Abstract
Parental care benefits offspring through maternal effects influencing their development, growth and survival. However, although parental care in general is likely the result of adaptive evolution, it does not follow that specific differences in the maternal effects that arise from care are also adaptive. Here, we used an interspecific cross-fostering design in the burying beetle species Nicrophorus orbicollis and N. vespilloides, both of which have elaborate parental care involving direct feeding of regurgitated food to offspring, to test whether maternal effects are optimized within a species and therefore adaptive. Using a full-factorial design, we first demonstrated that N. orbicollis care for offspring longer regardless of recipient species. We then examined offspring development and mass in offspring reared by hetero- or conspecific parents. As expected, there were species-specific direct effects independent of the maternal effects, as N. orbicollis larvae were larger and took longer to develop than N. vespilloides regardless of caregiver. We also found significant differences in maternal effects: N. vespilloides maternal care caused more rapid development of offspring of either species. Contrary to expectations if maternal effects were species-specific, there were no significant interactions between caretaker and recipient species for either development time or mass, suggesting that these maternal effects are general rather than optimized within species. We suggest that rather than coadaptation between parents and offspring performance, the species differences in maternal effects may be correlated with direct effects, and that their evolution is driven by selection on those direct effects.
Keywords: burying beetle, coadaptation, coleoptera, life-history, Nicrophorus, parental care
Introduction
Maternal (paternal, parental) effects arise when the traits of the mother (father, parent) exert a causal influence on the traits of an offspring, independent of the offspring’s genotype (Wolf & Wade, 2009). These effects, once considered a statistical nuisance in measurements of inheritance (Falconer & Mackay, 1996), are now considered fundamentally important to understanding evolution, but yet the evolutionary consequences of maternal effects are often nonintuitive (Kirkpatrick & Lande, 1989; Cheverud & Moore, 1994; Mousseau & Fox, 1998; Wade, 1998). Maternal effects are expected to be adaptive, evolving to allow organisms to ameliorate uncertain or inhospitable environments (Badyaev & Uller, 2009; Duckworth, 2009). However, the evolutionary trajectories involved in maternal effects are unusual. As traits expressed in one generation that influence the fitness of the next generation, maternal effects influence adaptive evolution through genetic changes in the parent rather than in the offspring but create a selective environment on the offspring (Kirkpatrick & Lande, 1989; Badyaev & Uller, 2009). By evolving adaptive maternal effects, mothers can adjust how they influence offspring to fit immediate circumstances and environmental variability. Implicit in this is the expectation that maternal effects are species-specific; that is, that maternal effects adaptively evolve to fit the specific ecology of the species studied.
Current understanding of adaptive maternal effects comes largely from work on the ability of parents to adjust offspring traits in response to variable environments. The evidence presented often consists of a common garden-type experiment, wherein offspring fitness is measured when the maternal effect is dissociated from its characteristic environment (e.g. Fox et al., 1997). However, maternal effects may still be adaptations even if they are not environmentally responsive. To assess whether this type of maternal effect is adaptive, the maternal environment needs to be dissociated from the offspring experiencing that environment. Post-zygotic–post-natal maternal effects (Wade, 1998) arising from parental care are especially amenable to this type of study, as opposed to prezygotic or prenatal maternal effects such as egg provisioning, where the maternal trait is physically linked to the offspring. Parental care is one of the traits expected to result in strong maternal effects (Cheverud & Moore, 1994) and is expected to evolve to allow species to exploit competitive environments, counter environmental adversity and defend resources (Tallamy, 1984; Tallamy & Wood, 1986; Royle et al., 2012). Parental care has costs (Royle et al., 2012) typically in terms of energy, exposure to predation and lost reproductive opportunities. Thus, although there is variation within a species, parental care is expected to be a species-specific adaptation (Dulac et al., 2014) responsive to the particular abiotic and social environment of a species (Royle et al., 2014). However, it is important to separate the evolution of parenting from the effects of parenting (maternal effects), as these are traits expressed in different generations and subject to different selection pressures (Cheverud & Moore, 1994). Thus, although parenting and maternal effects are linked, they are not necessarily simultaneously optimized (Marshall & Uller, 2007).
Burying beetles (Nicrophorus spp.) present an ideal system for experimentally examining the evolution of maternal effects and parental care. Beetles in the genus Nicrophorus provide extensive and elaborate parental care for their young, which are reared on vertebrate carcasses (Pukowski, 1933; Eggert & Müller, 1997; Scott, 1998). Adults process a carcass into a brood ball, partially digesting and manipulating the carrion, and upon hatching directly feed begging larvae regurgitated food. Highly developed parental care behaviour is conserved across the genus. Parental care (typically maternal care) has strong influences on offspring mass (Smiseth et al., 2003; Smiseth & Moore, 2004), development (Eggert et al., 1998; Meierhofer et al., 1999; Rauter & Moore, 2002a,b; Lock et al., 2004, 2007), which are offspring performance traits closely related to fitness (Lock et al., 2004), as well as survival (Trumbo, 1992; Eggert et al., 1998; Lock et al., 2004). There is, however, substantial variation among species in body size, habitat usage, duration of development, duration of care and other life-history characters (Eggert & Müller, 1997; Scott, 1998). Both males and females can provide care (Eggert & Müller, 1997; Walling et al., 2008), although most offspring receive only maternal care (A. J. Moore, unpub. data), and the addition of paternal care to maternal care does not seem to matter to offspring fitness (Bartlett, 1988; Scott, 1989; Trumbo, 1991; Müller et al., 1998). The extensive and easily observed parental care of Nicrophorus makes it an extremely useful system for examining maternal effects (Rauter & Moore, 2002a,b; Lock et al., 2004, 2007) and paternal effects (Head et al., 2012), not the least because they are easily cross-fostered because burying beetles use timing rather than kin recognition to direct their parental care (i.e. temporal kin recognition; Müller & Eggert, 1990; Eggert & Müller, 2000; Oldekop et al., 2007). This allows us to use an interspecific cross-fostering design to measure maternal effects in two burying beetle species, N. orbicollis and N. vespilloides.
We used both N. vespilloides and N. orbicollis in a full-factorial design, with both species acting as caregiver parents and recipient offspring. These two species differ substantially in size and mass (Scott, 1998), with N. orbicollis weighing on average more than twice as much as N. vespilloides. Furthermore, they differ behaviourally in that N. orbicollis larvae require feeding from birth (Trumbo, 1992) whereas N. vespilloides can survive without parental care (Eggert et al., 1998). They are also distantly related within the genus, with an estimated divergence time of over 85 million years (Sikes & Venables, 2013). We tested two hypotheses. First, we predicted that maternal care would differ between species. Given that parental care is clearly adaptive in burying beetles (Eggert & Müller, 1997; Scott, 1998), we next predicted that these differences would reflect adaptive divergence in maternal effects. That is, our second hypothesis was that if maternal effects were optimized to provide maximum benefit for a given species, and not just general benefits of care, then we should see a significant interaction between caregiver and recipient species for offspring performance traits. We found that although there were highly significant differences in care between the species, these do not necessarily result in optimal maternal effects within a species, as we found no coadaptation between levels of care and maternal effect on development or mass. This suggests that species differences may be more related to life-history differences than adaptation of maternal effects.
Materials and methods
We collected N. orbicollis from Whitehall Forest, Athens GA in the spring of 2013. These individuals were used to start an outbred colony maintained under temperature and light control (21 °C; 14: 10 light : dark) for five generations before the start of this experiment. Individuals were kept in isolated, plastic boxes (9 cm diameter, 4 cm deep; Eco Products, Boulder, CO, USA) half-filled with soil and fed two decapitated mealworms (Tenebrio) twice a week.
Nicrophorus vespilloides used in the experiment were taken from a population originating from Cornwall, UK (Head et al., 2012) and maintained at the University of Georgia (Cunningham et al., 2014). All experimental trials were performed at 21 °C under a 14:10 light cycle as described above for N. orbicollis.
All individuals in the experiment were at least 14 days old post-eclosion and were bred in plastic boxes (17.2 × 12.7 × 6.4 cm; Pioneer Plastics, Dixon, KY, USA) filled with approximately 2 cm of soil. Each plastic box contained a thawed mouse weighing between 22 and 26 g (RodentPro, Evansville, IN, USA). All individuals were weighed and measured before breeding. Nicrophorus orbicollis takes significantly longer to produce hatched offspring than does N. vespilloides. Therefore, we bred N. orbicollis pairs about 48 hours before breeding N. vespilloides pairs. After pairing, each box was checked twice daily for eggs. Timing of egg appearance was used to determine the parents to be switched. Intraspecific switches (controls) were made between mothers whose eggs appeared at the same time. Interspecific switches were made between N. orbicollis mothers that laid eggs 16–24 h before the corresponding N. vespilloides mother, to account for longer hatching time in the former.
We transferred both the mother and the carcass she prepared into a box containing foster eggs to control for prenatal maternal effects. At this point, we also removed the male to avoid possible post-hatching paternal effects. The removal of males does not affect either female behaviour or offspring fitness (Smiseth et al., 2005). Before transferring, the mouse was checked, and any larvae that had hatched and crawled onto the mouse before we switched parents were removed. These larvae would have been very newly arrived and we never observed any receiving parental care from their biological parents before they were removed. Such early larvae typically die and do not receive any care (Eggert & Müller, 2000).
After switching caretakers, we checked pairs twice daily (morning and afternoon) to determine duration of parental care and timing of dispersal of offspring (Head et al., 2012). We considered a mother to have abandoned the brood when we did not observe her on the carcass for two consecutive observations (Benowitz et al., 2013). At dispersal, we removed the mother, counted the number of offspring per brood, and weighed each larva individually to 0.1 mg using an electronic balance (Mettler-Toledo, Columbus, OH, USA). Thus, we measured two offspring traits: duration of development on the resource and mass at dispersal. These traits influence fitness (Lock et al., 2004) as the only feeding that occurs is during the development on the carcass, so mass at dispersal reflects the final size that can be attained (although mass can change after adult emergence as adults can and do feed). We analysed these traits using a Model 1 two-way ANOVA with SAS type III sums of squares, with the fixed effects being recipient species, caregiver species and their interaction. Mouse mass was used as a covariate in all analyses. However, as it was never a statistically significant effect (a common result when variation in mass of mouse is kept to a minimum; Moore, pers. obs.), we do not report any statistics for mouse mass or discuss it further. Our first test was for influences on parental care, measured as duration of maternal care. We then examined how caregiver species, recipient species or their interaction influenced average larvae mass, development time (hatching to dispersal from the carcass) and number of larvae dispersed. We used JMP (v11.0.0; SAS Institute, Cary, NC, USA) for all statistical analyses.
Results
The duration of care depended only on maternal traits; that is, which species was caring (Fig. 1). Nicrophorus orbicollis mothers remained present on the carcass longer than N. vespilloides mothers (F1,131 = 7.687, P = 0.006). However, duration of maternal care was neither affected by offspring species (F1,131 = 2.032, P = 0.156) nor by the interaction between caregiver and offspring species (F1,131 = 1.555, P = 0.215).
Fig. 1.
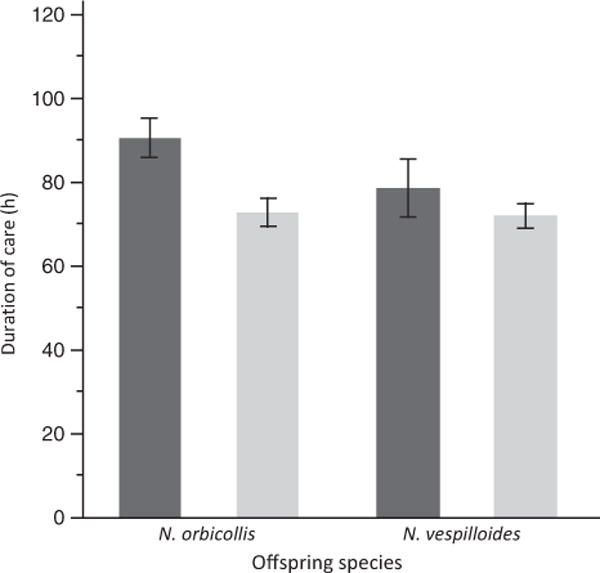
Mean (± SE) duration of maternal care measured from larval hatching to carcass abandonment. Dark grey bars indicate Nicrophorus orbicollis caretakers; light grey bars indicate N. vespilloides caretakers.
Both direct and maternal effects influenced development time (Fig. 2). Nicrophorus vespilloides offspring developed faster than N. orbicollis offspring regardless of the species that provided maternal care (F1,131 = 22.415, P < 0.0001). Broods raised by N. vespilloides parents developed faster than those raised by N. orbicollis (F1,131 = 12.607, P = 0.0005). However, there was no statistically significant interaction between caregiver and offspring species on development time (F1,131 = 0.343, P = 0.559).
Fig. 2.
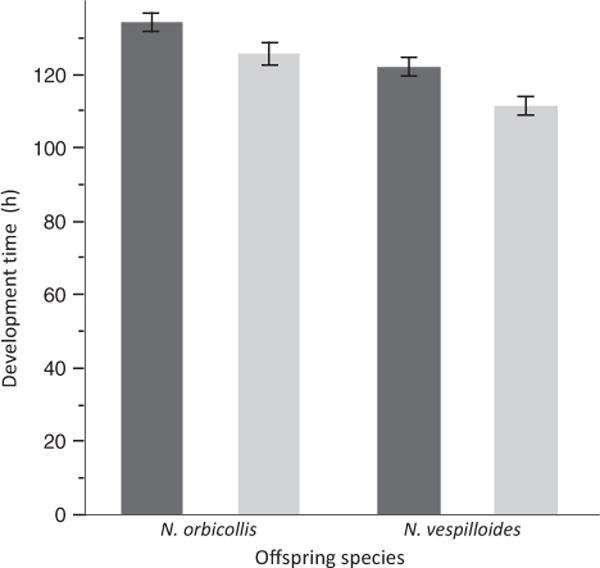
Mean (± SE) development time of larval broods, measured from hatching to dispersal from the carcass. Dark grey bars indicate Nicrophorus orbicollis caretakers; light grey bars indicate N. vespilloides caretakers.
Offspring mass was determined almost entirely by direct effects (Fig. 3).Regardless of parent, N. orbicollis larvae were much larger than N. vespilloides larvae (F1,127 = 307.777, P < 0.0001), and there was no statistically significant effect of caregiver species (F1,127 = 2.297, P = 0.132). The interaction was again not statistically significant (F1,126 = 1.896, P = 0.1709).
Fig. 3.
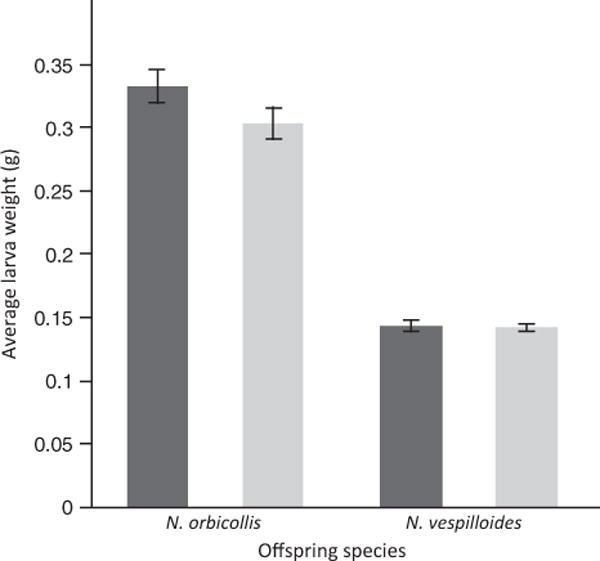
Mean (± SE) of larval weight at dispersal. Average larval weight of each dispersing brood was calculated. Dark grey bars indicate Nicrophorus orbicollis caretakers; light grey bars indicate N. vespilloides caretakers.
Discussion
A trait can be considered an adaptation if its function is tied to the selective forces that led to its evolution (Gould & Lewontin, 1979) or in the words of Williams (1966, p. 9) ‘… the machinery involved was fashioned by the selection for the goal attributed to it.’ Useful (or good) and adaptive are not equivalent (Williams, 1966). Maternal effects are common and important in phenotypic evolution and therefore have been suggested to be selected and adaptive (Mousseau & Fox, 1998). Parenting is an especially important source of environmental influences on offspring. If species differences in maternal effects arsing from parenting are adaptive, we predicted that parenting differences between species would reflect optimization for within-species performance and a coadaptation between offspring performance and parenting. That is, the maximum benefit should occur when there is a match between caretaker and recipient species. To test this, we used cross-fostering across species; if our hypothesis was correct, we expected significant interactions between the caregiver and recipient species on offspring performance. As expected, the effects of parenting were strong and important, but contrary to our prediction we found little evidence for species-specific optimization of maternal effects. In N. vespilloides and N. orbicollis, the lack of coadaptation between care and development and mass suggests that species differences in maternal effects on these traits are not adaptations, even if parental care itself is adaptive.
In a study that used a similar design to address how differences in social interactions contribute to species differences, Linksvayer (2007) examined worker (subsocial) care in ants to test for coevolution between brood genotype (direct effects) and worker genotype (social effects). He measured offspring performance in three reciprocally cross-fostered ant species and found both direct genetic effects and an effect of social environment, resulting in significant interactions between offspring and worker species and indicating a complex relationship between giving and receiving of care. As in our study, direct genetic effects predominated and the direct-by-indirect covariance could contribute to species differences. Interestingly, this was only true when including the most divergent species (Temnothorax longispinosus), which has much larger workers that were larger regardless of the foster sibs. For the two more closely related species (T. ambiguus and T. curvispinosus), there was only an interaction effect. As Linksvayer (pers. comm.) suggests to us, perhaps more closely related Nicrophorus spp. would also show the expected interaction effect.
Coadaptation is only one possible outcome when there are parent-offspring interactions. Following Kölliker et al. (2005) who modelled coadaptation between parental provisioning and offspring solicitation, we suggest that the existence, sign and extent of the species differences in coadaptation between direct and maternal effects for each trait may reflect the nature of phenotypic control – is the extent of parenting determined by the parent, offspring or both? For example, we found that the species of caregiver did not affect the mass of the offspring. Given reduced parental care can result in smaller offspring in Nicrophorus, we expected to observe smaller N. orbicollis when raised by N. vespilloides as the latter spend less time parenting. The fact that we did not observe this may reflect the ability of offspring to influence maternal behaviour. Burying beetle parents respond to offspring begging by increasing provisioning (Smiseth & Moore, 2002, 2008; Lock et al., 2004) and offspring appear to control food allocation (Smiseth et al., 2003), although parents influence sibling competition (Smiseth et al., 2007a,b). Anecdotally, we observed that N. orbicollis larvae beg much more aggressively, which could manipulate parents of either species to provide the requisite feeding for normal growth. Another possibility for why caregiver species did not affect offspring mass is that burying beetle larvae can partially compensate for differences in parental care through self-feeding (Smiseth et al., 2003, 2006; Smiseth & Moore, 2004). Thus, as long as they receive some food during a critical period of growth, they may be able to reach their optimal weight without additional parental help. If this was the case, we might expect to see costs in later life-history stages for individuals raised by the wrong species. However, maternal effects tend to act most strongly early in development (Cheverud & Moore, 1994).
Further support for the importance of the direct–indirect genetic covariance comes from the other performance trait we measured, development time, where we found a different pattern. Regardless of offspring species, broods cared for by N. vespilloides mothers dispersed earlier than those that received care from N. orbicollis. This indicates the presence of a positive maternal effect in burying beetles; in other words, for development time, maternal effects are correlated with direct genetic effects (Lande & Price, 1989). To the extent that this covariance reflects genetic variation in the two traits, this should enhance the evolution of this trait (Kirkpatrick & Lande, 1989) and could reflect strong selection in N. vespilloides for broods to leave the carcass as early as possible because it is an inferior competitor (Scott, 1998), resulting in phenotypic change in both parent and offspring phenotypes. However, the maternal effect appears to have evolved independently from the offspring trait, as development time is not affected by any mother–offspring interactions.
A possible explanation for the lack of coadaptation in maternal effects is that there is an absence of species differences in ecology or the costs and benefits of care. This seems unlikely as the ecology and behaviour between the species are strikingly different, suggesting that they can and do differ on many axes. In nature, N. orbicollis typically exploit larger carcasses (> 30 g) whereas N. vespilloides use smaller (< 20 g) resources, although there is overlap in the carcass size that these species will exploit and no evidence for a preference (Scott, 1998). We found differences in carcass processing, which may be related to differences in typical resource size between these species. Nicrophorus orbicollis parents formed the mouse into almost perfect spheres, whereas N. vespilloides parents may not have been able to completely process the larger carcasses used in this study (Trumbo, 1992). These species also differ in burial depth (N. orbicollis buries the mouse about 10 cm underground, whereas N. vespilloides does not completely bury the mouse, but rather uses a shallow depression; Eggert & Müller, 1997; K. M. Benowitz pers. obs.). Moreover, given N. orbicollis is a North American species and N. vespilloides is found in Europe, offspring may be adapted to different fauna. The microbiota associated with different species appears to be more influenced by environment than phylogenetic relationship among burying beetle species (Kaltenpoth & Steiger, 2014). It does not appear, however, that transfer of symbionts by parents is important in burying beetles (Eggert et al., 1998). Perhaps most importantly, N. orbicollis mothers remain with their broods longer than N. vespilloides mothers. This difference in duration of care is potentially a necessary consequence of the increased development time and mass in N. orbicollis, as females may need to remain longer on the carcass to protect and feed their offspring.
Our work suggests that direct genetic effects in response to these selection pressures associated with differences in ecology and life-history may have a stronger influence on species differences than maternal effects and that any evolution of maternal effects would reflect indirect selection and a correlated response. The ultimate causes of species differences are likely ecological pressures and subsequent life-history trade-offs. Maternal effects may have evolved to influence life-history trade-offs in N. vespilloides (Steiger, 2013), but such a relationship is less clear for N. orbicollis. Based on general species differences, it appears that size trades off with development time and offspring number in burying beetles (Bartlett & Ashworth, 1988; Trumbo, 1990; Smiseth et al., 2014). However, these trade-offs are less stringent in broods raised by N. vespilloides, as they are able to speed up development and raise more larvae without a cost to body size at dispersal. Why one species should be more efficient in raising offspring is not clear. This may be due to the fact that the carcasses used in this experiment were large enough to relax trade-offs in the smaller N. vespilloides, but not N. orbicollis as the size-number trade-off is only seen on small carcasses with N. vespilloides (Smiseth et al, 2014), but is seen across all carcass sizes in N. orbicollis (Trumbo, 1990). Another potential explanation is that N. vespilloides mothers suffer a direct cost to future reproductive potential by expending more energy during care (Wade, 1998), despite a shorter time on the carcass. Thus, if N. orbicollis reproduce multiple times, they may not be less efficient parents over their lifetime.
It is clear that species differences in maternal effects are widespread and that maternal effects can be adaptive (Mousseau & Fox, 1998). Parenting is also adaptive (Royle et al, 2012). However, our results show that it does not necessarily follow that the differences between species in maternal effects arising from care reflect differences in direct selection on specific aspects of parental care. It seems more likely that the species differences can reflect a correlated response to other traits, especially given a covariance between direct and maternal genetic effects is expected to be ubiquitous (Cheverud & Moore, 1994; Wilson & Réale, 2007), reflecting selection arising from differences in the species’ life-history.
Acknowledgments
We thank C. Cunningham, E. Roy-Zokan, L. McKinney, A. Gardner, S. Trumbo and T. Linksvayer for helpful comments on the manuscript, and B. Hsu and M. Douthit for help in beetle rearing. Funding from NERC (UK) and the Office of the Vice-President for Research at the University of Georgia to A.J.M., and an NIH Training Grant (T32GM007103) to K.M.B, supported this research.
References
- Badyaev AV, Uller T. Parental effects in ecology and evolution: mechanisms, processes and implications. Philos Trans R Soc B. 2009;364:1169–1177. doi: 10.1098/rstb.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. Male mating success and paternal care in Nicrophorus vespilloides (Coleoptera: Silphidae) Behav Ecol Sociobiol. 1988;23:297–303. [Google Scholar]
- Bartlett J, Ashworth CM. Brood size and fitness in Nicrophorus vespilloides (Coleoptera: Silphidae) Behav Ecol Sociobiol. 1988;22:429–434. [Google Scholar]
- Benowitz KM, Head ML, Williams CA, Moore AJ, Royle NJ. Male age mediates reproductive investment and response to paternity assurance. Proc R Soc B. 2013;280:20131124. doi: 10.1098/rspb.2013.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheverud JM, Moore AJ. Quantitative genetics and the role of the environment provided by relatives in the evolution of behavior. In: Boake CRB, editor. Quantitative Genetic Studies of Behavioral Evolution. University of Chicago Press; Chicago: 1994. pp. 67–100. [Google Scholar]
- Cunningham CB, Douthit MK, Moore AJ. Octopaminergic gene expression and flexible social behaviour in the subsocial burying beetle Nicrophorus vespilloides. Ins Mol Biol. 2014;23:391–404. doi: 10.1111/imb.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth RA. Maternal effects and range expansion: a key factor in a dynamic process? Philos Trans R Soc B. 2009;364:1075–1086. doi: 10.1098/rstb.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert AK, Müller JK. Biparental care and social evolution in burying beetles: lessons from the larder. In: Choe JC, Crespi BJ, editors. The Evolution of Social Behavior in Insects and Arachnids. Cambridge University Press; Cambridge: 1997. pp. 216–236. [Google Scholar]
- Eggert A-K, Müller JK. Timing of oviposition and reproductive skew in cobreeding female burying beetles (Nicrophorus vespilloides) Behav Ecol. 2000;11:357–366. [Google Scholar]
- Eggert A-K, Reinking M, Müller JK. Parental care improves offspring survival and growth in a burying beetle. Anim Behav. 1998;55:97–107. doi: 10.1006/anbe.1997.0588. [DOI] [PubMed] [Google Scholar]
- Falconer DS, Mackay T. Introduction to Quantitative Genetics. 4th. Longman; New York: 1996. [Google Scholar]
- Fox CW, Thakar MS, Mousseau TA. Egg size plasticity in a seed beetle: an adaptive maternal effect. Am Nat. 1997;149:149–163. doi: 10.1111/j.1558-5646.1999.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc B. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- Head ML, Berry LK, Royle NJ, Moore AJ. Paternal care: direct and indirect genetic effects of fathers on offspring performance. Evolution. 2012;66:3570–3581. doi: 10.1111/j.1558-5646.2012.01699.x. [DOI] [PubMed] [Google Scholar]
- Kaltenpoth M, Steiger S. Unearthing carrion beetles’ microbiome: characterization of bacterial and fungal hindgut communities across the Silphidae. Mol Ecol. 2014;23:1251–1267. doi: 10.1111/mec.12469. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Kölliker M, Brodie ED, III, Moore AJ. The coadaptation of parental supply and offspring demand. Am Nat. 2005;166:506–516. doi: 10.1086/491687. [DOI] [PubMed] [Google Scholar]
- Lande R, Price T. Genetic correlations and maternal effect coefficients obtained from offspring-parent regression. Genetics. 1989;122:915–922. doi: 10.1093/genetics/122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linksvayer TA. Ant species differences determined by epistasis between brood and worker genomes. PLoS ONE. 2007;2:e994. doi: 10.1371/journal.pone.0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JE, Smiseth PT, Moore AJ. Selection, inheritance, and the evolution of parent-offspring interactions. Am Nat. 2004;164:13–24. doi: 10.1086/421444. [DOI] [PubMed] [Google Scholar]
- Lock JE, Smiseth PT, Moore PJ, Moore AJ. Coadaptation of prenatal and postnatal maternal effects. Am Nat. 2007;170:709–718. doi: 10.1086/521963. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, Uller T. When is a maternal effect adaptive? Oikos. 2007;116:1957–1963. [Google Scholar]
- Meierhofer I, Schwarz HH, Müller JK. Seasonal variation in parental care, offspring development, and reproductive success in the burying beetle, Nicrophorus vespillo. Ecol Entomol. 1999;24:73–79. [Google Scholar]
- Mousseau TA, Fox CW. Maternal Effects as Adaptations. Oxford University Press; New York: 1998. [Google Scholar]
- Müller JK, Eggert AK. Time-dependent shifts between infanticidal and parental behavior in female burying beetles - a mechanism of indirect mother-offspring recognition. Behav Ecol Sociobiol. 1990;27:11–16. [Google Scholar]
- Müller JK, Eggert AK, Sakaluk SK. Carcass maintenance and biparental brood care in burying beetles: are males redundant? Ecol Ent. 1998;23:195–200. [Google Scholar]
- Oldekop JA, Smiseth PT, Piggins HD, Moore AJ. Adaptive switch from infanticide to parental care: how do beetles time their behaviour? J Evol Biol. 2007;20:1998–2004. doi: 10.1111/j.1420-9101.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- Pukowski E. Ökologische untersuchungen an Necrophorus F. Z. Morphol Oekol Tiere. 1933;27:518–586. [Google Scholar]
- Rauter CM, Moore AJ. Evolutionary importance of parental care performance, food resources, and direct and indirect genetic effects in a burying beetle. J Evol Biol. 2002a;15:407–417. [Google Scholar]
- Rauter CM, Moore AJ. Quantitative genetics of growth and development time in the burying beetle Nicrophorus pustulatus in the presence and absence of post-hatching parental care. Evolution. 2002b;56:96–110. doi: 10.1111/j.0014-3820.2002.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Royle NJ, Smiseth PT, Kölliker M. The Evolution of Parental Care. Oxford University Press; Oxford; 2012. [Google Scholar]
- Royle NJ, Russell AF, Wilson AJ. The evolution of flexible parenting. Science. 2014;345:776–781. doi: 10.1126/science.1253294. [DOI] [PubMed] [Google Scholar]
- Scott MP. Male parental care and reproductive success in the burying beetle, Nicrophorus orbicollis. J Insect Behav. 1989;2:133–137. [Google Scholar]
- Scott MP. The ecology and behavior of burying beetles. Annu Rev Entomol. 1998;43:595–618. doi: 10.1146/annurev.ento.43.1.595. [DOI] [PubMed] [Google Scholar]
- Sikes DS, Venables C. Molecular phylogeny of the burying beetles (Coleoptera: Silphidae: Nicrophorinae) Mol Phylo Evol. 2013;69:552–565. doi: 10.1016/j.ympev.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Moore AJ. Does resource availability affect offspring begging and parental provisioning in a partially begging species? Anim Behav. 2002;63:577–585. [Google Scholar]
- Smiseth PT, Moore AJ. Signalling of hunger when offspring forage by both begging and self-feeding. Anim Behav. 2004;67:1083–1088. [Google Scholar]
- Smiseth PT, Moore AJ. Parental distribution of resources in relation to larval hunger and size rank in the burying beetle Nicrophorus vespilloides. Ethology. 2008;114:789–796. [Google Scholar]
- Smiseth PT, Darwell CT, Moore AJ. Partial begging: an empirical model for the early evolution of offspring signalling. Proc R Soc B. 2003;270:1773–1777. doi: 10.1098/rspb.2003.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiseth PT, Dawson C, Varley E, Moore AJ. How do caring parents respond to mate loss? Differential response by males and females. Anim Behav. 2005;69:551–559. [Google Scholar]
- Smiseth PT, Ward RJS, Moore AJ. Asynchronous hatching in Nicrophorus vespilloides, an insect in which parents provide food for their offspring. Funct Ecol. 2006;20:151–156. [Google Scholar]
- Smiseth PT, Ward RJS, Moore AJ. Parents influence asymmetric sibling competition: experimental evidence with partially dependent young. Ecology. 2007a;88:2174–3182. doi: 10.1890/06-1992.1. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Lennox L, Moore AJ. Interaction between parental care and sibling competition: parents enhance offspring growth and exacerbate sibling competition. Evolution. 2007b;61:2331–2339. doi: 10.1111/j.1558-5646.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- Smiseth PT, Andrews CP, Mattery SN, Mooney R. Phenotypic variation in resource acquisition influences trade-off between number and mass of offspring in a burying beetle. J Zool. 2014;293:80–83. [Google Scholar]
- Steiger S. Bigger mothers are better mothers: disentangling size-related prenatal and postnatal maternal effects. Proc R Soc B. 2013;280:20131225. doi: 10.1098/rspb.2013.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallamy DW. Insect parental care. Bioscience. 1984;34:20–24. [Google Scholar]
- Tallamy DW, Wood TK. Convergence patterns in subsocial insects. Annu Rev Entomol. 1986;31:369–390. [Google Scholar]
- Trumbo ST. Reproductive benefits of infanticide in a biparental burying beetle Nicrophorus orbicollis. Behav Ecol Sociobiol. 1990;27:269–273. [Google Scholar]
- Trumbo ST. Reproductive benefits and the duration of paternal care in a biparental burying beetle, Necrophorus orbicollis. Behaviour. 1991;117:82–105. [Google Scholar]
- Trumbo ST. Monogamy to communal breeding: exploitation of a broad resource base by burying beetles (Nicrophorus) Ecol Ent. 1992;17:289–298. [Google Scholar]
- Wade MJ. The evolutionary genetics of maternal effects. In: Mousseau TA, Fox CW, editors. Maternal Effects as Adaptations. Oxford University Press; New York: 1998. pp. 5–21. [Google Scholar]
- Walling CA, Stamper CE, Smiseth PT, Moore AJ. The quantitative genetics of sex differences in parenting. Proc Natl Acad Sci USA. 2008;105:18430–18435. doi: 10.1073/pnas.0803146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. Adaptation and Natural Selection. Princeton University Press; Princeton, NJ: 1966. [Google Scholar]
- Wilson AJ, Réale D. Ontogeny of additive and maternal genetic effects: lessons from domestic mammals. Am Nat. 2007;167:E23–E38. doi: 10.1086/498138. [DOI] [PubMed] [Google Scholar]
- Wolf JB, Wade MJ. What are maternal effects (and what are they not)? Philos Trans R Soc B. 2009;364:1107–1115. doi: 10.1098/rstb.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]


