Abstract
The LDL receptor (LDLR) and scavenger receptor class B type I (SR-BI) play physiological roles in LDL and HDL metabolism in vivo. In this study, we explored HDL metabolism in LDLR-deficient mice in comparison with WT littermates. Murine HDL was radiolabeled in the protein (125I) and in the cholesteryl ester (CE) moiety ([3H]). The metabolism of 125I-/[3H]HDL was investigated in plasma and in tissues of mice and in murine hepatocytes. In WT mice, liver and adrenals selectively take up HDL-associated CE ([3H]). In contrast, in LDLR−/− mice, selective HDL CE uptake is significantly reduced in liver and adrenals. In hepatocytes isolated from LDLR−/− mice, selective HDL CE uptake is substantially diminished compared with WT liver cells. Hepatic and adrenal protein expression of lipoprotein receptors SR-BI, cluster of differentiation 36 (CD36), and LDL receptor-related protein 1 (LRP1) was analyzed by immunoblots. The respective protein levels were identical both in hepatic and adrenal membranes prepared from WT or from LDLR−/− mice. In summary, an LDLR deficiency substantially decreases selective HDL CE uptake by liver and adrenals. This decrease is independent from regulation of receptor proteins like SR-BI, CD36, and LRP1. Thus, LDLR expression has a substantial impact on both HDL and LDL metabolism in mice.
Keywords: scavenger receptor class B type I, cholesteryl ester, selective uptake
Specific plasma membrane receptors play critical roles in lipoprotein metabolism (1). A well-established molecule is the LDL receptor (LDLR) (2). This protein mediates the cellular internalization of cholesterol-rich LDL particles, for instance by the liver (3). Another membrane protein, scavenger receptor class B type I (SR-BI), has a dominant role in the physiology of HDL (4). This receptor facilitates the selective uptake of HDL-associated cholesteryl esters (CEs) by liver and adrenals (i.e., cellular lipid uptake without holo-particle internalization) (5, 6).
The physiological significance of these receptors is illustrated by mutations in the respective genes. In mice, LDLR deficiency is associated with an increase in LDL cholesterol as well as accelerated atherosclerosis (7, 8). With respect to HDL, induced mutations in the murine gene encoding SR-BI induce an increase in plasma HDL cholesterol and accelerated atherosclerosis (9, 10). However, besides an increase in LDL cholesterol, a deficiency of the LDLR can modulate non-LDL lipoproteins in plasma as well. For instance, a rise in plasma HDL cholesterol has been reported in LDLR-deficient mice in some studies but not in others (7, 11, 12). These observations suggest that an LDLR deficiency can affect the metabolism of more than one lipoprotein fraction, at least to some extent. However, with respect to the mechanism, it is not known how an LDLR deficiency modifies HDL cholesterol.
In the current study, HDL metabolism was explored in LDLR-deficient mice and compared with WT animals that express functional LDLR (7). To explore the fate of distinct lipoprotein components, HDL particles were radiolabeled in both the protein as well as in the lipid moiety (13). In metabolic studies using double radiolabeled HDL, liver and adrenals of WT mice selectively take up HDL-associated CE. In LDLR−/− mice, however, selective HDL CE uptake is significantly reduced in liver and adrenals. In parallel, uptake of radiolabeled HDL by isolated primary hepatocytes in vitro was explored. Compared with WT and in line with the in vivo studies, an LDLR deficiency is associated with a decrease in selective HDL CE uptake. Notably, SR-BI expression in liver and adrenal membranes was identical in mice with and without LDLR even though HDL selective CE uptake was reduced. In summary, we demonstrate that LDLR expression has a substantial impact on HDL metabolism in vivo and in vitro. Remarkably, regulation of selective HDL CE uptake occurs independent from SR-BI protein expression in tissues.
MATERIALS AND METHODS
Materials
Primers were purchased from Metabion. Taq-DNA polymerase, culture media, sera, and supplements for cell culture were supplied by Invitrogen. Six-well tissue culture plates were obtained from Becton Dickinson. Collagenase was from Worthington. 125Iodine, [3H]cholesteryl oleyl ether ([3H]CEt), and ECL reagent were purchased from GE Healthcare. Protease inhibitor cocktail “complete” and enzymatic assays for cholesterol, HDL cholesterol, and triglyceride were supplied by Roche. Assays for phospholipid were obtained from Wako. BSA, tyloxapol, and standard laboratory chemicals were purchased from Sigma Aldrich. Scintillation cocktail was from PerkinElmer. Nitrocellulose membrane was obtained from Schleicher and Schuell. Films were supplied by Kodak. Rodent chow was purchased from Ssniff.
Mice
Mice lacking a functional LDLR gene (LDLR−/−) were purchased from The Jackson Laboratory (Bar Harbor, ME) (7). Male LDLR−/− mice on a C57BL/6J genetic background and the respective male littermate controls (WT) were used. The genotype of each mouse was analyzed by PCR from genomic DNA isolated from tail biopsies (7). Mice were maintained on a standard laboratory chow diet with unlimited access to food and water. The age of the rodents used in this study was between 20 and 40 weeks. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University Hospital Hamburg.
Lipoprotein preparation
Mice were fasted 4 h before blood harvest. Murine HDL (d = 1.063–1.21 g/ml) was isolated from WT (WT-HDL) plasma or from LDLR-deficient (LDLR−/−HDL) plasma by sequential ultracentrifugation (14). LDL (d = 1.020–1.050 g/ml) was prepared from plasma of LDLR−/− mice.
Murine WT-HDL or LDLR−/−HDL was double labeled with 125I-tyramine cellobiose (125I-TC) in the apo and with [3H]CEt in the CE moiety (6, 13). [3H]CEt was introduced in 125I-TC-HDL by exchange from donor liposomal particles, which contained [3H]CEt using human plasma cholesteryl ester transfer protein (CETP) (15). 125I-TC-LDL was prepared as outlined (13). The final 125I-TC-/[3H]CEt-WT-HDL, 125I-TC-/[3H]CEt-LDLR−/−HDL, and 125I-TC-LDL preparations were extensively dialyzed against PBS (pH 7.4, 4°C) containing EDTA (1 mM).
HDL metabolism in mice
For plasma decay analysis of radiolabeled WT-HDL or LDLR−/−HDL, mice were fasted for 4 h before tracer injection (5). Then 125I-TC-/[3H]CEt-WT-HDL or 125I-TC-/[3H]CEt-LDLR−/−HDL (30 µg HDL protein per mouse) was injected via tail vein, and thereafter blood samples (30 µl per time point) were collected periodically (10 and 30 min; 2, 5, 9, 22, and 24 h) for 24 h after injection. Animals were fasted throughout the 24 h study period but had unlimited access to water. Plasma aliquots were directly assayed for 125I radioactivity, and [3H] was analyzed after lipid extraction (16). Computer modeling was used to fit (by method of least squares) multiexponential curves, arising from a common two-pool model, simultaneously to both tracers’ plasma decay data, and to calculate plasma fractional catabolic rates (plasma-FCRs) for each tracer (17). The modeling was done separately for the data from each mouse, so that individual plasma-FCRs for both tracers were calculated for each animal. In some cases, HDL metabolism in mice was explored for a 2 h interval only.
Tissue sites of uptake of HDL-associated tracers were determined 2 h or 24 h after injection of radiolabeled WT-HDL or LDLR−/−HDL (5). Finally, animals were anesthetized, the abdomen and chest were opened, and a catheter was inserted into the heart. The inferior vena cava was cut, and the mice were perfused extensively with saline (50 ml per animal). After perfusion, liver, adrenals, kidneys, brain, heart, lungs, spleen, stomach, intestine, and carcass from each mouse were harvested and homogenized. Homogenates from each tissue and from carcass were directly assayed for 125I radioactivity, and aliquots were analyzed for [3H] after lipid extraction (16).
Total radioactivity recovered from all tissues and from the carcass of each mouse was calculated (5). The fraction of total tracer uptake attributed to a specific organ was calculated as the radioactivity recovered in that organ divided by the total radioactivity recovered from all tissues and carcass. Thus the % of recovered extravascular radioactivity in tissues is determined 2 h or 24 h after injection of labeled HDL.
To allow comparison of the specific activities of various tissues in HDL internalization and to directly compare the rates of uptake of the apo component and the CE moiety of HDL, the data are expressed as organ fractional catabolic rates (organ-FCRs) (5). These rates are calculated as follows: (Organ-FCR in Tissue X) = (Plasma-FCR) × (Fraction [%] of Total Body Tracer Recovery in Tissue X). This organ-FCR represents the fraction of the plasma pool of either HDL tracer cleared by an organ per hour. 125I-TC represents the uptake of HDL holo-particles by tissues (18). Selective HDL CE uptake is calculated as the difference in organ-FCR between [3H]CEt and 125I-TC.
LDL metabolism in mice
125I-TC-LDL metabolism in plasma and tissue sites of uptake of this lipoprotein were investigated in mice analogously as outlined for radiolabeled HDL.
Preparation of murine hepatocytes
Primary hepatocytes were isolated from murine liver by perfusion (37°C, 18 min) with Hanks’ balanced salt solution supplemented with collagenase (0.3 mg/ml, type I), HEPES (10 mM), and protease inhibitor mixture “complete” (19). Thereafter, these cells were seeded (37°C, 2.0 h) in DMEM containing FBS (5%, v/v), penicillin (100 µg/ml), and streptomycin (100 µg/ml). Finally, the culture medium was aspirated, and the cells were washed (PBS, 3×). Hepatocytes were used for 125I-TC-/[3H]CEt-WT-HDL uptake or 125I-TC-WT-HDL binding assays.
Uptake and binding assay for radiolabeled HDL
To determine uptake of radiolabeled HDL, hepatocytes were incubated (37°C, 2.0 h) in DMEM containing BSA (5 mg/ml), penicillin (100 µg/ml), streptomycin (100 µg/ml), and 125I-TC-/[3H]CEt-WT-HDL (20). Finally, cells were harvested by trypsin/EDTA (1×, trypsin 0.05%, EDTA 0.53 mM) treatment, and cellular uptake of HDL tracers was measured. 125I was directly radioassayed, and [3H] was analyzed after lipid extraction (16). Uptake of 125I-TC-/[3H]CEt-WT-HDL by cells is shown in terms of apparent HDL particle uptake, expressed as HDL protein (5, 20). This is done to compare the uptake of both tracers on a common basis. The uptake of HDL holo-particles is represented by 125I-TC, and the difference between [3H]CEt and 125I-TC yields apparent selective HDL CE uptake by cells (18).
To investigate binding of radiolabeled HDL, hepatocytes were incubated (4°C, 2.0 h) in medium containing 125I-TC-WT-HDL (21). Thereafter, the medium was removed, and the cells were washed (PBS, 4°C). 125I-TC-WT-HDL binding to the cells was finally analyzed as outlined (21).
Immunoblots
Membrane fractions from murine liver or adrenals were prepared (22). Protease inhibitor cocktail “complete” was present during the entire preparation. Membrane fractions were boiled (93°C, 10 min) and separated by SDS polyacrylamide gel (10%) electrophoresis under reducing conditions (mercaptoethanol) and thereafter transferred to a nitrocellulose membrane (6). Finally, the blots were probed with anti-SR-BI (rabbit polyclonal antibody to murine SR-BI, Novus Biologicals), anti-LDL receptor-related protein 1 (LRP1) (sheep anti-LRP1 antibody, raised against a synthetic peptide from human LRP1) (23), anti-cluster of differentiation 36 (CD36) (rabbit polyclonal antiserum against murine CD36) (24), anti-ABCA1 (rabbit polyclonal antibody, Novus Biologicals), or anti-β-actin (monoclonal anti-β-actin, mouse, Sigma Aldrich) as primary antibodies. β-Actin was used as loading control. Finally, the blots were incubated with respective horseradish peroxidase-conjugated secondary antibodies (Sigma Aldrich) and developed with ECL. Blots were exposed to Bio Max MR films and quantified using Image Quant software, version 5.2 (GE Healthcare).
Miscellaneous
Routinely, all mice were fasted for 4 h before blood harvest for analytical or preparative purposes. Total cholesterol, HDL cholesterol, and triglycerides from plasma were measured using enzymatic assays. Plasma lipoproteins were fractionated by fast performance LC (FPLC) (25). In order to measure VLDL production, the nonionic detergent Tyloxapol (Triton WR 1339) was used to inhibit VLDL catabolism (26). Protein was analyzed as outlined (27).
Statistics
Values are means ± SEM. All statistical analyses were performed using Student’s t-test. Probability values <0.05 were considered statistically significant.
RESULTS
As expected, total plasma cholesterol was significantly higher in male LDLR−/− mice (284%) compared with their WT littermates (100%) (Table 1). HDL cholesterol was elevated (111%) in LDLR-deficient male mice compared with WT animals (100%); however, this difference was not statistically significant. Triglycerides were significantly higher (235%) in LDLR−/− mice compared with WT (100%). Plasma lipids were also analyzed in female mice (Table 1). In female LDLR−/− animals, total cholesterol and triglycerides increased to a similar extent compared with male LDLR−/− mice, whereas no increase in HDL cholesterol was detected.
TABLE 1.
Plasma cholesterol, HDL cholesterol, and triglycerides of WT or LDLR−/− mice
| Total Cholesterol | HDL Cholesterol | Triglycerides | |
| Males | mg/dl | mg/dl | mg/dl |
| WT | 93.3 ± 3.8 (30) | 83.8 ± 3.6 (27) | 105.8 ± 7.0 (20) |
| LDLR−/−−/− | 264.8 ± 16.9 (31) | 92.7 ± 4.7 (28) | 249.1 ± 26.1 (23) |
| P | <0.0001 | 0.0705 | <0.0001 |
| Females | |||
| WT | 89.9 ± 4.4 (30) | 78.2 ± 3.0 (30) | 122.5 ± 8.2 (24) |
| LDLR−/−−/− | 229.7 ± 8.8 (22) | 75.1 ± 3.3(22) | 161.2 ± 8.5 (17) |
| P | <0.0001 | 0.251 | 0.0014 |
Male or female WT or LDLR−/− mice were fasting for 4 h. Thereafter, blood was harvested and plasma was analyzed as outlined in Materials and Methods. Values are means ± SEM; the number of mice is given in parentheses.
To determine the distribution of cholesterol with respect to lipoprotein fractions, murine plasma was fractionated by FPLC (Fig. 1) (25). The major change due to the LDLR deficiency was an increase in cholesterol corresponding to particles of the LDL/IDL fractions. However, in LDLR−/− mice, a small increase in plasma cholesterol in the size range of HDL could be detected. These results on plasma lipids and on HDL cholesterol in LDLR−/− mice are in line with previous studies (7, 12). The compositional analysis of HDL isolated by sequential ultracentrifugation from murine plasma showed that LDLR−/−HDL is significantly enriched in triglycerides and depleted in phospholipids compared with WT-HDL (Table 2).
Fig. 1.
FPLC analysis of plasma cholesterol from WT or from LDLR−/− mice. After fasting for 4 h, blood was harvested from 4 WT or from 4 LDLR−/− male mice. Finally, the pooled plasma was subjected to FPLC, and cholesterol was analyzed in each fraction as outlined in Materials and Methods. Shown is a representative experiment from a total of n = 2.
TABLE 2.
Chemical composition of murine HDL
| WT | LDLR−/−−/− | P | |
| % of Total Mass | % of Total Mass | ||
| Cholesterol | 21.1 ± 0.3 | 20.4 ± 0.4 | 0.25 |
| Phospholipids | 28.0 ± 0.6 | 24.2 ± 0.5 | 0.0039 |
| Triglycerides | 3.4 ± 0.07 | 7.9 ± 0.1 | <0.0001 |
| Protein | 47.5 ± 0.9 | 47.6 ± 1.0 | 0.98 |
Blood was harvested in parallel from fasted (4 h) WT or LDLR−/− male mice. Subsequently, from plasma, HDL (d = 1.063–1.21 g/ml) was isolated by sequential ultracentrifugation. Thereafter, cholesterol, phospholipids, triglycerides, and protein were analyzed. Values are means ± SEM of n = 5 independent determinations; four independent preparations yielded qualitatively identical results.
The metabolism of 125I-TC-/[3H]CEt-WT-HDL was investigated in WT and in LDLR−/− mice (Fig. 2). This murine HDL preparation was injected intravenously in mice, and thereafter, blood samples were harvested during a 24 h interval (5). In these studies, [3H]CEt represents the metabolism of HDL-associated CE, and 125I-TC shows HDL holo-particle clearance (18). The difference between both tracers ([3H]CEt − 125I-TC) represents selective CE removal. In WT mice, the plasma decay of HDL-associated [3H]CEt is faster compared with 125I-TC; the difference in decay between both tracers yields selective CE removal from the HDL plasma pool by tissues in WT animals. In parallel, 125I-TC-/[3H]CEt-WT-HDL was injected in LDLR−/− mice. In this case, no difference in plasma decay between both HDL tracers can be detected. These data suggest that selective CE removal from the HDL plasma pool does not occur in LDLR−/− mice.
Fig. 2.
Plasma decay kinetics of 125I-TC-/[3H]CEt-WT-HDL in WT mice or in LDLR−/− mice. 125I-TC-/[3H]CEt-WT-HDL was injected intravenously in a WT or LDLR−/− male mouse. Thereafter, during a 24 h interval, periodic blood samples were harvested, and plasma was analyzed for 125I-TC (125I) and [3H]CEt ([3H]). The y-axis represents the fraction of the tracer in plasma (%). Shown are typical experiments of n = 13 WT mice and n = 9 LDLR−/− mice.
From decay curves shown in Fig. 2, plasma-FCRs for both HDL-associated tracers were calculated (Fig. 3) (17). In WT animals, the plasma-FCR for WT-HDL-associated [3H]CEt is substantially higher compared with 125I-TC; the difference between both rates ([3H]CEt − 125I-TC) yields selective CE removal from the plasma HDL pool by tissues. In LDLR−/− mice, no significant difference in plasma-FCR for 125I-TC is detected compared with WT. In contrast, in LDLR−/− mice, a substantial reduction in plasma-FCR for [3H]CEt is observed. Calculation of the difference between [3H]CEt and 125I-TC yields no difference, again showing that there is no selective CE removal from plasma WT-HDL by tissues in LDLR−/− mice.
Fig. 3.
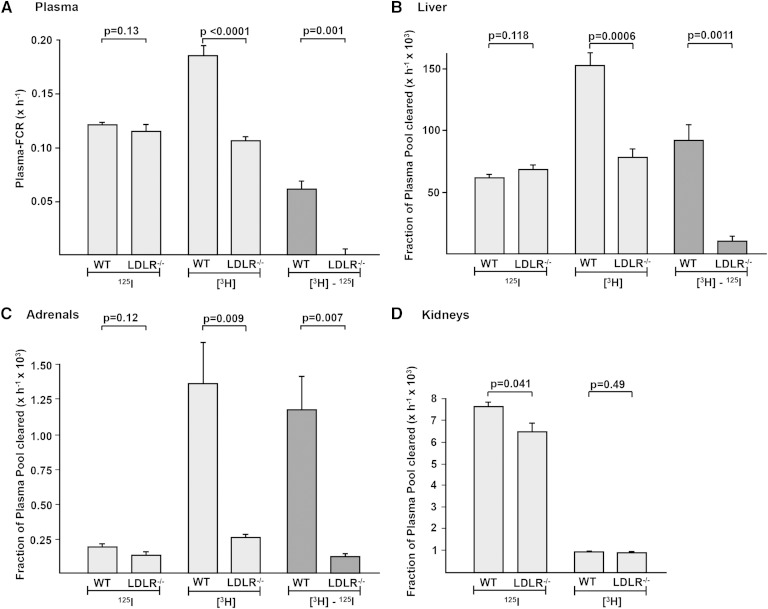
Plasma-FCRs and tissue tracer uptake rates for 125I-TC-/[3H]CEt-WT-HDL in WT mice or LDLR−/− mice. 125I-TC-/[3H]CEt-WT-HDL was injected intravenously in WT or LDLR−/− male mice. A: During the subsequent 24 h interval, blood was harvested periodically to determine the plasma decay of both tracers. 125I-TC (125I) and [3H]CEt ([3H]) were analyzed, and plasma-FCRs for 125I-TC (125I) and [3H]CEt ([3H]) were calculated. The difference in plasma-FCRs between [3H]CEt and 125I-TC was calculated. Twenty-four hours after tracer injection, the animals were euthanized, and tissues were analyzed for both tracers. Liver (B), adrenal (C), and kidney (D) organ-FCRs for 125I-TC (125I) and [3H]CEt ([3H]) and the difference in organ-FCRs between [3H]CEt and 125I-TC were calculated. All calculations were done as described in Materials and Methods. A: Values are means ± SEM of n = 13 (WT) or n = 9 (LDLR−/−) mice. B–D: Values are means ± SEM of n = 7 (WT) or n = 5 (LDLR−/−) mice. An independent experiment yielded qualitatively identical results.
Organ-specific HDL catabolism was determined. Twenty-four hours after 125I-TC-/[3H]CEt-WT-HDL injection, tracer content of each tissue was analyzed, and HDL uptake was calculated and expressed in terms of organ-FCRs (5). HDL tracer uptake by the liver is shown in Fig. 3. In WT mice, the hepatic organ-FCR for [3H]CEt is higher compared with 125I-TC; the difference between both rates ([3H]CEt − 125I-TC) yields selective CE uptake by the liver from WT-HDL. This result is in line with earlier studies in WT mice in which similar levels of selective CE uptake from HDL by the liver were observed (6, 31). In LDLR−/− mice, the hepatic uptake rate for 125I-TC was similar to the respective rate in WT animals (Fig. 3). However, in LDLR−/− mice, the hepatic organ-FCR for [3H]CEt decreased significantly compared with WT, indicating that although holo-particle uptake was similar in both LDLR−/− and WT murine livers, selective CE uptake from WT-HDL ([3H]CEt − 125I-TC) was decreased significantly in livers of LDLR−/− mice.
Uptake of 125I-TC-/[3H]CEt-WT-HDL by adrenal glands was explored (Fig. 3). In WT adrenals, the organ-FCR for [3H]CEt is substantially higher compared with 125I-TC, and the difference between both rates yields substantial selective CE uptake from HDL ([3H]CEt − 125I-TC) in glands with LDLR protein expression (5, 6). In adrenals from LDLR−/− mice, the organ-FCR for 125I-TC was not significantly different compared with WT, indicating normal holo-particle uptake. Similar to the results for liver, adrenal organ-FCR for [3H]CEt decreased significantly in LDLR−/− glands compared with WT. This decrease in HDL lipid internalization by LDLR−/− glands yielded a significant reduction in selective CE uptake ([3H]CEt − 125I-TC). Uptake of 125I-TC-/[3H]CEt-WT-HDL by murine kidneys was investigated (Fig. 3). The kidney organ-FCR for HDL-associated 125I-TC is higher compared with the respective rate for [3H]CEt, and this result is consistent with a physiological role of the kidneys in HDL apo catabolism (28). Kidney organ-FCRs for both HDL-associated tracers did not differ between WT and LDLR−/− mice.
The composition of WT-HDL and LDLR−/−HDL is different (Table 2). Therefore, the metabolism of 125I-TC-/[3H]CEt-LDLR−/−HDL was explored by the identical approach as with labeled WT-HDL (Fig. 4; supplementary Fig. I). In WT mice, the plasma-FCR for LDLR−/−HDL-associated [3H]CEt is substantially higher compared with 125I-TC; the difference between both tracers ([3H]CEt − 125I-TC) yields selective CE removal from the plasma HDL pool by tissues (17). Comparing WT and LDLR−/− mice, no significant difference in plasma-FCR for 125I-TC is detected. In LDLR−/− mice, however, a significant reduction in plasma-FCR for [3H]CEt is observed. The difference between [3H]CEt and 125I-TC yields a significant reduction in selective CE removal from the plasma LDLR−/−HDL pool by tissues in LDLR−/− mice.
Fig. 4.
Plasma-FCRs and liver tracer uptake rates for 125I-TC-/[3H]CEt-LDLR−/−HDL in WT mice or in LDLR−/− mice. 125I-TC-/[3H]CEt-LDLR−/−HDL was injected intravenously in WT or in LDLR−/− male mice. During the subsequent 24 h interval, blood was drawn periodically, and 24 h after tracer injection, the animals were euthanized and tissues were harvested. Plasma-FCRs and liver organ-FCRs for 125I-TC (125I), for [3H]CEt ([3H]), and for selective uptake ([3H]CEt − 125I-TC) were analyzed and calculated as outlined in Fig. 3. All values are means ± SEM of n = 5 (WT) or n = 5 (LDLR−/−) mice. Where no error bars are shown, the SEM is on the respective line.
To explore the metabolism of LDLR−/−HDL by tissues, tracer content was analyzed 24 h after injection of 125I-TC-/[3H]CEt-LDLR−/−HDL (5). In WT mice, the hepatic organ-FCR for [3H]CEt is higher than the one due to 125I-TC; the difference between both rates ([3H]CEt − 125I-TC) yields selective CE uptake by the liver from LDLR−/−HDL (Fig. 4). In LDLR−/− mice, the hepatic uptake rate for 125I-TC was similar to the respective rate in WT animals. However, in these LDLR-deficient mice, the hepatic organ-FCR for [3H]CEt decreased significantly compared with WT. Although holo-particle uptake was similar in livers of both genotypes, selective CE uptake from LDLR−/−HDL ([3H]CEt − 125I-TC) decreased significantly in the livers of LDLR−/− mice.
HDL-associated apos can exchange in plasma between lipoprotein fractions (1). In some species, CE can be transferred from HDL to more buoyant lipoproteins, and this reaction is mediated by CETP (15). However, mice have no CETP activity in the circulation, and therefore, no mechanism exists that mediates a transfer of HDL-associated lipids to more buoyant lipoproteins (36). To address the question of whether initially HDL-associated tracers can be transferred into non-HDL lipoproteins in plasma during a 24 h period, 125I-TC-/[3H]CEt-LDLR−/−HDL was injected in WT and in LDLR−/− mice (supplementary Table I). After 40 min, 8 h, and 24 h, blood was harvested and lipoproteins were separated using FPLC. Tracer in fractions corresponding to triglyceride-rich lipoproteins, LDL, and HDL were analyzed. In WT mice, during the 24 h time course both LDLR−/−HDL-associated tracers remained predominantly in the HDL fraction. In LDLR−/− mice, however, [3H]CEt tracer initially associated with LDLR−/−HDL was detected after 8 h and after 24 h to a substantial extent in fractions with a lower density than HDL. Qualitatively identical results were obtained with 125I-TC-/[3H]CEt-WT-HDL in both groups of mice (data not shown). Because there is no CETP in murine plasma, we investigated the mechanism of this [3H]CEt recovery in non-HDL lipoproteins (supplementary Table II). We speculated that HDL-derived radiolabeled tracers are partially reused for VLDL assembly and secretion. In contrast to the WT situation, the LDLR-dependent uptake of VLDL and LDL is reduced in LDLR−/− mice, and consequently, small amounts of recycled tracers can be found in non-HDL lipoproteins. To test this hypothesis, 125I-TC-/[3H]CEt-LDLR−/−HDL was injected in WT or in LDLR−/− mice (supplementary Table II). First, blood was harvested from these animals 24 h after tracer injection. Thereafter, Tyloxapol (Triton WR 1339), an inhibitor of lipoprotein catabolism, was injected. This experimental setup allows us to determine the reutilization of radiolabeled tracers for VLDL production (26). In WT and in LDLR−/− mice, Tyloxapol induced a substantial increase in plasma triglyceride, indicating an inhibition of VLDL clearance. With respect to tracer recovery in lipoprotein fractions, Tyloxapol induced a substantial increase in [3H]CEt tracer content in the VLDL gradient fraction in LDLR−/− mice and only a small increase in WT littermates (14). In contrast, Tyloxapol had virtually no effect on the distribution of the 125I-TC tracer between the VLDL and the non-VLDL fractions. This experiment indicates that, following uptake of 125I-TC-/[3H]CEt-LDLR−/−HDL by the liver, there is substantial resecretion of the [3H]CEt tracer in VLDL particles in LDLR−/− mice. This observation explains at least to some extent the recovery of the initially HDL-associated [3H]CEt tracer in non-HDL lipoproteins during the time course in LDLR−/− mice.
To ensure that the observed differences in HDL catabolism in LDLR−/− mice are independent from the resecretion of lipid tracers into non-HDL lipoprotein fractions, we determined the plasma decay and tissue uptake of radiolabeled HDL in a short-term 2 h study. In this period, all HDL-associated tracers remained within the plasma HDL fraction. 125I-TC-/[3H]CEt-LDLR−/−HDL was injected in WT or in LDLR−/− mice (supplementary Fig. II). In WT animals, the initial plasma-FCR for LDLR−/−HDL-associated [3H]CEt is substantially higher compared with 125I-TC; the difference between both rates ([3H]CEt − 125I-TC) yields selective CE removal from the plasma HDL pool by tissues. In LDLR−/− mice, a quantitative minor difference in initial plasma-FCR for 125I-TC is detected compared with WT. In LDLR−/− mice, a significant reduction in initial plasma-FCR for [3H]CEt and a significant decrease in selective CE removal ([3H]CEt − 125I-TC) from plasma LDLR−/−HDL by tissues are detected during the 2 h period. In WT mice, 2 h after 125I-TC-/[3H]CEt-LDLR−/−HDL injection we observed selective CE uptake ([3H]CEt − 125I-TC) by the liver. In LDLR-deficient mice, however, selective CE uptake from LDLR−/−HDL ([3H]CEt − 125I-TC) is decreased significantly. In summary, plasma decay and liver uptake of HDL-associated tracers during a 2 h period are qualitatively consistent with the results for the 24 h experiments.
The liver is composed of distinct cell types; however, hepatocytes quantitatively dominate in this organ (29). To investigate the role of the LDLR in cellular HDL uptake in a less complex system compared with an animal, hepatocytes were isolated from WT and LDLR−/− mice. Following a 4 h seeding period, liver cells were incubated with medium containing 125I-TC-/[3H]CEt-WT-HDL. Uptake of 125I-TC-/[3H]CEt-WT-HDL by WT hepatocytes is shown in Fig. 5. The uptake of both HDL-associated tracers increased in a dose-dependent manner, but internalization of [3H]CEt was higher compared with 125I-TC; the difference in uptake between both tracers yields selective CE uptake from HDL by WT hepatocytes. The uptake of both HDL tracers into hepatocytes isolated from LDLR−/− mice was significantly lower compared with WT cells. Accordingly, WT-HDL holo-particle uptake (125I-TC) and selective CE uptake ([3H]CEt − 125I-TC) were decreased in the LDLR-deficient hepatocytes. In contrast to uptake, no significant differences in 125I-TC-WT-HDL binding to hepatocytes isolated from WT and LDLR−/− mice were detected (supplementary Fig. III), indicating that double labeled HDL does not interact directly with LDLR.
Fig. 5.
Uptake of 125I-TC-/[3H]CEt-WT-HDL by hepatocytes isolated from WT or LDLR−/− mice. Hepatocytes were isolated from a WT or an LDLR−/− male mouse. These cells were incubated (37°C, 2.0 h) in medium containing 125I-TC-/[3H]CEt-WT-HDL, and the respective concentrations are given in the abscissae. Finally, cells were harvested, and apparent HDL particle uptake was analyzed as outlined in Materials and Methods. Values are means of n = 3 (WT) or n = 2 (LDLR−/−) independent experiments; within each experiment, incubations were done in triplicates. Comparing all data from WT and LDLR−/− hepatocytes, P < 0.05; two (WT) and two (LDLR−/−) independent similar experiments yielded qualitatively identical results.
To validate our experimental model, we investigated the metabolism of 125I-TC-LDL in WT and in LDLR−/− mice in a manner analogous as outlined for 125I-TC-/[3H]CEt-HDL (supplementary Fig. IV). Following intravenous injection of murine 125I-TC-LDL, periodic blood samples were harvested during a 24 h period. The plasma decay of 125I-TC, representing LDL clearance, was substantially attenuated in LDLR-deficient mice compared with WT animals. As expected, the plasma-FCR for 125I-TC-LDL decreased significantly in LDLR-deficient mice compared with WT (supplementary Table III). The organ-FCRs for 125I-TC-LDL uptake by liver and adrenals were significantly reduced in LDLR−/− mice compared with the respective tissues with LDLR expression (WT). In summary, these results demonstrate the appropriateness of the experimental model used in the current study (7).
HDL selective CE uptake by the liver and adrenals in vivo and by hepatocytes in vitro is reduced in LDLR−/− mice compared with WT animals. Because SR-BI mediates the selective CE uptake from HDL, we next addressed whether a downregulation of this receptor is responsible for the decrease in HDL CE uptake detected in tissues of LDLR−/− rodents (4). Murine liver membranes were immunoblotted using antibodies specific for SR-BI or LDLR (Fig. 6). The signal for SR-BI protein was identical in membranes prepared from WT or LDLR−/− liver, suggesting that altered SR-BI protein expression did not contribute to the reduction in selective uptake of HDL CE in the LDLR−/− mice. As expected, no signal for the LDLR was detected in membranes isolated from LDLR−/− liver confirming the correct genotype. Besides SR-BI, scavenger receptor CD36 and LRP1 have been implicated in selective HDL CE uptake in rodent liver and in hepatocytes, respectively (30–32). To determine whether these receptors played a role in the diminished selective CE uptake of LDLR−/− mice, expression levels of CD36 and LRP1 were determined by immunoblots (Fig. 6). In liver membranes from WT or LDLR−/− mice, the signals for CD36 and LRP1 proteins were nearly identical. Thus, these data suggest that altered protein expression of neither SR-BI, CD36, nor LRP1 accounts for the reduced HDL CE uptake observed in the livers of LDLR−/− mice. Similarly, no obvious differences in SR-BI, CD36, or LRP1 signals were detected in adrenal membrane preparations isolated from WT or from LDLR−/− mice (supplementary Fig. V).
Fig. 6.
SR-BI, LDLR, LRP1, and CD36 expression in liver membranes prepared from WT or LDLR−/− mice. Membrane fractions were isolated from livers originating from WT or LDLR−/− male mice. The indicated mass of protein was subjected to electrophoresis and transfer to a membrane. Finally, the proteins were immunoblotted using SR-BI- or LDLR-specific (A), LRP1-specific (B), and CD36- or β-actin-specific (C) antibodies. β-actin was used as loading control. Representative blots are shown. D: Three independent blots were quantified by densitometric scanning; P < 0.05 for all blots.
ABCA1 is a membrane protein that controls the rate-limiting step in HDL particle assembly by mediating the efflux of cholesterol and phospholipid from cells to lipid-free apoA-I, which forms nascent HDL particles (33). ABCA1 expressed by the liver has a substantial quantitative effect on HDL biogenesis and on HDL levels in plasma (34). In the studies presented previously, HDL selective CE uptake by tissues is reduced without concomitant increase in plasma HDL cholesterol, suggesting diminished HDL biogenesis. To test the hypothesis that a reduced hepatic expression of ABCA1 is responsible for a diminished HDL formation, the expression of ABCA1 protein was explored in liver membranes from WT or LDLR−/− mice (Fig. 7). The signal for ABCA1 was identical in membranes isolated from WT and LDLR−/− mice. This result argues against an altered HDL biogenesis in LDLR-deficient mice compared with WT, and this is true at least for the liver.
Fig. 7.
ABCA1 expression in liver membranes prepared from WT or LDLR−/− mice. Membrane fractions were isolated from livers originating from WT or LDLR−/− male mice. The indicated mass of protein was subjected to electrophoresis and transfer to a membrane. Finally, the proteins were immunoblotted using ABCA1- or β-actin-specific antibodies. β-actin was used as loading control. A: A typical blot is shown; three independent blots yielded qualitatively identical results. B: Densitometric scanning of three blots, P < 0.05.
DISCUSSION
A major consequence of a deficiency of functional LDLR in mice is an increase in plasma LDL cholesterol (7). Distinct from this change, a small or a more substantial increase in HDL cholesterol in LDLR−/− mice has been reported (7, 11, 12). We found a minor increase in plasma HDL cholesterol in male LDLR−/− mice compared with WT littermates, although this difference was not statistically significant. The explanation for these differences in HDL cholesterol may be the different genetic backgrounds of the mice or the feeding conditions. In contrast to HDL levels, the composition of LDLR−/−HDL is significantly different compared with WT-HDL (i.e., HDL from LDLR-deficient mice is enriched in triglyceride and depleted in phospholipid).
Mechanisms underlying the increased plasma HDL and LDL cholesterol in LDLR−/− mice were explored in this study in vivo. As expected, the decay of 125I-TC-LDL in the circulation was decreased in LDLR−/− mice compared with WT animals, and this result is in line with an earlier investigation (7). The liver is a major organ for LDL catabolism in vivo, and adrenals internalize LDL for steroidogenesis (3, 35). As predicted, the LDLR deficiency yielded a decreased rate of 125I-TC-LDL uptake by liver and adrenal glands in this study.
The protein and the lipid moieties of HDL particles can be metabolized at different rates in vivo and in vitro (5). A previous study used protein-iodinated HDL in LDLR−/− mice, and therefore, no information was obtained with respect to the turnover of the lipid component of HDL (7). To explore HDL metabolism in more detail, we radiolabeled the lipid and the protein moieties of these particles. With this approach, the fate of the distinct HDL components can be explored simultaneously (5). The composition of WT-HDL and LDLR−/−HDL is different; therefore, HDL preparations from both WT and LDLR−/− mice were used for these studies. Using double radiolabeled murine WT-HDL or LDLR−/−HDL in WT or in LDLR−/− mice, the major findings of this study are as follows: a) selective CE removal from the plasma HDL pool by tissues is reduced in rodents with an LDLR deficiency; b) selective CE uptake from HDL is diminished in liver and adrenals of LDLR−/− mice; and c) the reduced uptake of HDL-associated CE by tissues is not mediated by changes in membrane protein expression of SR-BI, CD36, or LRP1.
HDL-associated 125I-TC tracks the metabolism of HDL holo-particles (18). In vivo, the plasma decay of HDL-associated 125I-TC and liver and adrenal uptake of this tracer were not different between LDLR−/− and WT mice during the 24 h experiments. During the 2 h turnover, a quantitatively very small decrease in 125I-TC decay in plasma was detected in LDLR−/− mice; however, the biological relevance of this reduction presumably is minor. In vitro, in isolated LDLR-deficient hepatocytes, a decrease in uptake of HDL-associated 125I-TC was shown, suggesting reduced HDL holo-particle internalization. In parallel, 125I-TC-HDL binding (4°C) to WT liver cells or to LDLR−/− hepatocytes was identical. This observation suggests that the reduced HDL holo-particle uptake of LDLR−/− hepatocytes is not due to reduced HDL binding to the cell membrane. Thus, the interaction between HDL and the plasma membrane is not necessarily followed by hepatocellular HDL uptake. Compared with these results obtained in vitro, the HDL experiments performed in vivo are presumably physiologically more relevant. In summary, an LDLR deficiency has no substantial effect on HDL holo-particle metabolism in plasma and by tissues.
These observations on HDL holo-particle metabolism are in strong contrast to the selective CE pathway. A lack of LDLR is associated with a significant decrease of selective CE uptake from HDL by liver and adrenals. This was true for WT-HDL and for LDLR−/−HDL, as well as for the 2 h and the 24 h metabolic studies. Quantitatively, the decrease in selective CE uptake by liver and adrenals in LDLR−/− mice was smaller in the case of radiolabeled LDLR−/−HDL compared with WT-HDL. The explanation for this difference may be the different composition of both HDL preparations; a similar result has been obtained previously (6). Consistent with these observations on HDL metabolism in vivo, in cultured murine hepatocytes with an LDLR deficiency, a significant decrease in selective HDL CE uptake is observed compared with WT liver cells. This regulation of the HDL CE selective uptake pathway in the absence of LDLR has not been established previously, and our data represent a novel finding.
SR-BI can bind and mediate selective CE uptake from both LDL and HDL (4). LDLR−/− mice have a substantial increase in cholesterol-rich lipoprotein particles in plasma. Therefore, the question has to be raised as to whether a competition between LDL and HDL for SR-BI-mediated selective lipid uptake in vivo explains the decreased selective CE uptake in LDLR-deficient mice. However, the experiments with murine hepatocytes in vitro are a strong argument against this possibility.
With respect to intravascular lipoprotein metabolism, it is established that some apos, for instance HDL-associated apoA-I, are mobile and exchange between lipoprotein fractions (1). Concerning CE, in murine plasma no CETP activity is detectable; therefore, a lipid exchange reaction is unlikely in the circulation (36). To address the issue of an exchange of HDL-associated tracers with non-HDL lipoprotein, FPLC analysis of plasma lipoproteins after HDL tracer injection was done. In WT and LDLR−/− mice, no transfer of 125I-TC tracer (i.e., no apo transfer) out of the HDL fraction could be detected. In contrast, during the time course of 24 h after injection of radiolabeled LDLR−/−HDL, initially HDL-associated [3H]CEt tracer could be detected in FPLC fractions corresponding to non-HDL lipoproteins in LDLR−/− mice. Considering the CETP deficiency of mice (36), the hypothesis emerged of a resecretion of initially HDL-associated [3H]CEt by the liver, for instance in VLDL particles. In fact, an increased secretion of apoB-containing lipoproteins by LDLR−/− hepatocytes has been reported (37). Experiments in which the clearance of VLDL was inhibited suggest that there is indeed substantial resecretion of [3H]CEt tracer by the liver, and this is pronounced in LDLR−/− mice.
The question had to be raised as to whether the recovery of the initially HDL-associated [3H]CEt tracer in non-HDL fractions in plasma yields the decreased selective CE uptake, for instance by the liver. Remarkably, during the initial period of the 24 h plasma decay experiments, no HDL lipid label is detected in a non-HDL lipoprotein fraction. To address this issue in more detail, short-term experiments over a period of 2 h demonstrated reduced selective CE removal from the HDL plasma pool and decreased selective CE uptake by the liver in LDLR−/− mice, a result that is consistent with the 24 h studies. Besides, in the less complex system of cultured murine hepatocytes, qualitatively identical results were obtained as in mice. Based on the concurrence of the in vivo and in vitro observations, it is unlikely that the decrease in the selective uptake of HDL CE that we observed in vivo is significantly modified by the resecretion of HDL tracer in non-HDL particles.
SR-BI, CD36, and LRP1 mediate the selective uptake of HDL CE by the liver (4, 6, 30–32). Considering the decrease in selective CE uptake from HDL in LDLR−/− mice, the hypothesis emerged that a downregulation of these receptors may be responsible for the decrease in selective CE uptake under conditions of an LDLR deficiency. However, the protein expression of SR-BI, CD36, and LRP1 was not significantly different in our study with LDLR−/− mice compared with WT littermates. Consistent with our results, a previous study found no difference in LRP1 expression between LDLR−/− and WT mice (7). Thus, even though downregulation of the selective HDL CE uptake pathway is observed in tissues of LDLR−/− mice, this is independent from established receptors that play a role in cellular HDL uptake. In summary, there is no evidence that regulation of SR-BI, CD36, or LRP1 is responsible for the decrease in selective CE uptake in liver or adrenals in mutant mice.
What is an explanation for the discrepancy between a downregulation of selective HDL CE uptake by tissues and a lack of regulation of receptors like SR-BI? A substantial increase in liver and adrenal cholesterol in LDLR−/− mice compared with WT animals is established (11, 12). In familial hypercholesterolemia, cholesterol synthesis is enhanced (38). Thus, an increase in tissue cholesterol is detected in LDLR-deficient organs. Morrison and coworkers (39) suggested that lipid-lipid interactions between a lipoprotein particle and a membrane play a role in the selective transfer of CE from the HDL particle to a cell. Cholesterol is an important component of membranes, and this compound may modulate membrane function substantially (40). Considering the lack of altered receptor expression in LDLR-deficient tissues, it is speculated that the mechanism of the decrease in selective HDL CE uptake may be due to an increase in plasma membrane lipid content of the respective cells.
In this study, no significant change in HDL cholesterol in LDLR−/− mice was detected when compared with WT rodents. Generally, decreased HDL catabolism as observed here results in an increase in plasma HDL cholesterol (6). How can this discrepancy in our findings be explained? The steady-state concentration of plasma HDL cholesterol is the result of HDL biogenesis and HDL catabolism (1). A quantitatively dominant organ in HDL catabolism in rodents is the liver, and HDL lipid uptake by this organ is decreased in LDLR−/− mice (5). A possible explanation for the discrepancy between the essentially unchanged HDL cholesterol in plasma and the decreased selective HDL CE uptake by tissues is that HDL synthesis is reduced in LDLR−/− mice. Hepatic ABCA1 is a key regulator of plasma HDL cholesterol (34). Therefore, the hypothesis was tested as to whether a reduced hepatic expression of ABCA1 protein mediates a decrease in cellular lipid efflux in LDLR−/− mice. However, ABCA1 protein expression was unchanged in LDLR-deficient liver membranes compared with those from WT mice. This finding suggests that a difference in cholesterol efflux and a modified HDL biogenesis between both groups of animals is unlikely.
LDLR−/− mice are a frequently used model for studies on atherosclerosis (8, 41). Usually, this increased atherosclerotic burden is attributed to the increase in plasma cholesterol contained in apoB-containing lipoproteins. However, our studies point to an additional mechanism that may be relevant for the observed susceptibility for atherosclerosis. Substantial changes in HDL metabolism are detected in the presence of an LDLR deficiency, and HDL plays a key role in reverse cholesterol transport, i.e., the flux of lipid from peripheral tissues to the liver for excretion via bile (42). Therefore, it is suggested that the LDLR modulates both LDL-mediated cholesterol delivery to cells as well as HDL-mediated reverse cholesterol transport to the liver, and both pathways may be relevant for the pathogenesis of atherosclerosis at least in mice.
Supplementary Material
Acknowledgments
The authors thank S. Ehret, B. Schulz, and M. Thiel for technical assistance. Dr. Richard E. Morton and Diane Greene donated CETP for radiolabeling, and Dr. Martin Merkel injected mice intravenously.
Footnotes
Abbreviations:
- CD36
- cluster of differentiation 36
- CE
- cholesteryl ester
- CEt
- cholesteryl oleyl ether
- CETP
- cholesteryl ester transfer protein
- FPLC
- fast performance LC
- LDLR
- LDL receptor
- LRP1
- LDL receptor-related protein 1
- organ-FCR
- organ fractional catabolic rate
- plasma-FCR
- plasma fractional catabolic rate
- SR-BI
- scavenger receptor class B type I
- TC
- tyramine cellobiose
This study was supported by Research Grant Ri 436/8-1 from Deutsche Forschungsgemeinschaft (Bonn) and funds from German Diabetes Association, Werner Otto Stiftung (Hamburg), and Gertraud und Heinz Rose Stiftung (Hamburg). J. Heeren is supported by EU FP7 project RESOLVE Grant FP7-HEALTH-2012-305707.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures and three tables.
REFERENCES
- 1.Eisenberg S. 1984. High density lipoprotein metabolism. J. Lipid Res. 25: 1017–1058. [PubMed] [Google Scholar]
- 2.Brown M. S., Goldstein J. L. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science. 232: 34–47. [DOI] [PubMed] [Google Scholar]
- 3.Carew T. E., Pittman R. C., Steinberg D. 1982. Tissue sites of degradation of native and reductively methylated [14C]sucrose-labeled low density lipoprotein in rats. J. Biol. Chem. 257: 8001–8008. [PubMed] [Google Scholar]
- 4.Acton S., Rigotti A., Landschulz K. T., Xu S., Hobbs H. H., Krieger M. 1996. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 271: 518–520. [DOI] [PubMed] [Google Scholar]
- 5.Glass C., Pittman R. C., Civen M., Steinberg D. 1985. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro. J. Biol. Chem. 260: 744–750. [PubMed] [Google Scholar]
- 6.Brundert M., Ewert A., Heeren J., Behrendt B., Ramakrishnan R., Greten H., Merkel M., Rinninger F. 2005. Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl esters by the liver in mice. Arterioscler. Thromb. Vasc. Biol. 25: 143–148. [DOI] [PubMed] [Google Scholar]
- 7.Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. 1993. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92: 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibashi S., Goldstein J. L., Brown M. S., Herz J., Burns D. K. 1994. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 93: 1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigotti A., Trigatti B. L., Penman M., Rayburn H., Herz J., Krieger M. 1997. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl. Acad. Sci. USA. 94: 12610–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun A., Trigatti B. L., Post M. J., Sato K., Simons M., Edelberg J. M., Rosenberg R. D., Schrenzel M., Krieger M. 2002. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 90: 270–276. [DOI] [PubMed] [Google Scholar]
- 11.Osono Y., Woollett L. A., Herz J., Dietschy J. M. 1995. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across tissues of the mouse. J. Clin. Invest. 95: 1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton J. D., Shimano H., Hamilton R. L., Brown M. S., Goldstein J. L. 1999. Disruption of LDL receptor gene in transgenic SREBP-1a mice unmasks hyperlipidemia resulting from production of lipid-rich VLDL. J. Clin. Invest. 103: 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pittman R. C., Taylor C. A. 1986. Methods for assessment of tissue sites of lipoprotein degradation. Methods Enzymol. 129: 612–628. [DOI] [PubMed] [Google Scholar]
- 14.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morton R. E., Zilversmit D. B. 1983. Inter-relationship of lipids transferred by the lipid-transfer protein isolated from human lipoprotein-deficient plasma. J. Biol. Chem. 258: 11751–11757. [PubMed] [Google Scholar]
- 16.Dole V. P. 1956. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Invest. 35: 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le N. A., Ramakrishnan R., Dell R. B., Ginsberg H. N., Brown W. V. 1986. Kinetic analysis using specific radioactivity data. Methods Enzymol. 129: 384–395. [DOI] [PubMed] [Google Scholar]
- 18.Pittman R. C., Knecht T. P., Rosenbaum M. S., Taylor C. A. 1987. A nonendocytotic mechanism for the selective uptake of high density lipoprotein-associated cholesterol esters. J. Biol. Chem. 262: 2443–2450. [PubMed] [Google Scholar]
- 19.Silver D. L., Wang N., Tall A. R. 2000. Defective HDL particle uptake in ob/ob hepatocytes causes decreased recycling, degradation, and selective lipid uptake. J. Clin. Invest. 105: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinninger F., Brundert M., Jäckle S., Galle P. R., Busch C., Izbicki J. R., Rogiers X., Henne-Bruns D., Kremer B., Broelsch C. E., et al. 1994. Selective uptake of high-density lipoprotein-associated cholesteryl esters by human hepatocytes in primary culture. Hepatology. 19: 1100–1114. [PubMed] [Google Scholar]
- 21.Oram J. F. 1986. Receptor-mediated transport of cholesterol between cultured cells and high density lipoproteins. Methods Enzymol. 129: 645–659. [DOI] [PubMed] [Google Scholar]
- 22.Jokinen E. V., Landschulz K. T., Wyne K. L., Ho Y. K., Frykman P. K., Hobbs H. H. 1994. Regulation of the very low density lipoprotein receptor by thyroid hormone in rat skeletal muscle. J. Biol. Chem. 269: 26411–26418. [PubMed] [Google Scholar]
- 23.Heeren J., Grewal T., Jäckle S., Beisiegel U. 2001. Recycling of apolipoprotein E and lipoprotein lipase through endosomal compartments in vivo. J. Biol. Chem. 276: 42333–42338. [DOI] [PubMed] [Google Scholar]
- 24.Moore K. J., Rosen E. D., Fitzgerald M. L., Randow F., Andersson L. P., Altshuler D., Milstone D. S., Mortensen R. M., Spiegelman B. M., Freeman M. W. 2001. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat. Med. 7: 41–47. [DOI] [PubMed] [Google Scholar]
- 25.Yokode M., Hammer R. E., Ishibashi S., Brown M. S., Goldstein J. L. 1990. Diet-induced hypercholesterolemia in mice: prevention by overexpression of LDL receptors. Science. 250: 1273–1275. [DOI] [PubMed] [Google Scholar]
- 26.Merkel M., Loeffler B., Kluger M., Fabig N., Geppert G., Pennacchio L. A., Laatsch A., Heeren J. 2005. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J. Biol. Chem. 280: 21553–21560. [DOI] [PubMed] [Google Scholar]
- 27.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275. [PubMed] [Google Scholar]
- 28.Glass C. K., Pittman R. C., Keller G. A., Steinberg D. 1983. Tissue sites of degradation of apoprotein A-I in the rat. J. Biol. Chem. 258: 7161–7167. [PubMed] [Google Scholar]
- 29.McKay I. R. 2002. Hepatoimmunology: from horizon to harborside. In Liver Immunology. M. E. Gershwin, J. M. Vierling, and M. P. Manns, editors. Hanley and Belfus. Philadelphia, PA. 15–29. [Google Scholar]
- 30.Febbraio M., Abumrad N. A., Hajjar D. P., Sharma K., Cheng W., Pearce S. F. A., Silverstein R. L. 1999. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274: 19055–19062. [DOI] [PubMed] [Google Scholar]
- 31.Brundert M., Heeren J., Merkel M., Carambia A., Herkel J., Groitl P., Dobner T., Ramakrishnan R., Moore K. J., Rinninger F. 2011. Scavenger receptor CD36 mediates uptake of high density lipoproteins in mice and by culture cells. J. Lipid Res. 52: 745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassiliou G., McPherson R. 2004. A novel efflux-recapture process underlies the mechanism of high-density lipoprotein cholesteryl ester-selective uptake mediated by the low-density lipoprotein receptor-related protein. Arterioscler. Thromb. Vasc. Biol. 24: 1669–1675. [DOI] [PubMed] [Google Scholar]
- 33.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., Van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O. F., et al. 1999. Mutations in ABC1 in tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. [DOI] [PubMed] [Google Scholar]
- 34.Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovanen P. T., Faust J. R., Brown M. S., Goldstein J. L. 1979. Low density lipoprotein receptors in bovine adrenal cortex. I. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid hormone synthesis in cultured adrenocortical cells. Endocrinology. 104: 599–609. [DOI] [PubMed] [Google Scholar]
- 36.Guyard-Dangremont V., Desrumaux C., Gambert P., Lallemant C., Lagrost L. 1998. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 120: 517–525. [DOI] [PubMed] [Google Scholar]
- 37.Twisk J., Gillian-Daniel D. L., Tebon A., Wang L., Barrett P. H. R., Attie A. D. 2000. The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 105: 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldstein J. L., Brown M. S. 1973. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. Natl. Acad. Sci. USA. 70: 2804–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison J. R., Silvestre M. J., Pittman R. C. 1994. Cholesteryl ester transfer between high density lipoprotein and phospholipid bilayers. J. Biol. Chem. 269: 13911–13918. [PubMed] [Google Scholar]
- 40.Zhang X., Hurng J., Rateri D. L., Daugherty A., Schmid-Schönbein G. W., Shin H. Y. 2011. Membrane cholesterol modulates the fluid shear stress response of polymorphonuclear leukocytes via its effects on membrane fluidity. Am. J. Physiol. Cell Physiol. 301: C451–C460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zadelaar S., Kleemann R., Verschuren L., De Vries-Van der Weij J., Van der Hoorn J., Princen H., Kooistra M. 2007. Mouse models for atherosclerosis and pharmaceutical modifiers. Arterioscler. Thromb. Vasc. Biol. 27: 1706–1721. [DOI] [PubMed] [Google Scholar]
- 42.Glomset J. A. 1970. Physiological role of lecithin-cholesterol acyltransferase. Am. J. Clin. Nutr. 23: 1129–1136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.