Abstract
Research increasingly suggests that subjective cognitive decline (SCD) in older adults, in the absence of objective cognitive dysfunction or depression, may be a harbinger of non-normative cognitive decline and eventual progression to dementia. Little is known, however, about the key features of self-report measures currently used to assess SCD. The Subjective Cognitive Decline Initiative (SCD-I) Working Group is an international consortium established to develop a conceptual framework and research criteria for SCD (Jessen et al., 2014, Alzheimers Dement 10, 844–852). In the current study we systematically compared cognitive self-report items used by 19 SCD-I Working Group studies, representing 8 countries and 5 languages. We identified 34 self-report measures comprising 640 cognitive self-report items. There was little overlap among measures—approximately 75% of measures were used by only one study. Wide variation existed in response options and item content. Items pertaining to the memory domain predominated, accounting for about 60% of items surveyed, followed by executive function and attention, with 16% and 11% of the items, respectively. Items relating to memory for the names of people and the placement of common objects were represented on the greatest percentage of measures (56% each). Working group members reported that instrument selection decisions were often based on practical considerations beyond the study of SCD specifically, such as availability and brevity of measures. Results document the heterogeneity of approaches across studies to the emerging construct of SCD. We offer preliminary recommendations for instrument selection and future research directions including identifying items and measure formats associated with important clinical outcomes.
Keywords: Cognition, cognitive complaints, dementia, early detection, memory complaints, subjective cognition, mild cognitive impairment, preclinical Alzheimer’s disease, questionnaire, subjective cognitive impairment, subjective memory complaints
INTRODUCTION
Subjective cognitive decline (SCD) in older adults is increasingly recognized as a potential indicator of non-normative cognitive decline and eventual progression to dementia [1–5]. Moreover, there is emerging evidence of associations of SCD with Alzheimer’s disease (AD) biomarkers and neuroimaging markers [6–16], such as gray matter volume loss [6–8], cerebral hypometabolism [10], and amyloid deposition [12–14], in the absence of objective cognitive dysfunction or depression. Given research supporting SCD as a risk factor for AD in some individuals, the National Institute on Aging–Alzheimer’s Association preclinical AD working group has included SCD as a feature, highlighting its importance in disease detection and prevention [17]. In addition to preclinical AD, SCD is relevant to other conditions affecting older adults, for example: depression and anxiety [18–19], physical health problems and common chronic diseases [18, 20], and non-AD dementias [1, 21].
By definition, SCD is contingent on self-report of cognition, and this assessment approach is associated with certain practical advantages such as brevity, ease of administration, and low cost [22]. However, the field currently lacks a single accepted approach to the assessment of SCD, including the fundamental nature of the questions (present status versus decline), cognitive domains of greatest interest, and optimal items for each domain [22, 23]. Also, since the prevalence of cognitive complaints in the general adult population is relatively high [24] and can be affected by factors such as mood and personality [25], it becomes difficult to ascertain which complaints indicate underlying AD versus other neurodegenerative pathologies or psychological conditions. This underscores the need for a high degree of rigor in deriving self-report items with concurrent validity for the construct of SCD and predictive validity for future cognitive and clinical outcomes. The definition of the external validators for SCD is itself the subject of discussion [22, 23].
In contrast to extensive efforts to refine and standardize AD biomarkers [26–28] and criteria for conditions such as mild cognitive impairment (MCI) [29–33], research on the standardization and quantification of SCD is relatively limited. Many approaches are used to define and quantify SCD and related constructs, such as cognitive complaints, memory complaints, subjective memory impairment, and subjective cognitive impairment, with no agreed-upon standards [22, 23]. The variability in definition and large number of operational procedures for assessing SCD makes it difficult to compare findings and refine the construct for use in clinical and research settings.
A review of both the SCD and broader cognitive aging literature suggests heterogeneity in important aspects of cognitive self-report measures including mode of administration [34–41], number of items [42–53], and response option types [38, 54–59]. Other dimensions on which self-report measures vary are item content and complexity including whether items relate to memory exclusively [57, 60–62] or include additional cognitive domains [50, 63, 64] or non-cognitive items [51, 55, 65], whether items tap current cognitive ability or disability/impairment [65–67] versus intraindividual change [57, 68], and whether items inquire about general versus specific aspects of cognition. In terms of the origin of instruments, while some studies use complete published questionnaires [54–60, 65], others use groups of items from existing measures [69–71] or develop new items for a specific study [12, 72–77]. Finally, while selecting appropriate measures stands among the most critical decisions made in clinical research contexts, the SCD literature in many cases does not thoroughly discuss selection decisions.
An international consortium known as the Subjective Cognitive Decline Initiative (SCD-I) Working Group recently was established to develop common terminology and basic criteria for SCD to enable joint research efforts across studies and settings [23]. The current study describes the characteristics of self-report measures employed by studies represented by SCD-I investigators. Specifically, we focus on key structural and content validity issues and investigate content overlap among the questionnaire items as well as questionnaire selection decisions. Our goals are to identify areas of variability and consistency, raise issues related to the adequacy of assessment approaches, and ultimately develop improved tools for diverse research and clinical settings.
MATERIALS AND METHODS
The SCD-I
The SCD-I was launched in October 2012 and formed a working group that included AD researchers with a specific interest in SCD. Members were identified by a systematic literature search in addition to targeted inclusion of leading researchers in the field of preclinical AD assessment. The working group’s first project established a conceptual framework and research criteria for SCD [23]. Members of the SCD-I were subsequently invited to participate in the current item analysis project.
Questionnaire characteristics and coding procedures
Based on our review of the SCD literature, considerations of relevance to the field, and feasibility, we investigate the following ten issues through descriptive and content analysis of SCD-I Working Group measures: (1) number of self-report measures used by participating working group studies; (2) origin of instruments and mode of administration; (3) format and range of response options; (4) timeframe referenced by the items; (5) prevalence of items related to specific cognitive domains; (6) prevalence of items tapping cognitive ability/disability-impairment (and referents of frequency, severity, and impact) versus change or decline (and referents of temporal and atemporal); (7) item specificity and complexity; (8) overlap in measures used by working groups; (9) most commonly occurring items; and (10) considerations driving measure selection and the grouping of questionnaires within a given study. Coding of cognitive self-report items occurred prior to data analysis for research issues 1, 5, 6, 7, 9, and 10. For all coding procedures, working group member LAR carried out the initial coding. Two additional expert raters (working group members CMS and SAMS) subsequently identified items that they considered to be miscoded. Items in dispute were then reviewed and discussed until consensus was reached, occasionally after discussing disputed items with working group member PKC.
Selection of cognitive items
SCD-I Working Group members sent electronic copies of their subjective self-report measures to author LAR. For studies conducted in a language other than English, working group members sent a translated version of their measures. The raters then reviewed all measures to determine whether they merited inclusion in the current study by virtue of containing items that assess the self-perception of memory and other cognitive abilities. Notably, while the raters recognized that mood, anxiety, health complaints, and other variables are relevant to SCD and often included in cognitive self-report measures, they are not the focus of the current study.
Categorization by cognitive domain
The raters next classified items by cognitive domain. They first developed a list of cognitive domain categories under which to subsume all items. The list was revised following feedback from additional working group members and included the following categories: (1) memory (short-term/long-term/episodic/semantic/prospective memory and learning new information); (2) attention/working memory/processing speed (also includes basic attention, sustained attention, focused attention, concentration, divided attention, and alertness); (3) language (expressive and receptive language, word finding, reading, and spelling); (4) executive function (organizing, planning, switching, initiating, multi-tasking, reasoning, judgment, problem solving, decision-making, handling emergencies, impulsivity and self-regulation, clarity of mind, motor programming, handling money, and self-awareness of problems); (5) basic calculation and arithmetic tasks; (6) orientation to person, time, place, or situation; (7) general cognitive ability (memory and other thinking abilities grouped together in a single item); and (8) visuospatial skills (visuoperception, route finding, and directional orientation). The raters assigned each item to a single category using the widely-recognized neuropsychological framework suggested by Lezak and colleagues [78]. Though many items arguably could be categorized into more than one cognitive domain, the raters assigned a primary category for each item.
Categorization by ability/disability and change
The raters considered each cognitive item (both stem and response options) to determine whether the item inquired about a participant’s ability to perform a given cognitive task or whether the item inquired about change (improvement or decline). For items that assess ability versus disability/impairment, items could portray the intensity or severity of problems, their frequency, or impact on everyday life. The raters therefore coded for referents of severity (degree of seriousness), frequency (rate of occurrence), and impact (effect on real-world outcomes). For items that assess change, items could portray temporal change (relative to a general or specific timeframe) or atemporal change (relative to peers, a previous state, or rate of change). The raters therefore coded for both temporal and atemporal referents.
Categorization by item specificity and complexity
To address issues of item specificity and complexity, the raters coded memory items according to generality versus specificity and whether they were double-barreled (i.e., involved multiple sub-questions within the one item). A general memory item was defined as a cognitive self-report item that inquires about memory functioning without specifying a particular memory activity, problem, or task. The raters did not extend the coding of general versus specific items to cognitive domains other than memory because of the complexity of establishing coding criteria for non-memory domains. A double-barreled cognitive item was defined as an item that asks respondents to rate or respond to two or more different issues while allowing for only one response.
Most commonly occurring items
After close review of all items across the 34 questionnaires, the raters identified items with overlapping content (e.g., memory for recent events, memory for names, losing one’s train of thought) despite nonidentical item wording (as there were no items with verbatim wording for both item stems and response options). Subsequently, the raters generated a list of the 10 most common item themes.
Selection decisions
To understand the decision-making behind each research group’s choice of measures or development of new measures, the raters distributed a brief survey to working group members. The survey asked the following:
(1) for working groups utilizing existing cognitive self-report measures, respondents were asked to identify the “primary” self-report measure and state why the measure was initially selected for use, endorsing all responses that applied from among various options; (2) for working groups that developed their own cognitive self-report measures, respondents were asked to state why they chose to develop a new measure (free response); and (3) for working group studies that administered more than one cognitive self-report measure, respondents were asked to describe the rationale for including the specific number of measures in the study (free response).
RESULTS
Table 1 lists the names, institutional affiliations, and other key features of the 19 working group studies including country, research environment, approximate number of study participants, and number of SCD measures utilized. The United States houses the greatest number of working group studies (n = 7), followed by Germany (n = 3), Australia (n = 2), France (n = 2), and Spain (n = 2). Canada and the Netherlands house one study each, and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) has multiple international locations including the United States, Canada, Australia, Germany, and the United Kingdom. There was substantial variability in terms of research environment, with representation of memory clinics (n = 4), volunteer samples (n = 3), community-based samples (n = 3), population-based samples (n = 2), a general practice registry (n = 1), and mixed sampling approaches (n = 6).
Table 1.
Participating Subjective Cognitive Decline Initiative (SCD-I) working group studies
| Study name | Institutional affiliation | Country | Research environment | Approximate number of participants | Number of measures |
|---|---|---|---|---|---|
| AgeCoDe Study | University of Bonn and 5 other German universities | Germany | General practice registry-based | 3,327 | 2 |
| Alzheimer’s Disease Center Clinical Core and Center for Brain Health | New York University School of Medicine | United States | Volunteer | 525 | 1 |
| Alzheimer’s Disease Neuroimaging Initiative (ADNI) | Over 100 international affiliates: adcs.org/Research/ClinicalSite.aspx | United States, Canada, Australia, Germany, United Kingdom | Volunteer and memory clinic | 1,204 | 1 |
| Amsterdam Dementia Cohort | VU University Medical Center | Netherlands | Memory clinic | 1,244 | 2 |
| Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing (AIBL) | University of Melbourne | Australia | Volunteer and physician referred | 851 | 2 |
| Barcelona Group | IDIBAPS, Hospital Clinic, Barcelona | Spain | Volunteer and memory clinic | 400 | 1 |
| Bonn Memory Clinic | University of Bonn and the German Center for Neurodegenerative Diseases, Bonn | Germany | Memory clinic | 120 | 1 |
| Dartmouth-Indiana Longitudinal Cohort | Dartmouth Medical School, Indiana University School of Medicine | United States | Volunteer and physician referred | 269 | 8* |
| Einstein Aging Study | Albert Einstein College of Medicine | United States | Community-based | 2,500 | 7** |
| Harvard Aging Brain Study | Harvard Medical School, Brigham and Women’s Hospital and Massachusetts General Hospital | United States | Volunteer | 280 | 3 |
| IMAP Caen Group | Inserm, University and Hospital of Caen | France | Memory clinic | 86 | 1 |
| Leipzig Longitudinal Study of the Aged (LEILA 75+) | University of Leipzig | Germany | Population-based | 1,265 | 1 |
| Mayo Clinic Study of Aging (MCSA) | Mayo Clinic | United States | Population-based | 4,500 | 2 |
| Memory Clinic -Fundació ACE | Fundació ACE. Barcelona Alzheimer Treatment and Research Center | Spain | Community-based | 660 | 1 |
| PreAl Study | Hôpital de la Salpêtrière, AP-HP | France | Memory clinic | 180 | 1 |
| Sydney Memory and Ageing Study | University of New South Wales | Australia | Community-based | 873 | 3 |
| University of Pittsburgh Study/Monongahela-Youghiogheny Health Aging Team (MYHAT) | University of Pittsburgh | United States | Clinic-community volunteer sample and population-based | 2,100 | 3 |
| Vanderbilt Memory and Alzheimer’s Center (VMAC) | Vanderbilt University School of Medicine | United States | Memory clinic, physician referred, and community-based | 158 | 1 |
| Victoria Subjective Cognitive Decline Study (SCDS) | University of Victoria | Canada | Volunteer | 42 | 5 |
A subset of participants received all 8 questionnaires. The remaining participants received 5 questionnaires.
A subset of participants received all 7 questionnaires. The remaining participants received 3 questionnaires.
Below we present results related to the 10 instrument dimensions outlined in the Methods section.
(1) Number of self-report measures and items used
Table 2 presents the names and key structural and administrative features of the 34 self-report measures used by the 19 studies. The number of measures used per study ranged from 1 to 8 with a mean of 2.4 (SD = 2.1) and a mode of 1. While some measures included cognitive items exclusively, other measures primarily focused on mood or activities of daily living but included one or more cognitive items highly relevant to the current analysis. There were a total of 922 items across all measures. Of the 34 measures, 61.8% (n = 21) contained items that did not involve specific cognitive complaints. This included items related to the use of memory/other cognitive strategies, emotional and psychological functioning, reactions to cognitive changes, personality and interpersonal functioning, physical and motor functioning, vocational and social tasks, general health, fatigue or sleep behaviors, apraxia, basic activities of daily living, general health, general beliefs about memory and aging, and items that were not self-report. These non-SCD items were identified with <4% discrepancies between raters, eliminated for the purposes of the current study, and not considered further. All subsequent results relate to the subset of 640 cognitive items that tap the subjective perception of memory and other cognitive abilities, representing 69.4% of the total items across the 34 questionnaires. The number of cognitive items per questionnaire ranged from 1 to 56 with a mean of 18.8 (SD = 15.5). Table 2 shows the percentage of cognitive self-report items within each questionnaire.
Table 2.
Key structural and administrative features of self-report measures used by Subjective Cognitive Decline Initiative (SCD-I) Working Group studies
| Questionnaire/item subset abbreviation | Language | 5Administration mode | Total number of items | Number (percentage) of items relevant to analysis* | Response type and options | Timeframe |
|---|---|---|---|---|---|---|
| ADL | English | 5Self-administered paper questionnaire | 63 | 33 (52.4%) | 7 pts. “Above average ability” to “Severe disability”, plus “Not applicable” | Current |
| ADL Abbrev | English | 5Self-administered paper questionnaire | 50 | 45 (90.0%) | 7 pts. “Much better” to “Much worse” | Compared to 5 years ago |
| AgeCoDe MQ | German | 5Examiner- administered in person | 2 | 2 (100%) | Yes/No/Don’t know; 3 pts. “No” to “Yes, that worries me seriously”, plus “Don’t know” and “Not applicable” | Unspecified and current |
| AIBL Screen | English | 5Examiner- administered by telephone | 1 | 1 (100%) | Yes/No | Current |
| Blessed | English | 5Self-administered paper questionnaire | 6 | 5 (83.3%) | Yes/No/Don’t know; 4 pts. “Better than when I was younger” to “Definitely worse than when I was younger” | Compared to when “younger” |
| BRIEF-A | English | 5Self-administered paper questionnaire | 75 | 36 (48.0%) | 3 pts. “Never” to “Often” | Past month |
| CAPM-C | English | 5Self-administered paper questionnaire | 15 | 13 (86.7%) | 4 pts. “Strongly disagree” to “Strongly agree” | Current |
| CCI | English | 5Self-administered paper questionnaire | 20 | 20 (100%) | 5 pts. “No change” to “Much worse” | Compared to 5 years ago |
| CDS-Q | French | 5Self-administered paper questionnaire | 39 | 33 (84.6%) | 5 pts. “Never” to “Very often” | Current |
| CERAD-Self | English | 5Self-administered paper questionnaire | 36 | 22 (61.1%) | Yes/No/Don’t know; 4 pts. “Less than six months ago” to “More than two years ago”; 3 pts. “Very gradually” to “Suddenly”, plus “Other”; 3 pts. “Steadily worsened” to “Got worse then leveled off”, plus “Other” and “Don’t know” | Current |
| CFQ | English | 5Self-administered paper questionnaire | 25 | 17 (68.0%) | 5 pts. “Never” to “Very often” | Past 6 months |
| ECog Self | English, German, French | 5Self-administered paper questionnaire | 40 | 40 (100%) | Yes/No; 4 pts. “Better or no change” to “Consistently much worse”, plus “Don’t know” | Compared to 10 years ago |
| Einstein HSA | English | 5Self-administered paper questionnaire | 20 | 6 (30.0%) | Yes/No/Don’t know; 4 pts. “Frequently” to “Never”; 3 pts. “More often” to “About the same”; Free response (percent) | Unspecified; Past year; Compared to 1 and 10 years ago |
| GDS Long | English | 5Self-administered paper questionnaire | 30 | 4 (13.3%) | Yes/No | Past week |
| GDS Short | English, Dutch | 5Self-administered paper questionnaire | 15 | 1 (6.7%) | Yes/No | Past week |
| IQCODE Short | English | 5Self-administered paper questionnaire | 16 | 15 (93.8%) | 5 pts. “Much improved” to “Much worse” | Compared to 10 years ago |
| LEILA 75+ Questions | German | 5Examiner- administered in person | 4 | 4 (100%) | 4 pts. “No problems” to “Always”, plus “Don’t know”; 4 pts. “Not at all” to “Highly”, plus “Don’t know” and “Not applicable”; Free response (age), plus “Not applicable” | Current |
| MAC-Q (a) | English | 5Self-administered paper questionnaire | 6** | 6 (100%) | 5 pts. “Much better now” to “Much poorer now” | Compared to high school or college |
| MAC-Q (b) | English | 5Self-administered paper questionnaire | 6 | 6 (100%) | 5 pts. “Much better now” to “Much poorer now” | Compared to 5 years ago |
| MATS | English | 5Examiner- administered by telephone | 13 | 13 (100%) | Yes/No/Don’t know; The Same/Worse/Don’t know; Free response (age of onset) | Current |
| MFE-30 | Spanish | 5Self-administered paper questionnaire | 30 | 29 (96.7%) | 5 pts. “Never or almost never” to “Always or almost always” | Current |
| MFQ*** | English | 5Self-administered paper questionnaire | 63 or 64 | 49 (77.8%) or 50 (78.1%) | 7 pts. “Major Problems” to “No problems”; 7 pts. “Always” to “Never”; 7 pts. “Very poor” to “Very well”; 7 pts. “Very serious” to “Not serious”; 7 pts. “Much worse” to “Much better” | Current; Compared to 1, 5, 10, and 20 years ago; Compared to age 18 |
| MIA | English | 5Self-administered paper questionnaire | 108 | 35 (32.4%) | 5 pts. “Strongly disagree” to “Strongly agree” | Current |
| MMQ | German | 5Self-administered paper questionnaire | 57 | 32 (56.1%) | 5 pts. “Strongly agree” to “Strongly disagree”; 5 pts. “Always” to “Never” | Past 2 weeks |
| SCCS | English | 5Self-administered paper questionnaire | 30 | 27 (90.0%) | Yes/No; 4 pts. “Excellent” to “poor”; 2 pts. “Suddenly/Gradually” and “Other”; 3 pts. “Better” to “The Same”, plus “Never performed this activity”; Free response (age) | Multiple† |
| SCD-SID | English | 5Examiner- administered by telephone | 1 | 1 (100%) | Yes/No | Current |
| SCD-Q: Part 1 MyCog | Spanish | 5Self-administered paper questionnaire | 27 | 26 (96.3%) | Yes/No | Current and compared to 2 years ago |
| SCF | Dutch | 5Self-administered paper questionnaire | 4 | 2 (50.0%) | 7 pts. “Very strong improvement” to “Very sharp decline” | Past year |
| SCQ | French | 5Self-administered paper questionnaire | 14 | 14 (100%) | 4 pts. “The same” to “Much worse”; 4 pts. “No” to “Most of the time”; 4 pts. “No” to “Definitely”; 4 pts. “No” to “Very much” | Past year |
| SMDS | German | 5Examiner- administered in person | 4 | 4 (100%) | 3 pts. “No” to “Yes, a lot worse” | Compared to 10 years ago |
| Squire Memory Self-Rating | English | 5Self-administered paper questionnaire | 18 | 18 (100%) | 9 pts. “Worse than ever before” to “Better than ever before” | Compared to “ever before” |
| STDA | English | 5Self-administered paper questionnaire | 7 | 7 (100%) | Yes/No | Current |
| Sydney– SCQ Wave 1 | English | 5Self-administered paper questionnaire | 18 | 16 (88.9%) | Yes/No/Don’t know; 4 pts. “No, less difficulty” to “Yes, a lot more difficulty”, plus “Not applicable” | Unspecified and compared to 5 years ago |
| VMAC Cognitive Complaint Questionnaire | English | 5Self-administered paper questionnaire | 57 | 56 (98.2%) | Yes/No/Prefer not to answer; 3 pts. “Never” to “Always”; 3 pts. “No problems” to “Major problems” | Multiple‡ |
Items relevant for the current analysis relate to the subjective perception of one’s cognition (see text for details about eliminated items).
The NYU version of the MAC-Q contains a different item stem for item 4 (bringing the total number of overall items to one more than the totals displayed in columns 3 and 4). Also, the MAC-Q was separated into versions a and b because of the different timeframe references.
The University of Pittsburgh’s version of the questionnaire contains one additional item.
Timeframe includes current, compared to 1 year ago, and compared to how “you used to be”, and some items have unspecified time frames.
Timeframe includes current, compared to 1 year ago, compared to 2 years ago, compared to 5 years ago, compared to 10 years ago, and compared to 20 years ago, and some items have unspecified time frames.
AIBL Screen, Melbourne Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing (AIBL) Memory Screen Question [99]; ADL, Activities of Daily Living Rating Scale Self-Version [100]; ADL Abbrev, Activities of Daily Living Rating Scale Self-Version, Abbreviated (Saykin, unpublished); AgeCoDe MQ, AgeCoDe Study Memory Questions [101]; Blessed, Blessed Memory Test [102]; BRIEF-A, Behavioral Rating Inventory of Executive Function-Adult Version [103]; CAPM-C, Comprehensive Assessment of Prospective Memory-Section C [66]; CCI, Cognitive Change Index [7]; CDS-Q, Cognitive Difficulties Scale [58]; CERAD-Self, Consortium to Establish a Registry for Alzheimer’s Disease-Self Version [104]; CFQ, Cognitive Failures Questionnaire [55]; ECog Self, Everyday Cognition-Subject/Self-Report [68]; GDS Long, Geriatric Depression Scale-Long Version [105]; GDS Short, Geriatric Depression Scale-Short Version [106]; HSA, Albert Einstein Health Self-Assessment [107]; IQCODE Short, Short Form of the Informant Questionnaire on Cognitive Decline in the Elderly [108]; LEILA 75 + Questions, Leipzig Longitudinal Study of the Aged Questionnaire [109–110]; MAC-Q(a), Memory Complaint Questionnaire [57]; MAC-Q(b), Memory Complaint Questionnaire [57]; MATS, Memory and Aging Telephone Screen [67]; MFE-30, Memory Failures Everyday [111]; MFQ, Memory Functioning Questionnaire [60]; MIA, Metamemory in Adulthood Questionnaire [65]; MMQ, Multifactorial Memory Questionnaire [54]; SCCS, Subjective Cognitive Complaint Scale-(also referred to as the Subjective Memory Scale) [74,112]; SCD-SID, Subjective Cognitive Decline Self-Identification Item [47]; SCD-Q: Part 1 MyCog, Subjective Cognitive Decline Questionnaire: Part 1 My Cognition [50]; SCF, Subjective Cognitive Functioning [113]; SCQ, Self-Evaluation of Cognition Questionnaire [114]; SMDS, Subjective Memory Decline Scale [115]; Squire Memory Self-Rating Questionnaire [116]; STDA, Adapted from Structured Telephone Dementia Assessment [13, 38]; Sydney-SCQ, Sydney Subjective Complaint Questions, Wave 1 [92]; VMAC Cognitive Complaint Questionnaire, Vanderbilt Memory and Alzheimer’s Center Cognitive Complaint Questionnaire [40].
(2) Origin of instruments and mode of administration
With respect to the origin of their instruments, working group studies utilized existing published questionnaires and instruments developed specifically for their research studies with equal frequency (15 each, accounting for 88% of all measures). The remaining 12% of measures (n = 4) comprised subsets of items from lengthier published measures. The vast majority of measures (82.4%, n = 28) were self-administered via paper questionnaire. The remaining measures were examiner-administered by telephone (8.8%, n = 3) or in person (8.8%, n = 3); in both cases, examiners read the questions aloud to participants and recorded responses.
(3) Format and range of response options
There was considerable variability in response options with 21 measures (61.8%) having a single type of response option and the remaining 13 having between 2 to 5 different option types within a single measure (see Table 2). Traditional Likert and rank-ordered/categorical item scales were the most common, appearing on 27 (79.4%) measures. Dichotomous (yes/no) response options were also common, with 8 (23.5%) measures employing this format. An additional 7 (20.6%) measures used response options with yes/no/don’t know or yes/no/prefer not to answer formats. Four measures (11.8%) used free response options, which were primarily for reporting the age of onset of cognitive symptoms.
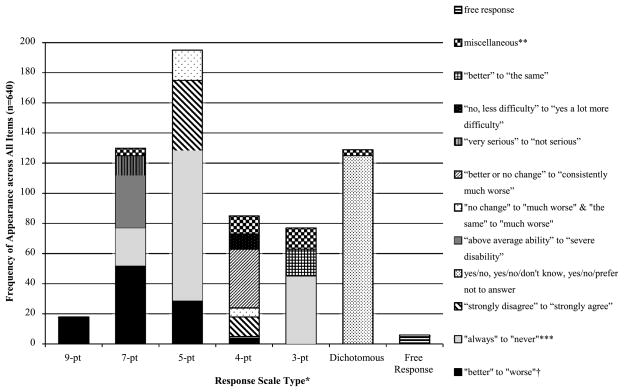
Figure 1 presents information related to response options in terms of the scaling methods and item response content. Five- and 7-point scales were used most often, accounting for 30.5% (n = 195) and 20.3% (n = 130) of items, respectively. We categorized response options into 12 types. Scales from “always” to “never” (or similar) were used most often, comprising 26.6% (n = 170) of all items in the form of 7-point, 5-point, 4-point, and 3-point scales. Other commonly used response options were yes/no, yes/no/don’t know, and yes/no/prefer not to answer (19.5%, n = 125); “better” to “worse” (or similar) in the form of 9-point, 7-point, 5-point, and 4-point scales (15.6%, n = 100); and “strongly disagree” to “strongly agree” in the form of 5-point and 4-point scales (9.4%, n = 60). All content categories are represented in Fig. 1.
Fig. 1.
Summary of response types and options.
Notes: *14 out of 34 questionnaires include multiple response types. **Includes: <10 each of “very poor” to “very well”; “major problems” to “no problems”; “no” to “most of the time”; “no” to “definitely”; “no” to “yes, a lot worse”; “no problems” to “always”; and “more often” to “about the same”. Includes one each of “no” to “yes, that worries me seriously”; “less than 6 months ago” to “more than two years ago”; “very gradually” to “suddenly”; “steadily worsened” to “got worse and then leveled off”; “not at all” to “highly”; “excellent” to “poor”; “no” to “very much”; agree/disagree; good/poor; same/worse; and suddenly/gradually”. ***Includes: “always” to “never”; “frequently” to “never”; “always or almost always” to “never or almost never”; “very often” to “never”, and “often” to “never”. †Includes: “better than ever before” to “worse than ever before”; “much better” to “much worse”; “very strong improvement” to “very sharp decline”; “ much improved” to “much worse”; “much better now” to “much poorer now”; and “better than when I was younger” to “definitely worse than when I was younger”.
(4) Timeframe
As shown in Table 2, the most common response timeframe referred to by the cognitive items was the recent past with 47.1% (n = 16) of measures asking participants to reflect about current functioning or functioning over the past week, 2 weeks, or 6 months. An additional 5.9% (n = 2) of measures referenced the past year. Other measures required comparisons to 5 years ago (n = 3), 10 years ago (n = 3), and lengthier timeframes including “younger,” “ever before”, and “high school or college” (n = 3). The remaining measures (n = 7) referenced more than one timeframe within a single questionnaire.
(5) Prevalence of items related to specific cognitive domains
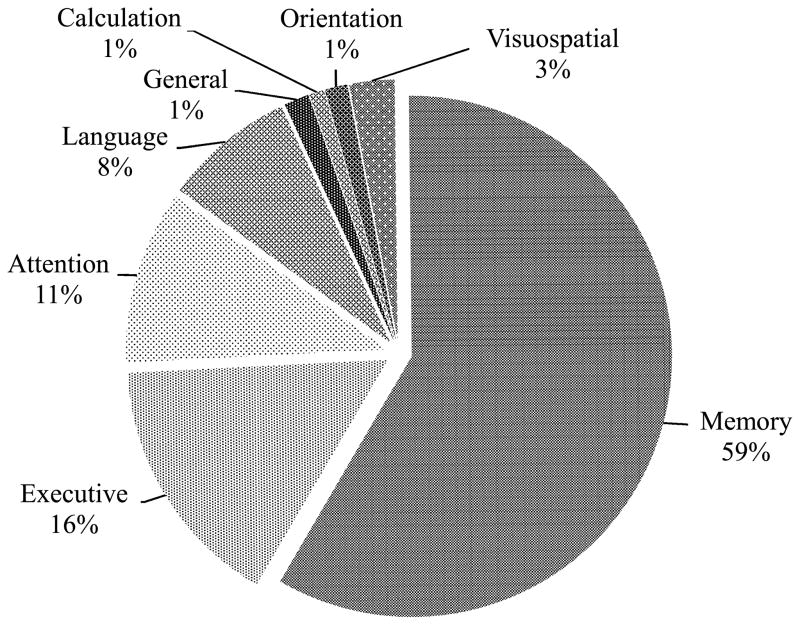
The raters categorized each item according to one of eight neuropsychological domains, with rater discrepancies arising in <4% of cases. As shown in Fig. 2, memory items represented the majority (58.6%, n = 375) of items, followed by executive function (15.8%, n = 101), attention/working memory/processing speed (10.8%, n = 69), and language (8.1%, n = 52). The remaining four domains of visuospatial skills, general cognitive ability, orientation, and basic calculation/arithmetic tasks together accounted for 6.7% of items (n = 43).
Fig. 2.
Percentage of items by cognitive domain.
Notes: Memory includes short-term, long-term, episodic, semantic, and prospective memory and learning new information; Attention includes basic, sustained, focused, and divided attention, working memory, concentration, processing speed, and alertness; Language includes expressive and receptive language, word finding, reading, and spelling; Executive includes executive functions such as organizing, planning, initiating, switching, multi-tasking, reasoning, problem solving, decision-making, impulsivity, and self-regulation; Calculation includes basic calculation and arithmetic; Orientation includes orientation to person, time, place, and situation; General refers to memory and other cognitive abilities grouped together in a single item; and Visuospatial includes visuoperception, route finding, and directional orientation.
(6) Prevalence of items tapping ability/disability-impairment versus change
We further investigated item content by categorizing items as ability/disability-impairment versus change; coding discrepancies between raters arose in <4% of cases. Of the 640 cognitive items, 58.4% (n = 374) tapped ability/disability-impairment and 41.6% (n = 266) assessed intraindividual change or decline. Of the 374 items tapping ability/disability-impairment, 49.5% (n = 185) assessed ability in terms of frequency, 44.7% (n = 167) assessed ability in terms of severity, and the remaining 5.9% (n = 22) of items assessed ability in terms of its impact on functioning. Of the 266 items assessing intraindividual change or decline, 97.7% (n = 260) assessed change in diachronic (or temporal) terms while 6 items (2.3%) assessed change in synchronic (or atemporal) terms. Table 3 presents example items within these various content designations.
Table 3.
Sample items categorized by ability or change
| Domain | Referent | Sample items |
|---|---|---|
| Ability (n = 374) | Severity | Please rate your current level of ability or functioning, or the severity of any problems: Ability to reason through a complicated problem 1 = above average ability; 2 = normal ability; 3 = mild disability; 4 = mild to moderate disability; 5 = moderate disability; 6 = moderate to severe disability; 7 = severe disability; not applicable How would you rate your memory in terms of the kinds of problems you have? 1, 2 = major problems; 3, 4, 5 = some minor problems; 6,7 = no problems |
| Frequency | How often does remembering or doing the following things present a problem for you? Losing the thread of thought in conversation 1, 2 = always; 3, 4, 5 = sometimes; 6, 7 = never I forget details of what I did or what happened the day before. 0 = never or almost never; 1 = rarely; 2 = sometimes; 3 = often; 4 = always or most always |
|
| Impact | Do you feel that your everyday life is difficult now due to your memory decline? 0 = no; 1 = yes; 2 = prefer not to answer How is your everyday life affected by the memory problems? 0 = not at all; 1 = a little; 2 = somewhat; 3 = highly |
|
| Change (n = 266) | Temporal | Compared to 10 years ago, has there been any change in verbally giving instructions to others? 1 = better or no change; 2 = questionable/occasionally worse; 3 = consistently a little worse; 4 = consistently much worse; don’t know My ability to pay attention to what goes on around me is: −4 = worse than ever before; −3, −2, −1, 0 = no change; 1, 2, 3, 4 = better than ever before |
| Atemporal | Are you worried or concerned about a decline in your thinking abilities, more than normal aging? 0 = no; 1 = yes Do you feel your memory got worse suddenly or gradually? 1 = suddenly; 2 = gradually; 3 = other, specify |
(7) Item specificity and complexity
We next considered item stem specificity, which refers to whether an item is general or specific, and complexity, which refers to whether items are double-barreled. As noted above, we limited our coding of general and specific items to memory items and did not extend this coding to other cognitive domains. There were discrepancies in <3% of cases for specificity and <2% for complexity. The vast majority of the 375 memory items were specific (70.7%, n = 265) as opposed to general (29.3%, n = 110). In addition, 98 of the 640 (15.3%) cognitive items were double-barreled. Tables 4 and 5 present examples of general and specific items and double-barreled items, respectively.
Table 4.
Sample memory items categorized by item stem specificity
| Variable | Sample item stems |
|---|---|
| General memory items (n = 110) | How would you rate your memory in terms of the kinds of problems you have? |
| Do you have problems with your memory compared to the way it was 5 years ago? | |
| Do you feel that you have more problems with memory than most? | |
| Any other problems with your memory? | |
| How long ago did your memory problems start? | |
| Specific memory items (n = 265) | How often do you leave something behind when you meant to bring it with you? |
| Do you have trouble recalling conversations a few days later? | |
| I am good at remembering birthdates. | |
| Compared to your peers, do you think you have more difficulty learning new information? | |
| I forget details about myself (age, telephone number). |
Table 5.
Sample cognitive items with double-barreled item stems
| Sample item stems |
|---|
| Are you concerned that you have a memory or other thinking problem? |
| Do you have any difficulty in understanding or following spoken instructions? |
| Do you talk less because of memory or word-finding difficulties? |
| Compared to 10 years ago, I am much worse at remembering titles of books, films or plays. |
| I find it harder to remember street and city names. |
| Forgets appointments, dates, or where things are stored. |
| Organizing daily activities and keeping a schedule. |
| I have trouble finding things in my room, closet, or desk. |
| Following a story in a book, movie, or TV. |
| Recalling telephone numbers or zip codes that you use on a daily or weekly basis. |
| Have you sought evaluation or treatment for your memory problems? |
| My ability to search through my mind and recall names or memories I know are there is. |
| Do you find you forget whether you’ve turned off a light or a fire or locked the door? |
| Remembering what day/date/month it is? |
| I get lost or I follow the wrong directions on trips, strolls, or buildings that I have previously been in. |
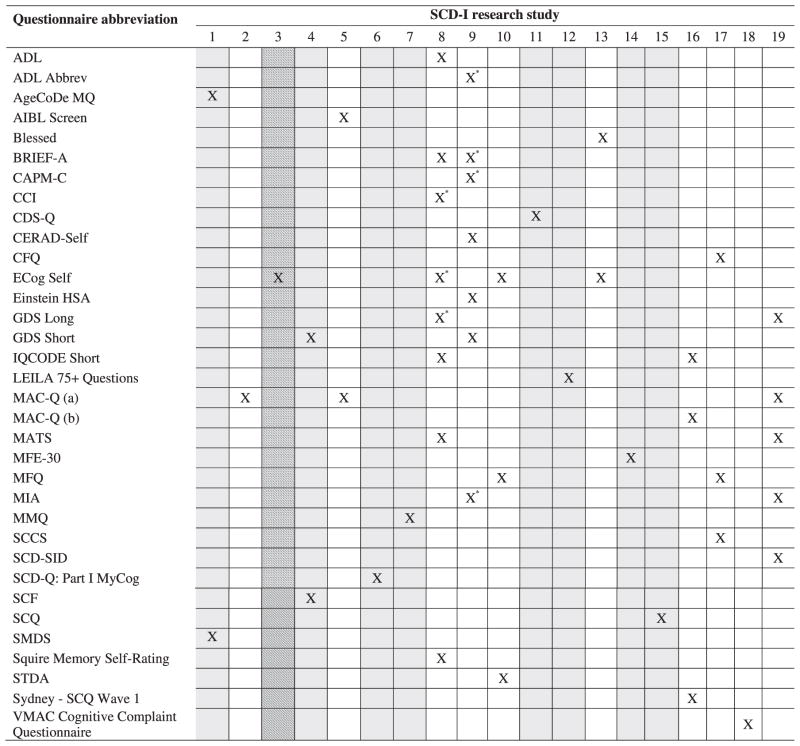
(8) Overlap in measures used by working groups
Table 6 shows the overlap in use of self-report measures by the working group studies. The 34 measures were used 46 times by the 19 working group studies; each study incorporated from 1 to 8 measures. Of the 34 measures, 25 (73.5%) were used only in a single study, 7 (20.6%) were used in 2 studies, the MAC-Q was used in 3 studies, and the ECog was used in 4 studies. Note that scales called that MAC-Q were actually used in 4 studies, but one study used a different timeframe so the raters designated the versions as MAC-Qa and MAC-Qb (see Tables 2 and 6).
Table 6.
Overlap in use of cognitive self-report measures by Subjective Cognitive Decline Initiative (SCD-I) Working Group studies
|
Questionnaire administered to a subset of participants. Columns with gray shading indicate measures administered in a language other than English, as specified below. Columns with line shading indicate measures administered in English plus one or more additional languages, as specified below. Working group study numbers: (1) AgeCoDe Study (German); (2) Alzheimer’s Disease Center Clinical Core and Center for Brain Health; (3) Alzheimer’s Disease Neuroimaging Initiative (ADNI; English, German, French); (4) Amsterdam Dementia Cohort (Dutch); (5) Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing (AIBL); (6) Barcelona Group (Spanish); (7) Bonn Memory Clinic (German); (8) Dartmouth-Indiana Longitudinal Cohort; (9) Einstein Aging Study; (10) Harvard Aging Brain Study; (11) IMAP Caen Group (French); (12) Leipzig Longitudinal Study of the Aged (LEILA 75+) (German); (13) Mayo Clinic Study of Aging (MCSA); (14) Memory Clinic -Fundació ACE (Spanish); (15) PreAl Study (French); (16) Sydney Memory and Ageing Study; (17) University of Pittsburgh Study/ Monongahela-Youghiogheny Health Aging Team (MYHAT); (18) Vanderbilt Memory and Alzheimer’s Center (VMAC); (19) Victoria Subjective Cognitive Decline Study (SCDS).
(9) Most commonly occurring items
We next coded the most commonly occurring items (i.e., items grouped together because they inquire about the same cognitive issue), with <4% discrepancies between raters. Table 7 presents the items that appear most frequently across the 34 questionnaires and their item counts. Note that in some cases a particular item is administered more than once within a measure. Items related to memory change and memory for the names of people were most common, followed by general memory problems and remembering where the participant had put common objects. We also determined item commonality by identifying items that appear on the most measures. For this analysis we only counted each item once per questionnaire. As shown in Table 7, items relating to memory for the names of people and remembering where the individual had put common objects appeared on the greatest number of self-report measures, followed by general memory problems, word finding, and remembering appointments.
Table 7.
Ten most frequently occurring cognitive self-report items
| Item theme and sample item stems | n (%) of total items | n (%) of measures that contain item |
|---|---|---|
| 1. Memory change | 36 (5.6%) | 14 (41.2%) |
| How is your memory compared to the way it was 10 years ago? | ||
| Do you feel you remember things less well than you did a year ago? | ||
| 2. Memory for names of people (longstanding and recently acquired names) | 32 (5.0%) | 19 (55.9%) |
| Do you find you forget people’s names? | ||
| How is your ability to remember the names of close friends and relatives? | ||
| 3. General memory problems | 30 (4.7%) | 16 (47.1%) |
| Have you noticed difficulty with your memory? | ||
| Do you feel like your memory has become worse? | ||
| 4. Remembering where you put common objects/finding familiar objects | 29 (4.5%) | 19 (55.9%) |
| Do you feel you are forgetting where things were placed? | ||
| Finding everyday objects? (e.g. keys, wallet) | ||
| 5. Word finding | 19 (3.0%) | 15 (44.1%) |
| I’m worse at finding the word I want to use in a conversation. | ||
| Do you have more difficulty finding the right words (e.g. feeling like the word is on the tip of your tongue)? | ||
| 6. Remembering appointments | 17 (2.7%) | 15 (44.1%) |
| How is your ability to remember appointments correctly? | ||
| When you actually forget in the following situations, how serious of a problem do you consider the memory problem to be?: appointments | ||
| 7. Remembering recent events | 16 (2.5%) | 13 (38.2%) |
| Do you have more trouble remembering things that have happened recently? | ||
| I forget details of what I did or what happened the day before. | ||
| 8. Remembering recent conversations/things told to you | 15 (2.3%) | 14 (41.2%) |
| Do you have difficulty remembering a conversation from a few days ago? | ||
| I forget things that I was told yesterday or a few days ago. | ||
| 9. Memory for intentions | 15 (2.3%) | 12 (35.3%) |
| Beginning to do something and forgetting what you were doing | ||
| Remembering what you entered a room to do | ||
| 10. Remembering phone numbers (frequently used or just checked) | 14 (2.2%) | 8 (23.5%) |
| Trouble recalling frequently used phone numbers | ||
| Having trouble remembering a telephone number you just looked up |
(10) Considerations driving questionnaire selection and the grouping of measures within a given study
For working groups that reported using an existing SCD measure as their “primary” cognitive self-report measure (n = 10), the most commonly endorsed reasons were the measure’s availability to the researchers, the measure’s brevity and convenience of administration, and the routine use of the measure in studies of cognitive impairment in aging (see Table 8). For working groups that reported developing their own cognitive self-report measure (n = 9), reasons cited related to one of three general themes: (1) categorization of participants into SCD versus non-SCD groups; (2) addressing concerns specific to SCD individuals not captured by other measures; and (3) enabling the researchers to tap cognitive domains of interest. For working groups that reported using more than one self-report measure within a specific study (n = 7), reasons cited related to one of four general themes: (1) use of one measure to classify SCD and additional measure(s) to quantify specific aspects of cognitive functioning; (2) for complete coverage of domains of interest; (3) to permit more variability in the distribution of responses; and (4) to replicate what is used by other studies with similar participant demographics.
Table 8.
Factors driving questionnaire selection decisions for existing measures (n = 10 SCD-I Working Group studies)
| Reason | Number (%) of responses |
|---|---|
| Readily available | 8 (19.5%) |
| Brief/convenient for administration (low participant or researcher burden) | 8 (19.5%) |
| Routinely used in studies of cognitive impairment in aging | 7 (17.1%) |
| Cost-effective (e.g., free/public domain) | 5 (12.2%) |
| Already being used in existing on-site studies | 4 (9.8%) |
| Useful in terms of tapping specific cognitive domains of interest relevant to SCD | 4 (9.8%) |
| Robust in terms of psychometric properties (i.e., reliability and validity) | 3 (7.3%) |
| Good for discriminating differing levels of cognitive impairment | 1 (2.4%) |
| Other considerations | 1 (2.4%) |
DISCUSSION
Overview of findings
The current study is the first to provide a systematic descriptive summary of the cognitive self-report measures used in 19 international cognitive aging studies affiliated with the Subjective Cognitive Decline Initiative(SCD-I)Working Group. Participating studies were diverse with respect to research environment, number of participants, number of self-report measures utilized, and language in which measures were administered. Results indicated that participating studies adopted a wide range of methods to assess SCD—specifically, 34 different self-report measures—the vast majority of which used only within a single study. Measures most commonly used across studies were the MAC-Q [57] and ECog [68], each used at four sites, though two different timeframes were used for the MAC-Q. Almost all self-report measures were self-administered in paper-and-pencil format. The cognitive domain most often targeted was memory, followed by executive function. Items related to memory for the names of people, remembering placement of common objects, and general memory problems occurred on the greatest percentage of measures. The vast majority of memory items tapped specific, rather than general, aspects of memory functioning.
Results highlight wide variation across studies ostensibly interested in the same construct and suggest that many factors may affect instrument choice beyond simple measurement of SCD. Most respondents indicated that measure selection decisions were based on accessibility and convenience of administration, as well as the frequency of use in the cognitive aging literature. Given that several sites reported very large sample sizes (i.e., >3,000 individuals), these decisions are understandable. Being mindful of participant and researcher burdens, this suggests that extensive batteries that fully characterize SCD may not be tractable for many sites to administer.
Our examination of currently used instruments demonstrated wide variation in the format, range, timeframe, and response options both within and across different measures. Items also varied as to whether they tapped ability/disability versus change or decline, with time referents for intraindividual change items ranging from the past 1, 2, 5, 10, or 20+ years to various other periods of life. Given that the subjective, first-person experience of SCD has been relatively understudied, such a broad swath of response options might increase the odds of successfully extracting the unique ways that various subgroups of individuals with SCD conceptualize their concerns.
There are limitations to quantifying an inherently subjective phenomenon such as a complaint. First, asking about complaints in such a large number of ways, largely a theoretical with regards to SCD, raises concern that group differences may emerge from type I error (i.e., statistical bias) incidence rather than actual distinction. In addition, large variability in reference periods has been shown to result in differing question interpretation and subsequent reporting by respondents [79, 80]. With shorter reference periods (e.g., over the past 6 months), respondents tend to report specific minor issues like forgetting names, whereas longer reference periods (e.g., over the past 5 years) tend to tap global, non-specific problems such as general age-related cognitive decline. In general, attentiveness to reference periods seems advisable [81]. Moreover, Likert (and related) formats used by many measures assume an experience of SCD continuous with that of healthy older adults, distinguished only by degree of frequency or severity of complaint, which has not been established as factually evident. Indeed the perception of severity may well vary by demographic factors, such as education [82, 83] at the very least, so endorsement of the same items may mean different things to people of different experiential backgrounds. It is also noteworthy that a significant proportion of items (15%) were double-barreled, where participants could respond to either or both semantic referents, with no systematic way to address resultant ambiguity. This is particularly problematic when directly comparing self- and informant-responses to the same subjective report items, where double-barreled items make it impossible to determine which element(s) of an item stem a given respondent has addressed [84, 85].
A previous review of the SCD literature indicated that SCD has primarily been examined in longitudinal clinical research that follows individuals who decline to MCI and dementia over the course of the studies [86]. Retrospective examination of these cases determined that individuals often complained about declines in cognition many years before decline was detected on standardized clinical assessment—as long as 15 years before manifest cognitive impairment [86, 87]. Given that episodic memory is the cognitive domain most often impaired in MCI patients who subsequently progress to AD [30, 88], one would expect cognitive self-report measures to focus heavily on memory. This was borne out by our data, where well over half of all items and the most frequently occurring items pertained to memory. However, these memory items are not necessarily the best items to include in SCD assessments. In fact, several population-based studies suggest that a large percentage of older adults will endorse some complaint about memory [24, 82], and it is questionable whether the most commonly occurring memory items have the requisite sensitivity and specificity to reliably identify individuals progressing toward MCI and dementia. A report from the Nurses’ Health Study indicated that trouble following a group conversation or finding one’s way around familiar streets were more highly associated with the risk of cognitive impairment than memory complaints such as forgetting things from one moment to the next [89]. Finding one’s way in a familiar place might be classified as orientation or as spatial memory, a specific type of memory, and following a conversation might not be considered memory at all, but rather attention or executive function. Reisberg and colleagues specifically recommended use of the term cognitive rather than memory impairment precisely because the unique phenomenology of SCD is not fully understood and may not be limited to memory [86]. Thus, memory as typically conceived might not be the primary complaint of individuals with SCD due to preclinical AD, and items from other domains may prove to have better discriminant validity at this early stage of non-normative cognitive decline.
Considerations
The current study was intended to serve as a selective overview of the cognitive self-report measures used in studies performed by SCD-I Working Group members, whose primary focus is on preclinical AD. Thus, we were unable to include every extant self-report measure, and this may have biased results to some extent. However, we feel confident that we have captured many of the instruments used to assess cognitive complaints in large aging cohort studies around the globe. In light of our findings, we provide several considerations for the measurement of SCD going forward:
First, the heterogeneity of measures used suggests the exercise of great caution in comparing findings across studies. This does not mean that every site investigating SCD should or could use exactly the same self-assessment battery. Research environment, for example, is an important consideration in selecting measures, as complaints likely mean something different in a population/volunteer sample as compared to a clinical sample. One might assume that individuals recruited from a clinic have specific concern or worry about their cognitive function, which is different from the report of complaints per se. While the literature tends to conflate complaints and concern, recent studies suggest that specific concern or worry about cognitive function has predictive value over and above complaints [21, 47].
Second, researchers might consider approaches from measurement science to inform proper selection of instruments to characterize SCD. Such approaches could be used to investigate the psychometric properties of existing measures, refine or shorten scales (by eliminating less informative items), or optimize measures for specific subgroups of the population of interest (e.g., a brief screen for SCD in a volunteer sample of highly educated older adults). Another application is computer adaptive testing, whereby participants are administered a set of self-report items tailored to their unique cognitive complaint profile estimated from previous item responses, resulting in use of the least number of items to estimate a person’s underlying subjective cognitive ability [90]. To our knowledge, computer algorithms to identify specific items to administer are not currently used. At a basic level it is necessary to investigate psychometric properties such as internal consistency, test-retest reliability, and content validity, as many of the instruments we reviewed were recently developed or not formally validated.
Third, more sophisticated and nuanced analytic approaches should be applied to the datasets on older adults available worldwide. At a minimum, this might include specificity and sensitivity analyses to derive items, for example, which discriminate SCD due to preclinical AD from healthy controls. Identifying items not only with sensitivity but also specificity for SCD is particularly salient in light of discussions about over-diagnosis and the potential to trigger a health crisis in the worried well if researchers over-screen for cognitive complaints [91].
Fourth, future research on SCD should include items related to mood, personality, and health. While the current study focused exclusively on cognitive self-report items, there is likely to be unique variance in perceived cognitive function explained by factors such as mood, personality, and health [69, 92–95]. Therefore, research on the predictive validity of self-report items should include non-cognitive measures to explain as much variance in the experience of SCD as possible. Additionally, we chose not to examine informant-report or clinician rating scales such as the Brief Cognitive Rating Scale, which incorporates various sources of information including self report of memory, objective observation of cognitive deficit on clinical interview, and assessment of functional ability [41]. These methods warrant investigation alongside self-report measures. Informant report, for example, may prove vital, not only in its own right, but also with respect to how it converges or diverges with self-report at different points along the spectrum of pathologic cognitive decline [96].
Fifth, the meaning of complaints tends to vary as a function of demographic characteristics such as level of education and age. Two previous studies [82, 83], for example, found that cognitive complaints in highly educated participants were associated with increased risk of decline to AD. In fact, self-report measures may provide the optimal approach for measuring and monitoring high-functioning individuals in the earliest stages of neurodegenerative cognitive decline, since these individuals may perform at ceiling level on neuropsychological tests [93]. Age of participants also may impact the meaning of complaints, with research suggesting that memory concerns in the young-old associate with anxiety, depression, or personality variables, while memory complaints expressed by older individuals may reflect actual impairment or decline [82]. Cross-site collaboration may be fruitful to the extent that sites share a common setting and common participant demographics. However, any sensitivity and specificity analyses may need to be conducted within the context of local norms for response frequency.
Finally, as noted by Reisberg and colleagues [86], the first-person experience of SCD remains largely unknown. While one might assume that SCD varies from MCI and AD only by degree, it may manifest as a phenomenologically distinct entity. This is likely to be true at least to the extent that individuals with SCD are assumed to have preserved awareness of their difficulties when compared to individuals further along the spectrum of pathologic cognitive decline. Likewise, the first-person experience of SCD due to mood or health complaints may differ from the preclinical AD form of SCD. Aside from the psychometric strategies already discussed, another approach to characterizing SCD could involve gathering qualitative data from persons classified as SCD to reveal their first-person experience, including the frequency and types of complaints most salient to them. This approach would be sensitive to possible cultural differences in the expression of SCD and allow researchers to develop measures specific to their sample demographic characteristics.
Conclusions and recommendations
SCD is rapidly becoming a topic of major interest in cognitive aging and dementia. The current study is the first of its kind, involving an in-depth review of self-report measures currently employed by 19 international sites focused on the study of SCD. Overall, our findings serve as a call for international collaboration to promote harmonization and pooling of cognitive self-report data and greater consistency in the measurement of SCD. The majority of instruments we reviewed were developed within the past 10 years, in some cases within the context of a specific research study, and have limited psychometric evidence. However, validation is an ongoing process, and psychometric data are hopefully forthcoming. With these caveats in mind, we offer the following recommendations:
-
Select measures with appropriate demographic characterization:
Ensure that your target population corresponds to the population for which the questionnaire was developed, understanding that response patterns may vary as a function of demography (e.g., general population versus clinic-based, young-old versus old-old participants). A good selection criterion is involvement of the target population in the questionnaire development process.
-
Select measures with adequate content coverage for the target population:
Ensure that item stems are simple and easy to understand. Questionnaires should not contain double-barreled items, which are known to confuse respondents and undermine the accuracy of responses [84, 97].
Combining multiple constructs within a single measure (e.g., items related to cognition in addition to aspects of mood, personality, or health status) may pose problems for calculating and interpreting scores and is generally not recommended.
Inquire about cognitive issues that older adults encounter frequently in their daily lives. For example, while older adults routinely face the task of recalling a loved one’s name, some have never attempted to handle financial affairs and may rely on ideas about related skills they do possess, such as basic mathematics [117]. An optimal approach may involve tailoring questions to individuals based on knowledge about their particular experiences or medical history [117].
Sample cognitive domains beyond episodic memory because items that tap into other aspects of memory, or other domains, might be more pathognomonic of non-normative decline at the early AD stages.
Utilize measures that contain a greater number of specific rather than general cognitive items. Specific items prompt older adults to search for explicit instances in which they experience memory problems in their daily lives, which leads to more accurate reporting. Broadly worded items, by contrast, may cause older adults to fall back upon global beliefs about their abilities and the cognitive aging process more generally [84, 117].
Ensure that response options correspond to the measurement purpose. When the primary purpose is to distinguish between groups, a dichotomous scale might be sufficient. For the measurement of change over time, a Likert scale might represent a better choice. Notably, to the extent that SCD implies a perceived decline, measures should include some items targeting cognitive change. Another consideration is whether response options should target the frequency of problems (how often) versus quality of performance (how well). To address frequency, it is essential to utilize manageable timeframes (e.g., number of times the individual misplaced car keys over the past week as opposed to the past year). To assess quality of performance, one should keep in mind that individuals will provide responses based on aggregated experience rather than specific problematic instances [117].
Ensure that the reference period is specific, appropriate, and narrow. Inquiring about experiences over the past month, week, or even several days is preferable to longer timeframes because these windows enable older adults to focus on concrete recent events rather than rely on beliefs about “typical” performance or the cognitive aging process as a whole [117, 118]. Commonly used longer timeframes (e.g., >1 year, 5 years) may seem face valid but could pose difficulties for older adults trying to recall specific recent events. Vague reference periods should never be used.
-
Consider issues of psychometric adequacy:
Select questionnaires that have been published, and for which there is at least some evidence for adequate reliability, construct validity, test-retest reliability, and differences between subgroups of interest.
Validate measures in the population of interest. For example, some instruments might have self and informant versions but have only been validated for one or the other population. One cannot assume that validity will generalize across populations, particularly as individuals and their informants contribute unique sources of variance to estimates of current cognitive function.
Consider whether items appropriately measure the cognitive domains of interest. This is especially important in cross-cultural collaborative research, where cognitive complaints might not mean the same thing in Western versus non-Western settings. Western conceptualizations of executive function, for example, often include time pressure/cognitive efficiency, which may not generalize. For questionnaires adapted for use in a different culture, language, or country, two types of validation must occur: linguistic validation (established equivalence of language) and cultural validation (item revision based on cultural appropriateness of wording and potential misinterpretation due to differences in ways of thinking about cognition) [98].
-
Develop new measures:
There is a need to derive a small number of well-constructed, easy-to-administer items with adequate reliability across diverse samples of older adults. Ideally, these items should discriminate subgroups of SCD, for example those with SCD due to preclinical AD from healthy older adults (concurrent validity), as well as indicate who of the SCD group is likely to decline to MCI and AD (predictive validity). A multi-method approach to assessment incorporating objective cognitive and biomarker measures might help establish the validity of self-report items.
Acknowledgments
LAR was supported by the National Institutes of Health (NIA/NIGMS grant SC2AG039235) and PSC-CUNY (Award #68859-00 46); CMS was supported by the Alzheimer Society of Canada (Young Investigator Award #1216); PKC was supported by the National Institute on Aging (NIA) grants U01 AG 006781 (E Larson, PI) and R01 AG 042437 (P Crane, PI); REA was supported by NIH grant K23AG044431 and Alzheimer’s Association grant NIRG-12-243012; RFB was supported by Alzheimer’s Australia Dementia Research Foundation (Postdoctoral Fellowship); GC and AP report the following sources of financial support and funding: Fondation Plan Alzheimer (Alzheimer Plan 2008-2012), Programme Hospitalier de Recherche Clinique (PHRC National 2011, complément PHRC 2012), Agence Nationale de la Recherche (ANR LONGVIE 2007), Région Basse Normandie, and Institut National de la Santé et de la Recherche Médicale (Inserm); BD and CT (PréAl study) were supported by the national Programme Hospitalier de Recherche Clinique; KE, PM, and RFB (AIBL study) were supported by Commonwealth Scientific and Industrial Research Organisation (CSIRO), the Science and Industry Endowment Fund (http://www.sief.org.au), the National Health and Medical Research Council (NHMRC), and Dementia Collaborative Research Centres (DCRC), as well as industry, including Pfizer, Merck, Janssen, and GE Healthcare; MJK and RBL were supported, in part by National Institutes of Health grants NIA 2 P01 AG03949 and NIA R03 AG045474, the Leonard and Sylvia Marx Foundation, and the Czap Foundation; TL was supported by the Study on Needs, Health Service Use, Costs and Health-related Quality of Life in a large Sample of Oldest-old Primary Care Patients (85+) AgeQualiDe; funded by the German Federal Ministry of Education and Research Grant 01GY1322A; BR was supported by National Institute on Aging (NIA/NIH) grants P30 AG08051 and AG03051 and by the Stringer Foundation, the Louis and June Kay Foundation, the Hagedorn Fund, and gifts from Dr. Felix and Mrs. Miriam Glaubach; LR is the recipient of a Miguel Servet II grant as a senior investigator from the Spanish Ministry of Science (CP2/00023); SLR was supported by NIA grants R01 AG19771, NIA P30 AG10133, the Alzheimer’s Association, the Indiana University Health-Indiana University School of Medicine Strategic Research Initiative, and the Indiana Clinical and Translational Science Institute; RAS and DMR were supported by NIH grants P01AG036694, R01 AG027435, K24 AG035007, and U19 AG10483; BES was supported by National Institutes of Health grants K23 AG038479, R01 AG023651, P01 AG025204, and R37 AG025516; AJS was supported by grants: NIA R01 AG19771, NIA P30 AG10133, and R01 LM011360; ADNI data and sharing was funded by the Alzheimer’s Disease Neuroimaging Initiative (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Inc., Biogen Idec Inc., Bristol-Myers Squibb Company, Eisai Inc., Elan Pharmaceuticals, Inc., Eli Lilly and Company, EuroImmun, F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc., Fujirebio, GE Healthcare, IXICO Ltd., Janssen Alzheimer Immunotherapy Research & Development, LLC, Johnson & Johnson Pharmaceutical Research & Development LLC, Medpace, Inc., Merck & Co., Inc., Meso Scale Diagnostics, LLC, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc., Piramal Imaging, Servier, Synarc Inc., and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research provides funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego; SAMS was supported by Stichting VUmc fonds, the Innovatiefonds Zorgverzekeraars, and a fellowship program from Alzheimer Nederland (WE. 15-2012-02).
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/15-0154r1).
References
- 1.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jessen F, Wiese B, Bickel H, Eiffländer-Gorfer S, Fuchs A, Kaduszkiewicz H, Köhler M, Luck T, Mösch E, Pentzek M, Riedel-Heller SG, Wagner M, Weyerer S, Maier W, van den Bussche H for the AgeCoDe Study Group. Prediction of dementia in primary care patients. PLoS One. 2011;6:e16852. doi: 10.1371/journal.pone.0016852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amieva H, Le Goff M, Millet X, Orgogozo JM, Pérès K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer’s disease: Successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 4.Dufouil C, Fuhrer R, Alpérovitch A. Subjective cognitive complaints and cognitive decline: Consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr Scand. 2014;6:1–13. doi: 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- 6.Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, Suliman H, Wagner M, Schild HH, Jessen F. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord. 2010;29:75–81. doi: 10.1159/000264630. [DOI] [PubMed] [Google Scholar]
- 9.Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011;68:845–852. doi: 10.1001/archgenpsychiatry.2011.80. [DOI] [PubMed] [Google Scholar]
- 10.Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, Popp J, Daamen M, Gorris D, Heneka MT, Boecker H, Biersack HJ, Maier W, Schild HH, Wagner M, Jessen F. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 12.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: A Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, van der Flier WM. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Wan L, Schmitt FA. Self-reported memory complaints: Implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, Tsolaki M, Minthon L, Wallin AK, Hampel H, Bürger K, Pirttila T, Soininen H, Rikkert MO, Verbeek MM, Spiru L, Blennow K. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 17.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comijs HC, Deeg DJH, Dik MG, Twisk JWR, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics: A 6-year follow-up study. J Affect Disord. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 19.Steffens DC, Potter GG. Geriatric depression and cognitive impairment. Psychol Med. 2008;38:163–175. doi: 10.1017/S003329170700102X. [DOI] [PubMed] [Google Scholar]
- 20.Caracciolo B, Gatz M, Xu W, Marengoni A, Pedersen N, Fratiglioni L. Relation of multimorbidity to subjective and objective cognitive impairment: A population-based twin study. J Alzheimers Dis. 2013;36:275–284. [Google Scholar]
- 21.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, Luck T, Mösch E, van den Bussche H, Wagner M, Wollny A, Zimmermann T, Pentzek M, Riedel-Heller SG, Romberg HP, Weyerer S, Kaduszkiewicz H, Maier W, Bickel H German Study on Aging, Cognition and Dementia in Primary Care Patients Study Group. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 22.Abdulrab K, Heun R. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23:321–330. doi: 10.1016/j.eurpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, deLeon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M Subjective Cognitive Decline Initiative (SCD-I) Working, Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper C, Bebbington P, Lindesay J, Meltzer H, McManus S, Jenkins R, Livingston G. The meaning of reporting forgetfulness: A cross-sectional study of adults in the English 2007 Adult Psychiatric Morbidity Survey. Age Ageing. 2011;40:711–717. doi: 10.1093/ageing/afr121. [DOI] [PubMed] [Google Scholar]
- 25.Boone KB. Fixed belief in cognitive dysfunction despite normal neuropsychological scores: Neurocognitive hypochondriasis? Clin Neuropsychol. 2009;23:1016–1036. doi: 10.1080/13854040802441135. [DOI] [PubMed] [Google Scholar]
- 26.Mattsson N, Andreasson U, Persson S, Arai H, Batish SD, Bernardini S, Bocchio-Chiavetto L, Blankenstein MA, Carrillo MC, Chalbot S, Coart E, Chiasserini D, Cutler N, Dahlfors G, Duller S, Fagan AM, Forlenza O, Frisoni GB, Galasko D, Galimberti D, Hampel H, Handberg A, Heneka MT, Herskovits AZ, Herukka SK, Holtzman DM, Humpel C, Hyman BT, Iqbal K, Jucker M, Kaeser SA, Kaiser E, Kapaki E, Kidd D, Klivenyi P, Knudsen CS, Kummer MP, Lui J, Lladó A, Lewczuk P, Li QX, Martins R, Masters C, McAuliffe J, Mercken M, Moghekar A, Molinuevo JL, Montine TJ, Nowatzke W, O’Brien R, Otto M, Paraskevas GP, Parnetti L, Petersen RC, Prvulovic D, de Reus HP, Rissman RA, Scarpini E, Stefani A, Soininen H, Schröder J, Shaw LM, Skinningsrud A, Skrogstad B, Spreer A, Talib L, Teunissen C, Trojanowski JQ, Tumani H, Umek RM, Van Broeck B, Vanderstichele H, Vecsei L, Verbeek MM, Windisch M, Zhang J, Zetterberg H, Blennow K. The Alzheimer’s Association external quality control program for cerebrospinal fluid biomarkers. Alzheimers Dement. 2011;7:386–395. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer’s disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 28.Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, Perret-Liaudet A, Teunissen CE, Parnetti L. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimers Dement. 2014;10:808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriat Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bondi MW, Smith GE. Mild cognitive impairment: A concept and diagnostic entity in need of input from neuropsychology. J Int Neuropsychol Soc. 2014;20:129–134. doi: 10.1017/S1355617714000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Jak AJ, Au R, Salmon DP, Bondi MW. Are empirically derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc. 2013;19:635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Rockwood K, Xiao S, Li J, Li X, Brayne C, Matthews FE, Stephan BCM, Lipton RB, Katz MJ, Ritchie K, Carriére I, Ancelin ML, Seshadri S, Au R, Beiser AS, Lam LCW, Wong CHY, Fung AWT, Kim KW, Han JW, Kim TH, Petersen RC, Roberts RO, Mielke MM, Ganguli M, Dodge HH, Hughes T, Anstey KJ, Cherbuin N, Butterworth P, Ng TP, Gao Q, Reppermund S, Brodaty H, Meguro K, Schupf N, Manly J, Stern Y, Lobo A, Lopez-Anton R, Santabárbara J COSMIC. COSMIC (Cohort Studies of Memory in an International Consortium): An international consortium to identify risk and protective factors and biomarkers of cognitive ageing and dementia in diverse ethnic and sociocultural groups. BMC Neurol. 2013;13:165–172. doi: 10.1186/1471-2377-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallassi R, Oppi F, Poda R, Scortichini S, Maserati MS, Marano G, Sambati L. Are subjective cognitive complaints a risk factor for dementia? Neurol Sci. 2010;31:327–336. doi: 10.1007/s10072-010-0224-6. [DOI] [PubMed] [Google Scholar]
- 35.Guarch J, Marcos T, Salamero M, Blesa R. Neuropsychological markers of dementia in patients with memory complaints. Int J Geriatr Psychiatry. 2004;19:352–358. doi: 10.1002/gps.1074. [DOI] [PubMed] [Google Scholar]
- 36.von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. J Neuropsychiatry Clin Neurosci. 2000;12:493–498. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- 37.Hurt CS, Burns A, Brown RG, Barrowclough C. Why don’t older adults with subjective memory complaints seek help? Int J Geriatr Psychiatry. 2012;27:394–400. doi: 10.1002/gps.2731. [DOI] [PubMed] [Google Scholar]
- 38.Go RCP, Duke LW, Harrell LE, Cody H, Bassett SS, Folstein MF, Albert MS, Foster JL, Sharrow NA, Blacker D. Development and validation of a Structured Telephone Interview for Dementia Assessment (STIDA): The NIMH Genetics Initiative. J Geriatr Psychiatry Neurol. 1997;10:161–167. doi: 10.1177/089198879701000407. [DOI] [PubMed] [Google Scholar]
- 39.Samieri C, Proust-Lima C, Glymour MM, Okereke OI, Amariglio RE, Sperling RA, Rentz DM, Grodstein F. Subjective cognitive concerns, episodic memory, and the APOE ε4 allele. Alzheimers Dement. 2014;10:752–759. doi: 10.1016/j.jalz.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gifford KA, Liu D, Lu Z, Tripodis Y, Cantwell NG, Palmisano J, Kowall N, Jefferson AL. The source of cognitive complaints differentially predicts diagnostic conversion in nondemented older adults. Alzheimers Dement. 2014;10:319–327. doi: 10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reisberg B, Ferris SH. Brief Cognitive Rating Scale (BCRS) Psychopharmacol Bull. 1988;24:629–636. [PubMed] [Google Scholar]
- 42.Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: Depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127:344–350. doi: 10.1111/ane.12038. [DOI] [PubMed] [Google Scholar]
- 43.Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimers Dis. 2010;22:159–170. doi: 10.3233/JAD-2010-100972. [DOI] [PubMed] [Google Scholar]
- 44.Brucki SM, Nitri R. Subjective memory impairment in a rural population with low education in the Amazon rainforest: An exploratory study. Int Psychogeriatr. 2009;21:164–171. doi: 10.1017/S1041610208008065. [DOI] [PubMed] [Google Scholar]
- 45.Comijs HC, Deeg DJH, Dik MG, Twisk JWR, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics: A 6-year follow-up study. J Affect Disord. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 46.Pike KE, Ellis KA, Villemagne VL, Good N, Chételat G, Ames D, Szoeke C, Laws SM, Verdile G, Martins RN, Masters CL, Rowe CC. Cognition and beta-amyloid in preclinical Alzheimer’s disease: Data from the AIBL study. Neuropsychologia. 2011;49:2384–2390. doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Smart CM, Segalowitz SJ, Mulligan BP, MacDonald SWS. Attention capacity and self-report of subjective cognitive decline: A P3 ERP study. Biol Psychol. 2014;103:144–151. doi: 10.1016/j.biopsycho.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 48.St John P, Montgomery P. Are cognitively intact seniors with subjective memory loss more likely to develop dementia? Int J Geriatr Psychiatry. 2002;17:814–820. doi: 10.1002/gps.559. [DOI] [PubMed] [Google Scholar]
- 49.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rami L, Mollica MA, García-Sanchez C, Saldaña J, Sanchez B, Sala I, Valls-Pedret C, Castellví M, Olives J, Molinuevo JL. The Subjective Cognitive Decline Questionnaire (SCD-Q): A validation study. J Alzheimers Dis. 2014;41:453–466. doi: 10.3233/JAD-132027. [DOI] [PubMed] [Google Scholar]
- 51.Knight RG, McMahon J, Green TJ, Skeaff CM. Some normative and psychometric data for the geriatric depression scale and the cognitive failures questionnaire from a sample of healthy older persons. New Zeal J Psychol. 2004;33:163–170. [Google Scholar]
- 52.Van der Linden M, Wijns C, Von Frenkell R, Coyette F, Seron X. Un questionnaire d’auto-évaluation de la mémoire (qam) Brussels; Belgium, Editest: 1989. [Google Scholar]
- 53.Smith G, Della Sala S, Logie RH, Maylor EA. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory. 2000;8:311–321. doi: 10.1080/09658210050117735. [DOI] [PubMed] [Google Scholar]
- 54.Troyer AK, Rich JB. Psychometric properties of a new metamemory questionnaire for older adults. Gerontology. 2002;57:19–27. doi: 10.1093/geronb/57.1.p19. [DOI] [PubMed] [Google Scholar]
- 55.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychology. 1982;21:1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 56.Derouesné C, Dealberto MJ, Boyer P, Lubin S, Sauron B, Piette F, Kohler F, Alpérovitch A. Empirical evaluation of the ‘Cognitive Difficulties Scale’ for assessment of memory complaints in general practice: A study of 1628 cognitively normal subjects aged 45–75 years. Int J Geriatr Psychiatry. 1993;8:599–607. [Google Scholar]
- 57.Crook THE, Feher EP, Larrabee GJ. Assessment of memory complaint in age-associated memory impairment: The MAC-Q. Int Psychogeriatr. 1992;4:165–176. doi: 10.1017/s1041610292000991. [DOI] [PubMed] [Google Scholar]
- 58.McNair D, Kahn RJ. Self-assessment of cognitive deficits. In: Crook T, Ferris S, Bartus R, editors. Assessment in Geriatric Psychopharmacology. Mark Powley Associates Inc; CT: 1983. pp. 137–143. [Google Scholar]
- 59.Harwood D, Barker W, Ownby R, Duara R. Memory complaints in the elderly: A comparative analysis of informant and subjects reports among Hispanics and white Non-Hispanics. Clin Gerontologist. 1998;18:56–60. [Google Scholar]
- 60.Gilewski MJ, Zelinski EM, Schaie KW. The memory functioning questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 61.Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, Pentzek M, Riedel-Heller SG, Luck T, Fuchs A, Weyerer S, Werle J, van den Bussche H, Scherer M, Maier W, Wagner M German Study on Aging, Cognition and Dementia in Primary Care Patients. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014;10:76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 62.Crawford JR, Henry JD, Ward AL, Blake J. The Prospective and Retrospective Memory Questionnaire (PRMQ): Latent structure, normative data and discrepancy analysis for proxy-ratings. Br J Clin Psychol. 2006;45:83–104. doi: 10.1348/014466505X28748. [DOI] [PubMed] [Google Scholar]
- 63.Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. Int J Aging Hum Dev. 2006;63:139–151. doi: 10.2190/1EAP-FE20-PDWY-M6P1. [DOI] [PubMed] [Google Scholar]
- 64.Vestergren P, Rönnlund M, Nyberg L, Nilsson LG. Development of the Cognitive Dysfunction Questionnaire (CDQ) in a population based sample. Scand J Psychol. 2011;52:218–228. doi: 10.1111/j.1467-9450.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 65.Dixon RA, Hultsch DF, Hertzog C. The Metamemory in Adulthood (MIA) Questionnaire. Psychopharmacol Bull. 1988;24:671–688. [PubMed] [Google Scholar]
- 66.Chau LT, Lee JB, Fleming J, Roche N, Shum D. Reliability and normative data for the Comprehensive Assessment of Prospective Memory (CAPM) Neuropsychol Rehabil. 2007;17:707–722. doi: 10.1080/09602010600923926. [DOI] [PubMed] [Google Scholar]
- 67.Rabin LA, Saykin AJ, Wishart HA, Nutter-Upham KE, Flashman LA, Pare N, Santulli RB. The Memory and Aging Telephone Screen: Development and preliminary validation. Alzheimers Dement. 2007;3:109–121. doi: 10.1016/j.jalz.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, DeCarli C. The measurement of everyday cognition (ECog): Scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kliegel M, Zimprich D. Predictors of cognitive complaints in older adults: A mixture regression approach. Eur J Ageing. 2005;2:13–23. doi: 10.1007/s10433-005-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potter GG, Hartman M. Response inhibition and everyday memory complaints in older adult women. Int J Geriatr Psychiatry. 2006;21:1115–1120. doi: 10.1002/gps.1615. [DOI] [PubMed] [Google Scholar]
- 71.Rabin LA, Wang C, Katz MJ, Derby CA, Buschke H, Lipton RB. Predicting Alzheimer’s disease: Neuropsychological tests, self-reports, and informant reports of cognitive difficulties. J Am Geriatr Soc. 2012;60:1128–1134. doi: 10.1111/j.1532-5415.2012.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mascherek A, Zimprich D, Rupprecht R, Lang FR. What do cognitive complaints in a sample of memory clinic outpatients reflect? GeroPsych. 2011;24:187–195. [Google Scholar]
- 73.Singh-Manoux A, Dugravot A, Ankri J, Nabi H, Berr C, Goldberg M, Zins M, Kivimaki M, Elbaz A. Subjective cognitive complaints and mortality: Does the type of complaint matter? J Psychiatr Res. 2014;48:73–78. doi: 10.1016/j.jpsychires.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Snitz BE, Morrow LA, Rodriguez EG, Huber KA, Saxton JA. Subjective memory complaints and concurrent memory performance in older patients of primary care providers. J Int Neuropsychol Soc. 2008;14:1004–1013. doi: 10.1017/S1355617708081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stewart R, Dufouil C, Godin O, Ritchie K, Maillard P, Delcroix N, Crivello F, Mazoyer B, Tzourio C. Neuroimaging correlates of subjective memory deficits in a community population. Neurology. 2008;70:1601–1607. doi: 10.1212/01.wnl.0000310982.99438.54. [DOI] [PubMed] [Google Scholar]
- 76.Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, Dufouil C. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry. 2011;198:199–205. doi: 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- 77.Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 78.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- 79.Schwarz N. Self-reports: How the questions shape the answers. Am Psychol. 1999;54:93–105. [Google Scholar]
- 80.Schwarz N, Oyserman D. Asking questions about behavior: Cognition, communication, and questionnaire construction. Am J Eval. 2001;22:127–160. [Google Scholar]
- 81.Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to Their Development and Use. 4. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- 82.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 83.van Oijen M, de Jong F, Hofman A, Koudstaal P, Breteler M. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimer Dement. 2007;3:92–97. doi: 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 84.Dillman DA, Smyth JD, Christian LM. Internet, Mail, and Mix-Mode Surveys: The Tailored Design Method. John Wiley and Sons, Inc; Hoboken, NJ: 2009. [Google Scholar]
- 85.Martyr A, Nelis SM, Clare L. Predictors of perceived functional ability in early-stage dementia: Self-ratings, informant ratings and discrepancy scores. Int J Geriatr Psychiatry. 2014;29:852–862. doi: 10.1002/gps.4071. [DOI] [PubMed] [Google Scholar]
- 86.Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr. 2008;20:1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 87.Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, Monteiro I, Torossian C, Vedvyas A, Ashraf N, Jamil IA, deLeon MJ. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 2008;4:S98–108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 88.Blacker D, Lee H, Muzikansky A, Martin EC, Tanzi R, McArdle JJ, Moss M, Albert M. Neuropsychological measures in normal individuals that predict subsequent cognitive decline. Arch Neurol. 2007;64:862–871. doi: 10.1001/archneur.64.6.862. [DOI] [PubMed] [Google Scholar]
- 89.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59:1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Snitz BE, Yu L, Crane PK, Chang CC, Hughes TF, Ganguli M. Subjective cognitive complaints of older adults at the population level: An item response theory analysis. Alzheimer Dis Assoc Disord. 2012;26:344–351. doi: 10.1097/WAD.0b013e3182420bdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alzheimer’s Association International Conference. [Accessed on January 21, 2015];2014 http://www.alzheimers.org.uk/site/scripts/documentsinfo.php?documentID=2723&pageNumber=7, Last updated 2015.
- 92.Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, Sachdev PS. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18:701–710. doi: 10.1097/jgp.0b013e3181df49fb. [DOI] [PubMed] [Google Scholar]
- 93.Cargin JW, Collie A, Masters C, Maruff P. The nature of cognitive complaints in healthy older adults with and without objective memory decline. J Clin Exp Neuropsychol. 2008;30:245–257. doi: 10.1080/13803390701377829. [DOI] [PubMed] [Google Scholar]
- 94.Dux MC, Woodard JL, Calamari JE, Messina M, Arora S, Chik H, Pontarelli N. The moderating role of negative affect on objective verbal memory performance and subjective memory complaints in healthy older adults. J Int Neuropsychol Soc. 2008;14:327–336. doi: 10.1017/S1355617708080363. [DOI] [PubMed] [Google Scholar]
- 95.Comijs HC, Deeg DJH, Dik MG, Twisk JW, Jonker C. Memory complaints: The association with psychoaffective and health problems and the role of personality characteristics: A 6-year follow-up study. J Affect Disord. 2002;72:157–164. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- 96.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW Alzheimer’s Disease Neuroimaging Initiative. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J Int Neuropsychol Soc. 2014;20:836–847. doi: 10.1017/S135561771400068X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Vet HCW, Terwee CB, Mokkink LB, Knol DL. Measurement in Medicine. Cambridge University Press; NY: 2001. [Google Scholar]
- 98.Beaton DE, Bombardier C, Guillemin F, Marcos BF. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine. 2001;25:3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 99.Ellis KA, Bush AI, Darby D, De Fazio D, Foser J, Hudson P, Lautenschlager NT, Lenzo N, Martins RN, Maruff P, Masters C, Milner A, Pike K, Rowe C, Savage G, Szoeke C, Taddei K, Villemagne V, Woodward M, Ames D AIBL Research Group. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr. 2009;21:672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 100.Saykin AJ. Neurobehavioral Function and Activities of Daily Living Rating Scale (NBFADL-63 item version) Dartmouth Medical School; 1992. [Google Scholar]
- 101.Jessen F, Wiese B, Cvetanovska G, Fuchs A, Kaduszkiewicz H, Kölsch H, Luck T, Mösch E, Pentzek M, Riedel-Heller SG, Werle J, Weyerer S, Zimmermann T, Maier W, Bickel H. Patterns of subjective memory impairment in the elderly: Association with memory performance. Psychol Med. 2007;37:1753–1762. doi: 10.1017/S0033291707001122. [DOI] [PubMed] [Google Scholar]
- 102.Mielke MM, Wiste HJ, Weigand SD, Knopman DS, Lowe VJ, Roberts RO, Geda YE, Swenson-Dravis DM, Boeve BF, Senjem ML, Vemuri P, Petersen RC, Jack CR., Jr Indicators of amyloid burden in a population-based study of cognitively normal elderly. Neurology. 2012;79:1570–1577. doi: 10.1212/WNL.0b013e31826e2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roth RM, Isquith PK, Gioia GA. Behavioral Rating Inventory of Executive Function—Adult version. Psychological Assessment Resources, Inc; Lutz, FL: 2005. [Google Scholar]
- 104.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1159. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 105.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 106.Sheikh JA, Yesavage JA. Geriatric Depression Scale (GDS): Recent findings and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. Howarth Press; NY: 1986. pp. 165–173. [Google Scholar]
- 107.Derby CA, Burns LC, Wang C, Katz MJ, Zimmerman ME, L’italien G, Guo Z, Berman RM, Lipton RB. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80:1307–1314. doi: 10.1212/WNL.0b013e31828ab2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 109.Luck T, Luppa M, Briel S, Matschinger H, König HH, Bleich S, Villringer A, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: Incidence and risk factors: Results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc. 2010;58:1903–1910. doi: 10.1111/j.1532-5415.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 110.Riedel-Heller SG, Matschinger H, Schork A, Angermeyer MC. Do memory complaints indicate the presence of cognitive impairment? Results of a field study. Eur Arch Psychiatry Clin Neurosci. 1999;249:197–204. doi: 10.1007/s004060050087. [DOI] [PubMed] [Google Scholar]
- 111.Lozoya-Delgado P, Ruiz-Sanchez de Leon JM, Pedrero-Perez EJ. Validación de un cuestionario de quejas cognitivas para adultos jóvenes: Relación entre las quejas subjetivas de memoria, la sintomatología prefrontal y el estrés percibido. Rev Neurol. 2012;54:137–150. [PubMed] [Google Scholar]
- 112.Ganguli M, Chang C-CH, Snitz BE, Saxton JA, Vanderbilt J, Lee C-W. Prevalence of mild cognitive impairment by multiple classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18:674–683. doi: 10.1097/JGP.0b013e3181cdee4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aalten P, Ramakers IHGB, Biessels GJ, de Deyn PP, Koek HL, OldeRikkert MGM, Oleksik AM, Richard E, Smits LL, van Swieten JC, Teune LK, van der Lugt A, Barkhof F, Teunissen CE, Rozendaal N, Verhey FRJ, van der Flier WM. The Dutch Parelsnoer Institute - Neurodegenerative diseases; methods, design and baseline results. BMC Neuro. 2014;14:254. doi: 10.1186/s12883-014-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mahieux F, Onen F, Berr C, Volteau M, Habert MO, Legrain S, Dubois B. Early detection of patients in the pre demented stage of Alzheimer’s disease: The Pre-Al Study. J Nutr Health Aging. 2009;13:21–26. doi: 10.1007/s12603-009-0004-2. [DOI] [PubMed] [Google Scholar]
- 115.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: A longitudinal analysis over 7–8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 116.Squire LR, Wetzel CD, Slater PC. Memory complaint after electroconvulsive therapy: Assessment with a new self-rating instrument. Biol Psychiatr. 1979;14:791–801. [PubMed] [Google Scholar]
- 117.Hill NL, Mogle JM, Munoz E, Wion R, Colancecco EM. Assessment of subjective cognitive impairment among older adults. J Gerontol Nurs. 2015;41:28–35. doi: 10.3928/00989134-20150309-01. [DOI] [PubMed] [Google Scholar]
- 118.Robinson MD, Clore GL. Belief and feeling: Evidence for an accessibility model of emotional self-report. Psychol Bull. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]