SUMMARY
Actinetobacter baumannii is an important nosocomial pathogen that can cause a wide range of serious conditions including pneumonia, meningitis, necrotizing fasciitis and sepsis. It is also a major cause of wound infections in military personnel injured during the conflicts in Afghanistan and Iraq, leading to its popular nickname of ‘Iraqibacter’. Contributing to its success in clinical settings is resistance to environmental stresses such as desiccation and disinfectants. Moreover, in recent years there has been a dramatic increase in the number of A. baumannii strains with resistance to multiple antibiotic classes. Acinetobacter baumannii is an inhabitant of oral biofilms, which can act as a reservoir for pneumonia and chronic obstructive pulmonary disease. Subgingival colonization by A. baumannii increases the risk of refractory periodontitis. Pathogenesis of the organism involves adherence, biofilm formation and iron acquisition. In addition, A. baumannii can induce apoptotic cell death in epithelial cells and kill hyphal forms of Candida albicans. Virulence factors that have been identified include pili, the outer membrane protein OmpA, phospholipases and extracellular polysaccharide. Acinetobacter baumannii can sense blue light through a blue-light sensing using flavin (BLUF) domain protein, BlsA. The resulting conformational change in BlsA leads to changes in gene expression, including virulence genes.
Keywords: Acinetobacter, infection models, oral microbiology, periodontal disease, respiratory tract microbiology
INTRODUCTION
There are few organisms that can compare with Acinetobacter baumannii in terms of variety of associated diseases. Serious infections that are caused by A. baumannii include pneumonia, meningitis, necrotizing fasciitis, sepsis, urinary tract infections, skin and/or soft tissue infections, endocarditis and keratitis (Peleg et al., 2008a). This gram-negative organism emerged as an important hospital-acquired opportunistic pathogen in the 1970s (Peleg et al., 2008a), and more recently has been thrust into the public eye as a ‘superbug’, with the increasing incidence of multidrug-resistant strains. Moreover, A. baumannii has gained notoriety through frequent infections in wounded military personnel (Davis et al., 2005; Dijkshoorn et al., 2007; Perez et al., 2007), which has earned it the popular nickname, ‘Iraqibacter’ (Howard et al., 2012). Indeed, a study by the National Naval Medical Center (USA) of war wounds of US troops located in Iraq and Afghanistan from 2007 to 2008 determined that A. baumannii accounted for 63% of all bacterial isolates in tissue biopsies (Sheppard et al., 2010). Acinetobacter baumannii had the highest incidence rate, at 22% 1 week post-injury, while Enterococcus faecium had the second highest incidence rate at only 3.3% (Sheppard et al., 2010). Acinetobacter baumannii has an unfortunate predilection for the severely injured, compromised and elderly, and many of the infections in these cases are associated with the use of contaminated medical equipment such as catheters, ventilators, external ventricular drains and even gloves (Peleg et al., 2008a; Park et al., 2013). Mortality rates with bacteraemia-related diseases are around 35%; however, when accounting for just imipenem-resistant strains the mortality rate rises to an astonishing 70% (Park et al., 2013; Lee et al., 2014). Acinetobacter baumannii is a resilient organism that can resist desiccation and other stressors including disinfectants (Jawad et al., 1996, 1998; Wendt et al., 1997; Peleg et al., 2008a; Rajamohan et al., 2010). Combined with its ability to form biofilms on biotic and abiotic surfaces, the organism has an aptitude to persist in medical environments, making it especially dangerous for immune-compromised hospital patients (Gaddy et al., 2009). Bacterial transmission is primarily from contact with contaminated surfaces, but can also occur by person-to-person spread in hospitals. Traditionally, the habitat of A. baumannii was thought to be exclusively clinical settings (Perez et al., 2007; Towner, 2009); however, a comprehensive review by Eveillard et al. (2013) concluded that the idea that A. baumannii is isolated exclusively from hospitals is flawed and that extra-hospital reservoirs probably exist. Such reservoirs could include pets, slaughter animals, lice, or human carriage; as the presence of A. baumannii in these locations has been shown by improved identification methods (Turton et al., 2006; Gundi et al., 2009; Eveillard et al., 2013).
A. BAUMANNII IN THE ORAL CAVITY
An increasing range of medically relevant pathogens are recognized in the oral cavity. Respiratory and systemic pathogens that have been isolated from chronic periodontitis and aggressive periodontitis patients include Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecalis, along with A. baumannii (Didilescu et al., 2005; da Silva-Boghossian et al., 2011). Furthermore, A. baumannii was identified with a significantly higher prevalence in patients with chronic or aggressive periodontitis compared with healthy individuals or patients with gingivitis (Slots et al., 1991; Ali et al., 1996; Colombo et al., 2002; Souto et al., 2006; da Silva-Boghossian et al., 2011; Silva-Boghossian et al., 2013), particularly in patients with human immunodeficiency virus (Goncalves et al., 2007). While the role of A. baumannii in periodontal disease has yet to be investigated, the presence of the organism in conjunction with the traditional periodontal pathogens Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola and Aggregatibacter actinomycetemcomitans along with P. aeruginosa increases the likelihood of aggressive periodontitis (da Silva-Boghossian et al., 2011). Additionally, the odds of a subject being refractory to periodontal treatment increase when A. baumannii is present (Colombo et al., 1998). As A. baumannii is well-equipped to survive in polymicrobial communities (see below) further study of interspecies interactions involving the organism may begin to elucidate any contribution to periodontal disease.
The presence of respiratory pathogens establishes the oral microbiota as an extra-hospital reservoir, and aerosolization of these bacteria into the lower respiratory tract can cause pneumonia and chronic obstructive pulmonary disease (Scannapieco et al., 2003). Consistent with this, hospitalized chronic lung disease patients have a higher incidence of respiratory pathogens, including A. baumannii, present in supra-gingival plaque (Didilescu et al., 2005). In addition, due to the anatomical closeness, aerosolized bacteria can enter the bloodstream and cause septicemia (Scannapieco & Ho, 2001). A corollary to an oral reservoir for pulmonary disease is that efforts have been made to reduce respiratory diseases by addressing oral health. For example, A. baumannii is a major pathogen in ventilator-associated pneumonia, which is a large problem in hospitals, especially in intensive care units (Ayraud-Thevenot et al., 2012; Lee et al., 2012; Martinez-Lamas et al., 2014). Özçako et al. (2012) showed that by simply swabbing the teeth of ventilated patients with 0.2% chlorhexidine gluconate the risk of ventilator-associated pneumonia was reduced.
POLYMICROBIAL INTERACTIONS
In the host and environment A. baumannii encounters and interacts with other organisms. It colonizes the multispecies oral biofilms on tooth surfaces, and although individual synergistic or antagonistic inter-species interactions have yet to be examined in detail, Streptococcus sanguinis produces an extracellular compound that is bactericidal to A. baumannii (Watanabe et al., 2009). Antagonism between A. baumannii and the early-colonizing S. sanguinis may be one reason A. baumannii is often associated with the gram-negative anaerobic later colonizers. Mixed infections with A. baumannii have also been documented with other gram-negative pathogens in intensive care unit patients (Didilescu et al., 2005; Souto et al., 2006; Mammina et al., 2013). Moreover, carbapenem-resistant A. baumannii is commonly found in mixed infections with other carbapenem-resistant pathogens such as Klebsiella pneumoniae, Enterobacteriaceae and P. aeruginosa (Marchaim et al., 2012; Mammina et al., 2013). Carbapenem-resistant A. baumannii may also shelter carbapenem-susceptible bacteria in a polymicrobial infection, exacerbating disease progression during carbapenem treatment (Liao et al., 2014). Secondary bacterial infection with A. baumannii has also been seen in pandemic outbreaks of respiratory illness associated with the influenza A (H1N1) virus (Palacios et al., 2009; Champunot et al., 2010; Schoindre et al., 2011).
It was recently established that A. baumannii possess a type VI secretion system (T6SS), a bacterial protein export machine that resembles the tail assembly of contractile bacteriophages (Carruthers et al., 2013). T6SS are often used to inject toxic effector molecules into other bacteria (Carruthers et al., 2013), and the T6SS of A. baumannii allows the organism to outcompete E. coli in mixed cultures (Carruthers et al., 2013). In addition, A. baumannii has the ability to inhibit Candida albicans filamentous growth and biofilm formation (Peleg et al., 2008b). However, if C. albicans biofilm is first allowed to mature it can inhibit the growth of A. baumannii through the quorum-sensing molecule, farnesol (Peleg et al., 2008b). Attachment of A. baumannii to Candida is mediated by the outer membrane protein A (OmpA), and secretion of OmpA can kill Candida by the induction of apoptosis (Gaddy et al., 2009). These processes demonstrate unique cross-kingdom extracellular signaling, probably to control microbial composition in niches containing both organisms, and modulate the virulence of the mixed species community. Such antagonistic interactions also suggest the potential for novel therapeutic agents to combat diseases that are challenging to treat due to antibiotic resistance.
ANTIBIOTIC RESISTANCE
The prevalence of extensively drug-resistant and pan-drug-resistant strains is causing concern for the end of the ‘antibiotic era’ (Hsueh et al., 2002; Kuo et al., 2012). Acinetobacter baumannii is a member of the ESKAPE group of organisms (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa and Enterobacter species) which are (generally) nosocomially acquired pathogens that have a high rate of antibiotic resistance (Rice, 2008). The frequency of carbape-nem-resistant isolates is on the rise with the increased use of broad-spectrum antibiotics in hospitals (Towner, 2009). For example, imipenem, a carbapenem that is considered a last-resort drug, is now ineffective against ~15% of isolates (Towner, 2009). A study on US combat casualties from 2005 to 2007 observed that Acinetobacter baumannii–calcoaceticus complex was the most commonly recovered multi-drug-resistant pathogen of interest, and the only one to have increasing microbial resistance each year (Murray et al., 2009). Current antibiotic treatment regimens consist of combination therapy, which has so far been effective in eliminating multidrug-resistant strains (Perez et al., 2007); however, the failure of this approach seems only a matter of time.
An almost bewildering number of antibiotic resistance mechanisms has been acquired by A. baumannii (Table 1). High-density pyrosequencing of A. baumannii strain ATCC 17978 showed 75 potential drug-resistance genes composed of 32 efflux pumps and 11 permeases (Smith et al., 2007). Other mechanisms that A. baumannii strains have adopted include, but are not limited to; class A–D β-lactamases, modifications of outer-membrane proteins and penicillin-binding proteins, aminoglycoside-modifying enzymes, and modifications or loss of lipopolysaccharides (Fernandez-Cuenca et al., 2003; Perez et al., 2007; Moffatt et al., 2013). Many of the associated genes and other putative virulence genes are located within pathogenicity islands (Smith et al., 2007). Fournier et al. (2006) identified an 86-kb hotspot in A. baumannii strain AYE that has two different genomic island insertions containing 45 of its 52 drug-resistance genes. This hotspot, named AbaR1, is surrounded by broad-host-range mobile genetic elements such as insertion sequences, transposons and class 1 integrons (Fournier et al., 2006). These genes are predicted to have originated from other gram-negative organisms such as E. coli, Salmonella spp. and Pseudomonas spp. (Fournier et al., 2006). Clearly A. baumannii has an unparalleled ability to change, rearrange and acquire genetic elements, making it highly adaptable to its surroundings and causing variation among strains (Averhoff & Friedrich, 2003).
Table 1.
Drug resistance mechanisms of Acinetobacter baumannii1
| Drug class | Mechanism of resistance | Example of effector molecules | Reference(s) |
|---|---|---|---|
| Aminoglycosides | Modifying enzymes | ||
| Acetyltransferase | ACC | Bakour et al. (2013) | |
| 16S rRNA methylation | ArmA | Doi et al. (2007) | |
| Phosphotransferase | APHA1, StrA, StrB | McGann et al. (2014), Nigro et al. (2013) | |
| Adenylytransferase | AadAB | Bakour et al. (2013) | |
| Efflux pump | AdeABC, AdeM | Magnet et al. (2001), Su et al. (2005) | |
| Carbapenems | Carbapenemases | Oxa-23, -58, -64, -65, -66, -68, -70, -71, -78, -79, -80 | Perez et al. (2007) |
| Loss of protein function | CarO | Mussi et al. (2005) | |
| Decreased expression | 33–36 kDa OMP | Clark (1996) | |
| Efflux pump | AdeIJK | Coyne et al. (2011) | |
| Cephalosporins | Efflux pump | AdeIJK | Coyne et al. (2011) |
| β-lactamases | AmpC | Rezaee et al. (2013) | |
| Lincosamides | Efflux pump | AdeFGH, AdeIJK, MsrA, MsrB | Coyne et al. (2011), Taitt et al. (2014) |
| Macrolides | Efflux pump | AdeABC, MsrA, MsrB | Magnet et al. (2001), Taitt et al. (2014) |
| Monobactams | Efflux pump | AdeIJK | Coyne et al. (2011) |
| Penicillins | Altered penicillin-binding proteins | PBP1-3,5-8 | Cayo et al. (2011) |
| β-lactamases | PER-1, TEM-1, VEB-1, CTX-M | Bae et al. (2011), Perez et al. (2007), Walther-Rasmussen & Hoiby (2004) | |
| Metallo-β-lactamases | IMP-1, -2, -4, -5, -6, -11 VIM-2, NDM-1 | Walsh et al. (2005) | |
| Efflux pump | AdeABC, AdeIJK | Yum et al. (2002), Jones et al. (2014) | |
| Decreased expression | 46 kDa OMP | Magnet et al. (2001), Coyne et al. (2011) | |
| Polypeptides | Gene mutations | pmrAB | Lesho et al. (2013) |
| phosphoethanolamine modification of lipid A | PmrC | Arroyo et al. (2011) | |
| Quinolones/Fluoroquinolone | Efflux pump | AdeABC, AdeIJK, AdeM, AdeFGH | Magnet et al. (2001), Su et al. (2005), Coyne et al. (2011) |
| Production of protective proteins | QnrA | Touati et al. (2008) | |
| Gene mutations | gyrA, parC | Hamouda & Amyes, | |
| Rifamycin | Efflux pump | AdeIJK | Coyne et al. (2011) |
| ADP-ribosyltransferase | ARR-2 | Houang et al. (2003) | |
| Sulfonamides | Drug-resistant variant | Sul1, Sul2 | Nigro et al. (2013) |
| Tetracyclines | Efflux pump | AdeABC, AdeIJK | Magnet et al. (2001), |
| TetAB | Coyne et al. (2011) | ||
| Decreased expression | CarO, OmpA38, OmpA32, OmpW | Guardabassi et al. (2000) | |
| Other | Aminocoumarin | ||
| Efflux pump | AdeIJK | Coyne et al. (2011) | |
| Chloramphenicol | |||
| Efflux pump | AdeABC, AdeFGH, AdeIJK, CmlA, CraA | Magnet et al. (2001), Coyne et al. (2011) | |
| Acetyltransferase | Cat | Turton et al. (2005) | |
| Fusidic acid | |||
| Efflux acid | AdeIJK | Coyne et al. (2011) | |
| Streptogramin | |||
| Efflux pump | MsrA, MsrB | Taitt et al. (2014) | |
| Tigecycline | |||
| Efflux pump | AdeABC, AdeIJK | Coyne et al. (2011) | |
| Trimethoprim | |||
| Efflux pump | AdeABC, AdeFGH, AdeIJK, AdeM | Coyne et al. (2011) | |
| Drug-resistant variant | FolA, DrfA1, DrfA7, DrfA19 | Taitt et al. (2014) |
Drug-resistance mechanisms vary greatly among strains and combinations of drug resistance genes are different. Many of these genes have been acquired by A. baumannii to confer drug-resistance, while some occur naturally.
Acinetobacter baumannii is naturally transformable through type IV pili (TFP) mediated uptake of foreign DNA and incorporation into the genome via homologous recombination (Metzgar et al., 2004; Harding et al., 2013). This accounts for the large strain variability and rapid development of antibiotic resistance. Acinetobacter species do not produce flagella, which led to the genus name which means non-motile rod in Greek. However, this is a misnomer, as A. baumannii is capable of twitching motility via the extension and retraction of the TFP (Harding et al., 2013). The TFP also play a role in adherence to surfaces (Harding et al., 2013), and strikingly A. baumannii is capable of moving along wet surfaces and picking up DNA, a process dependent on functional TFP (Wilharm et al., 2013). As an adjunct to conventional horizontal gene transfer, A. bauamannii is also able to secrete outer membrane vesicles containing antibiotic resistance genes, which can be acquired by susceptible strains thereby providing them with protection (Rumbo et al., 2011).
PATHOGENIC MECHANISMS
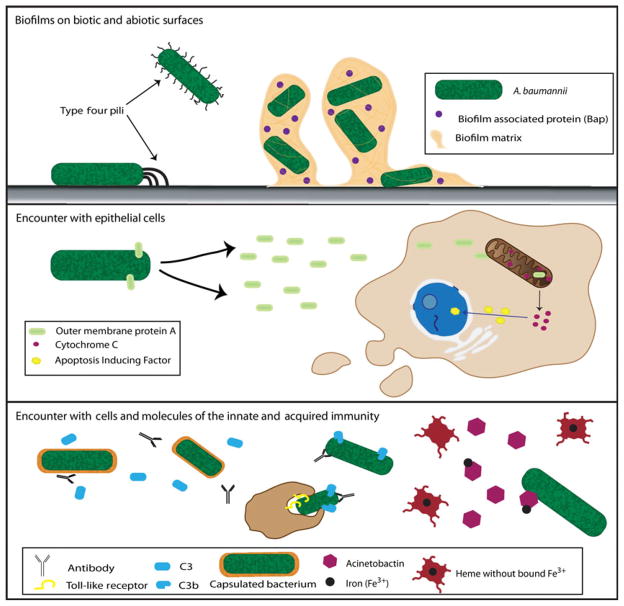
Despite the significant threat to human health posed by A. baumannii, comparatively little is known about its virulence mechanisms. The four main pathogenic mechanisms and factors described to date are biofilm formation, outer membrane protein A (OmpA or Omp38), the K1 capsule and a siderophore-mediated iron-acquisition system (Dorsey et al., 2003, 2004; Tomaras et al., 2003; Choi et al., 2005) (Fig. 1). Establishment of a biofilm is crucial to colonization by A. baumannii, and biofilm formation depends on pilus production mediated by the CsuA/BABCDE chaperone-usher assembly system (Tomaras et al., 2003; Gaddy et al., 2009). CsuA/B is thought to constitute the pilin subunit, and CsuE is the tip adhesin (de Breij et al., 2009). Transposon mutagenesis studies have also identified an RNase T2 family protein as a positive regulator of biofilm formation and motility (Jacobs et al., 2014). The biofilm-associated-protein, Bap which is homologous to the Staphylococcus aureus Bap protein, is required for maintenance and maturation of the biofilm (Loehfelm et al., 2008; Goh et al., 2013). Bap also plays an important role in the colonization of the host as it is involved in initial adherence to eukaryotic cells (Loehfelm et al., 2008; Brossard & Campagnari, 2012). Production of OmpA is necessary for the development of robust biofilms on abiotic surfaces and while the mechanistic basis is unclear, OmpA functions in conjunction with the pili (Gaddy et al., 2009). OmpA is essential for adherence to epithelial cells. Interestingly, when OmpA enters eukaryotic cells, via an unknown mechanism of entry, it localizes to the mitochondria, leading to the release of cytochrome c and apoptosis-inducing factor, and ultimately apoptotic cell death (Choi et al., 2005). Acinetobacter baumannii is resistant to serum killing, and OmpA contributes to serum resistance through binding and acquiring Factor H, an inhibitor of the alternative complement pathway (Kim et al., 2009). Biofilm formation, pilus and OmpA expression, along with serum sensitivity are regulated by the BfmS/R two-component system, which may sense and integrate signals derived from multiple environmental stimuli (McConnell et al., 2013).
Figure 1.
Virulence mechanisms of Acinetobacter baumannii. The organism is capable of forming biofilms on biotic and abiotic surfaces by attaching via its type IV pili. Subsequently Bap is secreted to help biofilm maturation and adherence to eukaryotic cells. Contact with host cells leads to secretion of OmpA, which induces apoptosis in the host by causing cytochrome c release from the mitochondria. This in turn stimulates to Apoptosis Inducing Factor localization in the nucleus. Capsulated A. baumannii are protected from detection by the host due to the inability of antibodies and complement to bind to the bacterial surface and diminished recognition by Toll-like receptors. To acquire the iron needed for survival, A. baumannii secretes Acinetobactin, a siderophore, which sequesters iron from the host.
Carbohydrates have a number of important functions for A. baumannii. Surface polysaccharide comprised poly-β-(1-6)-N-acetylglucosamine, the product of proteins encoded by the pgaABCD locus, contributes to biofilm development (Choi et al., 2009). The core sugars of both the lipopolysaccharide and capsular polysaccharide contribute to serum resistance and are necessary for full virulence in animal models (Luke et al., 2010; Russo et al., 2010). A recent study showed that type I capsular polysaccharide and O-glycoproteins are dependent on the activity of the PglC glycosyltransferase, and pentameric glycan subunits are used both individually for O-glycosylation, or polymerized for capsular polysaccharide. The synthesis of these structures appears to be common at the early stages; however the pathways bifurcate in the periplasm (Lees-Miller et al., 2013).
Acinetobacter baumannii produces phospholipases, lipolytic enzymes that can disrupt eukaryotic cell membranes. A phospholipase D is important for resistance to serum killing and epithelial cell invasion. In addition, in a murine model of pneumonia a phospholipase D mutant showed diminished dissemination from the lungs (Jacobs et al., 2010). Acinetobacter baumannii has two potential phospholipase C genes and disruption of one phospholipase C gene results in a decrease in A. baumannii-induced epithelial cell apoptosis (Camarena et al., 2010).
To enable survival in the iron-limiting conditions of the host, A. baumannii produces a unique catechol siderophore, which is structurally related to that of Vibrio anguillarum, termed acinetobactin (Yamamoto et al., 1994). Acinetobactin synthesis and export requires an 18-gene cluster organized into seven operons, some of which have been demonstrated to be upregulated under conditions of iron-limitation (Fiester & Actis, 2013). When iron conditions are plentiful the bacteria favor a planktonic lifestyle; while in the presence of iron-chelators, the bacteria increase attachment and biofilm formation, and decrease motility (Tomaras et al., 2003; McQueary et al., 2012). In the nosocomial environment A. baumannii will be exposed to iron-limiting conditions, thus promoting bio-film formation. Acinetobactin is important for virulence, as mutants deficient in acinetobactin production are compromised in their ability to persist and cause damage in epithelial cells, mice and caterpillars (Gaddy et al., 2012).
Acinetobacter baumannii is capable of quorum-sensing through the production of an N-acyl-homoserine lactone (AHL) by the synthase AbaI, which is homologous the LuxI family of molecules (Niu et al., 2008). An abaI mutant has a reduced biofilm-forming and motility phenotype, indicating a role for quorum-sensing in these processes (Niu et al., 2008; Clemmer et al., 2011). In a study of 32 Acinetobacter strains, 63% produced more than one AHL, suggesting that additional roles of quorum-sensing in A. baumannii remain to be discovered (Gonzalez et al., 2009). As mentioned, A. baumannii is frequently co-isolated with P. aeruginosa and the presence of these species together in the oral cavity increases the likelihood of aggressive periodontitis. AHL-dependent cross-talk between A. baumannii or P. aeruginosa can occur as the AHL of either species can induce the heterologous promoter in a mixed infection (Bhargava et al., 2012). In addition, the toxin, pyocyanin, produced by P. aeruginosa, does not affect the growth of A. baumannii, so removing a significant impediment to synergism between the organisms (Bhargava et al., 2012). Indeed, pyocyanin stimulates quorum-sensing-mediated tolerance to oxidative stress and increases the ‘persister’ cell population in A. baumannii (Bhargava et al., 2014). Mixed-species biofilms also have increased resistance to antibiotics compared with single-species biofilms (Burmolle et al., 2006). Such interspecies interactions may aid in the co-existence of A. baumannii with organisms in mixed infections and increase disease severity (Bhargava et al., 2012).
INTERACTIONS WITH THE IMMUNE SYSTEM
Immune responses to A. baumannii have yet to be extensively studied. Acinetobacter baumannii can incite a proinflammatory response in airway epithelial cells through recognition of microbe-associated molecular patterns such as lipopolysaccharide, and subsequent activation of mitogen-activated protein kinase and NF-κB signaling pathways (March et al., 2010). Innate immune mediators that are induced by A. baumannii include the neutrophil chemokine IL-8 and antimicrobial molecules such as β-defensins (March et al., 2010). Neutrophil recruitment to the lung is important in controlling multiplication and dissemination of A. baumannii (van Faassen et al., 2007), and both neutrophils and macrophages have been shown to internalize and kill the bacteria via reactive oxygen and nitrogen species (Qiu et al., 2009, 2012). Conflicting reports on the involvement of Toll-like receptors and their ability to recognize A. baumannii suggest that innate immunity against A. baumannii is strain variable and other innate immune factors are involved (Knapp et al., 2006; Erridge et al., 2007; Lin et al., 2012). One of those factors may be the NOD1/2 recognition and signaling pathway. NOD1/2 are intracellular pattern recognition receptors that signal downstream to Rip2 in the induction of NF-κB activation and apoptosis (Nembrini et al., 2009). Upon depletion of NOD1, NOD2 or Rip2 in lung epithelial cells, A. baumannii replication dramatically increased; however, this was not observed in macrophages, indicating cell-specific responses to the organism (Bist et al., 2013). OmpA is another immunomodulatory microbe-associated molecular pattern of A. baumannii that can upregulate nitric oxide synthase and Toll-like receptor 2 responses in laryngeal epithelial cells (Kim et al., 2008). At sublethal concentrations OmpA activates dendritic cells leading to differentiation of CD4+ T cells toward a T helper type 1 polarizing phenotype (Lee et al., 2007). Hence OmpA is an important determinant of the nature and extent of immune responses to A. baumannii.
No vaccine against Acinetobacter is currently available. Approaches in antigen-specific vaccine development include targeting OmpA, Bap, K1 capsule polysaccharide, and a membrane transporter Ata (Garcia-Quintanilla et al., 2013). Other strategies have involved inactivated whole cells, outer membrane complexes, and outer membrane vesicles (Garcia-Quintanilla et al., 2013). A mouse model has been developed, and has demonstrated the success of intranasal immunization with an inactivated whole cell vaccine against respiratory infection (Kuolee et al., 2014). Acinetobacter baumannii is generally viewed as an extracellular pathogen but more evidence is being discovered that an intracellular lifestyle can be supported, further complicating delivery of immune efforts required for a successful vaccine (Choi et al., 2008; Smani et al., 2012).
BLUE-LIGHT SENSING
Sensing of the surrounding environment is important to the survival of any organism. The environment provides crucial information that, in return, the organism will respond to, generally through a series of complex signal transduction pathways. It was recently discovered that A. baumannii ATCC 17978 and many other strains have the unique ability to sense blue light and alter virulence factors in response (Mussi et al., 2010). Current dogma limits light to be a driving force only in photosynthetic/phototropic organisms that are dependent on light for energy; however, that idea is quickly changing as new discoveries are made about the use of light by non-photosynthetic organisms, e.g. circadian rhythms and phototaxis. The original unexpected discovery was made in Brucella abortus, another non-photosynthetic pathogen capable of causing severe infection in humans; in response to visible light these bacteria become more virulent (Swartz et al., 2007). This response is mediated by a light, oxygen, or voltage (LOV) histidine kinase, a newly described light sensor and regulator, whose enzymatic activity is increased in the presence of light (Swartz et al., 2007). In B. abortus, the LOV domain containing protein is directly responsible for survival and replication within macrophages (Swartz et al., 2007). Conversely, the light-sensing protein discovered in A. baumannii ATCC 17978 does not contain a LOV domain but instead uses a blue-light sensing using flavin (BLUF) domain (Mussi et al., 2010) (Fig. 2). The BLUF domain, upon excitation by blue light at a wavelength of 470 nm, causes a conformational change in the protein, known as a red-shift (signaling state). This allows for the binding of the chromophore, flavin adenine dinuclecotide, between two α-helices, in a reversible process (Nagai et al., 2008; Mussi et al., 2010; Brust et al., 2014). The protein identified in A. baumannii, named Blue-light-sensing protein A (BlsA), is small and lacks an effector or output domain, making functional and binding predictions difficult (Mussi et al., 2010). The transcript level of blsA is upregulated in the dark (Mussi et al., 2010), and BlsA is involved in several virulence attributes of the organism (Mussi et al., 2010). In the presence of blue light, A. baumannii fails to produce biofilms and pellicles, does not move on semisolid media plates (Mussi et al., 2010), and exhibits an enhanced killing of C. albicans hyphae (Mussi et al., 2010). It is interesting to note that light regulation is only observed at the environmental temperature of 24° C rather than the pathologically relevant temperature of 37° C. Light regulation is not limited to A. baumannii but is widespread within the genus of Acinetobacter (Golic et al., 2013). Some species contain more than one BLUF-containing protein; many of these being environmental strains. Phylogenetic evidence suggests that the different BLUF proteins are derived from a common original predecessor (Golic et al., 2013). Adding to the difficulty in interpretation of the original role of blue-light sensing, A. baumannii regulation in response to light is different from most of the other Acinetobacter species in that it produces the opposite effect on bio-film formation (Golic et al., 2013). Also, many of the other species exhibit light regulation at 37° C, which could be a result of having multiple BLUF proteins (Golic et al., 2013).
Figure 2.
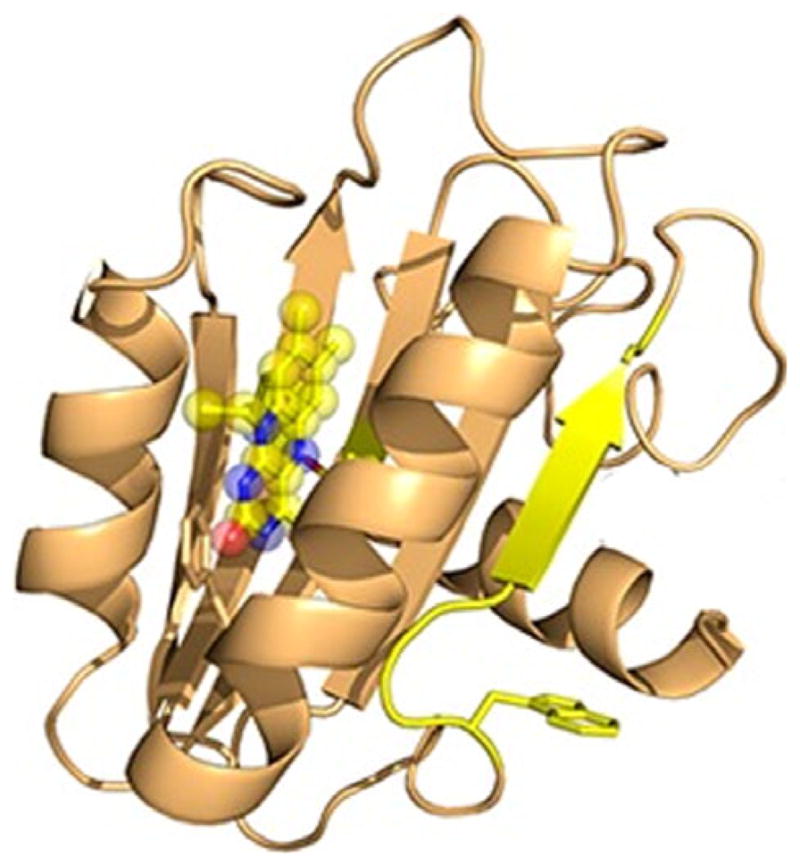
Structure of BlsA. The Blue-light sensing protein of Acine-tobacter baumannii. The protein consists of two α helices that bind flavin adenine dinucleotide upon excitation by blue light at a wavelength of 470 nm. From Brust et al. (2014).
CONCLUSIONS
Over the last few decades the importance of A. baumannii has increased as a result of its rapidly evolving antibiotic resistance, its predilection for infecting battlefield wounds and its persistence in hospital environments. Indeed, there are many factors that make A. baumannii a dangerous organism, and many more likely to be discovered. Pathogenicity is multifactorial involving specific virulence factors in combination with metabolic capabilities and resistance to environmental stresses. Adaptation to stress involves intricate and interconnected regulatory pathways that integrate environmental signals with growth and survival decisions that in turn impact pathogenic potential. The oral cavity can act as a reservoir for serious pulmonary infections, and sub-gingivally A. baumannii may increase the risk of aggressive periodontitis. A unique blue-light sensing and response system is present in A. baumannii, further study of which will reveal hitherto unrecognized aspects of the interface between bacteria and the environment. Given the versatility and pathogenic potential of the A. baumannii it is imperative that we make further progress understanding how to control its spread and render it incapable of damaging the host.
Acknowledgments
Preparation of the manuscript was supported by National Institutes of Health grants DE011111, DE012505, DE016690, DE017921, DE022867, DE023193 (RJL), AI069321 and AI107978 (YAK), and AMR was in receipt of an NSF fellowship.
References
- Ali RW, Velcescu C, Jivanescu MC, Lofthus B, Skaug N. Prevalence of 6 putative periodontal pathogens in subgingival plaque samples from Romanian adult periodontitis patients. J Clin Periodontol. 1996;23:133–139. doi: 10.1111/j.1600-051x.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Arroyo LA, Herrera CM, Fernandez L, Hankins JV, Trent MS, Hancock RE. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother. 2011;55:3743–3751. doi: 10.1128/AAC.00256-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averhoff B, Friedrich A. Type IV pili-related natural transformation systems: DNA transport in mesophilic and thermophilic bacteria. Arch Microbiol. 2003;180:385–393. doi: 10.1007/s00203-003-0616-6. [DOI] [PubMed] [Google Scholar]
- Ayraud-Thevenot S, Huart C, Mimoz O, et al. Control of multi-drug-resistant Acinetobacter baumannii outbreaks in an intensive care unit: feasibility and economic impact of rapid unit closure. J Hosp Infect. 2012;82:290–292. doi: 10.1016/j.jhin.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Bae IK, Jang SJ, Kim J, Jeong SH, Cho B, Lee K. Interspecies dissemination of the bla gene encoding PER-1 extended-spectrum beta-lactamase. Antimicrob Agents Chemother. 2011;55:1305–1307. doi: 10.1128/AAC.00994-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakour S, Touati A, Sahli F, Ameur AA, Haouchine D, Rolain JM. Antibiotic resistance determinants of multidrug-resistant Acinetobacter baumannii clinical isolates in Algeria. Diagn Microbiol Infect Dis. 2013;76:529–531. doi: 10.1016/j.diagmicrobio.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Bhargava N, Sharma P, Capalash N. N-acyl homoserine lactone mediated interspecies interactions between A. baumannii and P. aeruginosa. Biofouling. 2012;28:813–822. doi: 10.1080/08927014.2012.714372. [DOI] [PubMed] [Google Scholar]
- Bhargava N, Sharma P, Capalash N. Pyocyanin stimulates quorum sensing-mediated tolerance to oxidative stress and increases persister cells population in Acinetobacter baumannii. Infect Immun. 2014;82:3417–3425. doi: 10.1128/IAI.01600-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bist P, Dikshit N, Koh TH, Mortellaro A, Tan TT, Sukumaran B. Nod1, Nod2 and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection. Infect Immun. 2013;82:1112–1122. doi: 10.1128/IAI.01459-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breij A, Gaddy J, van der Meer J, et al. CsuA/BABCDE-dependent pili are not involved in the adherence of Acinetobacter baumannii ATCC19606(T) to human airway epithelial cells and their inflammatory response. Res Microbiol. 2009;160:213–218. doi: 10.1016/j.resmic.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Brossard KA, Campagnari AA. The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun. 2012;80:228–233. doi: 10.1128/IAI.05913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust R, Haigney A, Lukacs A, et al. Ultrafast structural dynamics of BlsA, a photoreceptor from the pathogenic bacterium. J Phys Chem Lett. 2014;5:220–224. doi: 10.1021/jz4023738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmolle M, Webb JS, Rao D, Hansen LH, Sorensen SJ, Kjelleberg S. Enhanced biofilm formation and increased resistance to antimicrobial agents and bacterial invasion are caused by synergistic interactions in multispecies biofilms. Appl Environ Microbiol. 2006;72:3916–3923. doi: 10.1128/AEM.03022-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010;6:e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers MD, Nicholson PA, Tracy EN, Munson RS., Jr Acinetobacter baumannii utilizes a type VI secretion system for bacterial competition. PLoS ONE. 2013;8:e59388. doi: 10.1371/journal.pone.0059388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayo R, Rodriguez MC, Espinal P, et al. Analysis of genes encoding penicillin-binding proteins in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:5907–5913. doi: 10.1128/AAC.00459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champunot R, Tanjatham S, Kerdsin A, et al. Impact of pandemic influenza (H1N1) virus-associated community-acquired pneumonia among adults in a tertiary hospital in Thailand. Jpn J Infect Dis. 2010;63:251–256. [PubMed] [Google Scholar]
- Choi CH, Lee EY, Lee YC, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- Choi CH, Lee JS, Lee YC, Park TI, Lee JC. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol. 2008;8:216. doi: 10.1186/1471-2180-8-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litran T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–5963. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RB. Imipenem resistance among Acinetobacter baumannii: association with reduced expression of a 33–36 kDa outer membrane protein. J Antimicrob Chemother. 1996;38:245–251. doi: 10.1093/jac/38.2.245. [DOI] [PubMed] [Google Scholar]
- Clemmer KM, Bonomo RA, Rather PN. Genetic analysis of surface motility in Acinetobacter baumannii. Microbiology. 2011;157:2534–2544. doi: 10.1099/mic.0.049791-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo AP, Haffajee AD, Dewhirst FE, et al. Clinical and microbiological features of refractory periodontitis subjects. J Clin Periodontol. 1998;25:169–180. doi: 10.1111/j.1600-051x.1998.tb02424.x. [DOI] [PubMed] [Google Scholar]
- Colombo AP, Teles RP, Torres MC, et al. Subgingival microbiota of Brazilian subjects with untreated chronic periodontitis. J Periodontol. 2002;73:360–369. doi: 10.1902/jop.2002.73.4.360. [DOI] [PubMed] [Google Scholar]
- Coyne S, Courvalin P, Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother. 2011;55:947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11:1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didilescu AC, Skaug N, Marica C, Didilescu C. Respiratory pathogens in dental plaque of hospitalized patients with chronic lung diseases. Clin Oral Investig. 2005;9:141–147. doi: 10.1007/s00784-005-0315-6. [DOI] [PubMed] [Google Scholar]
- Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- Doi Y, Adams JM, Yamane K, Paterson DL. Identification of 16S rRNA methylase-producing Acinetobacter baumannii clinical strains in North America. Antimicrob Agents Chemother. 2007;51:4209–4210. doi: 10.1128/AAC.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey CW, Tolmasky ME, Crosa JH, Actis LA. Genetic organization of an Acinetobacter baumannii chromosomal region harbouring genes related to siderophore biosynthesis and transport. Microbiology. 2003;149:1227–1238. doi: 10.1099/mic.0.26204-0. [DOI] [PubMed] [Google Scholar]
- Dorsey CW, Tomaras AP, Connerly PL, Tolmasky ME, Crosa JH, Actis LA. The sidero-phore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology. 2004;150:3657–3667. doi: 10.1099/mic.0.27371-0. [DOI] [PubMed] [Google Scholar]
- Erridge C, Moncayo-Nieto OL, Morgan R, Young M, Poxton IR. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J Med Microbiol. 2007;56:165–171. doi: 10.1099/jmm.0.46823-0. [DOI] [PubMed] [Google Scholar]
- Eveillard M, Kempf M, Belmonte O, Pailhories H, Joly-Guillou ML. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis. 2013;17:e802–e805. doi: 10.1016/j.ijid.2013.03.021. [DOI] [PubMed] [Google Scholar]
- van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun. 2007;75:5597–5608. doi: 10.1128/IAI.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cuenca F, Martinez-Martinez L, Conejo MC, Ayala JA, Perea EJ, Pascual A. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother. 2003;51:565–574. doi: 10.1093/jac/dkg097. [DOI] [PubMed] [Google Scholar]
- Fiester SE, Actis LA. Stress responses in the opportunistic pathogen Acinetobacter baumannii. Future Microbiol. 2013;8:353–365. doi: 10.2217/fmb.12.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier PE, Vallenet D, Barbe V, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy JA, Tomaras AP, Actis LA. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun. 2009;77:3150–3160. doi: 10.1128/IAI.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy JA, Arivett BA, McConnell MJ, Lopez-Rojas R, Pachon J, Actis LA. Role of acinetob-actin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect Immun. 2012;80:1015–1024. doi: 10.1128/IAI.06279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Quintanilla M, Pulido MR, McConnell MJ. First steps towards a vaccine against Acinetobacter baumannii. Curr Pharm Biotechnol. 2013;14:897–902. doi: 10.2174/1389201014666131226123511. [DOI] [PubMed] [Google Scholar]
- Goh HM, Beatson SA, Totsika M, et al. Molecular analysis of the Acinetobacter baumannii biofilm-associated protein. Appl Environ Microbiol. 2013;79:6535–6543. doi: 10.1128/AEM.01402-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic A, Vaneechoutte M, Nemec A, Viale AM, Actis LA, Mussi MA. Staring at the cold sun: blue light regulation is distributed within the genus Acinetobacter. PLoS ONE. 2013;8:e55059. doi: 10.1371/journal.pone.0055059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves LS, Soares Ferreira SM, Souza CO, Souto R, Colombo AP. Clinical and micro-biological profiles of human immunodeficiency virus (HIV)-seropositive Brazilians undergoing highly active antiretroviral therapy and HIV-seronegative Brazilians with chronic periodontitis. J Periodontol. 2007;78:87–96. doi: 10.1902/jop.2007.060040. [DOI] [PubMed] [Google Scholar]
- Gonzalez RH, Dijkshoorn L, Van den Barselaar M, Nudel C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Rev Argent Microbiol. 2009;41:73–78. [PubMed] [Google Scholar]
- Guardabassi L, Dijkshoorn L, Collard JM, Olsen JE, Dalsgaard A. Distribution and in-vitro transfer of tetracycline resistance determinants in clinical and aquatic Acinetobacter strains. J Med Microbiol. 2000;49:929–936. doi: 10.1099/0022-1317-49-10-929. [DOI] [PubMed] [Google Scholar]
- Gundi VA, Dijkshoorn L, Burignat S, Raoult D, La Scola B. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology. 2009;155:2333–2341. doi: 10.1099/mic.0.026054-0. [DOI] [PubMed] [Google Scholar]
- Hamouda A, Amyes SG. Novel gyrA and parC point mutations in two strains of Acinetobacter baumannii resistant to ciprofioxacin. J Antimicrob Chemother. 2004;54:695–696. doi: 10.1093/jac/dkh368. [DOI] [PubMed] [Google Scholar]
- Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS., Jr Acinetobacter baumannii strain M2 produces type IV pili which play a role in natural transformation and twitching motility but not surface-associated motility. MBio. 2013;4:e00360. doi: 10.1128/mBio.00360-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houang ET, Chu YW, Lo WS, Chu KY, Cheng AF. Epidemiology of rifampin ADP-ribosyltrans-ferase (arr-2) and metallo-beta-lactamase (blaIMP-4) gene cassettes in class 1 integrons in Acinetobacter strains isolated from blood cultures in 1997 to 2000. Antimicrob Agents Chemother. 2003;47:1382–1390. doi: 10.1128/AAC.47.4.1382-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh PR, Teng LJ, Chen CY, et al. Pan-drug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerg Infect Dis. 2002;8:827–832. doi: 10.3201/eid0808.020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AC, Hood I, Boyd KL, et al. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun. 2010;78:1952–1962. doi: 10.1128/IAI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AC, Blanchard CE, Catherman SC, Dunman PM, Murata Y. An ribonuclease T2 family protein modulates Acinetobacter baumannii abiotic surface colonization. PLoS ONE. 2014;9:e85729. doi: 10.1371/journal.pone.0085729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad A, Heritage J, Snelling AM, Gascoyne-Binzi DM, Hawkey PM. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J Clin Microbiol. 1996;34:2881–2887. doi: 10.1128/jcm.34.12.2881-2887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad A, Seifert H, Snelling AM, Heritage J, Hawkey PM. Survival of Acinetobacter baumannii on dry surfaces: comparison of outbreak and sporadic isolates. J Clin Microbiol. 1998;36:1938–1941. doi: 10.1128/jcm.36.7.1938-1941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LS, Toleman MA, Weeks JL, Howe RA, Walsh TR, Kumarasamy KK. Plasmid carriage of blaNDM-1 in clinical Acinetobacter baumannii isolates from India. Antimicrob Agents Chemother. 2014;58:4211–4213. doi: 10.1128/AAC.02500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SA, Yoo SM, Hyun SH, et al. Global gene expression patterns and induction of innate immune response in human laryngeal epithelial cells in response to Acinetobacter baumannii outer membrane protein A. FEMS Immunol Med Microbiol. 2008;54:45–52. doi: 10.1111/j.1574-695X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- Kim SW, Choi CH, Moon DC, et al. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett. 2009;301:224–231. doi: 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, Florquin S, et al. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med. 2006;173:122–129. doi: 10.1164/rccm.200505-730OC. [DOI] [PubMed] [Google Scholar]
- Kuo SC, Chang SC, Wang HY, et al. Emergence of extensively drug-resistant Acinetobacter baumannii complex over 10 years: nationwide data from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program. BMC Infect Dis. 2012;12:200. doi: 10.1186/1471-2334-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuolee R, Harris G, Yan H, et al. Intranasal immunization protects against Acinetobacter baumannii-associated pneumonia in mice. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.02.083. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee JC, Lee CM, et al. Outer membrane protein A of Acinetobacter baumannii induces differentiation of CD4− T cells toward a Th1 polarizing phenotype through the activation of dendritic cells. Biochem Pharmacol. 2007;74:86–97. doi: 10.1016/j.bcp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Lee YT, Fung CP, Wang FD, Chen CP, Chen TL, Cho WL. Outbreak of imipenem-resistant Acinetobacter calcoaceticus–Acinetobacter baumannii complex harboring different carbapenemase gene-associated genetic structures in an intensive care unit. J Microbiol Immunol Infect. 2012;45:43–51. doi: 10.1016/j.jmii.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Lee HY, Chen CL, Wu SR, Huang CW, Chiu CH. Risk factors and outcome analysis of Acinetobacter baumannii complex bacteremia in critical patients. Crit Care Med. 2014;42:1081–1088. doi: 10.1097/CCM.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Lees-Miller RG, Iwashkiw JA, Scott NE, et al. A common pathway for O-linked protein-glycosylation and synthesis of capsule in Acinetobacter baumannii. Mol Microbiol. 2013;89:816–830. doi: 10.1111/mmi.12300. [DOI] [PubMed] [Google Scholar]
- Lesho E, Yoon EJ, McGann P, et al. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis. 2013;208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- Liao YT, Kuo SC, Lee YT, et al. Sheltering effect and indirect pathogenesis of carbapenem resistant Acinetobacter baumannii in polymicrobial infection. Anti-microb Agents Chemother. 2014;57:3983–3990. doi: 10.1128/AAC.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Tan B, Pantapalangkoor P, et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. MBio. 2012;3:e00312. doi: 10.1128/mBio.00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehfelm TW, Luke NR, Campagnari AA. Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol. 2008;190:1036–1044. doi: 10.1128/JB.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke NR, Sauberan SL, Russo TA, et al. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun. 2010;78:2017–2023. doi: 10.1128/IAI.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S, Courvalin P, Lambert T. Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob Agents Chemother. 2001;45:3375–3380. doi: 10.1128/AAC.45.12.3375-3380.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammina C, Bonura C, Vivoli AR, et al. Co-colonization with carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii in intensive care unit patients. Scand J Infect Dis. 2013;45:629–634. doi: 10.3109/00365548.2013.782614. [DOI] [PubMed] [Google Scholar]
- March C, Regueiro V, Llobet E, et al. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS ONE. 2010;5:e10033. doi: 10.1371/journal.pone.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa. Am J Infect Control. 2012;40:830–835. doi: 10.1016/j.ajic.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lamas L, Constenla-Carames L, Otero-Fernandez S, Alvarez-Fernandez M. New clone of ST-187 Acinetobacter baumannii responsible for an outbreak in an intensive care unit. Enferm Infecc Microbiol Clin. 2014;32:242–245. doi: 10.1016/j.eimc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Actis L, Pachon J. Acineto-bacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev. 2013;37:130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]
- McGann P, Courvalin P, Snesrud E, et al. Amplification of aminoglycoside resistance gene aphA1 in Acinetobacter baumannii results in tobramycin therapy failure. MBio. 2014;5:e00915. doi: 10.1128/mBio.00915-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueary CN, Kirkup BC, Si Y, et al. Extracellular stress and lipopolysaccharide modulate Acinetobacter baumannii surface-associated motility. J Microbiol. 2012;50:434–443. doi: 10.1007/s12275-012-1555-1. [DOI] [PubMed] [Google Scholar]
- Metzgar D, Bacher JM, Pezo V, et al. Acinetobacter sp. ADP1: an ideal model organism for genetic analysis and genome engineering. Nucleic Acids Res. 2004;32:5780–5790. doi: 10.1093/nar/gkh881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JH, Harper M, Mansell A, et al. Lipopolysaccharide-deficient Acinetobacter baumannii shows altered signaling through host Toll-like receptors and increased susceptibility to the host antimicrobial peptide LL-37. Infect Immun. 2013;81:684–689. doi: 10.1128/IAI.01362-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CK, Yun HC, Griffith ME, et al. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil Med. 2009;174:598–604. doi: 10.7205/milmed-d-03-8008. [DOI] [PubMed] [Google Scholar]
- Mussi MA, Limansky AS, Viale AM. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: natural insertional inactivation of a gene encoding a member of a novel family of beta-barrel outer membrane proteins. Antimicrob Agents Chemother. 2005;49:1432–1440. doi: 10.1128/AAC.49.4.1432-1440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi MA, Gaddy JA, Cabruja M, et al. The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. J Bacteriol. 2010;192:6336–6345. doi: 10.1128/JB.00917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Fukushima Y, Okajima K, Ikeuchi M, Mino H. Formation of interacting spins on flavosemiquinone and tyrosine radical in photoreaction of a blue light sensor BLUF protein TePixD. Biochemistry. 2008;47:12574–12582. doi: 10.1021/bi8010187. [DOI] [PubMed] [Google Scholar]
- Nembrini C, Kisielow J, Shamshiev AT, et al. The kinase activity of Rip2 determines its stability and consequently Nod1- and Nod2-mediated immune responses. J Biol Chem. 2009;284:19183–19188. doi: 10.1074/jbc.M109.006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro SJ, Farrugia DN, Paulsen IT, Hall RM. A novel family of genomic resistance islands, AbGRI2, contributing to aminoglycoside resistance in Acinetobacter baumannii isolates belonging to global clone 2. J Antimicrob Chemother. 2013;68:554–557. doi: 10.1093/jac/dks459. [DOI] [PubMed] [Google Scholar]
- Niu C, Clemmer KM, Bonomo RA, Rather PN. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J Bacteriol. 2008;190:3386–3392. doi: 10.1128/JB.01929-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özçako O, Basoglu OK, Buduneli N, Tasbakan MS, Bacakoglu F, Kinane DF. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodont Res. 2012;47:584–592. doi: 10.1111/j.1600-0765.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- Palacios G, Hornig M, Cisterna D, et al. Streptococcus pneumoniae coinfection is correlated with the severity of H1N1 pandemic influenza. PLoS ONE. 2009;4:e8540. doi: 10.1371/journal.pone.0008540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Choo JW, Kwon SH, et al. Risk factors for mortality in patients with Acinetobacter baumannii Bacteremia. Infect Chemother. 2013;45:325–330. doi: 10.3947/ic.2013.45.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008a;21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Tampakakis E, Fuchs BB, Eliopoulos GM, Moellering RC, Jr, Mylonakis E. Prokaryote–eukaryote interactions identified by using Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2008b;105:14585–14590. doi: 10.1073/pnas.0805048105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Kuolee R, Harris G, Chen W. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun. 2009;77:1015–1021. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS ONE. 2012;7:e40019. doi: 10.1371/journal.pone.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohan G, Srinivasan VB, Gebreyes WA. Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:1919–1925. doi: 10.1093/jac/dkq195. [DOI] [PubMed] [Google Scholar]
- Rezaee MA, Pajand O, Nahaei MR, et al. Prevalence of Ambler class A beta-lactamases and ampC expression in cephalosporin-resistant isolates of Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2013;76:330–334. doi: 10.1016/j.diagmicrobio.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKA-PE. J Infect Dis. 2008;197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- Rumbo C, Fernandez-Moreira E, Merino M, et al. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:3084–3090. doi: 10.1128/AAC.00929-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Luke NR, Beanan JM, et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010;78:3993–4000. doi: 10.1128/IAI.00366-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72:50–56. doi: 10.1902/jop.2001.72.1.50. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8:54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- Schoindre Y, Bollee G, Dumont MD, Lesavre P, Servais A. Cold agglutinin syndrome associated with a 2009 influenza A H1N1 infection. Am J Med. 2011;124:e1–e2. doi: 10.1016/j.amjmed.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Sheppard FR, Keiser P, Craft DW, et al. The majority of US combat casualty soft-tissue wounds are not infected or colonized upon arrival or during treatment at a continental US military medical facility. Am J Surg. 2010;200:489–495. doi: 10.1016/j.amjsurg.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Silva-Boghossian CM, Neves AB, Resende FA, Colombo AP. Suppuration-associated bacteria in patients with chronic and aggressive periodontitis. J Periodontol. 2013;84:e9–e16. doi: 10.1902/jop.2013.120639. [DOI] [PubMed] [Google Scholar]
- da Silva-Boghossian CM, do Souto RM, Luiz RR, Colombo AP. Association of red complex, A. actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch Oral Biol. 2011;56:899–906. doi: 10.1016/j.archoralbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Slots J, Rams TE, Feik D, Taveras HD, Gillespie GM. Subgingival microfiora of advanced periodontitis in the Dominican Republic. J Periodontol. 1991;62:543–547. doi: 10.1902/jop.1991.62.9.543. [DOI] [PubMed] [Google Scholar]
- Smani Y, Docobo-Perez F, Lopez-Rojas R, Dominguez-Herrera J, Ibanez-Martinez J, Pachon J. Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells. J Biol Chem. 2012;287:26901–26910. doi: 10.1074/jbc.M112.344556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MG, Gianoulis TA, Pukatzki S, et al. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 2007;21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto R, Andrade AFBD, Uzeda M, Colombo APV. Prevalence of “non-oral” pathogenic bacteria in subgingival biofilm of subjects with chronic periodontitis. Braz J Microbiol. 2006;37:208–215. [Google Scholar]
- Su XZ, Chen J, Mizushima T, Kuroda T, Tsuchiya T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob Agents Chemother. 2005;49:4362–4364. doi: 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz TE, Tseng TS, Frederickson MA, et al. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science. 2007;317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- Taitt CR, Leski TA, Stockelman MG, et al. Antimicrobial resistance determinants in Acinetobacter baumannii isolates taken from military treatment facilities. Antimicrob Agents Chemother. 2014;58:767–781. doi: 10.1128/AAC.01897-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras AP, Dorsey CW, Edelmann RE, Actis LA. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: involvement of a novel chaperone-usher pili assembly system. Microbiology. 2003;149:3473–3484. doi: 10.1099/mic.0.26541-0. [DOI] [PubMed] [Google Scholar]
- Touati A, Brasme L, Benallaoua S, Gharout A, Madoux J, De Champs C. First report of qnrB-producing Enterobacter cloacae and qnrA-producing Acinetobacter baumannii recovered from Algerian hospitals. Diagn Microbiol Infect Dis. 2008;60:287–290. doi: 10.1016/j.diagmicrobio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73:355–363. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- Turton JF, Kaufmann ME, Glover J, et al. Detection and typing of integrons in epidemic strains of Acinetobacter baumannii found in the United Kingdom. J Clin Microbiol. 2005;43:3074–3082. doi: 10.1128/JCM.43.7.3074-3082.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. Identification of Acinet-obacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther-Rasmussen J, Hoiby N. Cefotaximases (CTX-Mases), an expanding family of extended-spectrum beta-lactamases. Can J Microbiol. 2004;50:137–165. doi: 10.1139/w03-111. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Senba M, Ichinose A, Yamamoto T, Ariyoshi K, Matsumoto K. Bactericidal activity in filtrated supernatant of Streptococcus sanguinis against multidrug-resistant Pseudomonas aeruginosa. Tohoku J Exp Med. 2009;219:79–84. doi: 10.1620/tjem.219.79. [DOI] [PubMed] [Google Scholar]
- Wendt C, Dietze B, Dietz E, Ruden H. Survival of Acinetobacter baumannii on dry surfaces. J Clin Microbiol. 1997;35:1394–1397. doi: 10.1128/jcm.35.6.1394-1397.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilharm G, Piesker J, Laue M, Skiebe E. DNA uptake by the nosocomial pathogen Acinetobacter baumannii occurs during movement along wet surfaces. J Bacteriol. 2013;195:4146–4153. doi: 10.1128/JB.00754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Okujo N, Sakakibara Y. Isolation and structure elucidation of acinetobactin, a novel siderophore from Acinetobacter baumannii. Arch Microbiol. 1994;162:249–254. doi: 10.1007/BF00301846. [DOI] [PubMed] [Google Scholar]
- Yum JH, Yi K, Lee H, et al. Molecular characterization of metallo-beta-lactamase-producing Acineto-bacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the bla(VIM-2) gene cassettes. J Antimicrob Chemother. 2002;49:837–840. doi: 10.1093/jac/dkf043. [DOI] [PubMed] [Google Scholar]