts. Significantly, the addition of purified, active PPAD to the culture medium in an amount equivalent to that carried by wt-Pg reestablished the adhesive/invasive phenotype of the mutant, eliminating the possibility that the attenuated adhesion/invasion phenotype is due to a polar effect of ppad gene deletion. This argues for the genuine involvement of PPAD activity in Pg invasion of fibroblasts.
To date, Pg major fimbriae are believed to mediate the bacterial invasion of several human epithelial cell lines and contribute to the persistence of Pg at intracellular locations in vitro (Yilmaz et al., 2006; Nagano et al., 2013). Moreover, different types of fimbriae possess various potential levels of virulence. Pg fimbriae are classified into six types (types I to V and Ib) based on the genes encoding FimA, a major subunit of fimbriae. Nakagawa et al. (2006) reported that amongst six representative strains possessing the different types of fimbriae, strain OMZ314, which has type II fimbriae, adhered to and invaded epithelial cells to a significantly greater degree than the other strains. In this study, we used the strain ATCC 33277, which expresses less virulent type I fimbriae, but nevertheless, actively participating in the adhesion to and invasion of host cells (Lamont et al., 1995; Nakagawa et al., 2006; Wang et al., 2007). In this context, our finding that PPAD knockout strongly attenuated the capacity of Pg to adhere to and invade fibroblasts, regardless of unaffected fibrination (data not shown), is very interesting and leads us to speculate that citrullination of fimbriae significantly contributes to the fimbriae-mediated effects of Pg interaction with fibroblasts. Experiments to verify this assumption are in progress in our laboratories.
It has been shown that both dead and viable Pg (Steffen et al., 2000), as well as Pg-derived LPS (Noguchi et al., 1996), elicited the production of PGE2 in human gingival fibroblast cultures. We confirmed this finding by showing that P. gingivalis strongly stimulated the expression of COX-2 and mPGES-1, two key enzymes in the prostaglandin synthesis pathway, enhanced the synthesis of PGE2 in infected fibroblasts. Interestingly, this stimulation was dependent on PPAD activity, since the Pg strain (C351A) expressing a catalytically inactive form of the enzyme was as ineffective for activation of the prostaglandin synthesis pathway as the PPAD-null mutant (Fig. 3). The difference was highly significant for each tested component of the prostaglandin pathway but most profound in the difference between COX-2 stimulation by wt-Pg and the PPAD activity-deficient mutants. Significantly, supplementation of the infection medium with purified active enzyme restored the expression of COX-2 and mPGES-1 as well as production of PGE2 in mutant-infected fibroblasts to the levels induced by wt-Pg. Because the addition of active PPAD alone to sham-infected control fibroblast cultures did not induce the cells to produce PGE2, it appears that citrullinated Pg cell surface protein(s) act as ligand(s) for activating the prostaglandin synthesis pathway.
Citrullination-dependent activation of fibroblasts, the predominant cell type in periodontal connective tissue, produces excessive amounts of prostaglandins, in particular PGE2, which may have dire consequences on homeostasis in the periodontium. PGE2 plays a significant role in the inflammatory response by contributing to the pathogenesis of several chronic inflammatory conditions (Offenbacher et al., 1993; Yucel-Lindberg et al., 1999; Preshaw & Heasman, 2002). Specifically, PGE2 is implicated in the pathogenesis of PD. Increased levels of PGE2 are present in periodontal tissue and the GCF of patients suffering from periodontitis (Offenbacher et al., 1986; Offenbacher et al., 1993; Preshaw & Heasman, 2002) and strongly correlate with disease severity as measured by attachment loss. Furthermore, the involvement of PGE2 in periodontitis is supported by findings that treatment with non-steroidal anti-inflammatory drugs (NSAIDs), as well as selective COX-2 inhibitors known to inhibit PGE2 synthesis, decreased PD severity as measured by alveolar bone resorption (Offenbacher et al., 1986; Yucel-Lindberg et al., 1999; Lerner & Lundberg, 2002). Aspirin, a widely used NSAID, is an irreversible inhibitor of cyclooxygenases (Fuster & Sweeny, 2011). It was shown that aspirin stimulated an increase in attachment in a select group of adult smokers during long-term aspirin intake and non-surgical periodontal therapy (scaling and root planning) (Shiloah et al., 2014). In another study, aspirin was the most effective NSAID for reducing probing depth, gingival index and bleeding on probing (Funosas et al., 2012). In this context, our finding that aspirin obviates Pg-stimulated activation of the prostaglandin synthesis pathway in oral fibroblasts is of great importance since it provides insight into the mechanism of its beneficial effects on periodontal health.
In conclusion, we demonstrate here for the first time that PPAD activity contributes to Pg adherence to and invasion of gingival fibroblasts. Furthermore, we found that PPAD activity through the citrullination of presently unidentified Pg surface protein(s) activates the prostaglandin synthesis pathway and secretion of PGE2 in infected fibroblasts. Taking into account the proven role of PGE2 in bone resorption, PPAD emerges from this study as an important virulence factor and valid target for drug development.
Acknowledgments
We sincerely thank Professor Philip de Groot for his encouragement and stimulating discussion on the results of this study.
This work was supported on part by grants from the National Science Centre, Poland (2012/07/B/NZ6/03524) (to K.G.), and the Foundation for Polish Science (TEAM, DPS/424-329/10), National Science Center (Krakow, Poland) (2011/01/B/NZ6/00268), the National Institutes of Health (NIDCR, DE 022597) (all to J.P.). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from the European Union (POIG.02.01.00-12-064/08).
Footnotes
Conflicts of interest
None declared.
References
- Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front Biosci. 2007;12:3965–3974. doi: 10.2741/2363. [DOI] [PubMed] [Google Scholar]
- Bicker KL, Thompson PR. The protein arginine deiminases (PADs): Structure, Function, Inhibition, and Disease. Biopolymers. 2013;99:155–163. doi: 10.1002/bip.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostanci N, Belibasakis GN. Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol Lett. 2012;333:1–9. doi: 10.1111/j.1574-6968.2012.02579.x. [DOI] [PubMed] [Google Scholar]
- Boyde TR, Rahmatullah M. Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal Biochem. 1980;107:424–431. doi: 10.1016/0003-2697(80)90404-2. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum. 2007;56:910–923. doi: 10.1002/art.22445. [DOI] [PubMed] [Google Scholar]
- Chiu J, March PE, Lee R, Tillett D. Site-directed, ligase-independent mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4h. Nucleic Acids Res. 2004;32:e174. doi: 10.1093/nar/gnh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KY, Chow LN, Mookherjee N. Cationic host defence peptides: multifaceted role in immune modulation and inflammation. J Innate Immun. 2012;4:361–370. doi: 10.1159/000336630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28–44. doi: 10.1111/j.1600-0757.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, Beck James, Douglass Gordon, Page Roy, Slade Gray, Taylor George W, Borgnakke Wenche CDC Periodontal Disease Surveillance workgroup and representatives of the American Academy of Periodontology. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Funosas E, Feser G, Escovich L, Maestri L. Alteration of hemostasis in patients treated with subgingival NSAIDs during periodontal therapy. Acta Odontol Latinoam. 2012;25:103–108. [PubMed] [Google Scholar]
- Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123:768–778. doi: 10.1161/CIRCULATIONAHA.110.963843. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 2012;27:409–419. doi: 10.1111/j.2041-1014.2012.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilsgård O, Andersson P, Malmsten M, Nordin SL, Linge HM, Eliasson M, Sörenson E, Erjefält JS, Bylund J, Olin AI, Sørensen OE, Egesten A. Peptidylarginine deiminases present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am J Respir Cell Mol Biol. 2012;46:240–248. doi: 10.1165/rcmb.2010-0500OC. [DOI] [PubMed] [Google Scholar]
- Koziel J, Bryzek D, Sroka A, Maresz K, Glowczyk I, Bielecka E, Kantyka T, Pyrć K, Svoboda P, Pohl J, Potempa J. Citrullination alters immunomodulatory function of LL-37 essential for prevention of endotoxin-induced sepsis. J Immunol. 2014;192:5363–5372. doi: 10.4049/jimmunol.1303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Jenkinson HF. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner UH. Bradykinin synergistically potentiates interleukin-1 induced bone resorption and prostanoid biosynthesis in neonatal mouse calvarial bones. Biochem Biophys Res Commun. 1991;175:775–783. doi: 10.1016/0006-291x(91)91633-n. [DOI] [PubMed] [Google Scholar]
- Lerner UH, Lundberg P. Kinins and neuro-osteogenic factors. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. 2. San Diego, CA: Academic Press; 2002. pp. 773–799. [Google Scholar]
- Liao YF, Hsieh HC, Liu GY, Hung HC. A continuous spectrophotometric assay method for peptidylarginine deiminase type 4 activity. Anal Biochem. 2005;347:176–181. doi: 10.1016/j.ab.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Loos T, Mortier A, Gouwy M, Ronsse I, Put W, Lenaerts JP, Van Damme J, Proost P. Citrullination of CXCL10 and CXCL11 by peptidylarginine deiminase: a naturally occurring posttranslational modification of chemokines and new dimension of immunoregulation. Blood. 2008;112:2648–2656. doi: 10.1182/blood-2008-04-149039. [DOI] [PubMed] [Google Scholar]
- Loos T, Opdenakker G, Van Damme J, Proost P. Citrullination of CXCL8 increases this chemokine’s ability to mobilize neutrophils into the blood circulation. Haematologica. 2009;94:1346–1353. doi: 10.3324/haematol.2009.006973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, Gawron K, Mizgalska D, Marcinska KA, Benedyk M, Pyrc K, Quirke AM, Jonsson R, Alzabin S, Venables PJ, Nguyen KA, Mydel P, Potempa J. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD) Plos Pathogens. 2013;9:e1003627. doi: 10.1371/journal.ppat.1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw WT, Potempa J, Farley D, Travis J. Purification, characterization, and sequence analysis of a potential virulence factor from Porphyromonas gingivalis, peptidylarginine deiminase. Infect Immun. 1999;67:3248–3256. doi: 10.1128/iai.67.7.3248-3256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K, Abiko Y, Yoshida Y, Yoshimura F. Genetic and antigenic analyses of Porphyromonas gingivalis FimA fimbriae. Mol Oral Microbiol. 2013;28:392–403. doi: 10.1111/omi.12032. [DOI] [PubMed] [Google Scholar]
- Nakagawa I, Inaba H, Yamamura T, Kato T, Kawai S, Ooshima T, Amano A. Invasion of epithelial cells and proteolysis of cellular focal adhesion components by distinct types of Porphyromonas gingivalis fimbriae. Infect Immun. 2006;74:3773–3782. doi: 10.1128/IAI.01902-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse W, Westra J, van der Wal JE, Abbas F, Nicholas AP, Vissink A, Brouwer E. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J Clin Periodontol. 2012;39:599–607. doi: 10.1111/j.1600-051X.2012.01885.x. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Pistolic J, Filewod NC, Hancock RE. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J Innate Immun. 2012;4:377–386. doi: 10.1159/000335901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Ishikawa I. The roles of cyclooxygenase-2 and prostaglandin E2 in periodontal disease. Periodontol 2000. 2007;43:85–101. doi: 10.1111/j.1600-0757.2006.00170.x. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Shitashige M, Yanai M, Morita I, Nishihara T, Murota S, Ishikawa I. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–568. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Heasman PA, Collins JG. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, Heasman PA. Prostaglandin E2 concentrations in gingival crevicular fluid: observations in untreated chronic periodontitis. J Clin Periodontol. 2002;29:15–20. doi: 10.1034/j.1600-051x.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- Proost P, Loos T, Mortier A, Schutyser E, Gouwy M, Noppen S, Dillen C, Ronsse I, Conings R, Struyf S, Opdenakker G, Maudgal PC, Van Damme J. Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. J Exp Med. 2008;205:2085–2097. doi: 10.1084/jem.20080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido D, Nogués MV, Boix E, Torrent M. Lipopolysaccharide neutralization by antimicrobial peptides: a gambit in the innate host defense strategy. J Innate Immun. 2012;4:327–336. doi: 10.1159/000336713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K, Milewska A, Kantyka T, Sroka A, Maresz K, Koziel J, Nguyen KA, Enghild JJ, Knudsen AD, Potempa J. Inactivation of epidermal growth factor by Porphyromonas gingivalis as a potential mechanism for periodontal tissue damage. Infect Immun. 2012;81:55–64. doi: 10.1128/IAI.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez SB, Stitt BL, Ash DE. Expression of peptidylarginine deiminase from Porphyromonas gingivalis in Escherichia coli: enzyme purification and characterization. Arch Biochem Biophys. 2009;488:14–22. doi: 10.1016/j.abb.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säll J, Carlsson M, Gidlöf O, Holm A, Humlén J, Öhman J, Svensson D, Nilsson BO, Jonsson D. The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis. J Innate Immun. 2013;5:290–300. doi: 10.1159/000346587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Hallas J, Friis S. Potential of prescription registries to capture individual-level use of aspirin and other nonsteroidal anti-inflammatory drugs in Denmark: trends in utilization 1999–2012. Clin Epidemiol. 2014;6:155–168. doi: 10.2147/CLEP.S59156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple F, Dorin JR. β-Defensins: multifunctional modulators of infection, inflammation and more? J Innate Immun. 2012;4:337–348. doi: 10.1159/000336619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiloah J, Bland PS, Scarbecz M, Patters MR, Stein SH, Tipton DA. The effect of long-term aspirin intake on the outcome of non-surgical periodontal therapy in smokers: a double-blind, randomized pilot study. J Periodontal Res. 2014;49:102–109. doi: 10.1111/jre.12085. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Parker A, Rogers MB. Plasmid transformation of Bacteroides spp. by electroporation. Plasmid. 1990;24:100–109. doi: 10.1016/0147-619x(90)90012-2. [DOI] [PubMed] [Google Scholar]
- Steffen MJ, Holt SC, Ebersole JL. Porphyromonas gingivalis induction of mediator and cytokine secretion by human gingival fibroblasts. Oral Microbiol Immunol. 2000;15:172–180. doi: 10.1034/j.1399-302x.2000.150305.x. [DOI] [PubMed] [Google Scholar]
- Taxman DJ, Lei Y, Zhang S, Holley-Guthrie E, Offenbacher S, Ting JPY. ASC-dependent RIP2 kinase regulates reduced PGE2 production in chronic periodontitis. J Dent Res. 2012;91:877–882. doi: 10.1177/0022034512454541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shakhatreh MAK, James D, Liang S, Nishiyama S, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen Ky-Anh, Lundberg K, Kinloch A, Culshaw S, Potempa J, Venables PJ. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, Lamont RJ, Jenkinson HF. Microbial interactions in building of communities. Mol Oral Microbiol. 2013;28:83–101. doi: 10.1111/omi.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz Ö, Verbeke P, Lamont RJ, Ojcius DM. Intercellular spreading of Porphyromonas gingivalis infection in primary gingival epithelial cells. Infect Immun. 2006;74:703–710. doi: 10.1128/IAI.74.1.703-710.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel-Lindberg T, Nilsson S, Modéer T. Signal transduction pathways involved in the synergistic stimulation of prostaglandin production by interleukin-1 beta and tumor necrosis factor alpha in human gingival fibroblasts. J Dent Res. 1999;78:61–68. doi: 10.1177/00220345990780010901. [DOI] [PubMed] [Google Scholar]
- Zhang S, Barros SP, Niculescu MD, Moretti AJ, Preisser JS, Offenbacher S. Alteration of PTGS2 promoter methylation in chronic periodontitis. J Dent Res. 2011;89:133–137. doi: 10.1177/0022034509356512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Slade GD, Beck JD, Offenbacher S. Gingival crevicular fluid interleukin-1beta, prostaglandin E2 and periodontal status in a community population. J Clin Periodontol. 2007;34:285–293. doi: 10.1111/j.1600-051X.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- Zhou XY, Gao JL, Hunter N, Potempa J, Nguyen KA. Sequence independent processing site of the C-terminal domain (CTD) influences maturation of the RgpB protease from Porphyromonas gingivalis. Mol Microbiol. 2013;89:903–917. doi: 10.1111/mmi.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]