Abstract
Increasing evidence indicates that transcription and alternative splicing are coordinated processes; however, our knowledge of specific factors implicated in both functions during the process of adipocyte differentiation is limited. We have previously demonstrated that the zinc finger protein ZNF638 plays a role as a transcriptional coregulator of adipocyte differentiation via induction of PPARγ in cooperation with CCAAT/enhancer binding proteins (C/EBPs). Here we provide new evidence that ZNF638 is localized in nuclear bodies enriched with splicing factors, and through biochemical purification of ZNF638’s interacting proteins in adipocytes and mass spectrometry analysis, we show that ZNF638 interacts with splicing regulators. Functional analysis of the effects of ectopic ZNF638 expression on a minigene reporter demonstrated that ZNF638 is sufficient to promote alternative splicing, a function enhanced through its recruitment to the minigene promoter at C/EBP responsive elements via C/EBP proteins. Structure-function analysis revealed that the arginine/serine-rich motif and the C-terminal zinc finger domain required for speckle localization are necessary for the adipocyte differentiation function of ZNF638 and for the regulation of the levels of alternatively spliced isoforms of lipin1 and nuclear receptor co-repressor 1. Overall, our data demonstrate that ZNF638 participates in splicing decisions and that it may control adipogenesis through regulation of the relative amounts of differentiation-specific isoforms.
Keywords: transcriptional coactivator, minigene reporter, nuclear speckles, adipocyte differentiation
The process of adipocyte differentiation is initiated and controlled by a number of transcription factors, including CCAAT/enhancer binding protein (C/EBP) β and C/EBPδ, and by cofactors (1), such as the recently characterized zinc finger protein ZNF638, which regulates the expression of the master controller of adipogenesis, PPARγ, in conjunction with C/EBP proteins (2). The gene expression program occurring during differentiation includes the engagement and cooperation of these tissue-selective factors and cofactors with components of the RNA polymerase II machinery at active transcription sites, initiation of transcription followed by mRNA processing, through RNA capping, polyadenylation, and removal of noncoding intronic sequences, prior to protein translation (3). The process of splicing gives rise to the mature mRNA though the sequential succession of several complexes, starting from the precomplex in which sites of splicing are chosen, to the catalytic removal of introns executed by complex C components (4). During differentiation and development, tissue-specific enrichment of splicing factors ensures that alternative splicing is achieved to generate tissue-specific, temporally and developmentally regulated isoforms required to confer the specific phenotype (4–6). It has been shown that the relative abundance or activity of splicing regulators that have opposing effects determines the use of competing splice sites, ultimately controlling the exon composition of tissue-specific isoforms (4, 5). In adipocytes, alternatively spliced isoforms such as those of the insulin receptor (7), lipin (8), mitochondrial oxodicarboxylate carrier (9), nuclear receptor co-repressor 1 (NCoR) (10), preadipocyte factor 1 (11), and mechanistic target of rapamycin (12) have been shown to play a role in the differentiation process.
Serine/arginine-rich (SR) proteins are a major class of splicing regulators. These proteins contain RNA recognition motifs (RRMs) at the amino terminus and arginine/serine-rich (RS) repeats at the carboxy terminus (13). SR proteins reside in discrete subnuclear regions, which represent storage areas for splicing factors serving nearby active transcription sites (14, 15). Furthermore, SR proteins bind to exonic splicing enhancer sequences and recruit spliceosomal ribonucleoproteins (RNPs) and non-RNPs to the pre-mRNA (16), thereby affecting the selection of splice sites already during the process of transcription. In addition to SR proteins, heterogeneous nuclear ribonucleoproteins (HNRNPs) bind to pre-mRNA transcripts and influence alternative splicing (17–19). It has been shown that pre-mRNA splicing is coupled to transcription both by the function of the C-terminal domain (CTD) of RNA polymerase II (3) and by a number of cofactors involved in both processes (20–23), as demonstrated for the metabolic coactivator PPAR gamma coactivator 1-alpha (PGC1α), which exerts a dual function when recruited at promoters containing direct repeat-1 responsive elements (23).
We have recently identified ZNF638 as a novel coactivator with domain homology to PGC1α and shown that ZNF638 functions as a transcriptional cofactor regulating the early stages of adipocyte differentiation through protein-protein interaction with C/EBP family members at the PPARγ promoter (2). ZNF638 is composed of RS domains and RRMs, in addition to a homology region to the matrix-bound protein matrin 3 (MH1), two C2H2 zinc finger (ZF) motifs (ZF1 and ZF2), a putative DNA binding domain (DBD), and a region with acidic repeats (ARs) (24, 25). This characteristic domain composition of ZNF638, reminiscent of RNA binding proteins and of splicing regulators, and its dotted nuclear localization pattern in adipocytes (2), led us to hypothesize that ZNF638 may also participate in alternative splicing, as previously demonstrated for the coactivator PGC1α (23). In this study, we tested this hypothesis using several experimental approaches, including confocal microscopy, mutagenesis, mass spectrometry analysis, and functional studies.
MATERIALS AND METHODS
Plasmids
Full-length murine ZNF638.pCR3.1 plasmid (2) was used as a template to generate eight deletion mutants by whole vector mutagenesis (Genewiz). ZNF638-ΔZF1 contains a deletion from amino acid 424 to amino acid 456, eliminating the N-terminal ZF motif, the ΔRS construct lacks the RS motif present from amino acids 470 to 573, the ΔRRM1 has a deletion of the RRM1 motif from amino acids 676 to 751, ΔRRM2 has a deletion of the RRM2 motif from amino acids 901 to 981, and ΔRRM1-3 has a deletion encompassing all three RRM motifs, from amino acids 676 to 1,077. The ZNF638-ΔDBD construct has a deletion of the DBD, encompassing amino acids 1,353 to 1,482, the ΔAR contains a deletion of the ARs from amino acids 1,538 to 1,770, and ΔZF2 lacks amino acids 1,876 to 1,908, eliminating the C-terminal ZF domain. The ΔRS plasmid served as a template to generate the ΔRS/ZF2 mutant lacking both the RS and ZF2 motifs, with two deletions, one from amino acids 470 to 573 and one from 1,876 to 1,908. The primer sequences to generate the previous mutants are listed in Table 1. The empty vector backbone pCR3.1 served as control. glutathione S-transferase (GST)-ZNF638ZF2 and green fluorescent protein (GFP)-ZNF638ZF2 constructs expressing only the carboxy-terminal region of ZNF638, from amino acids 1,773 to 1,926, containing the C-terminal ZF domain were previously described (2). The fibronectin minigene reporter 7iBi89 plasmid (26) was obtained from Addgene (14065). This minigene construct contains exons 24 through 28 of the rat fibronectin gene including introns 24-25 and 25-26 flanking the alternatively spliced exon 25. The gene cassette is under the control of a human β-actin promoter and contains a human growth hormone polyadenylation signal. To generate a fibronectin minigene reporter containing C/EBP responsive elements, a 46 bp sequence of the PPARγ2 promoter containing C/EBP responsive elements (5′-TTTTACTGCAATTTTAAAAAGCAATCAATATTGAACAATCTCTGCT-3′) (27) was inserted between the β-actin promoter and the beginning of the fibronectin minigene cassette. The C/EBP responsive element sequence was synthesized including XhoI and BsrGI restriction sites and cloned into the minigene plasmid at those sites (Genewiz). C/EBPβ and C/EBPδ plasmids were obtained from Addgene, and the C/EBPα construct was a gift of Kai Ge (National Institutes of Health).
TABLE 1.
Primers used to generate ZNF638 deletion mutants
| ZNF638 Mutants | Primer Sequences (5′ → 3′) |
| ΔZF1 | Forward: GAATATTTCCACAACAATACCCTGATTG |
| Reverse: GGTATTGTTGTGGAAATATTCTTGGAG | |
| ΔRS | Forward: CCTCCCATCCGATAGGAAAAAGGCATTAG |
| Reverse: CTTTTTCCTATCGGATGGGAGGATCTC | |
| ΔRRM1 | Forward: AAAAAGCCACAGAACAAAGAAATGAAG |
| Reverse: GTTGGAACTCAAAGACTGATCCTTC | |
| ΔRRM2 | Forward: GAATCGGAGGAAGATGAGGAAGCTCTC |
| Reverse: CTTCCTCATCTTCCTCCGATTCCTTGTTC | |
| ΔRRM1-3 | Forward: CCAAAGACTGACTCAGAGGTTCAAAG |
| Reverse: GTTGGAACTCAAAGACTGATCCTTCTG | |
| ΔDBD | Forward: GAGAAAAGCCAATAACAAAACAGTCTC |
| Reverse: GTTTTGTTATTGGCTTTTCTCTTATTG | |
| ΔAR | Forward: GACAATGATTCAAAAGTTGAGTTAG |
| Reverse: TTCATCCAAATTAAATGTAAATAATG | |
| ΔZF2 | Forward: GCCAAGCAAAGAAAGGAAAAGGAGC |
| Reverse: TCCAGCCTTCGGAACAAGAAAGTCC |
Antibodies
Rabbit anti-ZNF638 antibody (Bethyl, A301-548A) was used to detect ZNF638. Mouse anti-pre-mRNA-splicing factor 2 (SF2)/alternative splicing factor (ASF) antibody (Invitrogen, 32-4600), Alexa Fluor 488 goat anti-rabbit antibody (Invitrogen, A-11008), and Alexa Fluor 594 goat anti-mouse antibody (Invitrogen, A-11005) were used for immunofluorescence staining. Mouse anti-β-actin antibody (Sigma, A5316), anti-GFP antibody (Invitrogen, A-11122), anti-HNRNPA1 antibody (Abcam, ab5832), anti-HNRNPA2B1 antibody (Abcam, ab31645), anti-HNRNPLL antibody (Santa Cruz, sc-132712), anti-non-POU domain-containing octamer binding protein (NONO) antibody (Santa Cruz, sc-136296), anti-polyadenylate binding protein 1 (PABP1) antibody (Cell Signaling, 4992), anti-C/EBPα antibody (Santa Cruz, sc-61), anti-C/EBPβ antibody (Santa Cruz, sc-7962), anti-C/EBPδ antibody (Santa Cruz, sc-636), anti-mouse-HRP antibody (Santa Cruz, sc-2055), anti-rabbit-HRP antibody (Santa Cruz, sc-2054), and anti-goat-HRP antibody (Santa Cruz, sc-2020) were used for Western blotting.
Cell culture and transfections
The 3T3-L1, 10T1/2, U2OS, and HEK-293 cells (ATCC), were cultured in high-glucose DMEM medium (Invitrogen), supplemented with 10% fetal bovine serum (HyClone) and 1% penicillin/streptomycin (Mediatech) at 37°C and 5% CO2. The 3T3-L1 cells were cultured and differentiated as previously described (2). For minigene assays and transient transfections, cells were transfected with Xtreme Gene HP (Roche) and analyzed 24 h later.
Differentiation assays
The 10T1/2 cells were electroporated (Amaxa) with either control, full-length ZNF638, or mutant plasmids, induced to differentiate in culture medium supplemented with 5 µg/ml insulin, 0.5 mM 3-isobutyl-1-methylxanthine, 10 µM dexamethasone, and 10 µM troglitazone for 2 days and subsequently maintained in culture medium supplemented with 5 µg/ml insulin (28). Gene expression was assayed by real-time PCR 3 days after induction of differentiation, following RNA extraction (TRIzol) and reverse transcription (Roche). The sequences of the primers used were the following: for 36B4, forward 5′-GCTTCATTGTGGGAGCAGAC-3′, reverse 5′-ATGGTGTTCTTGCCCATCAG-3′ for CD36, forward 5′-TTTGGAGTGGTAGTAAAAAGGGC-3′, reverse 5′-TGACATCAGGGACTCAGAGTAG-3′ for aP2, forward 5′-ACACCGAGATTTCCTTCAAACTG-3′, reverse 5′-CCATCTAGGGTTATGATGCTCTTC-3′ for Pparγ2, forward 5′-GATGCACTGCCTATGAGCACTT-3′, reverse 5′-AGAGGTCCACAGAGCTGATTC-3′ for ZNF638, forward 5′-TCCCAGTTGAGAGTGGAACC-3′, reverse 5′-TGTGAGATCCGCTCTTGTTG-3′ for Lipin1α, forward 5′-GGTCCCCCAGCCCCAGTCCTT-3′, reverse 5′-GCAGCCTGTGGCAATTCA-3′ for Lipin1β, forward 5′-CAGCCTGGTAGATTGCCAGA-3′, reverse 5′-GCAGCCTGTGGCAATTCA-3′ for NCoR, forward 5′-CTGACAGGCCTCAAGAAAGG-3′, reverse 5′-AACCTGTTCCAGACGTGGTC-3′ and for NCoRω, forward 5′-CTGGCTGCTCTTGTGGATGCT-3′, reverse 5′-CTGTCCCATTCCCTCTGACTG-3′. To quantify the extent of lipid accumulation, cells were stained with Oil Red O at day 6 of differentiation, and the dye was extracted with isopropanol and absorbance measured at 520 nm, as described previously (29, 30).
Immunofluorescence
U2OS cells were plated on chamber slides (Labtek) and transfected with either vector, full-length ZNF638, or one of the eight ZNF638 deletion mutants. Twenty-four hours after transfection, cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% (w/v) Triton X-100 in PBS, blocked with 1% nonfat dry milk, and incubated with primary antibody for 1 h at room temperature or overnight at 4°C and with secondary antibodies for 1 h at room temperature. DAPI (4’,6-diamidino-2-phenylindole, Invitrogen, D1306) was used for nuclear counterstaining. Confocal imaging was performed with an LSM 5 live DuoScan (Zeiss) laser scanning microscope with a 63× oil objective, using ZEN 2009 acquisition software (Zeiss).
Biochemical purification of ZNF638 interacting proteins present in adipocytes and mass spectrometry analysis
Purification of the GST-ZNF638ZF2 fusion protein from BL21 bacterial cells and preparation of nuclear extracts from differentiating 3T3-L1 cells 2 days after induction with 0.5 mM 3-isobutyl-1-methylxanthine (Sigma), 1 μM dexamethasone (Fluka), and 5 μg/ml insulin (Sigma) were performed as previously described (2). ZNF638 interacting proteins purified from nuclear lysates obtained from differentiating 3T3-L1 adipocytes were separated by SDS-PAGE and stained with Coomassie blue. Prominent bands were excised and subjected to mass spectrometry analysis (Taplin Biological Mass Spectrometry Facility, Harvard Medical School, Boston, MA).
Immunoprecipitation assays
HEK-293 cells were transfected with GFP-ZNF638ZF2.pAcGFP or pAcGFP vector. Immunoprecipitation assays were performed using GFP-trap agarose beads (Chromotek, Allele), according to the manufacturer’s instructions. Protein lysates were resolved by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore). After blocking in TBS with Tween containing 2% nonfat dry milk, membranes were incubated with primary antibodies for 1 h at room temperature or overnight at 4°C and subsequently exposed to secondary HRP-conjugated antibodies for 1 h. Blots were incubated with ECL substrate (Pierce) and exposed to film (Kodak).
Minigene splicing assays
HEK-293 cells were plated on 12-well plates and transfected at 70–80% confluency with 1 µg of fibronectin minigene reporter plasmid and either 5 µg of ZNF638.pCR3.1 or pCR3.1 vector. For C/EBP coexpression studies, cells were cotransfected with 1 µg fibronectin minigene plasmid containing C/EBP responsive elements and with 1 µg of C/EBPα, C/EBPβ, or C/EBPδ plasmids or vector, and 3 µg of vector control or ZNF638. Twenty-four hours after transfection, cells were harvested, and the extracted mRNA (RNeasy, Qiagen) was reverse transcribed using anchored oligo dT primer (Transcriptor First Strand cDNA Synthesis Kit, Roche). Ratio of exon inclusion (EI) to exon skipping (ES) was assessed by real-time PCR. The EI primers specifically amplified the longer isoform including the alternatively spliced exon 25, and the ES primers amplified the short isoform, skipping exon 25, as previously described (31). The EI and ES primers utilized were the following: EI forward 5′-CCGTCATCCCAGAGGTGCCCCA-3′, EI reverse 5′-GGAGGGACGGCCGTTTGCTGTG-3′ and ES forward 5′-CCCCTATCTCTGATACCGTCATCCC-3′, ES reverse 5′-GTTCGTACACGCTGGAGACACTGAC-3′. The following real-time PCR conditions were used: 10 min 95°C, 35 cycles of 10 s at 95°C, 30 s at 67°C, and 30 s at 72°C. PCR products corresponding to EI or ES were further confirmed by sequencing (Genewiz). Statistical analysis was performed by the two-sided Student’s t-test with unequal variance on three replicates as indicated. A P value < 0.05 was considered statistically significant.
RESULTS
The adipogenic cofactor ZNF638 localizes in nuclear bodies enriched in splicing factors
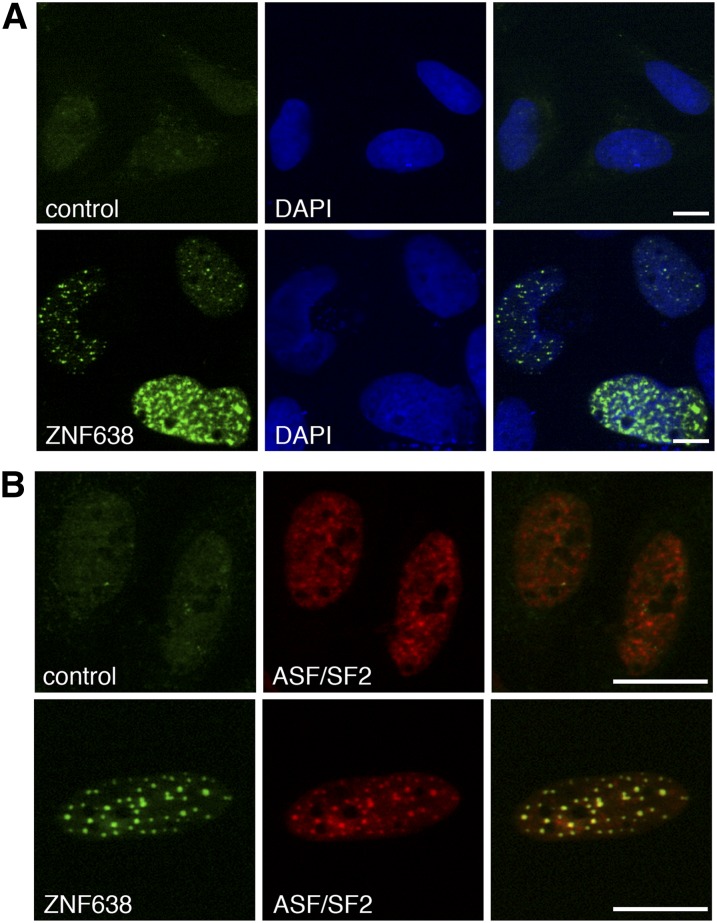
We previously identified ZNF638 as a transcriptional cofactor implicated in the regulation of PPARγ expression and adipocyte differentiation through interaction with the C/EBP family of transcription factors. In addition, we showed that ZNF638 is localized in the nucleus of differentiating 3T3-L1 cells in a punctate pattern (2). To better characterize the identity of the nuclear bodies in which ZNF638 resides, we performed immuofluorescence staining using antibodies against ZNF638 and the splicing factor ASF/SF2, which served as a marker for nuclear speckles (32). Confocal imaging analysis of U2OS cells transiently expressing ZNF638 revealed that ZNF638 colocalizes with endogenous ASF/SF2 in nuclear speckles (Fig. 1A, B).
Fig. 1.
ZNF638 colocalizes with the splicing factor ASF/SF2 in nuclear speckles. Subcellular localization of transiently expressed ZNF638 in U2OS cells detected by indirect immunofluorescence and by overlay with DAPI as nuclear counterstaining (A). Double immunofluorescence staining of transiently expressed ZNF638 in U2OS cells and overlay with endogenous ASF/SF2, a marker for nuclear speckles (B). A, B: Confocal imaging; scale bars: 10 µm.
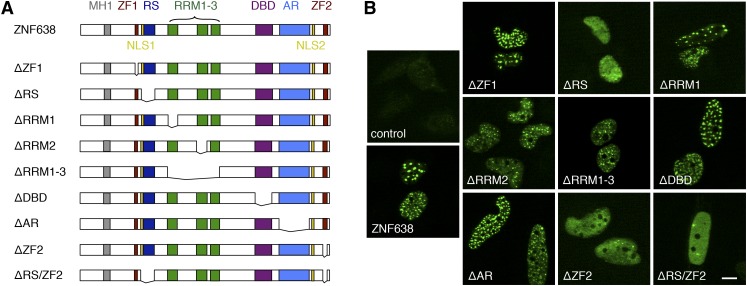
The RS and the C-terminal ZF domains are required for ZNF638’s localization to nuclear speckles
To investigate the contribution of the domains present in ZNF638 to the speckled localization observed, we generated deletion mutants, as shown in the schematic representation in Fig. 2A. Indirect immunofluorescence staining of the ectopically expressed full-length ZNF638 and mutants followed by confocal imaging demonstrated that the ablation of either the RS domain (ZNF638-ΔRS) or the C-terminal ZF domain (ZNF638-ΔZF2), or of both domains (ZNF638-ΔRS/ZF2), abolished ZNF638’s localization to speckles (Fig. 2B). These findings indicate that the RS domain and the C-terminal ZF domain are required for ZNF638’s localization to nuclear speckles.
Fig. 2.
The RS domain and the C-terminal ZF motif are required for ZNF638 localization to nuclear speckles. Schematic illustration of the domains present in full-length ZNF638 and schematic representation of ZNF638 deletion mutants analyzed in this study (A). NLS, nuclear localization signal. Subcellular localization of ectopically expressed full-length ZNF638 and ZNF638 deletion mutants in U2OS cells, detected by indirect immunofluorescence staining and confocal imaging (B). Scale bar, 10 µm.
ZNF638 interacts with regulators of pre-mRNA splicing present in adipocytes
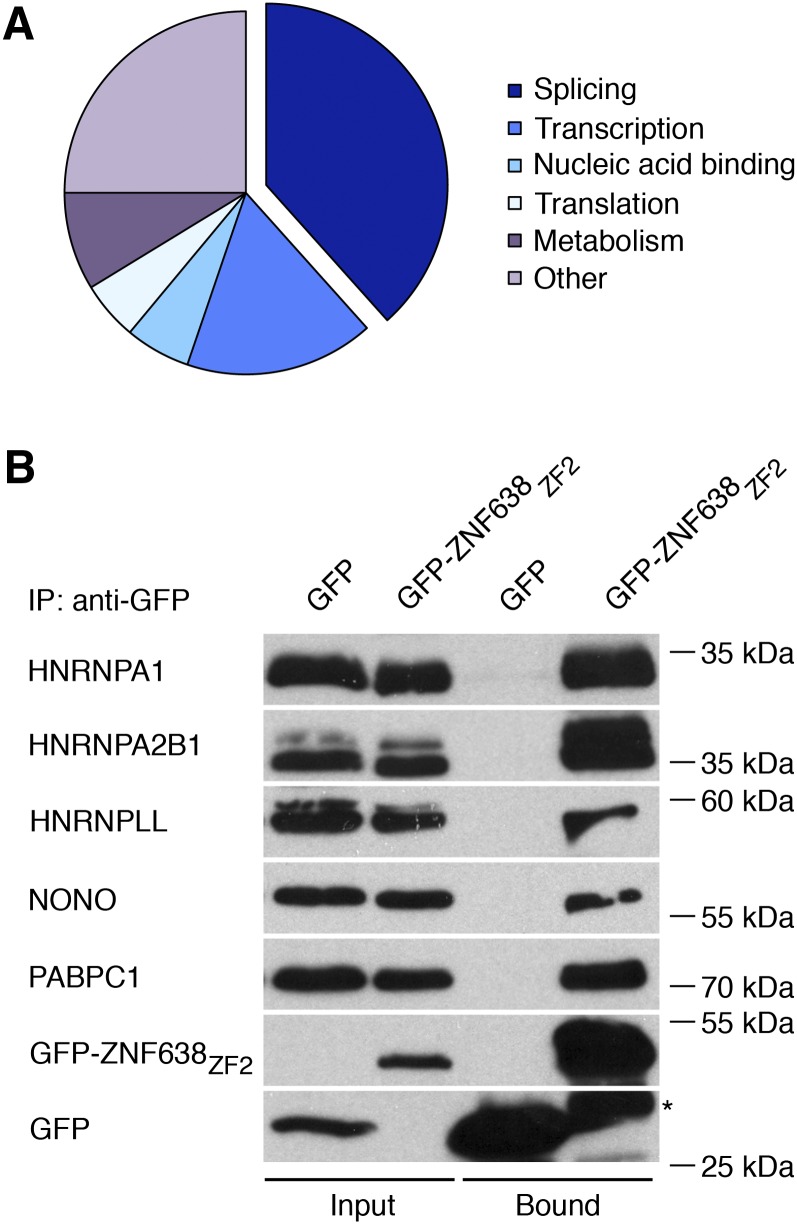
Given the punctate nuclear localization of ZNF638 and the evidence that speckles are nuclear bodies enriched in pre-mRNA splicing factors (14), we hypothesized that ZNF638 may complex with spliceosomal proteins. To assess this, we performed biochemical purification of ZNF638 interacting proteins from differentiating 3T3-L1 adipocytes using a GST fusion protein expressing the region containing the ZF domain present at the carboxy terminus (GST-ZNF638ZF2) required for speckled localization. Mass spectrometry analysis identified 172 novel ZNF638 interactors (Fig. 3A), and their clustering according to function revealed that 38% of these interacting proteins have been previously shown to be either associated with constitutive and alternative splicing, reported in interchromatin granule clusters or present in early or late spliceosomal complexes (33–36) (Fig. 3A, Table 2). Mass spectrometry analysis also revealed that the novel ZNF638 interacting proteins identified are involved in transcription, translation, nucleic acid binding, and metabolism, according to their annotation in the Uniprot database (Fig. 3A). Furthermore, we detected ZNF638 peptide sequences, which were not part of the ZNF638-GST fusion protein used for affinity purification (data not shown). To validate the results obtained via mass spectrometry analysis, we performed coimmunoprecipitation assays. As shown in Fig. 3B, ectopically expressed GFP-ZNF638ZF2 was able to immunoprecipitate endogenous HNRNPA1, HNRNPA2B1, HNRNPLL, NONO, and PABP1. Taken together, our data indicate that ZNF638 can complex with factors implicated in alternative splicing expressed in differentiating adipocytes.
Fig. 3.
ZNF638 interacts with factors involved in pre-mRNA splicing present in differentiating adipocytes. Graphical representation of the clustering of the 172 novel ZNF638 interacting proteins present in differentiating adipocytes identified by mass spectrometry analysis (A). Interacting proteins purified from nuclear extracts obtained from differentiating 3T3-L1 cells using a GST-ZNF638ZF2 fusion protein were resolved by SDS-PAGE and subjected to mass spectrometry analysis. Coimmunoprecipitation assays were performed to validate mass spectrometry data (B). GFP-ZNF638ZF2 fusion protein was expressed in HEK-293 cells, and endogenous interacting proteins were detected by Western blot after GFP immunoprecipitation. Asterisk indicates nonspecific band.
TABLE 2.
ZNF638 interacting proteins implicated in pre-mRNA splicing identified by mass spectrometry analysis
| Gene Symbol | Protein Name | Uniprot Accession Number | Number of Peptide Matches | References |
| Cpsf6 | Cleavage and polyadenylation specificity factor subunit 6 | Q6NVF9 | 1 | (36) |
| Ddx1 | ATP-dependent RNA helicase DDX1 | Q91VR5 | 30 | (36) |
| Ddx17 | Probable ATP-dependent RNA helicase DDX17 | Q501J6 | 10 | (33, 36) |
| Ddx3× | ATP-dependent RNA helicase DDX3× | Q62167 | 4 | (33, 36) |
| Ddx46 | Probable ATP-dependent RNA helicase DDX46 | Q569Z5 | 3 | (33, 34, 36, 53) |
| Ddx5 | Probable ATP-dependent RNA helicase DDX5 | Q61656 | 20 | (33–36, 54) |
| Ddx50 | ATP-dependent RNA helicase DDX50 | Q99MJ9 | 3 | (36) |
| Dhx15 | Putative pre-mRNA-splicing factor ATP-dependent RNA helicase DHX15 | O35286 | 6 | (33, 36, 55) |
| Dhx30 | Putative ATP-dependent RNA helicase DHX30 | Q99PU8 | 4 | (36) |
| Dhx9 | ATP-dependent RNA helicase A | O70133 | 5 | (33, 34, 36) |
| Elavl1 | ELAV-like protein 1 | P70372 | 2 | (34, 36) |
| Ewsr1 | RNA binding protein EWS | Q61545 | 10 | (33, 36) |
| Fbl | rRNA 2’-O-methyltransferase fibrillarin | P35550 | 2 | (33) |
| Fus | RNA binding protein FUS | P56959 | 18 | (33, 34, 36) |
| Gpatch1 | G patch domain-containing protein 1 | Q9DBM1 | 1 | (35) |
| Hist1 h1c | Histone H1.2 | P15864 | 2 | (33) |
| Hnrnpa0 | Heterogeneous nuclear ribonucleoprotein A0 | Q9CX86 | 1 | (33, 36) |
| Hnrnpa1 | Heterogeneous nuclear ribonucleoprotein A1 | P49312 | 14 | (33–38) |
| Hnrnpa2b1 | Heterogeneous nuclear ribonucleoproteins A2/B1 | O88569 | 25 | (33–36, 39, 40) |
| Hnrnpa3 | Heterogeneous nuclear ribonucleoprotein A3 | Q8BG05 | 15 | (33–36) |
| Hnrnpab | Heterogeneous nuclear ribonucleoprotein A/B | Q99020 | 8 | (36) |
| Hnrnpd | Heterogeneous nuclear ribonucleoprotein D0 | Q60668 | 4 | (33, 36) |
| Hnrnpl | Heterogeneous nuclear ribonucleoprotein L | Q8R081 | 6 | (33, 35, 36, 56) |
| Hnrnpm | Heterogeneous nuclear ribonucleoprotein M | Q9D0E1 | 4 | (33, 35, 36, 57) |
| Hnrnpr | Putative uncharacterized protein | Q3UZI0 | 10 | (36) |
| Hnrnpu | Heterogeneous nuclear ribonucleoprotein U | Q8VEK3 | 49 | (33–36) |
| Hnrpll | Heterogeneous nuclear ribonucleoprotein L-like | Q921F4 | 1 | (41, 42) |
| Hspa5 | 78 kDa glucose-regulated protein | P20029 | 19 | (33) |
| Hspa8 | Heat shock cognate 71 kDa protein | P63017 | 3 | (36) |
| Igf2bp3 | Insulin-like growth factor 2 mRNA binding protein 3 | Q9CPN8 | 10 | (36) |
| Ilf3 | Interleukin enhancer binding factor 3 | Q9Z1 × 4 | 3 | (33, 34, 36) |
| Matr3 | Matrin-3 | Q8K310 | 1 | (33, 36) |
| Ncbp1 | Nuclear cap binding protein subunit 1 | Q3UYV9 | 1 | (33–36, 58) |
| Ncl | Nucleolin | P09405 | 23 | (36) |
| Nono | Non-POU domain-containing octamer binding protein | Q99K48 | 1 | (33, 36, 57, 59, 60) |
| Nop56 | Nucleolar protein 56 | Q9D6Z1 | 3 | (33) |
| Nop58 | Nucleolar protein 58 | Q6DFW4 | 1 | (33) |
| Npm1 | Nucleophosmin | Q61937 | 2 | (36) |
| Nxf1 | Nuclear RNA export factor 1 | Q99JX7 | 1 | (33, 36) |
| Pabpc1 | Polyadenylate binding protein, cytoplasmic 1 | P29341 | 4 | (35, 61) |
| Ppp1ca | Serine/threonine-protein phosphatase PP1-α catalytic subunit | P62137 | 2 | (36) |
| Ppp1r10 | Serine/threonine-protein phosphatase 1 regulatory subunit 10 | Q80W00 | 12 | (33) |
| Rbm15 | Putative uncharacterized protein | Q3THK4 | 1 | (36) |
| Rbm39 | RNA binding protein 39 | Q8VH51 | 2 | (21, 33, 34) |
| Rbmx | Heterogeneous nuclear ribonucleoprotein G | O35479 | 1 | (33, 35, 36, 62) |
| Rpl7a | 60S ribosomal protein L7a | P12970 | 4 | (33) |
| Rplp0 | 60S acidic ribosomal protein P0 | P14869 | 4 | (33) |
| Rps2 | 40S ribosomal protein S2 | P25444 | 2 | (33) |
| Rps3a | 40S ribosomal protein S3a | P97351 | 3 | (33) |
| Safb2 | Scaffold attachment factor B2 | Q80YR5 | 7 | (36) |
| Sf1 | Splicing factor 1 | Q64213 | 2 | (33, 36, 63) |
| Sf3b1 | Splicing factor 3B subunit 1 | Q99NB9 | 1 | (33–36, 64) |
| Sfpq | Splicing factor, proline and glutamine rich | Q8VIJ6 | 34 | (33, 36, 65) |
| Sltm | SAFB-like transcription modulator | Q8CH25 | 1 | (33) |
| Srpk1 | Serine/threonine-protein kinase SRPK1 | O70551 | 1 | (66) |
| Srsf4 | Serine/arginine-rich splicing factor 4 | Q8VE97 | 1 | (33, 36, 67) |
| Srsf7 | Serine/arginine-rich splicing factor 7 | Q8BL97 | 1 | (33, 34, 36, 68) |
| Syncrip | Heterogeneous nuclear ribonucleoprotein Q | Q7TMK9 | 23 | (35, 36, 69) |
| Taf15 | TAF15 RNA polymerase II, TATA box binding protein-associated factor | Q8BQ46 | 3 | (36) |
| Tdrd3 | Tudor domain-containing protein 3 | Q91W18 | 2 | (34) |
| Tmpo | Lamina-associated polypeptide 2, isoforms α/zeta | Q61033 | 1 | (33) |
| U2surp | U2 snRNP-associated SURP motif-containing protein | Q6NV83 | 1 | (33, 34) |
| Wbp11 | WW domain binding protein 11 | Q923D5 | 1 | (36, 70) |
| Xrn2 | 5′-3′ exoRNase 2 | Q9DBR1 | 2 | (36) |
| Zfr | Zinc finger RNA binding protein | O88532 | 6 | (33, 36) |
| Znf638 | Zinc finger protein 638 | Q61464 | 27 | (33) |
ZNF638 modulates alternative splicing decisions on a minigene reporter
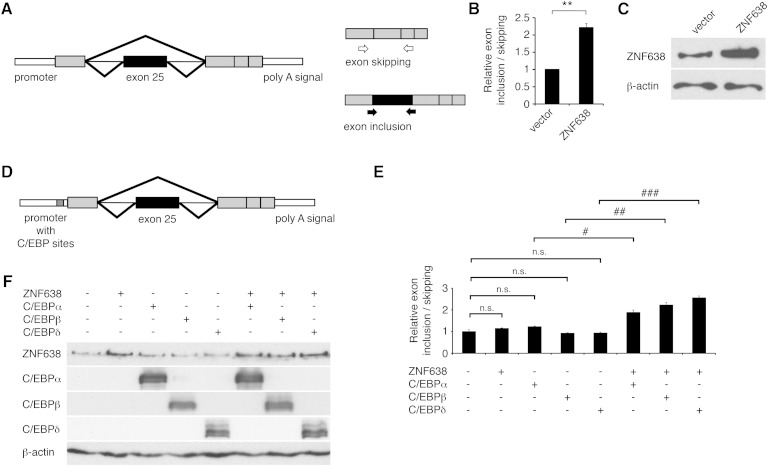
In order to test the hypothesis that ZNF638 may participate in the regulation of pre-mRNA splicing, we investigated the effects of coexpressing ZNF638 with a minigene reporter containing a genomic exon cassette derived from the rat fibronectin gene. This reporter system is used for the quantification of alternative splicing through measurements of an alternatively spliced exon (Fig. 4A) (26). When ZNF638 was coexpressed with the minigene reporter, we observed an increase in the ratio of EI to ES, as compared with the ratio observed in control cells (Fig. 4B, C). Exon inclusion and exclusion were further confirmed by direct sequencing of PCR products (data not shown).
Fig. 4.
ZNF638 modifies splicing of minigene reporter transcripts. Schematic representation of the fibronectin minigene reporter and of the assay performed (A). EI-specific primers (filled arrows) and ES primers (empty arrows) were used to quantify the mRNA isoforms retaining the alternative spliced exon 25 (black boxes) and the short isoform skipping the alternatively spliced exon. Gray boxes indicate constitutively retained exons; thin lines, introns; and thick lines, spliced isoforms. Quantification of the ratio of EI to ES in HEK-293 cells transiently expressing the minigene plasmid with ZNF638 or vector control (B). Mean ± SEM. Statistical analysis was performed on three independent experiments. ** P < 0.01. Western blot of ZNF638 expression levels from a representative experiment (C). β-actin was used as loading control. Schematic representation of the fibronectin minigene containing C/EBP responsive elements in the promoter (D). Ratio of EI over ES in HEK-293 cells transiently expressing the minigene containing C/EBP binding sites, in the presence or absence of ZNF638 and C/EBP factors (E). Experiments were repeated at least three times. A representative experiment is shown. Mean ± SEM; n.s., not statistically significant; # P < 0.05, ## P < 0.01, ### P < 0.001. Western blot analysis to assess protein levels of transiently expressed ZNF638, C/EBPα, C/EBPβ, and C/EBPδ in a representative experiment. β-actin was used as loading control (F).
Given our previously published data demonstrating a function for ZNF638 as a coactivator of C/EBP proteins at C/EBP responsive elements present in the PPARγ promoter, we assessed the effect of ZNF638 on splicing of the fibronectin minigene when a C/EBP responsive element cassette was introduced in the fibronectin minigene reporter (Fig. 4D). As shown in Fig. 4E, F, low amounts of ectopically expressed ZNF638 were able to increase the ratio of EI to ES only when C/EBPα, C/EBPβ, or C/EBPδ were coexpressed, suggesting that loading of ZNF638 on promoters enhances its action on splicing. These data indicate that ZNF638 is able to influence alternative splicing of a minigene reporter and that this process is facilitated in the presence of C/EBP responsive elements and C/EBP proteins.
The RS motif and the C-terminal ZF domain are necessary for the proadipogenic function of ZNF638
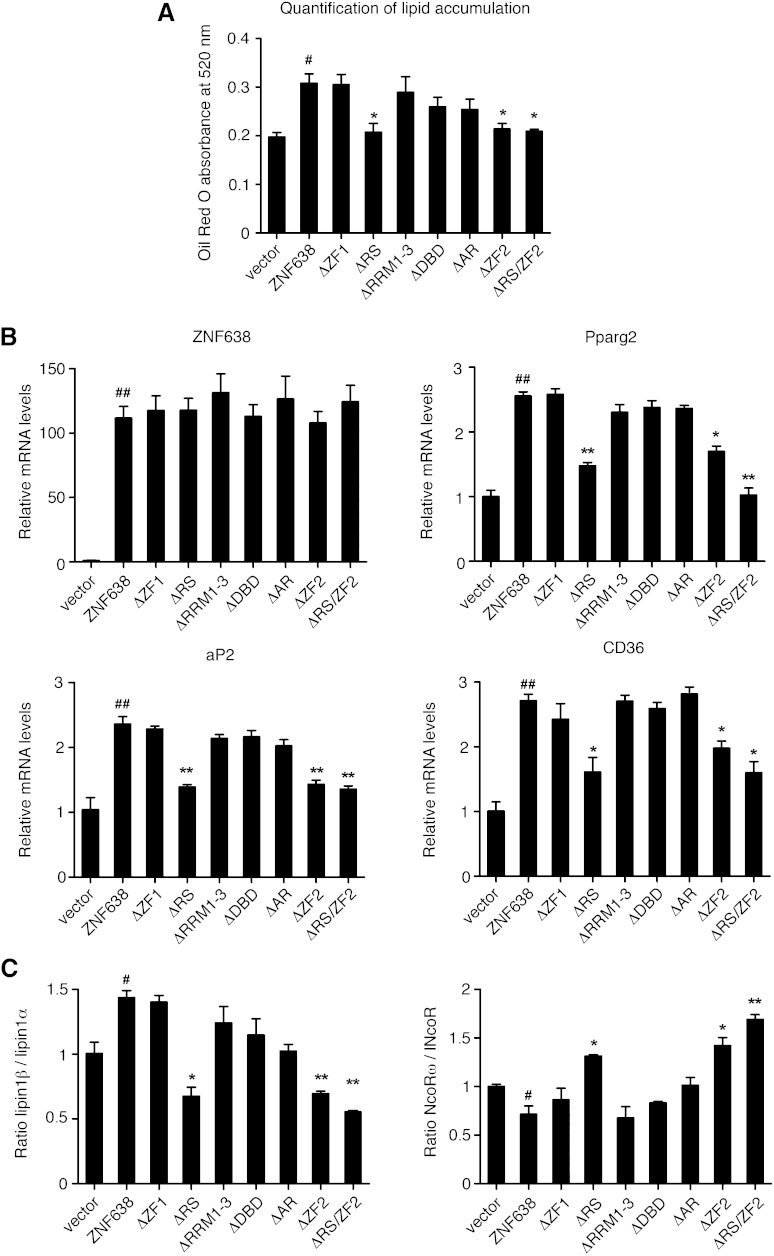
Given our previous characterization of ZNF638 as a coregulator of adipocyte differentiation, we assessed the requirement of each domain of ZNF638 for this process. We therefore ectopically expressed either vector, full-length ZNF638, or each deletion mutant in the mesenchymal cell line 10T1/2 and induced their differentiation. As shown in Fig. 5A, B, while 10T1/2 cells expressing full-length ZNF638 showed increased lipid accumulation and induced classic adipocyte markers, cells expressing ZNF638 mutants lacking either the RS, the C-terminal ZF, or both domains showed a decreased ability to induce adipocyte differentiation compared with full-length ZNF638, even though these mutants were expressed at the same levels as full-length ZNF638.
Fig. 5.
Requirements of ZNF638 domains in adipocyte differentiation. Quantification of lipid accumulation in 10T1/2 cells expressing vector, full-length ZNF638, or deletion mutants at day 6 after induction of differentiation measured through quantification of Oil Red O extracted from stained cells (A). mRNA levels of adipocyte markers in 10T1/2 cells expressing either vector, full-length ZNF638, or deletion mutants, after 3 days of differentiation (B). Ratio of alternatively spliced isoforms during adipocyte differentiation in 10T1/2 cells expressing either vector, full-length ZNF638, or deletion mutants, after 3 days of induction of differentiation (C). A, B, C: Mean ± SEM. Full-length ZNF638 compared with vector control: # P < 0.05, ## P < 0.01. Mutants compared with full-length ZNF638: * P < 0.05, ** P < 0.01.
Given that alternatively spliced isoforms of lipin1 and NCoR have been previously shown to be differentially regulated during the adipogenic process (8, 10), we determined whether their levels were altered in 10T1/2 cells expressing ZNF638 or its deletion mutants. As shown in Fig. 5C, we observed an altered ratio of lipin1β/lipin1α and of NcoRω/NcoR in cells expressing the mutants lacking the RS or the C-terminal ZF domains compared with cells expressing full-length ZNF638. Overall, these data demonstrate that the RS and the C-terminal ZF domains are necessary for the proadipogenic function of ZNF638 and for the regulation of alternatively spliced isoforms present in adipocytes.
DISCUSSION
The ZF protein ZNF638 is a multidomain protein initially cloned in the mid-1990s whose function has remained unknown for more than a decade. We have recently characterized ZNF638 as a transcriptional cofactor involved in adipocyte differentiation acting in cooperation with C/EBPβ and C/EBPδ to regulate the expression of the nuclear receptor PPARγ in adipocytes (2). In the present study, we have provided novel evidence that ZNF638 localizes in nuclear regions enriched in splicing factors, that it interacts with splicing regulatory proteins present in adipocytes, and that it participates in the modulation of alternative splicing of a pre-mRNA transcript derived from a minigene reporter.
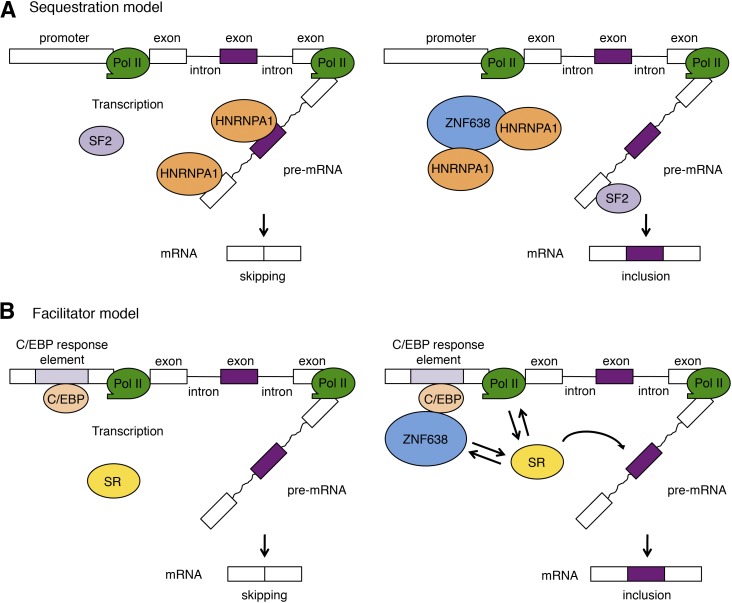
Our systematic analysis of ZNF638 interactors in adipocytes revealed that ZNF638 complexes with 16 factors shown to be part of the early prespliceosomal complex, including U2SURP, SRSF7, TDRD3, SF3B1, FUS, DDX46, DDX5, DHX9, ELAVL1, ILF3, NCBP1, RBM39, and the HNRNP proteins A1, A2/B1, A3, and U (34). These interactions suggest an early association of ZNF638 with the splicing machinery and its participation in splice site selection during prespliceosomal assembly (4). Particularly noteworthy is the interaction of ZNF638 with HNRNPA1, HNRNPA2B1, and HNRNPLL, which have been critically implicated in alternative splicing (37–42). Given that HNRNPA1 functions by antagonizing the splicing factor ASF/SF2 on splice site selection (37), it is possible that ZNF638 may affect the relative local abundance of HNRNPA1 through binding and sequestration, shifting the cellular balance in favor of the splicing factor SF2, as proposed in Fig. 6A.
Fig. 6.
Proposed models of ZNF638’s mode of action on alternative splicing. Sequestration model (A). ZNF638 may affect splicing decisions by altering the balance between available inhibitory and activating splicing factors competing for their binding to the pre-mRNA. Via sequestration of HNRNP proteins like HNRPA1, ZNF638 may prevent their binding to splice sites on the pre-mRNA, increasing the availability of activating splicing factors, such as SF2. Facilitator model (B). The loading of ZNF638 on the promoters of its target genes via C/EBP responsive elements may facilitate the recruitment of SR proteins to the CTD of the polymerase II and to the nearby nascent pre-mRNA. A, B: Purple exon is an alternatively spliced exon. Pol II, RNA polymerase II.
Our mass spectrometry analysis has also revealed that the C-terminal portion of ZNF638 interacts with other ZNF638 proteins present in adipocytes, suggesting that either multiple ZNF638 molecules may be recruited to protein complexes formed by ZNF638 or that ZNF638 may be able to di- or multimerize. Direct verification of the functional significance of these findings in adipocyte differentiation will require further investigation.
The present data indicating that ZNF638 affects alternative splicing of a minigene containing C/EBP binding sites combined with the previous reported function of ZNF638 in transcription through interaction with C/EBP proteins at C/EBP responsive elements (2) suggest that ZNF638 may act as a dual function regulator. This double role is consistent with emerging evidence indicating that cofactors can participate in both transcription and splicing, as described for p52, RNA binding motif protein 39, RNA binding motif protein 14, and PGC1α (20–23). It is plausible that when loaded on a promoter through C/EBP responsive elements, ZNF638 could facilitate the recruitment of the splicing factor(s) required for splice choice to the CTD of the largest subunit of RNA polymerase II (3, 43) and to the proximity of the nascent pre-mRNA (Fig. 6B). This model is supported by the identification through our mass spectrometry analysis of SRSF4, a splicing factor previously shown to be involved in alternative splice site selection during pre-mRNA processing (44, 45), as a novel ZNF638 interactor. This putative dual function of ZNF638 is reminiscent of the splicing role played by the coactivator PGC1α when loaded on the promoter of its target genes (23).
Through mutagenesis and confocal microscopy analysis, we showed that the RS domain and the C-terminal ZF motif are required for ZNF638’s localization in nuclear bodies enriched in splicing factors. While it has been previously recognized that RS domains can confer speckle targeting (46–48), our data indicate that also the U1/matrin-like ZF (C-X2-C-X(12,16)-H-X5-H) (49) present at the C terminus of ZNF638 is required for speckle localization. Interestingly, this type of ZF motif has been previously identified in other proteins present in nuclear speckles and shown to be required for their localization, as in the case of SF3A2 and SF3A3 (50). These observations suggest a possible contribution of matrin-like ZFs in targeting proteins to nuclear speckles.
Our structure-function analysis has revealed that the RS and the C-terminal ZF domains required for speckle localization are also necessary for the differentiation function of ZNF638. While it is well established that RS domains play a role in splicing (51), the requirement of SR domains in adipocyte differentiation has been only recently identified through the analysis of the RS domain in the function of the CDC-like kinase 1) kinase in adipocyte differentiation (52). Further studies will determine whether the RS and C-terminal ZF domains present in ZNF638 are necessary for the generation of adipocyte-specific isoforms through their function as splicing activation domains via interactions with spliceosomal components or whether their role in adipocyte differentiation is through the recruitment of cofactors involved in transcription.
Acknowledgments
The authors would like to thank Yun-Ping Wu for help with confocal imaging and Pasha Sarraf for reading the manuscript.
Footnotes
Abbreviations:
- AR
- acidic repeat
- C/EBP
- CCAAT/enhancer binding protein
- CTD
- C-terminal domain
- DBD
- DNA binding domain
- EI
- exon inclusion
- ES
- exon skipping
- GFP
- green fluorescent protein
- GST
- Glutathione S-transferase
- HNRNP
- heterogeneous nuclear ribonucleoprotein
- NCoR
- nuclear receptor co-repressor 1
- NONO
- non-POU domain-containing octamer binding protein
- PABP1
- polyadenylate binding protein 1
- PGC1α
- PPAR gamma coactivator 1-alpha
- RRM
- RNA recognition motif
- RS
- arginine/serine rich
- ASF/SF2
- pre-mRNA-splicing factor 2/alternative splicing factor
- SR
- serine/arginine rich
- ZF
- zinc finger
- ZNF638
- zinc finger protein 638
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
REFERENCES
- 1.Mueller E. 2014. Understanding the variegation of fat: novel regulators of adipocyte differentiation and fat tissue biology. Biochim. Biophys. Acta. 1842: 352–357. [DOI] [PubMed] [Google Scholar]
- 2.Meruvu S., Hugendubler L., Mueller E. 2011. Regulation of adipocyte differentiation by the zinc finger protein ZNF638. J. Biol. Chem. 286: 26516–26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cramer P., Srebrow A., Kadener S., Werbajh S., de la Mata M., Melen G., Nogues G., Kornblihtt A. R. 2001. Coordination between transcription and pre-mRNA processing. FEBS Lett. 498: 179–182. [DOI] [PubMed] [Google Scholar]
- 4.Chen M., Manley J. L. 2009. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat. Rev. Mol. Cell Biol. 10: 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luco R. F., Allo M., Schor I. E., Kornblihtt A. R., Misteli T. 2011. Epigenetics in alternative pre-mRNA splicing. Cell. 144: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cáceres J. F., Kornblihtt A. R. 2002. Alternative splicing: multiple control mechanisms and involvement in human disease. Trends Genet. 18: 186–193. [DOI] [PubMed] [Google Scholar]
- 7.Entingh A. J., Taniguchi C. M., Kahn C. R. 2003. Bi-directional regulation of brown fat adipogenesis by the insulin receptor. J. Biol. Chem. 278: 33377–33383. [DOI] [PubMed] [Google Scholar]
- 8.Péterfy M., Phan J., Reue K. 2005. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J. Biol. Chem. 280: 32883–32889. [DOI] [PubMed] [Google Scholar]
- 9.Niimi M., Tao L., Lin S. H., Yin J., Wu X., Fukui H., Kambayashi J., Ye J., Sun B. 2009. Involvement of an alternatively spliced mitochondrial oxodicarboxylate carrier in adipogenesis in 3T3–L1 cells. J. Biomed. Sci. 16: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodson M. L., Mengeling B. J., Jonas B. A., Privalsky M. L. 2011. Alternative mRNA splicing of corepressors generates variants that play opposing roles in adipocyte differentiation. J. Biol. Chem. 286: 44988–44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei B., Zhao L., Chen L., Sul H. S. 2002. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: role of alternative splicing. Biochem. J. 364: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huot M. É., Vogel G., Zabarauskas A., Ngo C. T., Coulombe-Huntington J., Majewski J., Richard S. 2012. The Sam68 STAR RNA-binding protein regulates mTOR alternative splicing during adipogenesis. Mol. Cell. 46: 187–199. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeois C. F., Lejeune F., Stevenin J. 2004. Broad specificity of SR (serine/arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog. Nucleic Acid Res. Mol. Biol. 78: 37–88. [DOI] [PubMed] [Google Scholar]
- 14.Misteli T., Caceres J. F., Spector D. L. 1997. The dynamics of a pre-mRNA splicing factor in living cells. Nature. 387: 523–527. [DOI] [PubMed] [Google Scholar]
- 15.Lamond A. I., Spector D. L. 2003. Nuclear speckles: a model for nuclear organelles. Nat. Rev. Mol. Cell Biol. 4: 605–612. [DOI] [PubMed] [Google Scholar]
- 16.Graveley B. R. 2000. Sorting out the complexity of SR protein functions. RNA. 6: 1197–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreyfuss G., Matunis M. J., Pinol-Roma S., Burd C. G. 1993. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62: 289–321. [DOI] [PubMed] [Google Scholar]
- 18.Han S. P., Tang Y. H., Smith R. 2010. Functional diversity of the hnRNPs: past, present and perspectives. Biochem. J. 430: 379–392. [DOI] [PubMed] [Google Scholar]
- 19.Krecic A. M., Swanson M. S. 1999. hnRNP complexes: composition, structure, and function. Curr. Opin. Cell Biol. 11: 363–371. [DOI] [PubMed] [Google Scholar]
- 20.Auboeuf D., Dowhan D. H., Li X., Larkin K., Ko L., Berget S. M., O’Malley B. W. 2004. CoAA, a nuclear receptor coactivator protein at the interface of transcriptional coactivation and RNA splicing. Mol. Cell. Biol. 24: 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowhan D. H., Hong E. P., Auboeuf D., Dennis A. P., Wilson M. M., Berget S. M., O’Malley B. W. 2005. Steroid hormone receptor coactivation and alternative RNA splicing by U2AF65-related proteins CAPERalpha and CAPERbeta. Mol. Cell. 17: 429–439. [DOI] [PubMed] [Google Scholar]
- 22.Ge H., Si Y., Wolffe A. P. 1998. A novel transcriptional coactivator, p52, functionally interacts with the essential splicing factor ASF/SF2. Mol. Cell. 2: 751–759. [DOI] [PubMed] [Google Scholar]
- 23.Monsalve M., Wu Z., Adelmant G., Puigserver P., Fan M., Spiegelman B. M. 2000. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell. 6: 307–316. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki H., Matsushima Y., Nakamura K., Ohshima M., Kadowaki T., Kitagawa Y. 1996. A large DNA-binding nuclear protein with RNA recognition motif and serine/arginine-rich domain. J. Biol. Chem. 271: 12525–12531. [DOI] [PubMed] [Google Scholar]
- 25.Matsushima Y., Ohshima M., Sonoda M., Kitagawa Y. 1996. A family of novel DNA-binding nuclear proteins having polypyrimidine tract-binding motif and arginine/serine-rich motif. Biochem. Biophys. Res. Commun. 223: 427–433. [DOI] [PubMed] [Google Scholar]
- 26.Huh G. S., Hynes R. O. 1993. Elements regulating an alternatively spliced exon of the rat fibronectin gene. Mol. Cell. Biol. 13: 5301–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y., Qi C., Korenberg J. R., Chen X. N., Noya D., Rao M. S., Reddy J. K. 1995. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc. Natl. Acad. Sci. USA. 92: 7921–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q. A., Scherer P. E., Gupta R. K. 2014. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J. Lipid Res. 55: 605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehlem A., Hagberg C. E., Muhl L., Eriksson U., Falkevall A. 2013. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc. 8: 1149–1154. [DOI] [PubMed] [Google Scholar]
- 30.Younce C. W., Azfer A., Kolattukudy P. E. 2009. MCP-1 (monocyte chemotactic protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3–L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 284: 27620–27628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du K., Peng Y., Greenbaum L. E., Haber B. A., Taub R. 1997. HRS/SRp40-mediated inclusion of the fibronectin EIIIB exon, a possible cause of increased EIIIB expression in proliferating liver. Mol. Cell. Biol. 17: 4096–4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spector D. L. 2006. SnapShot: cellular bodies. Cell. 127: 1071. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh N., Spahr C. S., Patterson S. D., Bubulya P., Neuwald A. F., Spector D. L. 2004. Proteomic analysis of interchromatin granule clusters. Mol. Biol. Cell. 15: 3876–3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmuth K., Urlaub H., Vornlocher H. P., Will C. L., Gentzel M., Wilm M., Luhrmann R. 2002. Protein composition of human prespliceosomes isolated by a tobramycin affinity-selection method. Proc. Natl. Acad. Sci. USA. 99: 16719–16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurica M. S., Licklider L. J., Gygi S. R., Grigorieff N., Moore M. J. 2002. Purification and characterization of native spliceosomes suitable for three-dimensional structural analysis. RNA. 8: 426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y-I. G., Moore R. E., Ge H. Y., Young M. K., Lee T. D., Stevens S. W. 2007. Proteomic analysis of in vivo-assembled pre-mRNA splicing complexes expands the catalog of participating factors. Nucleic Acids Res. 35: 3928–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai Y., Lee D., Yu T., Chasin L. A. 1999. Control of 3′ splice site choice in vivo by ASF/SF2 and hnRNP A1. Nucleic Acids Res. 27: 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayeda A., Krainer A. R. 1992. Regulation of alternative pre-mRNA splicing by hnRNP A1 and splicing factor SF2. Cell. 68: 365–375. [DOI] [PubMed] [Google Scholar]
- 39.Golan-Gerstl R., Cohen M., Shilo A., Suh S. S., Bakacs A., Coppola L., Karni R. 2011. Splicing factor hnRNP A2/B1 regulates tumor suppressor gene splicing and is an oncogenic driver in glioblastoma. Cancer Res. 71: 4464–4472. [DOI] [PubMed] [Google Scholar]
- 40.Chen Z. Y., Cai L., Zhu J., Chen M., Chen J., Li Z. H., Liu X. D., Wang S. G., Bie P., Jiang P., et al. 2011. Fyn requires HnRNPA2B1 and Sam68 to synergistically regulate apoptosis in pancreatic cancer. Carcinogenesis. 32: 1419–1426. [DOI] [PubMed] [Google Scholar]
- 41.Topp J. D., Jackson J., Melton A. A., Lynch K. W. 2008. A cell-based screen for splicing regulators identifies hnRNP LL as a distinct signal-induced repressor of CD45 variable exon 4. RNA. 14: 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oberdoerffer S., Moita L. F., Neems D., Freitas R. P., Hacohen N., Rao A. 2008. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science. 321: 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornblihtt A. R. 2005. Promoter usage and alternative splicing. Curr. Opin. Cell Biol. 17: 262–268. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Wang J., Gao L., Stamm S., Andreadis A. 2011. An SRp75/hnRNPG complex interacting with hnRNPE2 regulates the 5′ splice site of tau exon 10, whose misregulation causes frontotemporal dementia. Gene. 485: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tranell A., Fenyo E. M., Schwartz S. 2010. Serine- and arginine-rich proteins 55 and 75 (SRp55 and SRp75) induce production of HIV-1 vpr mRNA by inhibiting the 5′-splice site of exon 3. J. Biol. Chem. 285: 31537–31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H., Bingham P. M. 1991. Arginine/serine-rich domains of the su(wa) and tra RNA processing regulators target proteins to a subnuclear compartment implicated in splicing. Cell. 67: 335–342. [DOI] [PubMed] [Google Scholar]
- 47.Hedley M. L., Amrein H., Maniatis T. 1995. An amino acid sequence motif sufficient for subnuclear localization of an arginine/serine-rich splicing factor. Proc. Natl. Acad. Sci. USA. 92: 11524–11528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cáceres J. F., Misteli T., Screaton G. R., Spector D. L., Krainer A. R. 1997. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J. Cell Biol. 138: 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muto Y., Pomeranz Krummel D., Oubridge C., Hernandez H., Robinson C. V., Neuhaus D., Nagai K. 2004. The structure and biochemical properties of the human spliceosomal protein U1C. J. Mol. Biol. 341: 185–198. [DOI] [PubMed] [Google Scholar]
- 50.Nesic D., Tanackovic G., Kramer A. 2004. A role for Cajal bodies in the final steps of U2 snRNP biogenesis. J. Cell Sci. 117: 4423–4433. [DOI] [PubMed] [Google Scholar]
- 51.Philipps D., Celotto A. M., Wang Q. Q., Tarng R. S., Graveley B. R. 2003. Arginine/serine repeats are sufficient to constitute a splicing activation domain. Nucleic Acids Res. 31: 6502–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li P., Carter G., Romero J., Gower K. M., Watson J., Patel N. A., Cooper D. R. 2013. Clk/STY (cdc2-like kinase 1) and Akt regulate alternative splicing and adipogenesis in 3T3–L1 pre-adipocytes. PLoS ONE. 8: e53268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Will C. L., Urlaub H., Achsel T., Gentzel M., Wilm M., Luhrmann R. 2002. Characterization of novel SF3b and 17S U2 snRNP proteins, including a human Prp5p homologue and an SF3b DEAD-box protein. EMBO J. 21: 4978–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kar A., Fushimi K., Zhou X., Ray P., Shi C., Chen X., Liu Z., Chen S., Wu J. Y. 2011. RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5′ splice site. Mol. Cell. Biol. 31: 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fouraux M. A., Kolkman M. J., Van der Heijden A., De Jong A. S., Van Venrooij W. J., Pruijn G. J. 2002. The human La (SS-B) autoantigen interacts with DDX15/hPrp43, a putative DEAH-box RNA helicase. RNA. 8: 1428–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jønson L., Vikesaa J., Krogh A., Nielsen L. K., Hansen T., Borup R., Johnsen A. H., Christiansen J., Nielsen F. C. 2007. Molecular composition of IMP1 ribonucleoprotein granules. Mol. Cell. Proteomics. 6: 798–811. [DOI] [PubMed] [Google Scholar]
- 57.Marko M., Leichter M., Patrinou-Georgoula M., Guialis A. 2010. hnRNP M interacts with PSF and p54(nrb) and co-localizes within defined nuclear structures. Exp. Cell Res. 316: 390–400. [DOI] [PubMed] [Google Scholar]
- 58.Izaurralde E., Lewis J., McGuigan C., Jankowska M., Darzynkiewicz E., Mattaj I. W. 1994. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell. 78: 657–668. [DOI] [PubMed] [Google Scholar]
- 59.Peng R., Dye B. T., Perez I., Barnard D. C., Thompson A. B., Patton J. G. 2002. PSF and p54nrb bind a conserved stem in U5 snRNA. RNA. 8: 1334–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu L., Xie N., Rennie P., Challis J. R., Gleave M., Lye S. J., Dong X. 2011. Consensus PP1 binding motifs regulate transcriptional corepression and alternative RNA splicing activities of the steroid receptor coregulators, p54nrb and PSF. Mol. Endocrinol. 25: 1197–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosoda N., Lejeune F., Maquat L. E. 2006. Evidence that poly(A) binding protein C1 binds nuclear pre-mRNA poly(A) tails. Mol. Cell. Biol. 26: 3085–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J., Gao Q. S., Wang Y., Lafyatis R., Stamm S., Andreadis A. 2004. Tau exon 10, whose missplicing causes frontotemporal dementia, is regulated by an intricate interplay of cis elements and trans factors. J. Neurochem. 88: 1078–1090. [DOI] [PubMed] [Google Scholar]
- 63.Krämer A. 1992. Purification of splicing factor SF1, a heat-stable protein that functions in the assembly of a presplicing complex. Mol. Cell. Biol. 12: 4545–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang C., Chua K., Seghezzi W., Lees E., Gozani O., Reed R. 1998. Phosphorylation of spliceosomal protein SAP 155 coupled with splicing catalysis. Genes Dev. 12: 1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patton J. G., Porro E. B., Galceran J., Tempst P., Nadal-Ginard B. 1993. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 7: 393–406. [DOI] [PubMed] [Google Scholar]
- 66.Gui J. F., Tronchere H., Chandler S. D., Fu X. D. 1994. Purification and characterization of a kinase specific for the serine- and arginine-rich pre-mRNA splicing factors. Proc. Natl. Acad. Sci. USA. 91: 10824–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zahler A. M., Neugebauer K. M., Stolk J. A., Roth M. B. 1993. Human SR proteins and isolation of a cDNA encoding SRp75. Mol. Cell. Biol. 13: 4023–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavaloc Y., Popielarz M., Fuchs J. P., Gattoni R., Stevenin J. 1994. Characterization and cloning of the human splicing factor 9G8: a novel 35 kDa factor of the serine/arginine protein family. EMBO J. 13: 2639–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mourelatos Z., Abel L., Yong J., Kataoka N., Dreyfuss G. 2001. SMN interacts with a novel family of hnRNP and spliceosomal proteins. EMBO J. 20: 5443–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llorian M., Beullens M., Andres I., Ortiz J. M., Bollen M. 2004. SIPP1, a novel pre-mRNA splicing factor and interactor of protein phosphatase-1. Biochem. J. 378: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]