Abstract
Ketone bodies (KBs), acetoacetate and β-hydroxybutyrate (βHB), were considered harmful metabolic by-products when discovered in the mid-19th century in the urine of patients with diabetic ketoacidosis. It took physicians many years to realize that KBs are normal metabolites synthesized by the liver and exported into the systemic circulation to serve as an energy source for most extrahepatic tissues. Studies have shown that the brain (which normally uses glucose for energy) can readily utilize KBs as an alternative fuel. Even when there is diminished glucose utilization in cognition-critical brain areas, as may occur early in Alzheimer’s disease (AD), there is preliminary evidence that these same areas remain capable of metabolizing KBs. Because the ketogenic diet (KD) is difficult to prepare and follow, and effectiveness of KB treatment in certain patients may be enhanced by raising plasma KB levels to ≥2 mM, KB esters, such as 1,3-butanediol monoester of βHB and glyceryl-tris-3-hydroxybutyrate, have been devised. When administered orally in controlled dosages, these esters can produce plasma KB levels comparable to those achieved by the most rigorous KD, thus providing a safe, convenient, and versatile new approach to the study and potential treatment of a variety of diseases, including epilepsy, AD, and Parkinson’s disease.
Keywords: epilepsy; Alzheimer’s disease; Parkinson’s disease; ketoacidosis; hyperketonemia; mitochondrial dysfunction; histone acetylation; 1,3-butanediol monoester of β-hydroxybutyrate; glyceryl-tris-3-hydroxybutyrate
Acetoacetate (AcAc) and β-hydroxybutyrate (βHB) are collectively known as ketone bodies (KBs) (Although many consider acetone an authentic member of the KB family, its importance for purposes of this review is minimal.). KBs have been dubbed “metabolism’s ugly duckling” because, in the mid-19th century, they were first discovered in large quantities in the urine of patients succumbing to diabetic ketoacidosis. Thus, it is not surprising that physicians of the era considered KBs to be toxic by-products of impaired carbohydrate metabolism. It took almost half a century for medical scientists to understand that KBs are normal metabolites manufactured by the liver in increasing amounts when dietary sources of carbohydrate and glucogenic amino acids are in short supply (1). Unfortunately, some physicians still fail to distinguish between the safe “physiological” hyperketonemia that occurs in healthy individuals during fasting or adherence to a ketogenic diet (KD), and the pathological, out-of-control hyperketonemia associated with insulin-deficient diabetes.
When Owen et al. (2) reported that during a prolonged fast KBs can provide 60% or more of the brain’s daily energy requirement (thereby sparing ∼80 g/day of glucose that otherwise would have been derived largely from breakdown of the body’s limited protein stores), it was finally acknowledged that, as in Hans Christian Andersen’s 1843 fairy tale, the creature first thought to be an ugly duckling was turning out to be an emerging swan. It became evident that the ketogenic response to starvation is an indispensable metabolic adaptation designed by nature to preserve strength and prolong life during times when food is unavailable (3).
It is now known that (in nondiabetic individuals), owing to the blood’s efficient buffering capacity, plasma KB levels can increase to 6–8 mM during a prolonged fast without giving rise to clinically hazardous acidosis (4).
PHYSIOLOGY OF KETOGENESIS
Four physiological facts lie at the root of the ketogenic adaptation: i) the body’s small reserve supply of preformed carbohydrate (largely as glycogen), ii) the body’s limited protein stores, iii) the relative plenitude in human adipose tissue of stored TG [triacylglycerol (TAG)], and iv) the inability of long-chain FAs (≥C12) to cross the blood-brain barrier (BBB). Given these considerations, the evolutionary advantage of having a TAG-derived metabolite capable of crossing the BBB and nourishing the brain during times when food is unavailable is self-evident.
In a 70 kg male of normal body composition, the amount of fuel reserves in the form of TAG is ∼12 kg. Muscle protein is ∼6 kg, while the carbohydrate reserves (glycogen) in liver and muscle are ∼100 g and ∼400 g, respectively (5). Glucose is the brain’s usual fuel source. After an overnight fast, owing to increased glucagon secretion and diminished insulin release, amplified mobilization of FFAs from adipose tissue is associated with their increased utilization by muscle and enhanced hepatic ketogenesis. However, at this early stage of carbohydrate privation, while plasma KBs are still low, the brain remains heavily dependent on glucose.
During total caloric starvation, the only source of new glucose is that synthesized from the glycerol released from adipose tissue together with FFA and from glucogenic amino acids derived from the breakdown of stored protein. With continued starvation, gluconeogenesis is curtailed, and the liver shifts acetyl-CoA to KB synthesis (see below). During glucose scarcity, the astrocytes also may contribute to KB formation. Astrocytes in culture have been shown to produce KBs from FAs (6) and from leucine (7). The mechanism by which the astrocytes synthesize KBs is very similar to that of cultured hepatocytes. In a review of KB synthesis in the brain, it was suggested that production of KBs by astrocytes contributes to the survival of neurons subjected to hypoxia (8). Most studies in astrocyte ketogenesis come from cell culture experiments, and the extent of KB formation by astrocytes in vivo remains to be determined. Nevertheless, the major determinants of cerebral KB metabolism are the prevailing plasma KB concentrations and availability of suitable monocarboxylic acid transporter (MCT) isoforms (9).
Studies based on positron emission tomography (PET) imaging in rats found a 7- to 8-fold enhancement of brain uptake of ketones during a KD or fasting (10).
THE BRAIN’S HIGH ENERGY REQUIREMENT
Usually, the brain obtains its fuel mainly from glucose/pyruvate-derived substrate, which is almost completely oxidized in the mitochondria, generating CO2, water, and high energy phosphate bonds (principally ATP). The brain is responsible for ∼20% of the body’s total resting energy expenditure; yet, it represents only about 2% of adult body weight. The brain metabolizes ∼120 g of glucose per day under conditions of normal glucose availability. Studies have shown that most of the glucose-derived energy entering the brain is used to maintain pre- and postsynaptic ion gradients required for neurotransmission and for maintenance of the resting potential of neurons (11).
When glucose is in short supply, KBs serve as the brain’s principal alternative fuel. However, the brain can only use them in quantity if their levels in the plasma substantially exceed default concentrations (≤0.2 mM). In the postabsorptive state, for example in the morning upon awakening, there exists a mild degree of transient hyperketonemia, with plasma ketone levels of 0.1–0.3 mM. These concentrations drop precipitously after ingestion of a mixed meal, only to rise again in the next postabsorptive state. In diabetic ketoacidosis, plasma concentration of KBs can exceed 25 mM (12).
The liver forms KBs but lacks the enzymes to use them as energy substrates. Transfer of AcAc and βHB across cell membranes (including those of neurons) is enabled by MCTs. In the mitochondrial matrix, βHB is converted to AcAc by βHB dehydrogenase, and the resulting AcAc, together with any AcAc that has entered the matrix as such, is then transformed to AcAc-CoA by oxoacid-CoA transferase. AcAc-CoA is then converted to acetyl-CoA by acetoacetyl-CoA thiolase, with the resulting acetyl-CoA units entering the Krebs (tricarboxylic acid) cycle. In the cycle, they undergo oxidative degradation, with reduction of the electron carriers NAD+ and flavine adenine dinucleotide (FAD) to NADH and FADH2. NADH and FADH2 donate electrons to the protein complexes I and II of the electron transport chain (ETC). Energy derived from the transfer of electrons along the ETC to oxygen (O2) is used by the electron transport system to pump protons (H+) into the mitochondrial intermembrane space, thereby generating a gradient across the inner mitochondrial membrane (proton motive force) that provides energy to regenerate ATP from ADP and inorganic phosphate. The role of mitochondrial dysfunction in neuronal degeneration has been reviewed by Schon and Manfredi (13).
KB: SOURCE OF ENERGY FOR BRAIN, HEART, AND MUSCLE
There is evidence that the whole brain uses energy from KBs as a function of the blood (plasma) concentration, as shown in Table 1.
TABLE 1.
Proportion of brain energy metabolism supported by KB, as a function of plasma KB concentration (mM) (2, 3, 9, 10, 41)
| Plasma KB Concentration | Proportion of Brain Energy |
| 0.3–0.5 mM (12–24 h fast) | 3–5% |
| 1.5 mM (2–3 day fast) | 18% |
| 5 mM (8 day fast) | 60% |
| 7 mM (≥20 day fast) | >60% |
In the human brain, the transport system for KBs (unlike that for glucose) remains relatively intact with advancing age. Certain MCT isoforms are well expressed in neurons (MCT2), astrocytes (MCT4), and brain capillaries (MCT1). When glucose utilization is impaired in neurodegenerative diseases, transport of KBs into the brain appears to be less affected and their utilization for energy by the brain mitochondria is not impeded by such factors as local insulin resistance that, by interfering with the neuronal fuel supply, may contribute to the progressive nerve cell damage observed in Alzheimer’s disease (AD) (1, 5, 14–16).
The central actions of βHB have been reviewed by Laeger et al. (17). These include its sources; its metabolism during starvation and cellular signaling; its effects on food intake; its role in ATP production, energy metabolism, and thermogenesis; its neuroprotective effects; and its influence on pituitary hormone release. The authors cite studies indicating that all the enzymes needed for KB oxidation, such as βHB dehydrogenase, 3-ketoacid CoA transferase, and acetyl-CoA thiolase, are present in the brain.
REGULATION OF PLASMA KB CONCENTRATIONS
In the first few days of a prolonged fast, while the body’s carbohydrate stores are being rapidly depleted, the liver accelerates its manufacture of KBs from FFA released in increasing amounts from adipocytes. In the absence of dietary carbohydrate, and as depletion of the body’s stored glycogen continues, the liver also increases its production of new glucose. Krebs cycle intermediates, notably oxaloacetate, are diverted to gluconeogenesis, which entails conversion in the liver of pyruvate derived from the carbon skeletons of glucogenic amino acids to glucose. Glycerol released from adipocytes along with FFA is also converted to glucose in the liver.
At the same time, insulin production tends to wane as glucose availability diminishes. Reduced concentrations of circulating insulin result in attenuation of insulin’s inhibiting effect on FFA/glycerol release. At this point, because much of the limited supply of oxaloacetate is being used for gluconeogenesis, metabolism in the Krebs cycle of FA-derived acetyl-CoA is slowed, and the resulting accumulation of the two-carbon units is then redirected to production of KBs for export into the systemic circulation.
To promote regeneration of oxaloacetate and thereby allow restoration of earlier levels of gluconeogenesis, the intrahepatic accumulation of acetyl-CoA apparently stimulates pyruvate carboxylase activity, resulting in conversion of more pyruvate to oxaloacetate, a key intermediate in both the Krebs cycle and the gluconeogenic process.
As the liver increases its KB output, the plasma total KB concentration rises gradually to 5–7 mM, or even slightly higher, depending in considerable part on the duration of the fast. In individuals whose islet β-cells are intact and functional, an elevated plasma ketone concentration can directly stimulate the β-cells to increase insulin secretion. However, it should be kept in mind that much of the evidence for hyperketonemia-induced enhancement of insulin release was obtained from dog studies in which infusions of KBs produced plasma KB concentrations of ∼3 mM (18, 19, 21). The relatively brief time frame in which the infusion experiments took place is very different from the slow rate at which metabolic changes occur during the development of fasting-induced hyperketonemia. During a prolonged fast, blood glucose plateaus at a lower than usual level, with an associated reduction in insulin release (20).
Nevertheless, a KB-generated negative feedback effect could explain the fall in arterial glucose concentration; the gradual increase, followed by a leveling off, of plasma FFA levels; and the stabilization of plasma KB observed over time in fasting individuals. Reducing the quantity of FFA released from adipocytes decreases FFA traffic through the liver. Reduction in rate of FFA entry into the liver would be expected to cause a decrease in hepatic KB formation, in effect, closing the negative feedback loop that prevents plasma KBs from rising to unsafe levels during starvation. Moreover, hyperketonemia per se may limit FA release from adipose tissue. However, the presence of insulin may be necessary for this effect (21).
THERAPEUTIC USES OF KBs
Traditionally, physicians have been taught to fear ketosis because the marked hyperketonemia that results from insulin deficiency can cause severe acidosis and death in individuals with type 1 diabetes. Thus, in their description of the potential therapeutic uses of KBs, Veech et al. (14, 22) emphasize that, in marked contrast to the clinical picture in diabetic ketoacidosis, mild to moderate hyperketonemia (up to ∼8 mM) can materially prolong survival during periods of caloric starvation. As glucose availability diminishes, KBs manufactured in the liver from FAs mobilized from adipose tissue become major sources of energy for muscle, heart, and brain (23).
Veech et al. (14, 22) described “clinical maneuvers” for readily increasing blood levels of βHB to 2–8 mM, concentrations similar to those produced by starvation or various KDs. To achieve this objective, they recommended use of small synthetic, digestible KB polymers (including dimers) or esters of βHB administered orally at 100–150 g/day in divided doses. The goals were to i) obtain relatively high plasma KB levels, which might enhance the clinical effectiveness of KB therapy in some cases; and ii) provide a more efficient source of energy per unit oxygen consumed for the treatment of certain types of heart failure and neurodegenerative diseases characterized by focal brain hypometabolism, such as Parkinson’s disease (PD) and AD. The authors also suggested that the ability of βHB to reduce NADP+ might be important in decreasing the oxidative damage associated with various kinds of metabolic stress (14).
KBs ARE A “HIGH-OCTANE” FUEL FOR THE BODY
The effect of adding insulin or KBs (4 mM) to a buffer containing 10 mM of glucose in a perfused rat heart preparation was studied by Kashiwaya et al. (24) and by Sato et al. (25). The addition of either insulin or ketones increased the efficiency of the working heart (hydraulic work/energy from O2 consumed) by 25%. The addition of both insulin and KBs in combination increased heart efficiency by 36%. The authors concluded that moderate hyperketonemia (∼4 mM) may compensate for defects in mitochondrial transduction associated with insulin deficiency, local glucoprivation, or mitochondrial senescence. Later work by the same group showed that moderate hyperketonemia following ingestion of the 1,3-butanediol monoester of βHB [ketone monoester (KME)] significantly improved the endurance of rats on a treadmill and also the physical performance of competing university athletes (26).
AD
Possible triggering role of mitochondrial dysfunction
Mitochondrial dysfunction has been implicated in the etiology of mild cognitive impairment and AD (27). Such dysfunction, which may be related to diminished energy production from mitochondrial glucose/pyruvate oxidation, potentiates the pathological intraneuronal (and later extracellular) deposition of amyloid-β (Αβ) and hyperphosphorylated tau. The mechanism for the mitochondrial dysfunction is not certain. However, several possible explanations have been proposed and are discussed in recent reviews (28, 29). Manifestations of impaired mitochondrial function include a decrease in oxidative phosphorylation and ATP synthesis, increased superoxide anion production, evidence of oxidative damage, inhibition of mitochondrial pyruvate dehydrogenase complex activity, and functional impairment in the mitochondrial ETC, particularly involving cytochrome c oxidase. Magnetic resonance spectroscopy (MRS) has been used to access neuronal mitochondrial metabolism in healthy elderly and young volunteers (27). MRS studies in these two groups revealed that, in the aging subjects, there was a reduction in neuronal and glial mitochondrial metabolism compared with the healthy young subjects. In a mouse model of AD, Chou et al. (30) found that early dysregulation of the mitochondrial proteome precedes the development of plaque and tangle pathologies. A number of mitochondrial proteins were downregulated in the cerebral cortices of these mice, notably in complexes I and IV of the oxidative phosphorylation system. Other studies have provided strong evidence that the impaired glucose metabolism in certain parts of the brain, which is characteristic of AD, is related to mitochondrial dysfunction (31–37). In AD, changes in glucose metabolism in cognition-associated parts of the brain have been detected by PET imaging with 2-[18F]fluoro-2-deoxyglucose (FDG) decades before the appearance of typical AD dementia (38). Four apparently normal individuals with FDG-PET evidence of reduced glucose utilization in cognition-related brain sites were followed for 9–19 years to the onset of clinical symptoms of dementia and, subsequently, to postmortem confirmation of the diagnosis of AD.
Factors impeding glucose utilization by the brain may contribute to, or precipitate, AD neuropathology. This possibility is strengthened by evidence that diminished glucose utilization can be present well in advance of measurable cognitive decline (29).
Studies have shown that certain glucose transporters (GLUTs) in the brain (GLUT 1 and GLUT 2) may be diminished significantly in the AD brain (34). In addition, there is evidence that the concentration of GLUT 3, the principal neuronal GLUT, is diminished in the brains of AD patients (39). A decrease in GLUTs also correlates with abnormal hyperphosphorylation of tau in AD (40). Such GLUT deficiencies presumably contribute to the impaired glucose metabolism implicated in neuronal degeneration.
There is preliminary evidence that, unlike glucose, transport and metabolism of KBs are not diminished in the AD brain (41, 42). This finding underlines the importance of developing a safe, simple, and reliable way to provide the brain with KBs as an alternative fuel to glucose. The subject of brain fuel metabolism in aging and AD has been extensively reviewed by Cunnane et al. (41). In a more recent communication, Castellano et al. (42) reported that at the same time a diminished brain glucose utilization in AD could be demonstrated, ketone uptake was unchanged. In recent years, extensive evidence has accumulated suggesting that regional hypometabolism within the brain may be a root cause of cognitive decline in sporadic AD (15). For example, carriers of one copy of the APOE-ε4 allele (a situation that enhances risk of developing AD) exhibit abnormally low rates of glucose metabolism bilaterally in the posterior cingulate, parietal, temporal, and prefrontal cortex (15). Under normal conditions, the energy used by the adult human brain is derived almost exclusively from glucose (42, 43). In individuals with an increased risk of developing AD, glucose hypometabolism (manifested by a reduced cerebral metabolic rate for glucose) may occur in cognition-critical parts of the brain decades before symptoms of dementia become manifest and may precede intra- and extraneuronal deposition of abnormal proteins. These findings suggest that neuronal energy privation may be an important contributor to the decline in cognitive performance exhibited by patients with early AD. Early support for the concept that the AD brain may retain its ability to use KBs for energy even when glucose utilization is impaired was obtained by feeding a mildly ketogenic (0.5–0.8 mM) medium-chain TG (MCTG), tricaprylin, to AD patients. Even at such relatively low plasma KB concentrations, a modest rise in cognitive performance occurred transiently in a subset of the AD cohort under examination. Yet, despite the unspectacular nature of the improvement that occurred, the studies reviewed were well designed and the cognitive improvement measured following MCTG ingestion was statistically significant (15).
In a mouse model of AD, the feeding of a KME (composed of d-βHB and R-1,3-butanediol) as 21.5% of dietary calories was associated with lessening in anxiety and improvement in performance on learning and memory tests. Moreover, the mice fed the KME exhibited reduced Aβ peptide deposition in the hippocampus and amygdala and reduced levels of hyperphosphorylated tau deposits in the same areas and in the cortex (44).
Histone acetylation and deacetylation
During the past 10 years, a number of studies have addressed the phenomenon of histone acetylation and deacetylation and the role of these processes in cognitive impairment and AD. For example, degradation of histone acetylation is associated with age-dependent memory impairment in mice. In contrast, restoration of histone acetylation leads to recovery of cognitive performance (45). More recent studies suggest that there is an urgent need to develop additional selective histone deacetylase (HDAC) inhibitors (46).
Recently, βHB was found to inhibit HDACs 1, 3, and 4 at concentrations of 5.3, 2.4, and 4.5 mM, respectively. Thus, millimolar concentrations of βHB appeared to increase histone acetylation via inhibition of HDACs. Moreover, the same study provided evidence that βHB exerts a suppressive effect on oxidative stress (47). Inhibition of HDAC was also shown in mice that were protected from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic damage by feeding a TG of βHB (48).
The human and rodent genome encodes for 11 HDAC proteins that are divided into four classes (HDAC I–IV). There is evidence that inhibition of HDACs 1–3 (class I) reverses memory dysfunction in a mouse model of AD (49). Agents reported to inhibit HDAC include sodium butyrate, trichostatin A, suberoylanilide hydroxamic acid, and sodium phenylbutyrate. βHB also qualifies as an HDAC inhibitor (47, 48). Most HDAC inhibitors influence the activities of the HDAC isoforms and classes nonselectively, and the term “pan-inhibitor” has been used to distinguish them from inhibitors that are class selective or isoform selective.
PD
Although the pathogenesis of sporadic PD remains unresolved, numerous studies suggest that, at the least, impairment of mitochondrial function involving the substantia nigra pars compacta (SNpc) plays an important contributory role (50, 51). In 1983, Langston et al. (52) reported that four persons developed marked parkinsonism after taking an illicit drug intravenously. The drug, 4-propyloxy-4-phenyl-N-methylpiperidine (MPPP), was a meperidine (Demerol®) analog. A contaminant (and unwanted side product) resulting from apparently careless MPPP manufacture, MPTP, was found to be the likely culprit. It was the MPTP, after being oxidized in the brain to methylphenylpyridine, that presumably caused selective destruction of dopaminergic neurons in the SNpc, giving rise to the human PD-like syndrome described by Langston et al. (52). Subsequently, MPTP has been used extensively to produce animal models of PD.
Because a reduction in complex I activity and impaired mitochondrial function had been reported in the brain and other tissues of patients with PD (53, 54), Tieu et al. (55) reasoned that, inasmuch as the brain can utilize KB for energy via mitochondrial complex II, KBs might protect against MPTP induction of parkinsonism in mice. Indeed, infusion of βHB into mice was found to confer protection against the dopaminergic neurodegeneration and motor deficits induced by MPTP.
In a tissue culture study of rat neurons, βHB protected hippocampal neurons from Aβ 1–42 toxicity and mesencephalic neurons from MPTP toxicity. These findings suggest that KBs have the potential of preventing, or possibly treating, both AD and PD (56). In a later recent study, Cheng et al. (57) reported, in a rat model of PD, that a KD protected dopaminergic neurons of the SNpc against the neurotoxicity of 6-hydroxydopamine.
Recently, oral administration of glyceryl-tris-3-hydroxybutyrate (3GHB), the TG of βHB, was found to exert an extended neuroprotective action against MPTP-induced neuronal destruction in the SNpc of mice. It was shown that 3GHB protects these neurons in a dose-dependent manner (48). The study’s authors suggested that this protection might be mediated via inhibition of HDAC. They concluded that this new ketone ester (KE), 3GHB, represented a promising preventive and/or therapeutic strategy for a range of pathological conditions affecting the brain, including PD and AD (48).
Another study in mice demonstrated that βHB inhibits HDAC in vitro and in vivo (47). The in vivo studies involved producing hyperketonemia (1.5 mM) in mice by means of a 24 h fast, caloric restriction (0.6 mM), or infusion of buffered βHB (1.2 mM). A positive correlation was observed between serum βHB level and histone acetylation, promoted by the KB-induced inhibition of HDAC. Treatment of mice with βHB also conferred significant protection against oxidative stress. Other studies indicate that KBs are protective against oxidative stress in neocortical neurons (58). They also help protect against the neuronal synaptic dysfunction induced by respiratory complex inhibitors (59).
EPILEPSY
The anticonvulsant effect of fasting has been known for centuries (1). The KD for the treatment of epilepsy, which mimics the metabolic effects of fasting, was first conceived in 1921 by Wilder (60). In terms of energy distribution, the original KD was 90% fat, ∼8% protein, and ∼2% carbohydrate.
The very high-fat, very low-carbohydrate, low-protein KD can produce rises in plasma LDL cholesterol, uric acid, and FFAs. Occasionally, the KD may be associated with an increased incidence of nephrolithiasis and other serious complications (1). Some of these adverse effects can be prevented by guarding against chronic dehydration. Hyperlipidemia can be avoided in most cases by boosting the proportion in the diet of polyunsaturated (ω6 and ω3) and monounsaturated FAs (61). Also, incorporation of MCTG into the KD may be helpful in formulating more tolerable ketogenic regimens for the long-term treatment of drug-resistant epilepsy (62–65).
KDs have also been found therapeutically effective in approximately two-thirds of 104 patients with infantile spasm (66). In another study, at 1–3 months after the initiation of the KD in 26 patients with infantile spasm, 46% had a >90% reduction in symptoms (67).
The mechanism responsible for the beneficial effect of the KD in epilepsy is not known. Several explanations have been proposed: i) reduction in neural excitability, ii) changes in energy availability, and iii) direct anticonvulsion action. Another mechanism for the antiseizure action of the KD, suggested by Yudkoff et al. (68), pertains to decreased availability of excitatory neurotransmitters (aspartate and glutamate) and increased availability of the inhibitory neurotransmitters [γ-aminobutyric acid (GABA)], via stimulation of glutamic acid decarboxylase, which, in turn, increases GABA production from glutamate. Many studies have contributed in a variety of ways to our understanding of the beneficial effect of KDs on epilepsy (60, 62, 69–76). However, despite the abundance of hypotheses, the basis for the antiseizure action of KBs remains unclear.
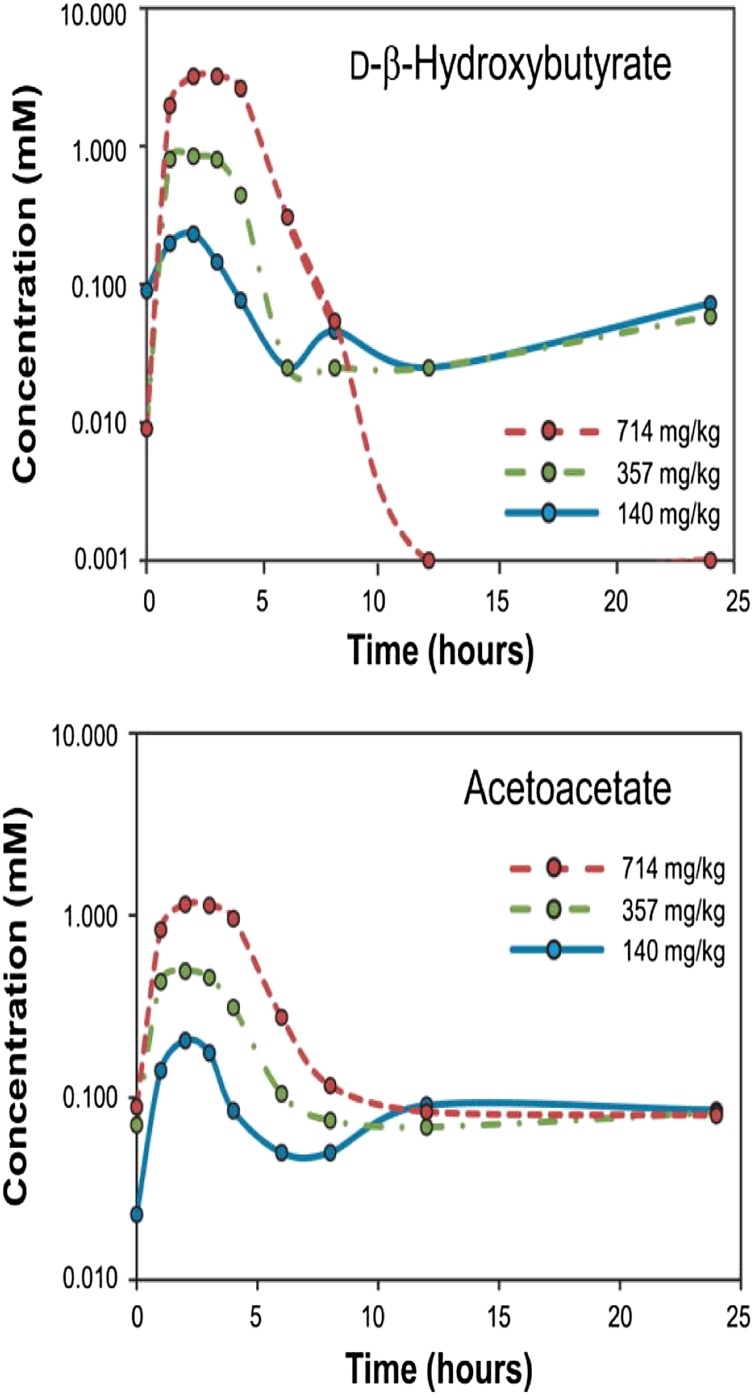
Because the new KEs (see Fig. 1) can elevate plasma KBs to concentrations comparable to those achieved during prolonged adherence to a KD, without the concurrent need to change the composition of the habitual diet, it should now be possible to determine conclusively whether hyperketonemia has an antiseizure effect in epileptic patients independent of any associated dietary change.
Fig. 1.
Changes in circulating d-βΗΒ and AcAc concentrations for 24 h following ingestion of a single dose of the ketone monoester. Note that concentrations reflect dose size. Reproduced from Clarke et al. (79).
A recent study of brain metabolism in normal Wistar rats fed a KME (1,3-butanediol monoester of βHB) may provide a possible explanation for the antiepileptic effect of KDs. Animals fed KME as 28% of daily calories for 14 days had their brain metabolites measured after removal of their brains by freeze blowing. The KME-supplemented animals had elevated blood KB levels in the 3.5 mM range and had a 2-fold decrease in food intake despite lowered plasma glucose, insulin, and leptin. The authors attributed the diminished food intake to increased malonyl-CoA and uncoupling proteins 4 and 5. Feeding the KME diet resulted in a significant decrease in both l-glutamate and GABA. This observation provides additional support for the notion that the antiepileptic effect of KDs may result from the reduction in the excitatory amino acid glutamate associated with their use (77).
The anticonvulsant effect of sustained hyperketonemia has also been studied in a rat model of CNS oxygen toxicity seizures (78). In an attempt to mimic the sustained therapeutic hyperketonemia (∼7 mM) that can be achieved by means of a strict KD, a single oral dose (10 g/kg) of a KE (R,S-1,3-butanediol AcAc diester) was administered to rats over a 30 min period before placing them in a seizure-inducing hyperbaric oxygen chamber. The KE treatment was associated with a substantial delay in occurrence of the CNS oxygen toxicity-induced seizures. Ingestion of the KE resulted in rapid and sustained elevations of βHB (>3 mM), AcAc (>3 mM), and acetone (∼0.7 mM). The KE had no effect on blood glucose, and the ketonemia was induced despite the fact that the rats had been fed a standard carbohydrate-containing diet.
KEs
Conversion of KBs to KEs eliminates KB acidity, making the KEs suitable vehicles for the delivery of KBs to the blood circulation via the gastrointestinal route. Ingestion of KE can directly increase plasma KBs to levels within the range achieved during fasting. The degree of KB elevation attained is readily controlled by the dose size (Fig. 1).
Two KEs are known to be under current study: a) 1,3-butanediol monoester of βHB (KME) (77, 79–84) and b) 3GHB (48, 85, 86). Studies have demonstrated that orally or intravenously administered 1,3-butanediol or glycerol esters of βHB are safe and well tolerated in animals (80, 86), and that the orally administered 1,3-butanediol monoester is also safe and well tolerated in humans (79).
Like other FA esters, KEs described herein are hydrolyzed in the intestine into ketoacids and the esterifying polyol (1,3-butanediol or glycerol). Early studies on polyols such as 1,2-, 1,4-, and 2,3-butanediols revealed that they had varying degrees of toxicity. In contrast, 1,3-butanediol was found to be nontoxic when fed to rats and dogs (87). When 1,3-butanediol was fed ad libitum to rats for 43 days as a replacement for carbohydrate (which was added to a high-fat diet at 23.4% of daily calories), it was shown that 1,3-butanediol was readily metabolized in a manner similar to ethanol, with subsequent conversion to βHB and, eventually (at the peripheral tissue level), to AcAc (88). A similar study in rats later confirmed the conversion of 1,3-butanediol to βHB when it was added as a replacement of up to 20% of dietary carbohydrate energy (89).
Desrochers et al. (81, 82) synthesized R,S-1,3-butanediol AcAc monoesters and diesters as totally or partially water-soluble compounds that could replace emulsions of long-chain TAG for total parenteral nutrition. In a follow-up study, continuous intravenous administration to pigs of R,S-1,3-butanediol AcAc esters in amounts providing up to 30% of the hourly energy requirement resulted in their complete utilization, leading to plasma concentrations of 1,3-butanediol of 0.1 mM and total KBs of 0.5 mM. In contrast, when the esters were given to pigs as intragastric boluses at 15% of daily calories, the blood 1,3-butanediol and KB levels were 2–3 mM and 5 mM, respectively (82).
Various investigators have used the term “therapeutic ketosis,” a term that implies achievement of plasma KB levels in the 2–7 mM range, comparable to concentrations found in subjects maintained on various KDs or in those undergoing a fast. Such degrees of hyperketonemia have been readily achieved by KE administration in rats, mice, pigs, and humans (78–86).
SUMMARY
The advent of the 1,3-butanediol and glycerol esters of AcAc and βHB has made feasible oral administration of KEs as food supplements capable of providing an alternative fuel source (namely, KBs) for cognition-critical parts of the brain that, for various reasons, are manifesting impairment of glucose utilization. However, such impairment does not necessarily extend to the utilization of KBs during aging and in certain types of early neurodegenerative disease. Given the high energy requirement of the brain and its critical dependence on the delivery of a constant supply of fuel, the consequences of leaving such an energy shortfall untreated can be dire. When the brain’s energy supply is insufficient to meet its metabolic needs, the neurons that work hardest, especially those concerned with memory and cognition, are among the first to exhibit functional incapacity (e.g., impairment of memory and cognitive performance). At the molecular level, neuronal energy deprivation is associated with impaired mitochondrial function, reduction in the efficiency of the ETC, overproduction of reactive oxygen species, and intraneuronal (followed by extraneuronal) accumulation of deposits of Aβ oligomers and (later) polymers and hyperphosphorylated tau. As energy privation continues and worsens, fuel-deprived brain cells (particularly neurons that function at a high synaptic activity level) exhibit a drop in cellular energy followed by an increase in intracellular Na+ and Ca2+, excessive release of neurotransmitters, and apoptosis.
If the foregoing scenario is credible, it would seem critically important to test whether the hyperketonemia readily achievable by ingestion of a Food and Drug Administration-approved KE can prevent or delay the occurrence of neuronal energy privation (and its pathological consequences) in individuals in whom preclinical AD or PD can be diagnosed.
It is also crucial to determine whether KE treatment per se is effective in the prevention and control of epileptic seizures.
Footnotes
Abbreviations:
- Aβ
- amyloid-β AcAc, acetoacetate
- AD
- Alzheimer’s disease
- βHB
- β-hydroxybutyrate
- ETC
- electron transport chain
- FAD
- flavine adenine dinucleotide
- GABA
- γ-aminobutyric acid
- GLUT
- glucose transporter
- HDAC
- histone deacetylase
- KB
- ketone body
- KD
- ketogenic diet
- KE
- ketone ester
- KME
- ketone monoester
- MCT
- monocarboxylic acid transporter
- MCTG
- medium-chain TG
- MPTP
- 1-methyl-4-phenol-1,2,5,6-tetrahydropyridine
- PD
- Parkinson’s disease
- PET
- positron emission tomography
- SNpc
- substantia nigra pars compacta
- TAG
- triacylglycerol
- 3GHB
- glyceryl-tris-3-hydroxybutyrate.
S. A. Hashim is the recipient of a patent involving the TG of β-hydroxybutyrate as a food supplement for use in disorders characterized by impairment of glucose utilization by the brain. T. B. VanItallie is a minority shareholder in a company that markets a product that yields medium-chain FAs.
REFERENCES
- 1.VanItallie T. B., Nufert T. H. 2003. Ketones: metabolism’s ugly duckling. Nutr. Rev. 61: 327–341. [DOI] [PubMed] [Google Scholar]
- 2.Owen O. E., Morgan A. P., Kemp H. G., Sullivan J. M., Herrera M. G., Cahill G. F., Jr 1967. Brain metabolism during fasting. J. Clin. Invest. 46: 1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cahill G. F., Jr 2006. Fuel metabolism in starvation. Annu. Rev. Nutr. 26: 1–22. [DOI] [PubMed] [Google Scholar]
- 4.Cahill G. F., Jr 1983. President’s address: starvation. Trans. Am. Clin. Climatol. Assoc. 94: 1–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Cahill G. F., Veech R. L. 2003. Ketoacids? Good medicine. Trans. Am. Clin. Climatol. Assoc. 114: 149–163. [PMC free article] [PubMed] [Google Scholar]
- 6.Auestad N., Korsak R. A., Morrow J. W., Edward J. 1991. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J. Neurochem. 56: 1376–1386. [DOI] [PubMed] [Google Scholar]
- 7.Bixel M. G., Hamprecht B. 1995. Generation of ketone bodies from leucine by cultured astroglial cells. J. Neurochem. 65: 2450–2461. [DOI] [PubMed] [Google Scholar]
- 8.Guzmán M., Blázquez C. 2004. Ketone bodies synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot. Essent. Fatty Acids. 70: 287–292. [DOI] [PubMed] [Google Scholar]
- 9.Morris A. A. 2005. Cerebral ketone metabolism. J. Inherit. Metab. Dis. 28: 109–121. [DOI] [PubMed] [Google Scholar]
- 10.Bentourkia M., Tremblay S., Pifferi F., Rousseau J., Leomte R., Cunnane S. 2009. PET study of 11C-acetoacetate kinetics in rat brain during dietary treatments affecting ketosis. Am. J. Physiol. Endocrinol. Metab. 296: E796–E801. [DOI] [PubMed] [Google Scholar]
- 11.Ruderman N. B., Ross P. S., Berger M., Goodman M. N. 1974. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem. J. 138: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laffel L. 1999. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 15: 412–426. [DOI] [PubMed] [Google Scholar]
- 13.Schon E. A., Manfredi G. 2003. Neuronal degeneration and mitochondrial dysfunction. J. Clin. Invest. 111: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veech R. L. 2004. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redux states insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids. 70: 309–319. [DOI] [PubMed] [Google Scholar]
- 15.Costantini L. C., Barr L. J., Vogel J. I., Henderson S. T. 2008. Hypometabolism as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 9 (Suppl. 2): S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbot K., Wang H-Y., Kazi H., Ham L-Y., Bakshi K. P., Stucky A., Fuino R. L., Kawaguchi K. R., Samoyedny A. J., Wilson R. S., et al. 2012. Demonstrated brain insulin resistance in Alzheimer’s disease is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 122: 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laeger T., Metges C. C., Kuhla B. 2010. Role of β-hydroxybutyrate in the central regulation of energy balance. Appetite. 54: 450–455. [DOI] [PubMed] [Google Scholar]
- 18.Madison L. L., Mebane D., Unger R. H., Lochner A. 1964. The hypoglycemic action of ketones II. Evidence for a stimulatory feedback of ketones on the pancreatic beta cells. J. Clin. Invest. 43: 408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi-Sunyer F. X., Campbell R. G., Hashim S. A. 1970. Experimentally induced hyperketonemia and insulin secretion in the dog. Metabolism. 19: 263–270. [DOI] [PubMed] [Google Scholar]
- 20.Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Kipnis D. M. 1966. Hormone-fuel interrelationships during fasting. J. Clin. Invest. 45: 1751–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garber A. J., Menzel P. H., Boden G., Owen O. E. 1974. Hepatic ketogenesis and gluconeogenesis in humans. J. Clin. Invest. 54: 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veech R. L., Chance B., Kashiwaya Y., Lardy H. A., Cahill G. F., Jr 2001. Ketone bodies, potential therapeutic uses. IUBMB Life. 51: 241–247. [DOI] [PubMed] [Google Scholar]
- 23.Cotter D. G., Schugar R. C., Crawford P. A. 2012. Ketone body metabolism and cardiovascular diseases. Am J Physiol. ePub ahead of print. DOI:10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashiwaya Y., Sato K., Tsuchiya N., Thomas S., Fell D. A., Veech R. L., Passonneau J. V. 1994. Control of glucose utilization in working perfused rat heart. J. Biol. Chem. 269: 25502–25514. [PubMed] [Google Scholar]
- 25.Sato K., Kashiwaya Y., Keon C. A., Tsuchiya N., King M. T., Radda G. K., Chance B., Clarke K., Veech R. L. 1995. Insulin, ketone bodies, and mitochondrial energy metabolism. FASEB J. 9: 651–658. [DOI] [PubMed] [Google Scholar]
- 26.Burne J. 2012. Could this elixir hold the key to weight loss? Experts hope it’ll also treat diabetes, epilepsy and Alzheimer’s. http://www.dailymail.co.uk/health/article-2238842/. [Google Scholar]
- 27.Boumezbeur F., Mason G. F., de Graaf R. A., Behar K. L., Cline G. W., Shulman G. I., Rothman D. L., Petersen K. F. 2010. Altered brain mitochondrial metabolism in healthy ageing as assessed by in vivo magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 30: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva D. F. F., Esteves A. R., Oliveira C. R., Cardoso S. M. 2011. Mitochondria: the common upstream driver of amyloid-β and tau pathology in Alzheimer’s disease. Curr Alzheimer Res. 8: 563–572. [DOI] [PubMed] [Google Scholar]
- 29.VanItallie T. B. 2013. Preclinical sporadic Alzheimer’s disease: target for personalized diagnosis and preventive intervention. Metabolism. 62 (Suppl. 1): S30–S33. [DOI] [PubMed] [Google Scholar]
- 30.Chou J. L., Shenoy D. V., Thomas N., Choudhary P. K., La Ferla F. M., Goodman S. R., Breen G. A. M. 2011. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. J. Proteomics. 74: 466–479. [DOI] [PubMed] [Google Scholar]
- 31.Hoyer S. 1991. Abnormalities of glucose metabolism in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 640: 53–58. [DOI] [PubMed] [Google Scholar]
- 32.Ishii K., Sasaki M., Kitagaki H., Yamaji S., Sakamoto S., Matsuda K., Mori E. 1997. Reduction of cerebral glucose metabolism in advanced Alzheimer’s disease. J. Nucl. Med. 38: 925–928. [PubMed] [Google Scholar]
- 33.Blass J. P. 2000. The mitochondrial spiral. An adequate cause of dementia in Alzheimer’s syndrome. Ann. N. Y. Acad. Sci. 924: 170–183. [DOI] [PubMed] [Google Scholar]
- 34.Hoyer S. 2004. Causes and consequences of disturbance of glucose metabolism in sporadic Alzheimer’s disease: therapeutic implications. Adv. Exp. Med. Biol. 541: 135–152. [DOI] [PubMed] [Google Scholar]
- 35.Reddy P. H., Beal M. F. 2008. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 14: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crouch P. J., Harding S-M. E., White A. R., Camakaris J., Bush A. I., Masters C. L. 2008. Mechanism of Aβ mediated neurodegeneration in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 40: 181–198. [DOI] [PubMed] [Google Scholar]
- 37.Moreira P. I., Duarte A. I., Santos M. S., Rego A. C., Olivera C. R. 2009. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer’s disease. J. Alzheimers Dis. 16: 741–761. [DOI] [PubMed] [Google Scholar]
- 38.Mosconi L., Mistur R., Switalski R., Tsui W. H., Glodzik L., Li Y., Pirraglia E., De Santi S., Reisberg B., Wisniewski T., et al. 2009. FDG-PET changes in brain glucose metabolism from normal to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol. Imaging. 36: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson I. A., Chundu K. R., Davies-Hill T., Honer W. G., Davies P. 1994. Decreased concentrations of GLUT 1 and GLUT 3 in brains of patients with Alzheimer’s disease. Ann. Neurol. 35: 546–551. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y., Liu F., Iqbal K., Grundke-Iqbal I., Gong C. X. 2008. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer’s disease. FEBS Lett. 582: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunnane S., Nugent S., Roy M., Courchesne-Loyer A., Croteau E., Tremblay S., Castellano A., Pifferi F., Bocti C., Paquet N., et al. 2011. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 27: 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castellano C. A., Nugent S., Tremblay S., Fortier M., Pacquet N., Bocti C., Lepage M., Turcotte E., Fulop T., Cunnane S. C. 2013. In contrast to lower glucose uptake, brain ketone uptake is unchanged in mild Alzheimer’s disease. A dual tracer study comparing 18FDG and 11C-acetoacetate (Abstract). J. Nutr. Health Aging. 17: 810–811. [Google Scholar]
- 43.Shestov A. A., Emir U. E., Kumar A., Henry P.-G., Seaquist E. R., Oz G. 2011. Simultaneous measurement of glucose transport and utilization in the human brain. Am. J. Physiol. Endocrinol. Metab. 301: E1040–E1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashiwaya Y., Bergman C., Lee J. H., Wan R., King M. T., Mughal M. R., Okun E., Clarke K., Mattson M. P., Veech R. L. 2013. A ketone ester diet exhibits anxiolytic and cognition properties and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 34: 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peleg S., Sananbenesi F., Zovoilis A., Bukhardt S., Bahari-Javan S., Agis-Balboa R. C., Cota P., Wittnam J. L., Gogol-Doering A., Opitz L., et al. 2010. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 328: 753–756. [DOI] [PubMed] [Google Scholar]
- 46.Agis-Balboa R. C., Pavelka Z., Kerimoglu C., Fischer A. 2013. Loss of HDAC 5 impairs memory function: implications for Alzheimer’s disease. J. Alzheimers Dis. 33: 35–44. [DOI] [PubMed] [Google Scholar]
- 47.Shimazu T., Hirschey M. D., Newman J., He W., Shirakawa K., Moan N. L., Grueter C. A., Lim H., Saunders L. R., Stevens R. D., et al. 2013. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 339: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blesa J., Jackson-Lewis V., Boaz N., Hashim S., Przedborski S. 2012. Glyceryl-tris-3-hydroxybutyrate protects dopaminergic neurons in a MPTP model of Parkinson’s disease. (Abstract in 2012 Neuroscience Meeting Planner, Society for Neuroscience. New Orleans, LA, October 17, 2012). [Google Scholar]
- 49.Kilgore M., Miller C. A., Fass D. M., Hennig K. M., Haggarty S. J., Sweatt J. D., Rumbaugh G. 2010. Inhibitors of class I histone deacetylase reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 35: 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanItallie T. B. 2008. Parkinson disease: primacy of age as a risk factor for mitochondrial dysfunction. Metabolism. 57 (Suppl. 2): S50–S55. [DOI] [PubMed] [Google Scholar]
- 51.Büeler H. 2009. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp. Neurol. 218: 235–246. [DOI] [PubMed] [Google Scholar]
- 52.Langston J. W., Tetrud J. W., Irwin I. 1983. Chronic Parkinsonism in humans due to a product of meperidine-analogue synthesis. Science. 219: 979–980. [DOI] [PubMed] [Google Scholar]
- 53.Nicklas W. J., Youngster S. K., Kindt M. V., Heikkila R. E. 1987. MPTP, MPP+ and mitochondrial function. Life Sci. 40: 721–729. [DOI] [PubMed] [Google Scholar]
- 54.Greenamyre J. T., Sherer T. B., Betarbet R., Pavov A. V. 2001. Complex I and Parkinson’s disease. IUBMB Life. 52: 135–141. [DOI] [PubMed] [Google Scholar]
- 55.Tieu K., Perier C., Casperson C., Teismann P., Wu D. C., Yan S. D., Naini A., Vila M., Jackson-Lewis V., Ramasamy R., et al. 2003. D-betahydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson’s disease. J. Clin. Invest. 112: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kashiwaya Y., Takeshima T., Mori N., Nakashima K., Clarke K., Veech R. L. 2000. D-beta-hydroxybutyrate protects neurons of Alzheimer’s and Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 97: 5440–5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng B., Yang X., Liangxiang A., Gao B., Liu X., Liu S. 2009. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 1286: 25–31. [DOI] [PubMed] [Google Scholar]
- 58.Kim D. Y., Davis L. M., Sullivan P. G., Maalouf M., Simeone T. A., van Breolerode J., Rho J. M. 2007. Ketone bodies are protective against oxidative stress in neocortical neurons. J. Neurochem. 101: 1316–1326. [DOI] [PubMed] [Google Scholar]
- 59.Kim D. Y., Vellejo J., Rho J. M. 2010. Ketones prevent synaptic dysfunction induced by respiratory complex inhibitors. J. Neurochem. 114: 130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilder R. M. 1921. The effects of ketonemia on the course of epilepsy. Mayo Clin Bull. 2: 307–308. [Google Scholar]
- 61.Fuehrlein B. S., Rutenberg M. S., Silver J. N., Warren M. W., Theriaque D. W., Duncan G. E., Stacpoole P. W., Brantly M. L. 2004. Differential metabolic effects of saturated versus polyunsaturated fats in ketogenic diets. J. Clin. Endocrinol. Metab. 89: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 62.Huttenlocher P. R., Wilbourn A. J., Signore J. M. 1971. Medium chain triglyceride as a therapy for intractable childhood epilepsy. Neurology. 21: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 63.Wu P. Y. K., Edmond J., Avestad N., Rambathla S., Benson J., Picone T. 1986. Medium chain triglycerides In infant formulas and their relation to plasma ketone body concentration. Pediatr. Res. 20: 338–341. [DOI] [PubMed] [Google Scholar]
- 64.Balietti M., Gasoli T., DiStefano G., Giorgetti B., Aicardi G., Pattovetti P. 2010. Ketogenic diets: an historical antiepileptic therapy with promising potentialities for the aging brain. Ageing Res. Rev. 9: 273–279. [DOI] [PubMed] [Google Scholar]
- 65.Neal E. G., Chaffe H., Schwartz R. H., Lawson M. S., Edwards N., Fitzsimmons G., Whitney A., Cross J. H. 2009. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of epilepsy. Epilepsia. 50: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 66.Hong A. M., Turner Z., Hamdy R. F., Kossoff E. H. 2010. Infantile spasms treated with the ketogenic diet: prospective single-center experience in 104 consecutive infants. Epilepsia. 51: 1403–1407. [DOI] [PubMed] [Google Scholar]
- 67.Numis A. L., Yellen M. B., Chu-Shore C. J., Pfeifer H., Theile E. A. 2011. The relationship of ketosis and growth to the efficacy of the ketogenic diet in infantile spasm. Epilepsy Res. 96: 172–175. [DOI] [PubMed] [Google Scholar]
- 68.Yudkoff M., Daikhin Y., Horyn O., Nissim I., Nissim I. 2008. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 49 (Suppl. 8): 73–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Freeman J. M., Viking E. P., Pillas D. L., Pyzik P. L., Casey J. C., Kelly L. M. 1998. The efficiency of the ketogenic diet-1998: a prospective evaluation of intervention in 150 children. Pediatrics. 102: 1358–1363. [DOI] [PubMed] [Google Scholar]
- 70.Likhodii S. S., Musa K., Meneonca A., Dell C., Burnham W. M., Cunnane S. C. 2000. Dietary fat, ketosis, and seizure resistance in rats on ketogenic diet. Epilepsia. 41: 1400–1410. [DOI] [PubMed] [Google Scholar]
- 71.Rho J. M., Anderson G. D., Donevan S. D., White H. S. 2002. Acetoacetate, acetone, and dibenzylamine (a contaminant in L-(+)- betahydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia. 43: 358–361. [DOI] [PubMed] [Google Scholar]
- 72.Stafstrom C. E., Bough K. J. 2003. The ketogenic diet for the treatment of epilepsy: a challenge for nutritional neuroscientists. Nutr. Neurosci. 6: 67–79. [DOI] [PubMed] [Google Scholar]
- 73.Likhodii S., Nylen K., Burnham W. M. 2008. Acetone as an anticonvulsant. Epilepsia. 49 (Suppl. 8): 83–86. [DOI] [PubMed] [Google Scholar]
- 74.Nei M., Ngo L., Sirven J. I., Sperling M. R. 2014. Ketogenic diet in adolescents and adults with epilepsy. Seizure. 23: 439–442. [DOI] [PubMed] [Google Scholar]
- 75.Kossoff E. H., Zupec-Kania B. A., Rho J. M. 2009. Ketogenic diets: an update for child neurologists. J. Child Neurol. 24: 979–988. [DOI] [PubMed] [Google Scholar]
- 76.McNally M. A., Hartman A. L. 2012. Ketone bodies in epilepsy. J. Neurochem. 121: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kashiwaya Y., Pawlosky R., Markis W., King M. T., Bergman C., Srivastava S., Murray A., Clarke K., Veech R. L. 2010. A ketone ester diet increased brain malony-CoA and uncoupling protein 4 and 5 while decreasing food intake in the normal Wistar rat. J. Biol. Chem. 285: 25950–25956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D’Agostino D. P., Pilla R., Held H. F., Landon C. S., Puchowicz M., Brunengraber H., Ari C., Arnold P., Dean J. B. 2013. Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304: R829–R836. [DOI] [PubMed] [Google Scholar]
- 79.Clarke K., Tchabanenko K., Pawlosky R., Carter E., King M. T., Musa-Veloso K., Ho M., Roberts A., Robertson J., VanItallie T. B., et al. 2012. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 63: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke K., Tchabamenko K., Pawlosky R., Carter E., Knight N. S., Murray A. J., Cochlin L. E., King M. T., Wong A. W., Roberts A., et al. 2012. Oral 28-day and developmental toxicity studies of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate. Regul. Toxicol. Pharmacol. 63: 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desrochers S., Quinze K., Dugas H., Debreuil P., Bomont C., David F., Agarwal K. C., Kumar A., Soloviev M. V., Powers L., et al. 1995. R,S-1,3-butanediol acetoacetate esters, potential alternates to lipid emulsions for parenteral nutrition. J. Nutr. Biochem. 6: 111–118. [Google Scholar]
- 82.Desrochers S., Dubreuil P., Brunet J., Jetté M., David F., Landau B. R., Brunengraber H. 1995. Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am J Physiol. 268: E660–E667. [DOI] [PubMed] [Google Scholar]
- 83.Srivastava S., Kashiwaya Y., King M. T., Baxa U., Tam J., Niu G., Chen X., Clarke K., Veech R. L. 2012. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 26: 2351–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srivastava S., Kashiwaya Y., King M. T., Baxa U., Tam J., Niu G., Chen X., Clarke K., Veech R. L. 2012. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 26: 2351–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birkhahn R. H., McCombs C., Clemens R., Hubbs J. 1997. Potential of the monoglyceride and triglyceride of DL-3-hydroxybutyrate for parenteral nutrition: synthesis and preliminary biological testing in the rat. Nutrition. 13: 213–219. [DOI] [PubMed] [Google Scholar]
- 86.Brunengraber H. 1997. Potential of ketone body esters for parenteral nutrition. Nutrition. 13: 233–235. [DOI] [PubMed] [Google Scholar]
- 87.Scala R. A., Paynter O. E. 1967. Chronic oral toxicity of 1,3-butanediol. Toxicol. Appl. Pharmacol. 10: 160–164. [DOI] [PubMed] [Google Scholar]
- 88.Tobin R. B., Garthoff L. H., Mehlman M. A., Veech R. L. 1978. Metabolite levels, redox states, and gluconeogenic enzyme activities in livers of rats fed diets containing 1,3-butanediol. J Environ Pathol Toxicol. 2: 389–398. [PubMed] [Google Scholar]
- 89.Romsos D. R., Belo P. S., Leveille G. A. 1975. Butanediol and lipid metabolism. Fed. Proc. 34: 2186–2190. [PubMed] [Google Scholar]