Abstract
Measles is a highly contagious viral disease in NHP. The infection can range from asymptomatic to rapidly fatal, resulting in significant morbidity and mortality in captive populations. In addition to appropriate quarantine practices, restricted access, the immunization of all personnel in contact with NHP, and the wearing of protective clothing including face masks, measles immunization further reduces the infection risk. Commercially available measles vaccines are effective for use in NHP, but interruptions in their availability have prevented the implementation of ongoing, consistent vaccination programs. This need for a readily available vaccine led us to perform a broad, multicenter safety and immunogenicity study of another candidate vaccine, MVac (Serum Institute of India), a monovalent measles vaccine derived from live Edmonston–Zagreb strain virus that had been attenuated after 22 passages on human diploid cells.
Abbreviations: MV, measles virus
Measles is an acute, highly contagious viral disease characterized by a generalized maculopapular eruption (rash).2,59 Measles virus (MV) is an enveloped, single-stranded RNA virus in the family Paramyxoviridae which, along with the closely related canine distemper virus, the Rinderpest virus, Peste des Petits Ruminants virus of ungulates, and some newly identified viruses of marine mammals, comprises the genus Morbillivirus.22,42
Humans and NHP are the only hosts susceptible to MV.2,33 Infection in NHP can range from asymptomatic to rapidly fatal. New World species are especially susceptible to MV.3,30,31 Clinical illness is characterized by a 2- to 3-d prodrome consisting of fever (which may exceed 105 °F), malaise, and anorexia followed by coryza, keratoconjunctivitis, and dry cough. In addition, generalized lymphadenopathy and splenomegaly are frequent findings at this stage, and the pathognomonic ‘Koplik spots’ may appear on the buccal mucosa. The measles rash usually appears from 3 to 5 d after the onset of clinical signs, developing first on the head and face. The rash becomes maculopapular and spreads rapidly down the neck, trunk, and extremities over several days. In late stages, the rash becomes a deep reddish-purple in color and may be associated with edema of the skin. After this stage, fever decreases and systemic manifestations begin to resolve. The rash fades in the same top-down sequence as it appeared and may be associated with fine powdery desquamation.7,20,32,36 Primary MV pneumonia, encephalitis, and enteropathy are all possible complications of acute infection. In addition, MV is highly immunosuppressive, causing dysfunction of both the humoral and cell-mediated immune systems that may last from weeks to months, thus predisposing infected subjects to several common complications of secondary bacterial infections, including bacterial pneumonia, bacterial enteritis, and systemic bacteremia.35,48,53
Although no true carrier state is recognized, humans with subclinical infections and marginal immunity can potentially serve as a source of MV introduction.10,60 The primary portal of entry of MV is the respiratory and conjunctival mucosae. MV is spread by direct contact, fomites, and aerosol. Transmission is highly efficient. Herd immunity levels exceeding 94% are required to prevent transmission in human populations.17,18 The period of communicability lasts from 1 d prior to the onset of the prodrome to 5 to 6 d after the appearance of the rash.41
Humans are the only reservoir host species of MV.2,33 Measles is endemic only in high-density human populations. There is a density threshold below which MV cannot be maintained in a population due to very efficient transmission and rapid exhaustion of susceptible hosts. However, apes and both Old World and New World monkeys are susceptible to MV infection. Because wild NHP do not live in populations large enough to exceed the endemic maintenance threshold, measles is not a naturally occurring infection.33
Historically, measles was a common infectious disease problem in NHP, primarily during capture and transport from the wild. In addition, many outbreaks in NHP colonies have been described.3,11,14,24,30,33,38,39,44,47,58,59 There are documented cases of measles epizootics brought by personnel into high-density NHP facilities. In 1986 to 1987 a large measles outbreak occurred at the California National Primate Research Center, with 147 recognized NHP cases (Macaca mulatta and M. fascicularis) and 28% mortality (not including stillbirths). The cost to treat infected animals was approximately US$187,000, not including animal loss due to secondary infection or abortion. During this same time period, 5 human cases among human contacts were documented. Epidemiologic evidence suggests instances of NHP-to-human MV transmission in this outbreak, raising occupational health concerns.49
The morbidity and mortality associated with measles infection in captive NHP populations is considerable. Appropriate quarantine practices, restricted access, immunization of all personnel in contact with NHP, and wearing of protective clothing including face masks, reduces the risk. Measles immunization further reduces the infection risk. Many primate facilities include measles vaccination as a component of their preventive medicine programs as part of an overall effort to enhance animal health, preserve research, and protect personnel.
To our knowledge, Attenuvax (Merck, Rahway, NJ) was the first commercial measles vaccine administered to NHP colonies. This live attenuated vaccine licensed for use in humans was moderately expensive and, after several years of usage, alternative vaccines were researched. In the mid1990s, scientists at the California National Primate Research Center performed a vaccine study to compare the Attenuvax vaccine with a modified live combination canine distemper virus–MV vaccine, Vanguard DM (Exter, PA). The Vanguard DM product achieved measles antibody titers that exceeded those of Attenuvax-immunized NHP and was deemed a cost-effective vaccine alternative.12 In light of these study results and lower costs, many NHP programs implemented the use of the Vanguard DM vaccine. In 2007, Pfizer altered the Vanguard DM vaccine by removing the measles component. This change in vaccine composition and the unavailability of Attenuvax resulted in the discontinuation of measles vaccination as part of routine preventive medicine programs for NHP at several institutions. Given the lack of a viable, domestic-source, monovalent measles vaccine and alternatives, a collaborative effort was established among the United States NIH P51-supported National Primate Research Centers to investigate the safety and efficacy of MVac, a live attenuated measles vaccine that is licensed by the World Health Organization for use in humans and produced by the Serum Institute of India.51 Here we report the results from our multicenter trial to assess the safety and immunogenicity of the MVac vaccine for NHP.
Materials and Methods
Animals.
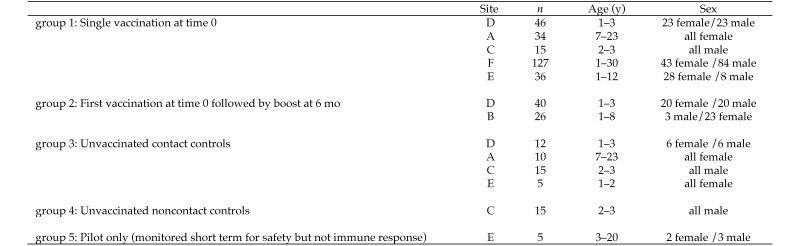
Rhesus macaques (Macaca mulatta) and pigtailed macaques (M. nemestrina) from the National Primate Research Center colonies located at Emory University, Texas Biomedical Research Institute, Tulane University of Louisiana, University of California at Davis, University of Washington, and University of Wisconsin were included in this study. Each center is a fully AAALAC-accredited facility, and all animals were maintained in accordance with the Animal Welfare Act, Regulations, and the Guide for the Care and Use of Laboratory Animals.4,5,23 All procedures involving animals used in this study were approved by each institution's IACUC. Experimental groups included male and female NHP from 1 to 30 y of age and housed in various configurations ranging from indoor pairs to small indoor groups (3 to 5 animals) and large (as many as 80 animals) outdoor groups housed in half-acre field cages. The inclusion of multiple sites with varying standard conditions and protocols allowed for the measurement of vaccine safety and immunogenicity across a range of normal colony situations. We compared 5 study groups and followed them for a maximum of 15 mo after vaccination (Figure 1): group 1, single vaccination at 0 mo (time 0); group 2, primary vaccination at time 0 with a boost at 6 mo; group 3, unvaccinated contact controls cohoused with vaccinated NHP; group 4, unvaccinated noncontact controls; and group 5, a pilot group of 5 monkeys (already destined for tissue collection and distribution) that were vaccinated and monitored daily for any adverse effects for 14 d prior to their prescheduled necropsy and tissue collection. Daily cageside monitoring assessed appetite, hydration, stool and urine, posture and attitude, respiration, skin, activity, and potential seizure events. All animals were monitored daily by trained animal care and veterinary technical staff, with abnormalities reported immediately to a veterinarian. Measurements included body condition scores, physical examination, complete blood counts, chemistry panels and urinalysis according to the standard protocols in place at each center. Blood samples for antibody testing were collected prior to vaccination and at regular intervals for a maximum of 15 mo (Figure 2).
Figure 1.
Description of the study population. All subjects were rhesus macaques, except for those at site E, which were M. nemastrina.
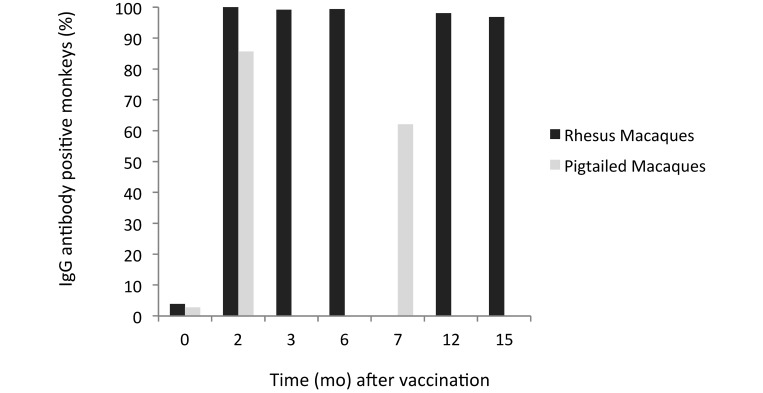
Figure 2.
Seroconversion. All macaques were sampled at time 0. Subsets of rhesus macaques were sampled at 2, 3, 6, 12, and 15 mo after vaccination. Pigtailed macaques were sampled at 2 and 7 mo after vaccination.
MVax product.
A monovalent measles vaccine derived from live Edmonston–Zagreb strain, which had been attenuated after 22 passages on human diploid cells (MRC5 human lung fibroblasts) was purchased from the Serum Institute of India.51 This vaccine has been licensed by the World Health Organization for use in humans. Each lyophilized dose (0.5 mL) contained more than 1000 CCID50 virus particles per dose.51 The route of administration was subcutaneous on the dorsum between or medial to the shoulders.
IgG antibody.
Reactivity to measles viral lysate and recombinant nucleocapsid antigens was measured on a liquid bead-based array platform (Luminex, Austin, Tx) by using multiplex microbead reagents (Charles River Laboratories, Wilmington, MA).26 Reactivity to the 2 types of MV antigen-coupled beads was assayed simultaneously in parallel, with reactivity to uninfected baculovirus, human IgG, and antihuman IgG antigen-coupled beads to function as internal negative and positive controls. In addition, beads coupled with the lysate from the vaccine-production cell line MRC5 were added to the panel as an additional negative-cell control. In this immunoassay, antigen-coupled microbeads of defined spectral properties were incubated with 1:100-diluted serum to capture the specific antibodies. After washing, the beads were reacted with antihuman (validated to crossreact with NHP) IgG biotinylated antibodies (Jackson Immunoresearch, West Grove, PA). After additional washing, the beads were reacted with Streptavidin-R-Phycoerythrin (Charles River Labs). After a final washing to remove all unbound material, the beads were analyzed spectrophotometrically. By measuring the spectral properties of the beads and the amount of associated Streptavidin-R-Phycoerthrin, the median fluorescence index for each specific antigen was determined. The sample median fluorescence index was compared against a previously validated known positive–negative cutoff value to determine qualitatively the presence or absence of antibody. This assay also was used to quantitate titers on a subset of samples.
Neutralization titers.
A plaque reduction–microneutralization assay was used to detect functional neutralizing antibodies in a subset of 122 serum samples (group 1 sites D and E; group 2 site D).21 The assay was performed in a 96-well microtiter plate containing DMEM with 2% fetal calf serum. Briefly, 50 μL serum (serially diluted from 1:20 to 1:640) was incubated with 50 μL of approximately 50 CCID50 MV MVvac2GFP, a molecular clone of the Moraten vaccine strain that expresses green fluorescent protein,16 at 37 °C, 95% humidity, and 5% CO2. After 1 h, 2 × 104 low-passage Vero cells were added to each well and incubated for 3 d. The wells were observed microscopically (magnification, 10×) and scored for the presence (no neutralization) or absence (neutralization) of fluorescence.16 Negative controls to assess cell confluence and toxicity and plaque-positive (30% to 75%) virus controls were run in addition. The data were analyzed by using the Reed Muench equation46 to compute a reciprocal titer for each sample. To standardize and compare results, a neutralizing antibody score [(sample titer / rhesus measles immune globulin control titer tested in parallel) × 100] was calculated for each sample.
Results
Safety.
Overall, 6 rhesus macaques from group 1, site D (single vaccination) and the 5 pigtailed macaques previously assigned for necropsy were vaccinated with MVax and monitored daily, with particular attention paid to the skin, dermis, and subcutis at the injection site. No adverse signs were noted. Health scores for all body systems were normal throughout. The rhesus macaques continued on the study as members of group 1, site D. The pigtailed macaques were euthanized at day 14, and no abnormalities were noted on gross necropsy. Results of CBC counts and serum chemistry panels were unremarkable. NHP in groups 1 (single dose) and 2 (2 doses: primary and boost) and the control animals from groups 3, 4, and 5 were all monitored daily by trained animal care and veterinary technical staff. Measurements including body condition scores, physical examination, CBC, serum chemistry panels, and urinalysis performed in accordance with standard protocols at each center revealed no remarkable conditions or clinical abnormalities that potentially might be related to vaccination.
Immunogenicity.
All but 2 of the 288 vaccinated rhesus macaques had seroconverted after primary immunization by the time of their first sample collection at 2, 3, or 6 mo after immunization. The macaques that were seronegative at their first postvaccination sampling point were seropositive when retested at the next time point. At 12 or 15 mo, antibody had waned below detectable limits in 7 of the group 1 monkeys that received only a single dose of vaccine. In contrast, all of the group 2 animals, which received an initial dose at 0 mo and a booster dose at 6 mo, were antibody-positive.
We found that 30 of the 35 pigtailed macaques that received a single vaccination were seropositive when tested at 2 mo after vaccination. Only 18 of the 29 macaques that were positive at 2 mo were still seropositive when retested at 7 mo. The remaining 6 NHP were lost to follow-up.
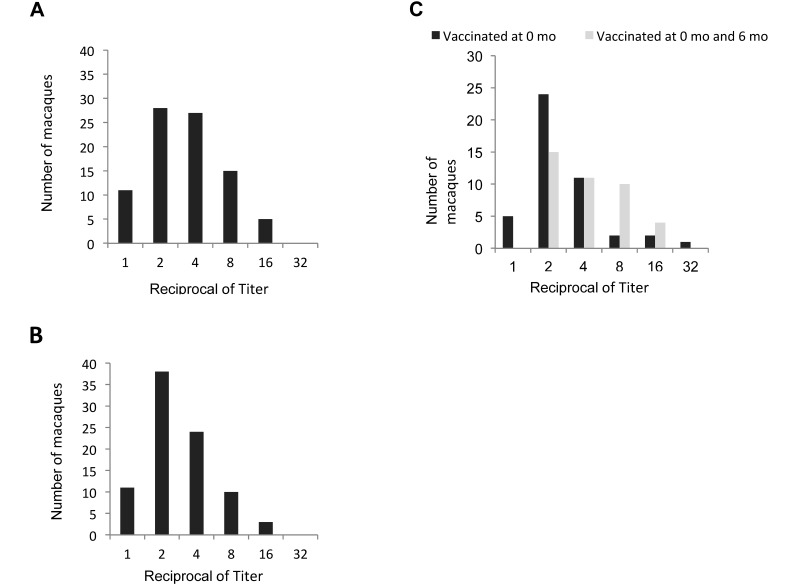
Immunogenicity data are summarized in Figure 2. Antibody titers were measured in a subset comprising 40 rhesus macaques from group 1 and 40 rhesus macaques from group 2. The titers at 3, 6, and 12 mo after initial vaccination with and without a second dose of vaccine, ranged from 1:2 to 1:16 of the initial 1:100 dilution used for qualitative screening. The range, median (1:2 or 1:4), and mode (1:2) titers did not vary by more than one two fold dilution factor in either single- or double-dose groups at all time points (Figure 3). Antibody was detected in the initial serum collected from 11 of the rhesus macaques and one pigtailed macaque at the time of vaccination. The reactivity for these time-0 samples was low, close to the positive–negative cutoff value, and increased at the next postvaccination time point.
Figure 3.
IgG antibody titers at (A) 3, (B) 6, and (C) 12 mo after vaccination with MVac determined for a subset of 40 rhesus macaques from group 1 site D that received a single vaccination and 40 rhesus macaques from group 2 site D that received 2 doses of vaccine. Because animals in both groups each had received only 1 dose of vaccine at the 3- and 6-mo time points, the groups are combined for those time points. The 2nd dose of vaccine was not admininistered to group 2 until after the collection of the 6-mo sample.
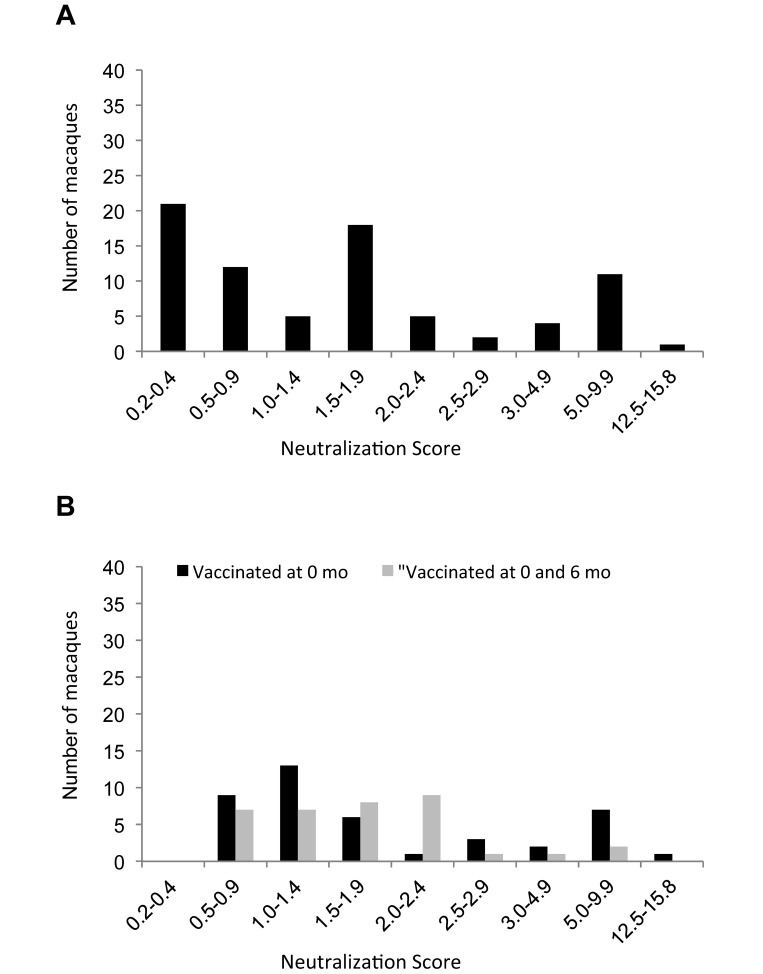
Neutralizing antibody titers were determined in a subset of 122 sera from vaccinated rhesus macaques (group 1, site D; Figure 4). At 6 mo after vaccination, neutralizing antibody was detected in 79 of 86 samples. At 12 mo, neutralizing antibody was detectable in 42 of 45 sera from the NHP that received a single dose of vaccine and in 35 of the 40 samples from macaques that also received a booster dose at 6 mo after the initial vaccination. The titers were low, ranging from 1:20 to 1:320, as compared with that for the measles immune globulin control, which was 1 to 2 logs higher, ranging from 1:2032 to 1:10,240. In the pigtailed macaques, neutralizing antibody was detectable in only 7 of the 35 sera at 2 mo and in 3 of the 28 sera at 7 mo after a single vaccination at time 0. No indication of vaccine virus shedding was demonstrated, given that no antibodies to MV were detected in the serum collected from the unvaccinated (group 3) contact controls at 3, 6, or 12 mo during the course of the study.
Figure 4.
Neutralization scores (ratio of sample to measles immune globulin control titer) at (A) 6 mo or (B) 12 mo after vaccination with MVac to a subset of 40 rhesus macaques from group 1 site D that received a single vaccination and 40 rhesus macaques from group 2 site D that received 2 doses of vaccine. Because animals in both groups each had received only 1 dose of vaccine at the 6-mo time point, the data are combined for that time points. The 2nd dose of vaccine was not administered to group 2 until after the collection of the 6-mo sample.
Discussion
Measles is a vaccine-preventable disease in both humans and NHP. Unfortunately, the high costs of immunization and implementation programs may deter the vaccination of NHP populations. Instead, facilities rely heavily on appropriate quarantine practices, restricted access, the immunization of all personnel in contact with NHP, and the wearing of personal protective equipment. These practices can still leave large, susceptible, MV-naïve NHP populations at risk. Because the immunity to measles is variable along a continuum of clinical illness, humans with subclinical infections and marginal immunity can serve as sources of virus introduction.10,60 Recent human measles outbreaks in the United States demonstrate the need for establishing immunity in NHP breeding and research colonies to decrease the risk to these populations from infected humans.63 Therefore, the goal of the current study was to test the efficacy of an economical measles vaccine (MVac) in NHP to provide the protection needed for valuable animal resources, decrease occupational health concerns for personnel, and prevent variables in ongoing research studies.
Safety concerns regarding attenuated vaccines include the possibilities of viral replication and associated disease and of viral shedding after immunization. In the current study, all vaccinated and control monkeys were observed and monitored regularly after immunization with MVac. Monitoring tools included routine health checks, observation of the immunization site, CBC, and serum chemistry values. No remarkable events emerged. No gross lesions, histologic findings, or other pathology were noted, either generally or at the injection site in tissues collected from the subset of pilot group 5 animals prescheduled for necropsy.
The lack of detectable antibody in any of the unvaccinated contact controls (group 3) provides evidence against viral shedding. Although these findings support vaccine safety, they also preclude a strategy of vaccinating several animals in a social group to build group immunity through viral shedding. In addition, antibody was not detected in the noncontact control NHP (group 4), as expected.
MVac demonstrated immunogenicity, as evidenced by seroconversion in all of the vaccinated rhesus macaques (groups 1 and 2) and in at least one serum sample collected after vaccination from 30 of the 35 pigtailed macaques. Published serologic studies have shown a lower prevalence of measles antibody in pigtailed compared with rhesus macaques.25 In SIV studies, pigtailed and rhesus macaques show different susceptibilities and disease features.25 In addition, these 2 species have shown differential immune responses to SIV vaccination, perhaps related to differences in the structure of the peptide-binding pockets in the MHC molecules.6,19,27,43 We speculate that these differences might affect MV replication.29
Similarly to previous studies, most vaccinees seroconverted quickly in the current study, with antibody present at the first time point after immunization (2, 3, or 6 mo).12 Although the median and mean titers were one dilution higher in the boosted compared with single-dose animals, both the highest and lowest titers occurred in the single-dose group. A 1-dilution variation is not considered to be biologically relevant. At the 2nd postvaccination time point, antibody levels had waned in the serum of a small group of 8 vaccinated rhesus macaques. There was no correlation with number of doses, age, or sex, given that the group included both male and female macaques.28,57 Macaques were not prescreened to determine antibody status prior to inclusion in this study, and antibody was detected in 1 pigtailed and 11 rhesus macaques on the day of immunization. This antibody reactivity was weak, with raw values within 20% of the positive–negative cutoff value. None of these sera reacted excessively to the internal cell controls as compared to other study samples. Further review of the animal histories revealed that 6 macaques had been vaccinated with other measles vaccines more than 5 to 7 y previous; histories for the other 6 animals were either unavailable or did not reveal any explanation for the reactivity. Routine measles vaccination is not an uncommon practice in some NHP production facilities.1 Because the specificity of a single assay never exceeds 100%, we speculate that some of these initial scores are false-positive scores from an undetermined cross reactivity.
The neutralizing antibody levels detected in the MVac macaques all were low compared with titers in measles immune globulin and in the sera from macaques infected with WT MV. However, these levels were in the same range as those in 2 macaques that previously received a similar live attenuated vaccine (Attenuvax) and that were protected from challenge.12 Without an MV challenge study, we lack sufficient data to determine the minimal antibody level required for protection; therefore we cannot judge the efficacy of this vaccine.
We recommend further studies, including infectious challenge to determine the minimal protective antibody titer and a longer follow-up period to ascertain the efficacy and optimal time interval of booster vaccination to maintain that titer. The completion of such studies is necessary before implementing a measles vaccination program utilizing MVac. The most appropriate age at which to vaccinate should also be determined. Young animals may have circulating maternal antibodies. Geriatric NHP may have low antibody titer, given that measles antibody titers tend to wane with age.13,28,57 This situation might be a cause of concern, because what constitutes a protective MV antibody titer in NHP is unknown.52 In addition, the immunosuppressive nature of MV affects an animal's immune response to other antigens. One NHP study found that 20 to 40% of animals (depending on the attenuated measles vaccine used) had intradermal tuberculin skin test values that were below baseline at 14 d after vaccination.52 Medical literature indicates that in humans, anergy or unresponsiveness to tuberculin skin testing can develop relatively slowly and last for as long as 14 d after measles vaccination.8,37,55 These results indicate the immunologic effects of measles immunization with a live attenuated vaccine and suggest that tuberculin skin testing should be deferred temporarily after MV vaccination.
Alternate strategies for inducing immunity to measles might include vaccination for other morbilliviruses, such as canine distemper virus. Adequate attenuation of live canine distemper virus for use in species other than those for which it originally was developed and intended is always a source of concern. In one study, canine distemper virus was inoculated intramuscularly and intracerebrally into susceptible cynomolgous macaques; these animals showed no signs of clinical disease after 2 injections and developed antibodies against distemper virus.50 However, in other studies, cynomolgous macaques and squirrel monkeys were susceptible to experimentally induced (cerebral) infection with canine distemper virus.34,40 In addition, there have been reports of naturally occurring canine distemper infections in NHP, including cases of fever, rashes, thickened footpads, and death.15,45,54,61,62
There are several differences between MVac and other measles vaccines that have been studied in NHP. Attenuvax contains 1000 CCID50 per 0.5 mL, whereas the MV–canine distemper virus vaccine contains a 10-fold higher antigen load per 1.0-mL dose.37 Both vaccines are derived from the Edmonston strain and are propagated in chick embryo fibroblasts.37 The MVac contains the attenuated Edmonston–Zagrreb strain that has been propagated on human diploid cells, and each 0.5-mL dose contains at least 1000 CCID50.51 It is theoretically possible that the MVac vaccine might carry some human antigen inadvertently, especially given that measles is an enveloped virus. The live measles–mumps–rubella virus vaccine (MMR II) vaccine would have the same issue.56 Contamination with human cell antigen might affect an animal's immune response to other antigens, an outcome that could be important for research studies requiring characterization of immune responses. Some SIV experiments have been affected by the sensitization of macaques to human antigens.9
Professionals managing NHP colonies continue to discuss and evaluate the costs and benefits of measles vaccination for maintaining colonies of healthy animals. This discussion includes the determination of methods to reduce the cost of a measles vaccination program yet still provide sufficient protection. The importation of nondomestically produced pharmaceuticals is complicated and time-consuming. It is prudent to identify efficacious and safe alternative products for use in protecting NHP production and research colonies. This study demonstrates the practical efficiency, economic advantages, scientific values, and other benefits of a multicenter collaborative study to evaluate alternative vaccines for measles protection of NHP.
Acknowledgments
Research reported in this publication was supported by the Office of the Director, NIH, under award numbers P51OD011107, P51ODO11104, P51OD010425, P51OD011133, P51OD011106, and P510D011132.
We thank Ann Rosenthal for optimizing the neutralization assays; Sarah Davis and Saverio Capuano for vaccine procurement; Charles River Labs for donation of measles antibody-bead reagents; the colony management staff at each location for administering vaccine, monitoring subjects, and collecting samples; and the staff of the Pathogen Detection Laboratory at the California National Primate Research Center for laboratory testing.
References
- 1.Abee CR, Mansfield K, Tardif SD, Morris T. 2012. Nonhuman primates in biomedical research: biology and management, 2nd ed San Francisco (CA): Academic Press. [Google Scholar]
- 2.Acha PN, Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals: parasitic zoonoses, 3rd ed Washington (DC): Pan American Health Organization. [Google Scholar]
- 3.Albrecht P, Lorenz D, Klutch M, Vickers J, Ennis F. 1980. Fatal measles infection in marmosets pathogenesis and prophylaxis. Infect Immun 27:969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Animal Welfare Act as Amended. 2013. 7 USC §2131–2159.
- 5.Animal Welfare Regulations. 2013. 9 CFR § 3.129.
- 6.Beck SE, Kelly KM, Queen SE, Adams RJ, Zink MC, Tarwater PM, Mankowski JL. 2015. Macaque species susceptibility to simian immunodeficiency virus: increased incidence of SIV central nervous system disease in pigtailed macaques versus rhesus macaques. J Neurovirol 21:148–158. [DOI] [PubMed] [Google Scholar]
- 7.Blake FG, Trask JD. 1921. Studies on measles: II. Symptomatology and pathology in monkeys experimentally infected. J Exp Med 33:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brody JA, Overfield T, Hammes LM. 1964. Depression of the tuberculin reaction by viral vaccines. N Engl J Med 271:1294–1296. [DOI] [PubMed] [Google Scholar]
- 9.Chan WL, Rodgers A, Hancock R, Taffs F, Kitchin P, Farrar G, Liew FY. 1992. Protection in SIV-vaccinated monkeys correlates with antiHLA class I antibody response. J Exp Med 176:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. 1990. Measles antibody: reevaluation of protective titers. J Infect Dis 162:1036–1042. [DOI] [PubMed] [Google Scholar]
- 11.Choi YK, Simon MA, Kim DY, Yoon BI, Kwon SW, Lee KW, Seo IB, Kim DY. 1999. Fatal measles virus infection in Japanese macaques (Macaca fuscata). Vet Pathol 36:594–600. [DOI] [PubMed] [Google Scholar]
- 12.Christe KL, McChesney MB, Spinner A, Rosenthal AN, Allen PC, Valverde CR, Roberts JA, Lerche NW. 2002. Comparative efficacy of a canine distemper–measles and a standard measles vaccine for immunization of rhesus macaques (Macaca mulatta). Comp Med 52:467–472. [PubMed] [Google Scholar]
- 13.Davidkin I, Peltola H, Leinikki P, Valle M. 2000. Duration of rubella immunity induced by 2-dose measles, mumps, and rubella (MMR) vaccination. A 15-year follow-up in Finland. Vaccine 18:3106–3112. [DOI] [PubMed] [Google Scholar]
- 14.de Swart RL. 2009. Measles studies in the macaque model. Curr Top Microbiol Immunol 330:55–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries RD, Ludlow M, Verburgh RJ, van Amerongen G, Yuksel S, Nguyen DT, McQuaid S, Osterhaus ADME, Duprex WP, de Swart RL. 2014. Measles vaccination of nonhuman primates provides partial protection against infection with canine distemper virus. J Virol 88:4423–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.del Valle JR, Devaux P, Hodge G, Wegner NJ, McChesney MB, Cattaneo R. 2007. A vectored measles virus induces hepatitis B surface antigen antibodies while protecting macaques against measles virus challenge. J Virol 81:10597–10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine PE. 1993. Herd immunity: history, theory, practice. Epidemiol Rev 15:265–302. [DOI] [PubMed] [Google Scholar]
- 18.Fox JP. 1983. Herd immunity and measles. Rev Infect Dis 5:463–466. [DOI] [PubMed] [Google Scholar]
- 19.Galvin TA, Muller J, Khan AS. 2000. Effect of different promoters on immune responses elicited by HIV1 gag–env multigenic DNA vaccine in Macaca mulatta and Macaca nemestrina. Vaccine 18:2566–2583. [DOI] [PubMed] [Google Scholar]
- 20.Hall WC, Kovatch RM, Herman PH, Fox JG. 1971. Pathology of measles in rhesus monkeys. Vet Pathol 8:307–319. [DOI] [PubMed] [Google Scholar]
- 21.Haralambieva IH, Ovsyannikova IG, Vierkant RA, Poland GA. 2008. Development of a novel efficient fluorescence-based plaque reduction microneutralization assay for measles virus immunity. Clin Vaccine Immunol 15:1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilleman MR. 2001. Current overview of the pathogenesis and prophylaxis of measles with focus on practical implications. Vaccine 20:651–665. [DOI] [PubMed] [Google Scholar]
- 23.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 24.Jones-Engel L, Engel GA, Schillaci MA, Babo R, Froehlich J. 2001. Detection of antibodies to selected human pathogens among wild and pet macaques (Macaca tonkeana) in Sulawesi, Indonesia. Am J Primatol 54:171–178. [DOI] [PubMed] [Google Scholar]
- 25.Kalter SS, Heberling RL, Cooke AW, Barry JD, Tian PY, Northam WJ. 1997. Viral infections of nonhuman primates. Lab Anim Sci 47:461–467. [PubMed] [Google Scholar]
- 26.Khan IH, Mendoza S, Yee J, Deane M, Venkateswaran K, Zhou SS, Barry PA, Lerche NW, Luciw PA. 2006. Simultaneous detection of antibodies to 6 nonhuman-primate viruses by multiplex microbead immunoassay. Clin Vaccine Immunol 13:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klatt NR, Canary LA, Vanderford TH, Vinton CL, Engram JC, Dunham RM, Cronise HE, Swerczek JM, Lafont BA, Picker LJ, Silvestri G, Brenchley JM. 2011. Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J Virol 86:1203–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremer JR, Schneider F, Muller CP. 2005. Waning antibodies in measles and rubella vaccinees—a longitudinal study. Vaccine 24:2594–2601. [DOI] [PubMed] [Google Scholar]
- 29.Lafont BA, Buckler-White A, Plishka R, Buckler C, Martin MA. 2003. Characterization of pigtailed macaque classical MHC class I genes: implications for MHC evolution and antigen presentation in macaques. J Immunol 171:875–885. [DOI] [PubMed] [Google Scholar]
- 30.Levy BM, Mirkovic RR. 1971. An epizootic of measles in a marmoset colony. Lab Anim Sci 21:33–39. [PubMed] [Google Scholar]
- 31.Lorenz D, Albrecht P. 1980. Susceptibility of tamarins (Saguinus) to measles virus. Lab Anim Sci 30:661–665. [PubMed] [Google Scholar]
- 32.Lowenstine LJ. 1993. Measles virus infection, nonhuman primates, p 108–118. In: Jones TC, Mohr U, Hunt RD. Nonhuman Primates I. New York (NY): Springer–Verlag. [Google Scholar]
- 33.MacArthur JA, Mann P, Oreffo V, Scott G. 2009. Measles in monkeys: an epidemiological study. J Hyg (Lond) 83:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsubara Y, Morikawa Y, Yoshikawa Y, Nagashima K, Yamanouchi K. 1985. Encephalitis induced in nonhuman primates by canine distemper virus adapted to human neural cells. Jpn J Exp Med 55:99–108. [PubMed] [Google Scholar]
- 35.McChesney MB, Fujinami RS, Lerche NW, Marx PA, Oldstone MB. 1989. Virus-induced immunosuppression: infection of peripheral blood mononuclear cells and suppression of immunoglobulin synthesis during natural measles virus infection of rhesus monkeys. J Infect Dis 159:757–760. [DOI] [PubMed] [Google Scholar]
- 36.McChesney MB, Miller CJ, Rota PA, Zhu YD, Antipa L, Lerche NW, Ahmed R, Bellini WJ. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 233:74–84. [DOI] [PubMed] [Google Scholar]
- 37.Merck. 2006. Attenuvax (measles virus vaccine live). Whitehouse Station (NJ): Merck Sharp and Dohme [Google Scholar]
- 38.Meyer HM, Jr, Brooks BE, Douglas RD, Rogers NG. 1962. Ecology of measles in monkeys. Am J Dis Child 103:307–313. [DOI] [PubMed] [Google Scholar]
- 39.Montrey RD, Huxsoll DL, Hildebrandt PK, Booth BW, Arimbalam S. 1980. An epizootic of measles in captive silvered leaf-monkeys (Presbytis cristatus) in Malaysia. Lab Anim Sci 30:694–697. [PubMed] [Google Scholar]
- 40.Nagata T, Ochikubo F, Yoshikawa Y, Yamanouchi K. 1990. Encephalitis induced by a canine distemper virus in squirrel monkeys. J Med Primatol 19:137–149. [PubMed] [Google Scholar]
- 41.Nahmais AJ, O'Reilly RJ. 1982. Immunology of human infection: part II. Viruses and parasites. In: Good RA, Day SB. Comprehensive immunology. New York (NY): Plenum Publishing. [Google Scholar]
- 42.Osterhaus AD, de Vries P, van Binnendijk RS. 1994. Measles vaccines: novel generations and new strategies. J Infect Dis 170:Suppl 1S42–S55. [DOI] [PubMed] [Google Scholar]
- 43.Polacino P, Larsen K, Galmin L, Suschak J, Kraft Z, Stamatatos L, Anderson D, Barnett SW, Pal R, Bost K, Bandivdekar AH, Miller CJ, Hu SL. 2008. Differential pathogenicity of SHIVSF162 P4 infection in pigtailed and rhesus macaques. J Med Primatol 37:Suppl 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potkay S, Ganaway JR, Rogers NG, Kinard R. 1966. An epizootic of measles in a colony of rhesus monkeys (Macaca mulatta). Am J Vet Res 27:331–334. [PubMed] [Google Scholar]
- 45.Qiu W, Zheng Y, Zhang S, Fan Q, Liu H, Zhang F, Wang W, Liao G, Hu R. 2011. Canine distemper outbreak in rhesus monkeys, China. Emerg Infect Dis 17:1541–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reed LJ, Muench H. 1938. A simple method of estimating 50% endpoints. Am J Hygiene 27:493–497. [Google Scholar]
- 47.Remfry J. 1976. A measles epizootic with 5 deaths in newly imported rhesus monkeys (Macaca mulatta). Lab Anim 10:49–57. [DOI] [PubMed] [Google Scholar]
- 48.Renne RA, McLaughlin R, Jenson AB. 1973. Measles virus-associated endometritis, cervicitis, and abortion in a rhesus monkey. J Am Vet Med Assoc 163:639–641. [PubMed] [Google Scholar]
- 49.Roberts J, Lerche N, Anderson J, Markovits J, Maul D. [Internet]. 1988. Epizootic measles at the CRPRC. Abstracts of scientific papers. Thirty-ninth annual meeting, American Association for Laboratory Animal Science; Detroit, MI, October 9–13, 1988. [Cited 30 Mar 2015]. Available at: http://www.unboundmedicine.com/medline/citation/3184866/. [PubMed] [Google Scholar]
- 50.Schwarz AJ, Boyer PA, Zirbel LW, York CJ. 1960. Experimental vaccination against measles. I. Tests of live measles and distemper vaccine in monkeys and 2 human volunteers under laboratory conditions. J Am Med Asoc 173:861–867. [DOI] [PubMed] [Google Scholar]
- 51.Serum Institute of India. 2014. Measles vaccine (live) IP MVac (freeze-dried). Hadapsar (India): Serum Institute of India. [Google Scholar]
- 52.Staley EC, Southers JL, Thoen CO, Easley SP. 1995. Evaluation of tuberculin testing and measles prophylaxis procedures used in rhesus macaque quarantine–conditioning protocols. Lab Anim Sci 45:125–130. [PubMed] [Google Scholar]
- 53.Steele MD, Giddens WE, Jr, Valerio M, Sumi SM, Stetzer ER. 1982. Spontaneous paramyxoviral encephalitis in nonhuman primates (Macaca mulatta and M. nemestrina). Vet Pathol 19:132–139. [DOI] [PubMed] [Google Scholar]
- 54.Sun Z, Li A, Ye H, Shi Y, Hu Z, Zeng L. 2010. Natural infection with canine distemper virus in hand-feeding rhesus monkeys in China. Vet Microbiol 141:374–378. [DOI] [PubMed] [Google Scholar]
- 55.Tamashiro VG, Perez HH, Griffin DE. 1987. Prospective study of the magnitude and duration of changes in tuberculin reactivity during uncomplicated and complicated measles. Pediatr Infect Dis J 6:451–453. [DOI] [PubMed] [Google Scholar]
- 56.U.S. Department of Health and Human Services [Internet]. 2014. Measles, mumps, and rubella virus vaccine, live. [Cited 30 Mar 2015]. Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm094050.htm.
- 57.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. 2008. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 46:1078–1084. [DOI] [PubMed] [Google Scholar]
- 58.Welshman MD. 1989. Measles in the cynomolgus monkey (Macaca fascicularis). Vet Rec 124:184–186. [DOI] [PubMed] [Google Scholar]
- 59.Willy ME, Woodward RA, Thornton VB, Wolff AV, Flynn BM, Heath JL, Villamarzo YS, Smith S, Bellini WJ, Rota PA. 1999. Management of a measles outbreak among Old World nonhuman primates. Lab Anim Sci 49:42–48. [PubMed] [Google Scholar]
- 60.Wintermeyer L, Myers MG. 1979. Measles in a partially immunized community. Am J Public Health 69:923–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamanouchi K, Yoshikawa Y, Sato TA, Katow S, Kobune F, Kobune K, Uchida N, Shishido A. 1977. Encephalomyelitis induced by canine distemper virus in nonhuman primates. Jpn J Med Sci Biol 30:241–257. [DOI] [PubMed] [Google Scholar]
- 62.Yoshikawa Y, Ochikubo F, Matsubara Y, Tsuruoka H, Ishii M, Shirota K, Nomura Y, Sugiyama M, Yamanouchi K. 1989. Natural infection with canine distemper virus in a Japanese monkey (Macaca fuscata). Vet Microbiol 20:193–205. [DOI] [PubMed] [Google Scholar]
- 63.Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K, Centers for Disease Control and Prevention (CDC). 2015. Measles outbreak—California, December 2014–February 2015. MMWR Morb Mortal Wkly Rep 64:153–154. [PMC free article] [PubMed] [Google Scholar]