Abstract
Little is known about the regulation of arthritis severity and joint damage in rheumatoid arthritis (RA). Fibroblast-like synoviocytes (FLS) have a central role in joint damage and express increased levels of the cation channel Trpv2. We aimed at determining the role of Trpv2 in arthritis. Treatment with Trpv2-specific agonists decreased the in vitro invasiveness of FLS from RA patients and arthritic rats and mice. Trpv2 stimulation suppressed IL-1β-induced expression of MMP-2 and MMP-3. Trpv2 agonists, including the new and more potent LER13, significantly reduced disease severity in KRN serum- and collagen-induced arthritis, and reduced histologic joint damage, synovial inflammation, and synovial blood vessel numbers suggesting anti-angiogenic activity. In this first in vivo use of Trpv2 agonists we discovered a new central role for Trpv2 in arthritis. These new compounds have the potential to become new therapies for RA and other diseases associated with inflammation, invasion and angiogenesis.
1. INTRODUCTION
Rheumatoid arthritis (RA) affects nearly 1% of the population [1] and is associated with reduced quality of living, increased risk for disability and reduced survival [2–4]. New and more effective therapies emerged during the past decade significantly improving disease control and quality of living. Yet, disease remission is rarely achieved in RA underscoring the need for developing more effective therapies. One way of identifying new targets for therapy is to better understand the processes regulating arthritis severity and articular damage, which are major predictors of disease outcome such as the risk of developing deformities and disability.
The hyperplastic synovial tissue in RA, also called pannus, has unique characteristics and like a cancer invades and destroys cartilage and bone. While the RA synovial tissue behavior is incompletely understood, the joint destruction it mediates correlates with increased disease severity and worse outcome. The fibroblast-like synoviocyte (FLS) has a central role in the formation of the RA synovial pannus [5], and the in vitro invasive properties of FLS correlate with radiographic and histologic damage in RA [6] and in animal models of arthritis such as pristane-induced arthritis (PIA) [7]. Therefore, understanding the regulation of the invasive properties of FLS has the potential to generate new therapies aimed at reducing articular damage [8, 9].
Trpv2 (Transient receptor potential vanilloid subfamily, type 2 channel) is a non-selective cation channel gene that we detected for the first time in highly invasive FLS from rats with PIA [10]. FLS are known to express other ion channels including those of the TRP family such as Trpv1, Trpv3, Trpv4 [11, 12], Trpc1, Trpc5 and Trpm3 [13, 14], but their role in FLS function remains unknown. Trpv2 is related to Trpv1, but has a higher temperature threshold for heat activation (>52°C) [15] and has not been implicated in the regulation of pain. Trpv2 is expressed by dorsal root ganglia, lung, placenta, myeloid cells such as macrophages and mast cells, NK and B cells. Trpv2 was recently implicated in macrophage phagocytosis [16], but other than that its role in human disease remains largely unknown.
The increased expression of Trpv2 in highly invasive FLS initially suggested that Trpv2 might have an invasion-inducing effect. However our studies with siRNA and agonists revealed that Trpv2 is in fact a suppressor of cell invasion, inflammatory cell infiltration, angiogenesis and arthritis severity. This is also the first time that Trpv2 agonists are used in vivo, including the new compound LER13.
2. MATERIAL AND METHODS
2.1. Synovial tissue collection from rats with pristane-induced arthritis (PIA) and mice with KRN serum-induced arthritis
Eight to 10 week-old inbred DA (DA/BklArbNsi, arthritis-susceptible) rats received 150 μl of pristane (2,6,10,14-tetramethylpentadecane) (MP Bio, Solon, OH) by intradermal injection [17, 18]. On day 21 post-pristane injection (a time-point when all DA rats have arthritis), animals were euthanized and synovial tissues collected from the ankle joints for FLS isolation. Synovial tissues from C57BL/6 mice (Jackson Laboratories) with KRN serum-induced arthritis (see below) were collected at the end of the arthritis observation period (day 10). All experiments involving animals were reviewed and approved by the Feinstein Institute Institutional Animal Care and Use Committee (IACUC).
2.2. RA patients and synovial tissues
Human synovial tissues were obtained from RA patients undergoing elective orthopedic surgery. All patients met the American College of Rheumatology criteria for RA [19]. Informed consent was obtained from all participating subjects under an Institutional Review Board-approved protocol through the Feinstein Institute’s Tissue Donation Program.
2.3. Isolation and culture of FLS
FLS were obtained as previously described [7]. Briefly, freshly obtained synovial tissues were minced and incubated with a solution containing DNase (0.15mg/ml), hyaluronidase type I-S (0.15 mg/ml), and collagenase type IA (1 mg/ml) (Sigma, St.Louis, MO) in DMEM (Invitrogen, Carlsbad, CA) for 1 hour at 37°C. Cells were washed and re-suspended in complete media containing DMEM supplemented with 10% FBS (Invitrogen), glutamine (300 ng/ml), amphotericin B (250 μg/ml) (Sigma), and gentamicin (20 ng/ml) (Invitrogen). After overnight culture, non-adherent cells were removed and adherent cells cultured. All experiments were performed with FLS after passage four (>95% FLS purity).
2.4. Invasion Assay
The in vitro invasiveness of FLS was assayed in a transwell system using Matrigel-coated inserts (BD Biosciences, Franklin Lakes, NJ), as previously described [6, 7]. Briefly, 70–80% confluent cells were harvested by trypsin-EDTA digestion, and re-suspended at 2.0 × 104 cells in 500 μl of serum-free DMEM. Cells were placed in the upper compartment of the Matrigel-coated inserts. Where indicated, O1821, LER13 or vehicle was added to the upper chamber. The lower compartment was filled with complete media (as described above) and the plates were incubated at 37°C for 24 hours. After 24 hours the upper surface of the insert was wiped with cotton-swabs to remove non-invading cells and the Matrigel layer. The opposite side of the insert was stained with Crystal Violet (Sigma) and the total number of cells that invaded through Matrigel counted at 100X magnification. Experiments were done in duplicate.
2.5. Trpv2 agonists
The Trpv2 agonist O-1821 (Cayman Chemicals) [20] is a synthetic cannabinoid that stimulates Trpv2, but does not stimulate Trpv1 or the cannabinoid receptors [20, 21]. We synthesized and screened a focused library around O-1821 and identified LER13 as a novel and potent Trpv2 agonist (see supplemental table 1; provisional patent application serial number 62/126,167).
2.6. siRNA knock-down
Dharmacon SMARTpool siRNA targeting either Trpv2, Gapdh, and a non-coding control were purchased from Thermo Scientific (Lafayette, CO) and transfected into DA FLS according to the manufacturer’s instructions. Cells were then incubated at 37°C for 72 hours prior to initiating the invasion assays. Knock-down was confirmed with qPCR and western blot.
2.7. MMP-2 and MMP-3 qPCR studies
The qPCR conditions used have been previously described [10]. Briefly, total RNA (200 ng) from each sample was reverse transcribed using Superscript III (Invitrogen). MMP-2 (forward-CGGTTTTCTCGAATCCATGA; reverse-GGTATCCATCGCCATGCT) and MMP-3 (forward-CCAGGTGTGGAGTTCCTGA; reverse-GCATCTTTTGGCAAATCTGG) primers were designed based on the Universal ProbeLibrary (Roche, Indianapolis, IN). Reactions were prepared with Absolute Blue qPCR Mix (Thermo Fisher, Waltham, MA), and run in duplicate on a LightCycler 480 thermocycler (Roche) using Relative Quantitative Software (Roche). Ct (threshold cycle) values were adjusted for GAPDH in each sample (ΔCt). Fold differences were calculated with the 2−ΔΔCt method [22].
2.8. Intracellular calcium influx
Intracellular calcium influx was measured using the Fluo-4 NW calcium assay kit (Molecular Probes/Invitrogen) according to the manufacturer’s instructions, as previously described [23]. Briefly, DA FLS were plated at a density of 4,500 cells/well in a 96-well plate (100 μl volume of complete media per well). After cell adhesion the media was changed to serum-free media for overnight culture. Serum-free media was then replaced with 50 μl of dye loading solution, followed by incubation at 37°C for 30 minutes and a baseline fluorimetric reading. Then, O1821, LER13 or vehicle were added to their respective wells at different concentrations and fluorescence read immediately in a Fluoroskan Ascent Microplate Fluorometer (Thermo Scientific) with settings appropriate for excitation at 494 nm and emission at 516 nm.
2.9. Single cell patch clamp studies
FLS obtained from DA rats with PIA were plated in culture medium onto glass coverslips and allowed to adhere at 37°C. Recordings were performed in the whole-cell configuration of the patch-clamp technique at room temperature using an EPC10-USB amplifier (HEKA instruments, Bellmore, NY). Patch pipettes had a resistance of 2–4 MΩ when filled with a solution containing 45mM CsCl, 100mM CsF, 5mM EGTA, 10mM HEPES, and 5mM glucose, pH 7.4. The bath solution contained 132mM NaCl, 5.4mM KCl, 1.8mM CaCl2, 0.8mM MgCl2, 10mM HEPES, and 10mM glucose, at pH 7.4. O1821-activated currents were elicited with a 600 ms ramp from −100 to +100 mV from a holding potential of −107 mV. Addition of O1821 was accomplished by complete bath exchange.
2.10. Immunofluorescence and confocal microscopy
Immunofluorescence was performed as previously reported [8, 24]. Briefly, FLS were cultured on coverslips to 10–20% confluence, starved overnight and then pre-treated for 30 minutes, 60 minutes or 24h with either DMSO or O1821, in the presence or absence of IL-1β 10–25 ng/ml or PDGF 50 ng/ml in complete media or in serum-free media. Cells were then fixed with 4% formaldehyde for 15 minutes at room temperature and permeabilized with PBS/Triton X-100 0.1% for 5 minutes. Non-specific binding was blocked with 5% nonfat milk. A rabbit anti-Trpv2 (Santa Cruz Biotechnology) was used as primary antibody, and a Alexa Fluor 488 (green) donkey anti-rabbit IgG (Invitrogen) used as secondary antibody. Alexa Fluor 594 (red) Phalloidin (Invitrogen) was used to stain the actin filament. The stained cells were mounted on a glass slide. A Zeiss Axiovert 200M fluorescent microscope was used for visualization with the appropriate filters, with Zeiss Axioversion 4.7 software. Immunofluorescence microscopy was used for cell cytoskeleton scoring as previously described [23]. An Olympus FluoView 300 was used for confocal microscopy with a 600X magnification.
2.11. MTT assay
4×104 FLS per well were plated in triplicate in 96-well plates in 100 μl of complete media. Cells were allowed to adhere for 24 hours. Media was then changed and either O1821, LER13 or vehicle added at the same concentrations used for the invasion studies. After 24 hours (same duration of the invasion experiments) viable cells were determined by the colorimetric MTT kit (Millipore) according to the manufacturer’s instructions.
2.12. Mice and in vivo testing of Trpv2 agonists
2.12.1. KRN serum transfer-induced arthritis
Seven to eight week-old C57BL/6 male mice (Jackson Laboratories) received 200 μl of KRN arthritogenic serum [25] (KBN TCR transgenic mice were a kind gift from Christophe Benoist and have been intercrossed with NOD at the Feinstein Institute to generate KRN mice and the arthritogenic serum) intraperitoneal (IP) on day zero (D0) and day 2 (D2). After the onset of clear ankle arthritis (day 3; D3), mice were started on O1821 10mg/Kg IP BID or control vehicle (DMSO in saline solution). Arthritis severity was scored according to the system reported by Jirholt et al, which includes number of involved joints and degree of inflammation (swelling/redness) in a scale of 0–12 per mouse per day [26].
2.12.2. Collagen-induced arthritis (CIA)
CIA was induced in 8-week-old male DBA1/J mice (Jackson Laboratories) using a standard protocol [27, 28]. Briefly, bovine type II collagen (Chondrex, Redmond, WA) was dissolved overnight in acetic acid 0.1N and then homogenized at 1:1 with Complete Freund’s Adjuvant (CFA, Difco, Detroit, MI). Anesthetized mice were injected intra-dermally with 100 μg of type II collagen emulsified in CFA in the tail. The new Trpv2 agonist LER13 10 mg/Kg or control vehicle (PBS 50%, PEG300 40%, PG 5%, PS80 5%) was started on day 10 (D10) and administered intra-peritoneal every-other-day (from D10 until day 55). On day 21 (D21), mice received a booster injection of 100 μg of type II collagen in incomplete Freund’s adjuvant. Clinical signs of arthritis were evident on day 24 (D24).
Arthritis severity in CIA mice was scored using a modified scoring system based on Jirholt et al. [26]. Briefly, the modified scoring system ranged from 0–16 per mouse per day and included a functional component of disease severity (non-weight bearing) in the maximum score of 4 per paw. The arthritis severity index (ASI), which is the sum of all scores obtained during the observation period, was used to compare treatment groups as it provides a comprehensive assessment of arthritis severity over time, and correlates with histologic damage [18, 29].
2.13. Histology scoring
Mice were euthanized at the end of the in vivo arthritis experiments (76 days), ankles fixed in formalin, decalcified and embedded in paraffin for H&E and Safranin-O staining. Histology slides were scored without knowledge of treatment and according to a comprehensive scoring system previously reported that includes parameters for synovial hyperplasia, inflammatory cell infiltration, and bone and cartilage erosions [18].
2.14. Statistics
Means were compared with the Student’s t-test or one-way ANOVA with a pairwise multiple comparison procedure (Holm-Sidak method) using SigmaStat 3.0 (SPSS, Chicago, IL).
3. RESULTS
3.1. Trpv2 is expressed in increased levels in highly invasive FLS
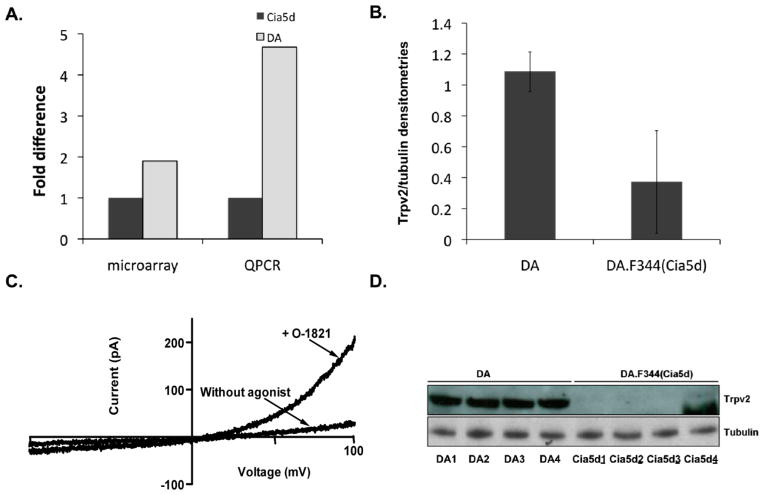
In microarray studies leading up to this study we analyzed the transcriptome of highly invasive FLS obtained from DA (severe and erosive arthritis) and of minimally invasive FLS from DA.F344(Cia5d) (mild and non-erosive arthritis) rat strains. We identified an expression signature that included genes implicated in cancer-related phenotypes such as invasion and metastasis [8, 10]. One of the most significantly differentially expressed genes was Trpv2. Trpv2 had a 1.9-fold increased expression in highly invasive DA FLS, compared with the minimally invasive FLS from DA.F344(Cia5d) FLS (P=0.0075, figure 1A), which initially suggested that this gene could have an invasion-favoring function. Levels of Trpv2 were confirmed with qPCR (4.68-fold; P=0.012, figure 1A) and Western blot (2.9-fold, figure 1B and D).
Figure 1. Trpv2 is functional and expressed in increased levels in invasive FLS from DA rats.
(A) Microarray analysis and QPCR confirmation showing increased mRNA levels of Trpv2 in DA FLS compared with DA.F344(Cia5d) (n=6 per group). (B) Trpv2 protein levels were also increased in DA FLS, compared with DA.F344(Cia5d), as shown on densitometries (normalized for tubulin±S.D) from Western blot (panel D). (C) FLS obtained from DA rats with PIA were patch-clamped in the absence of agonist, and after perfusion with O1821 30 μM, which induced channel opening. At positive potentials, the current is outward (upward), matching the Trvp2 pattern, thus confirming that FLS have functional Trpv2 channels. Currents are representative of 3–5 cells isolated from 3 different rats with PIA (n = 11 cells total). (D) Western blot image of four different DA and four different DA.F344(Cia5d) FLS cells lines (each from a different rat) demonstrating significantly higher Trpv2 protein levels in DA FLS (2.9-fold; densitometries on panel B).
3.2. Single cell patch clamp studies confirmed that Trpv2 is functional in FLS
At negative potentials, the current was inward (downward in the plot, figure 1C) and reverses at 0 mV, while at positive potentials, the current is outward (upward in the plot, figure 1C), matching the Trvp2 pattern [30], and confirming that the Trpv2 channel is not only expressed, but also functional in FLS and opened by O1821 (figure 1C).
3.3. Trpv2 stimulation increased intracellular calcium influx in FLS
Trpv2 stimulation is known to increase intracellular calcium influx [15, 31]. Therefore, two different DA FLS cells lines were stimulated in duplicate with increasing concentrations of the Trpv2 agonist O1821 (Supplemental figure 1A). O1821 induced a significant dose-dependent increase in intracellular FLS calcium influx, which was more pronounced and more sustained in the highest concentration (10μM).
3.4. O1821 significantly decreased rodent and RA FLS invasion
Overnight treatment with O1821 significantly reduced FLS invasion in a dose-dependent manner by 80% at 1μM, and by 99.4% at 10μM in DA cells (figure 2A). O1821 also reduced RA FLS invasion by 90% at 20μM and by 99.4% at 50μM concentrations compared with control vehicle (P≤0.001; figure 2B). O1821 also reduced invasion of FLS obtained from a C57BL/6 with KRN serum-induced arthritis (figure 2C).
Figure 2. Trpv2 stimulation decreases the invasiveness of FLS from rodents and RA patients.
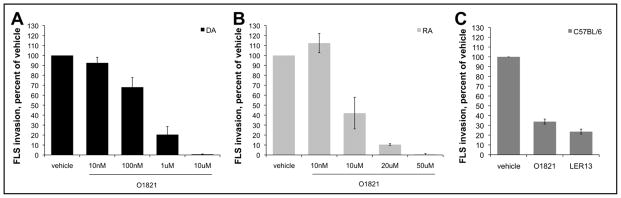
Invasiveness of FLS from both DA (A) and RA (B) treated with the Trvp2 agonist O1821 was significantly reduced in a dose-dependent manner by as much as 100% compared with controls treated with vehicle alone (24h treatment; four different DA FLS cell lines, and seven different RA FLS lines) (mean±S.E.M.; p≤0.001, Rank Sum Test). (C) FLS obtained from a single C57BL/6 mouse with KRN serum-induced arthritis had a reduction in invasion or 65% and 75% in the presence of O1821 10μM and LER13 10μM, respectively, compared with control vehicle (one cell line tested in triplicate).
We examined the possibility of cell mortality using the MTT assay over a 24-hour period (same duration of the invasion experiments) and identified similar numbers of dead cells in the O1821 and control vehicle groups. These observations suggested that O1821 was not toxic to FLS, and that the reduced invasion effect was not explained by increased cell mortality.
3.5. Trpv2 is diffusely expressed in FLS, including within lamellipodia
Confocal microscopy studies of three DA and three RA FLS lines revealed diffuse cytoplasmic and plasma membrane distribution of Trpv2 (Supplemental figure 2). Trpv2 was also expressed in lamellipodia (Supplemental figure 2, grey arrows), which are cell protrusions required for FLS invasion, raising the possibility of a localized effect on the production of proteases involved in cell invasion.
3.6. O1821 does not affect FLS morphology or the cell distribution of Trpv2
Stimulation with O1821 (10 μM) for 30 minutes to 24 hours had no significant effect on actin cytoskeleton rearrangements, number or location of lamellipodia or other cell morphology characteristics (Supplemental figure 2A) in cells stimulated with either PDGF or IL-1β in the presence or absence of 10% FBS. O1821 also did not significantly change the distribution of Trpv2 in FLS (Supplemental figure 2B).
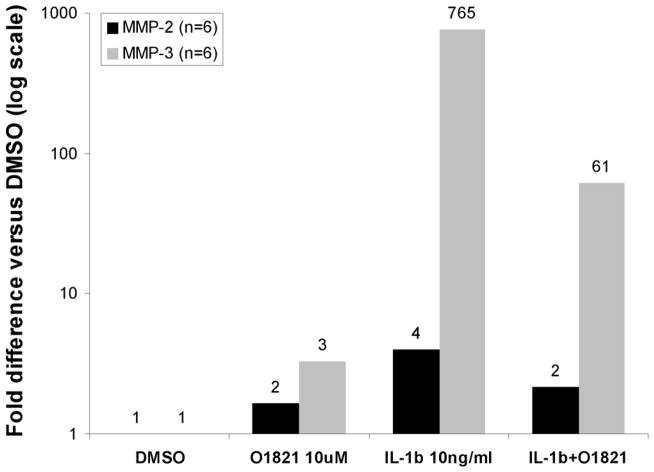
3.7. Trpv2 stimulation reduces IL-1β-induced expression of MMP-2 and MMP-3 in RA FLS
Treatment of RA FLS with O1821 significantly reduced the IL-1β-induced expression of MMP-2 by 45.6% (P=0.02, n=6 RA; figure 3) and MMP-3 by 92% (P<0.001, n=6 RA; figure 3).
Figure 3. Trpv2 agonist decreases IL-1β-induced expression of matrix metalloproteases in RA FLS.
FLS derived from synovial tissues from six different patients with RA were stimulated with IL-1β 10ng/ml for 48 hours in the presence or absence of the Trpv2 agonist O1821 10μM. qPCR demonstrated that IL-1β induced a significant 765-fold increase in MMP-3, and a 4-fold increase in MMP-2 expression. Trpv2 stimulation significantly decreased the IL-1β-induced expression of MMP-2 and MMP-3 by 50% and 90%, respectively (number on each bar is the fold-difference compared with the DMSO treatment control group; data shown in log scale).
3.8. The O1821-induced suppression of FLS invasion did not involve Akt, Erk or p65 NFκB
Akt, Erk and NFκB have been implicated in the regulation of FLS invasion. Akt and Erk had been suggested to mediate Trpv2 activity in experiments with endothelial [21] and glioma cells [32] that were unrelated to invasion, and the ankyrin domains in Trpv2 could potentially interact and interfere with NFκB activity [33]. Therefore, we considered that the Trpv2-dependent effect of O1821 might involve one of these cell signaling mediators. However, O1821 did not affect FLS levels of phosphorylated Akt or phosphorylated Erk1/2 (data not shown). O1821 did not change p65 NFκB activation based on nuclear location of p65 on immunofluorescence analyses (5, 10, 30 and 60 minutes, and 24 hours) (data not shown).
3.9. O1821 reduced disease severity, clinical signs of inflammation and histologic damage in established KRN serum transfer model of arthritis
C57BL/6 mice with KRN serum transfer-induced arthritis were started O1821 10mg/Kg IP BID or control vehicle (DMSO in saline solution) after the onset of arthritis on day three. By day five (D5), a small difference in arthritis severity was already detected and reached statistical significance on days seven and eight (D7 and D8) (figure 4A). The arthritis severity indices (ASI), which are the sum of all scores obtained during the observation period, were compared as they provide a more comprehensive assessment of arthritis over time, and correlate with histologic damage [18, 29]. O1821-treated mice had a significant 30% lower ASI, compared with control vehicle-treated mice (ASI mean±S.D.: O1821=38.8±6.3 and DMSO control vehicle=53.2±9.1; P=0.02, t-test).
Figure 4. Trpv2 agonists significantly reduced clinical arthritis inflammation and severity in vivo.
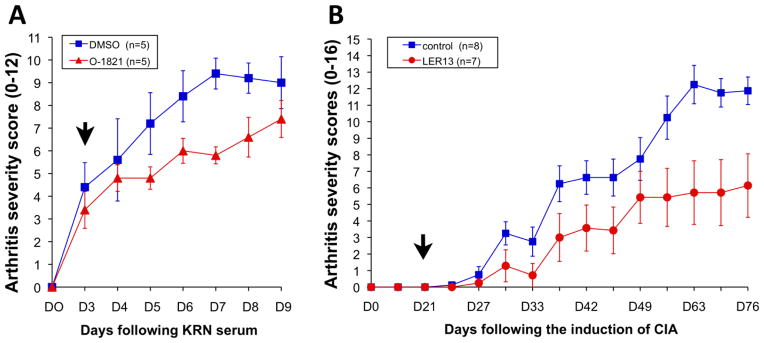
(A) C57BL/6 mice were injected with KRN arthritogenic serum. O1821 (10mg/Kg BID IP) was started after the onset of arthritis (day 3; D3, black arrow) and continued through day nine (D9). O1821-treated mice had reductions in arthritis severity scores that were evident as early as day five (D5) and reached statistical significance on days seven and eight (* D6: P=0.091; # D7: P=0.002; ¶ D8: P=0.045; mean±SEM ; t-test). (B) DBA1/J mice with collagen-induced arthritis (CIA) treated with LER13 (n=8 per treatment group) (treatment started on D10 following the first immunization, black arrow) had a significant reduction in arthritis severity and clinical signs of inflammation compared with the vehicle group (figure 6B). CIA onset is typically around D21 following immunization, and the disease-protecting effect was detected by day 31 (D31) and persisted through the end of the arthritis scoring period at day 76 (D76), with a reduction of 50% in mean cumulative arthritis scores (arthritis severity index, ASI; mean±S.D.: control=80±21.8 and LER18 40.7±36.8; p=0.023, t-test), compared with control vehicle.
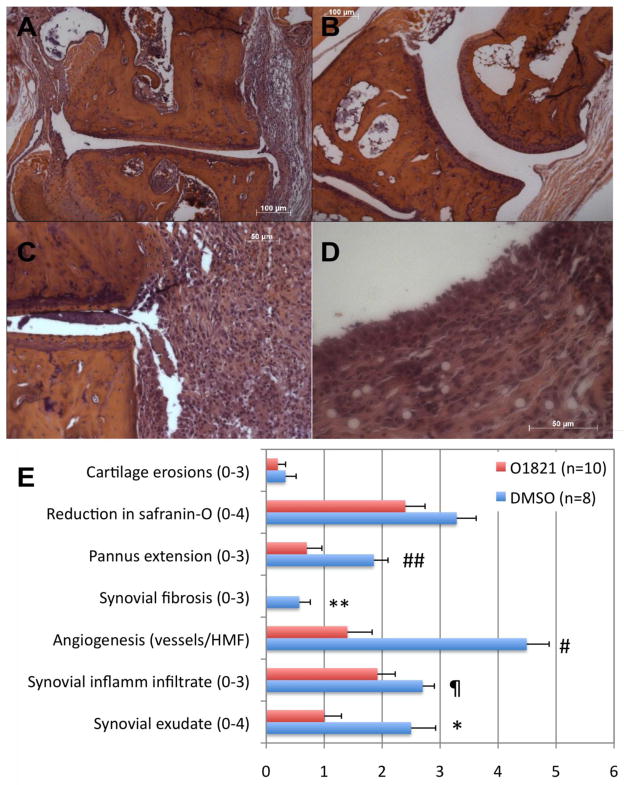
O1821 was studied in the KRN serum transfer model over a short period (nine days), but that was enough time to detect reduced synovial pannus formation, reduced synovial fibrosis and preservation of cartilage and proteoglycan staining (Safranin-O), compared with control vehicle (figure 5 panels A–E). O1821-treated mice also had reduced synovial tissue inflammatory infiltration, reduced synovial fluid exudates, and marked reduction in the number of vessels (figure 5B and 5E). These observations suggest that in addition to regulating the invasive properties of FLS and MMP expression, Trpv2 may be involved in the regulation of both angiogenesis and the influx of inflammatory cells into the synovial tissues. These are new discoveries as Trpv2 had not been previously implicated in these processes.
Figure 5. Arthritic mice treated with a Trpv2 agonist preserved a normal joint and synovial architecture.
(A) C57BL/6 mice with KRN serum transfer-induced arthritis treated with control vehicle developed pronounced ankle synovial hyperplasia and pannus formation. (B) Mice treated with O1821 had normal ankle joint and synovial histology with no pannus formation, and reduced inflammatory infiltration. (C and D) The synovial tissues from vehicle-treated mice had pronounced inflammation and hyperplasia (H&E staining). (E) At the end of the arthritis study (D10) both ankles (thus the higher sample size) were collected and prepared for histology (H&E and Safranin-O for proteoglycan analyses). Slides were scored without knowledge of treatment group (blindly), using a comprehensive histologic scoring system [18]. Treatment with O-1821 reduced several histologic parameters, including synovial fluid exudate (* P=0.008), angiogenesis (# P=0.00032), inflammatory infiltration (¶ P=0.06), pannus formation (## P=0.0082) and synovial fibrosis (** P=0.003).
3.10. A new and more potent Trpv2 agonist, LER13, significantly reduces FLS invasion, and disease severity in CIA
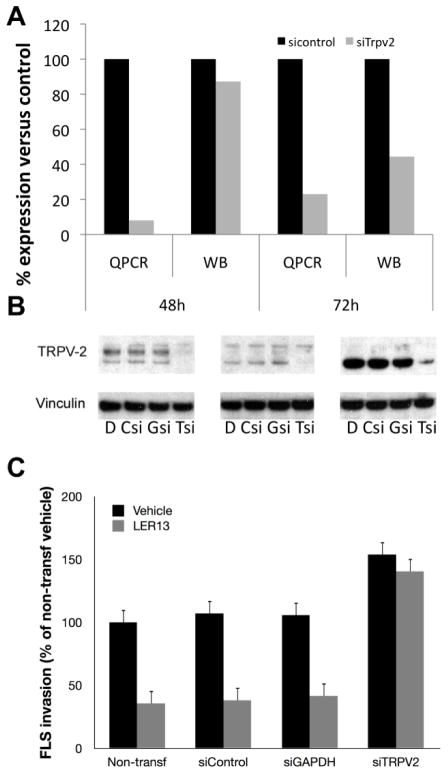
We synthesized and screened a focused library around O1821 for new Trpv2 agonists (Supplemental table 1). These compounds were tested for their ability to increase intracellular calcium influx. The five most potent compounds were then tested for their ability to suppress FLS invasion in vitro, and arthritis in vivo. LER13 was the most promising of these compounds and induced a nearly 30% higher calcium influx in FLS, compared with O1821 (Supplemental figure 1B), and significantly reduced FLS invasion by 65% both in cells from a mouse with KRN serum-induced arthritis (figure 2C) and rats with PIA (figure 6C). siRNA knock-down of Trpv2 (figure 6A–B) rendered FLS non-responsive to the invasion-suppressive effect of LER13 (figure 6C) demonstrating that the compound functions in a Trpv2-dependent manner. In fact, Trpv2 knock-down increased FLS invasiveness by nearly 60%, further supporting a role for this gene in invasion suppression (figure 6C).
Figure 6. LER13 suppresses FLS invasion in a Trpv2-dependent manner.
(A) siRNA targeting Trpv2 achieved an 80% reduction in mRNA levels and 50% reduction in protein levels 72 hours after transfection (shown as percentage of the control; QPCR=quantitative PCR and WB=Western blot, where levels were normalized for vinculin densitometries). (B) Western blot 72h after Trpv2 siRNA knockdown showing a 50% or greater reduction in protein levels (D=DMSO control; Csi=control siRNA; Gsi=GAPDH siRNA; Tsi=TRPV2 siRNA; each blot was done with FLS from a different DA rat). (C) LER13 10μM suppresses FLS invasion by 60% in non-transfected (non-transf), siRNA control (siControl) and in siRNA GAPDH (siGAPDH), but not in siRNA Trpv2 (siTRPV2) demonstrating that LER13 invasion-suppressive activity is dependent on the expression of Trpv2.
Treatment with LER13 significantly reduced arthritis severity and clinical signs of inflammation in DBA1/J mice with CIA (eight mice per group; figure 4B). The disease-protecting effect was detected by day 31 (D31) following the induction of CIA, and persisted though the end of the arthritis scoring period at day 76 (D76), with a reduction of 50% in mean cumulative arthritis scores, compared with control vehicle (arthritis severity index, ASI; mean±S.D.: control=80±21.8 and LER18 40.7±36.8; p=0.023, t-test; figure 4B). Maximum arthritis severity scores per mouse were also reduced by nearly 50% in LER13-treated mice compared with vehicle (mean±SD: LER13=7±4.2; vehicle control 13±2.44, p=0.004, t-test).
We also examined the number of joints with the maximum score (maximum score was 4 per paw per day and included avoidance of weight-bearing as a functional/disability component) during the 76 days of arthritis scoring as an estimate of the effect of LER13 at preventing the most severe and potentially disabling form of disease. The difference between vehicle-treated control and LER13 was significant at days 63 and 70 (D63: control=16 versus LER13=5, p=0.014; D70: control=12 versus LER13=1, p=0.001, Fisher’s exact test).
The pronounced arthritis severity-improving effect observed with LER13 10 mg every-other-day in CIA, as opposed to O1821 10 mg BID in vivo (four-fold difference in daily dose) suggests that LER13 is more potent and has a longer half-life.
4. DISCUSSION
While new and more effective therapies have been developed to treat RA, disease remission remains uncommon, and most patients only achieve modest to moderate reduction in disease activity. These observations underscore the need for more effective therapies. We have focused our efforts on the identification and characterization of genes implicated in disease severity and joint damage as potential new targets for therapy. In a combination of genetic studies in rodent models of arthritis and functional and gene expression studies using FLS we discovered a new role for the non-specific cation channel Trpv2 in the regulation of arthritis. Our initial observation of increased expression of Trpv2 in highly invasive FLS, compared with minimally invasive FLS, suggested that the expression of this channel might favor FLS invasion and joint damage. However, experiments with Trpv2 agonists established that this cation channel is in fact a suppressor of FLS invasion that reduces the expression of MMP-2 and MMP-3, two key proteases implicated in joint damage in RA and rodent models of arthritis. Knock-down of Trpv2 with siRNA also increased FLS invasion, further supporting its role in suppressing invasion.
The in vitro invasive properties of FLS have been shown to correlate with radiographic damage in RA, and with histologic damage and erosions in rodent models of arthritis [6, 7]. This in vitro model of invasion is also a highly useful screening method to identify relevant pathways and cellular processes for therapeutic targeting [8, 34–38]. Therefore, we initially used O1821, a commercially-available Trpv2-specific agonist. O1821 was effective at reducing invasiveness of FLS from patients with RA and FLS from DA rats with PIA by over 90%. O1821 was also effective at reducing mouse FLS invasion and clinical disease severity and histologic joint damage and pannus formation in vivo in the KRN serum transfer model where therapy was started after the onset of arthritis, thus mimicking the RA clinical setting.
While O1821 reduced disease severity in vivo, it required high-doses to obtain a modest effect. Therefore, we aimed at developing a new and more potent Trpv2 agonist. Twenty-one new compounds were developed and screened for Trpv2 agonistic activity based on their ability to increase calcium influx and inhibit FLS invasion. Based on those screening parameter five different compounds were selected and tested in vivo in CIA in mice, a chronic and well-established classic model of RA. One of these five compounds, LER13 was significantly effective and reduced arthritis severity scores, number of joints with functional impairment, and the maximum arthritis scores by 50%. Unlike the suppressive effect of O-1821 administration at 10mg/Kg BID, when administered at 10mg every-other-day this compound was not protective (data not shown), suggesting that LER13 is at least four times more potent, and/or has a longer half-life. Our studies are the first to use Trpv2 agonists in vivo, and we observed no evidence of significant toxicities, mortality, infections or tumors, suggesting that these might be a promising new category of therapeutic agents.
To understand the processes regulated by Trpv2 in arthritis severity and FLS invasion we examined several parameters, including FLS morphology, the actin cytoskeleton and the formation of lamellipodia, but none of those were affected by Trpv2 stimulation. However, the FLS expression of MMP-2 and MMP-3, two key mediators of invasion and joint damage, was significantly reduced by Trpv2 stimulation. These observations suggest that Trpv2 does not necessarily interferes with the FLS ability to move but may instead affect invasion and joint damage via interference with an effector pathway such as MMP expression or activation. Our discoveries raise the possibility that Trpv2 agonists such as O1821 and LER13 may be useful to treat not only arthritis, but also other diseases such as cancers where cell invasion has a central role in pathogenesis and outcome.
We considered that Trpv2 could be inhibiting MMP expression via interference with a signaling pathway implicated on MMP transcription or in cell invasion. Akt, Erk and NFκB were obvious candidates since they have been implicated in the regulation of cell invasion [39], and arthritis severity and joint damage [40]. Akt and Erk were previously shown to mediate Trpv2 activity in experiments unrelated to invasion with endothelial [21] and glioma cells [32]. Furthermore, the ankyrin domains in Trpv2 could potentially interact and interfere with NFκB activity [33]. However, we detected no significant difference or reduction on levels of activation of these signaling proteins. Several other signaling pathways could be involved in mediating the Trpv2-induced suppressive effect on FLS invasion and arthritis severity [8, 24, 41–47], and will be the subject of future work in our laboratory.
Trpv2 stimulation also reduced the numbers of synovial-infiltrating inflammatory cells and numbers of synovial vessels. These observations suggest that Trpv2 could interfere with the ability of inflammatory cells or endothelial cell precursors to infiltrate the synovial tissue via a direct effect or through the suppression of chemotactic and angiogenic factors. Therefore, Trpv2 agonists may have a future role in the treatment of other inflammatory and autoimmune diseases, as well as in diseases characterized by pronounced angiogenesis.
5. CONCLUSION
We have identified a new role for Trpv2 in the regulation of arthritis severity, pannus formation, FLS invasion and joint damage. Our observations also suggest a new role for Trpv2 in the regulation of angiogenesis. These new discoveries suggest that the use of Trpv2 agonists has the potential to become a novel strategy and a new class of drugs in the treatment of RA, and perhaps other diseases such as cancers where cell invasion and angiogenesis have a central role in pathogenesis.
Supplementary Material
HIGHLIGHTS.
The cation channel TRPV2 is expressed in synovial fibroblasts.
TRPV2 agonists, including the new LER13, reduce the invasiveness of synovial fibroblasts.
TRPV2 agonists reduce disease severity in two well-established models of rheumatoid arthritis.
TRPV2 agonists reduced joint damage, synovial inflammation and synovial angiogenesis in models of rheumatoid arthritis.
This is the first time that TRPV2 agonists are used in vivo, and there was no evidence of toxicity.
Acknowledgments
Funded by the National Institutes of Health grants R01-AR46213, R01-AR052439 (NIAMS) and R01-AI54348 (NIAID) to Percio S. Gulko, and T32 GM088129 training grant to Mark R. Tanner.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simonsson M, Bergman S, Jacobsson LT, Petersson IF, Svensson B. The prevalence of rheumatoid arthritis in Sweden. Scand J Rheumatol. 1999;28(6):340–343. doi: 10.1080/03009749950155319. [DOI] [PubMed] [Google Scholar]
- 2.van Zeben D, Breedveld FC. Prognostic factors in rheumatoid arthritis. J Rheumatol Suppl. 1996;44:31–33. [PubMed] [Google Scholar]
- 3.Gossec L, Dougados M, Goupille P, Cantagrel A, Sibilia J, Meyer O, Sany J, Daures JP, Combe B. Prognostic factors for remission in early rheumatoid arthritis: a multiparameter prospective study. Ann Rheum Dis. 2004;63(6):675–680. doi: 10.1136/ard.2003.010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37(4):481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 5.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, Breedveld FC, Toes RE, Huizinga TW. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52(7):1999–2002. doi: 10.1002/art.21118. [DOI] [PubMed] [Google Scholar]
- 7.Laragione T, Brenner M, Mello A, Symons M, Gulko PS. The arthritis severity locus Cia5d is a novel genetic regulator of the invasive properties of synovial fibroblasts. Arthritis Rheum. 2008;58(8):2296–2306. doi: 10.1002/art.23610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med. 2010;16(9–10):352–358. doi: 10.2119/molmed.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulko PS. Contribution of genetic studies in rodent models of autoimmune arthritis to understanding and treatment of rheumatoid arthritis. Genes Immun. 2007;8(7):523–531. doi: 10.1038/sj.gene.6364419. [DOI] [PubMed] [Google Scholar]
- 10.Laragione T, Brenner M, Li W, Gulko PS. Cia5d regulates a new fibroblast-like synoviocyte invasion-associated gene expression signature. Arthritis Res Ther. 2008;10(4):R92. doi: 10.1186/ar2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler A, Aeschlimann A, Simmen BR, Michel BA, Gay RE, Gay S, Sprott H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem Biophys Res Commun. 2007;359(4):884–888. doi: 10.1016/j.bbrc.2007.05.178. [DOI] [PubMed] [Google Scholar]
- 12.Kochukov MY, McNearney TA, Fu Y, Westlund KN. Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am J Physiol Cell Physiol. 2006;291(3):C424–432. doi: 10.1152/ajpcell.00553.2005. [DOI] [PubMed] [Google Scholar]
- 13.Ciurtin C, Majeed Y, Naylor J, Sukumar P, English AA, Emery P, Beech DJ. TRPM3 channel stimulated by pregnenolone sulphate in synovial fibroblasts and negatively coupled to hyaluronan. BMC Musculoskelet Disord. 2010;11:111. doi: 10.1186/1471-2474-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, et al. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451(7174):69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 16.Link TM, Park U, Vonakis BM, Raben DM, Soloski MJ, Caterina MJ. TRPV2 has a pivotal role in macrophage particle binding and phagocytosis. Nat Immunol. 2010;11 (3):232–239. doi: 10.1038/ni.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vingsbo C, Sahlstrand P, Brun JG, Jonsson R, Saxne T, Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149(5):1675–1683. [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner M, Meng H, Yarlett N, Griffiths M, Remmers E, Wilder R, Gulko P. The non-MHC quantitative trait locus Cia10 contains a major arthritis gene and regulates disease severity, pannus formation and joint damage. Arthritis Rheum. 2005;52 (1):322–332. doi: 10.1002/art.20782. [DOI] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31 (3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28(24):6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63(3):699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Laragione T, Brenner M, Sherry B, Gulko PS. CXCL10 and its receptor CXCR3 regulate synovial fibroblast invasion in rheumatoid arthritis. Arthritis Rheum. 2011;63 (11):3274–3283. doi: 10.1002/art.30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan A, Akhtar M, Brenner M, Zheng Y, Gulko PS, Symons M. The GTPase Rac Regulates the Proliferation and Invasion of Fibroblast-Like Synoviocytes from Rheumatoid Arthritis Patients. Mol Med. 2007;13(5–6):297–304. doi: 10.2119/2007-00025.Chan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji H, Gauguier D, Ohmura K, Gonzalez A, Duchatelle V, Danoy P, Garchon HJ, Degott C, Lathrop M, Benoist C, et al. Genetic influences on the end-stage effector phase of arthritis. J Exp Med. 2001;194(3):321–330. doi: 10.1084/jem.194.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jirholt J, Cook A, Emahazion T, Sundvall M, Jansson L, Nordquist N, Pettersson U, Holmdahl R. Genetic linkage analysis of collagen-induced arthritis in the mouse. Eur J Immunol. 1998;28(10):3321–3328. doi: 10.1002/(SICI)1521-4141(199810)28:10<3321::AID-IMMU3321>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 27.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 28.Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW, Thorbecke GJ. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci U S A. 1986;83(19):7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner M, Meng HC, Yarlett NC, Joe B, Griffiths MM, Remmers EF, Wilder RL, Gulko PS. The Non-MHC Quantitative Trait Locus Cia5 Contains Three Major Arthritis Genes That Differentially Regulate Disease Severity, Pannus Formation, and Joint Damage in Collagen- and Pristane-Induced Arthritis. J Immunol. 2005;174(12):7894–7903. doi: 10.4049/jimmunol.174.12.7894. [DOI] [PubMed] [Google Scholar]
- 30.Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J Neurosci. 2010;30(49):16536–16544. doi: 10.1523/JNEUROSCI.4426-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwata Y, Katanosaka Y, Arai Y, Shigekawa M, Wakabayashi S. Dominant-negative inhibition of Ca2+ influx via TRPV2 ameliorates muscular dystrophy in animal models. Hum Mol Genet. 2009;18(5):824–834. doi: 10.1093/hmg/ddn408. [DOI] [PubMed] [Google Scholar]
- 32.Nabissi M, Morelli MB, Amantini C, Farfariello V, Ricci-Vitiani L, Caprodossi S, Arcella A, Santoni M, Giangaspero F, De Maria R, et al. TRPV2 channel negatively controls glioma cell proliferation and resistance to Fas-induced apoptosis in ERK-dependent manner. Carcinogenesis. 2010;31(5):794–803. doi: 10.1093/carcin/bgq019. [DOI] [PubMed] [Google Scholar]
- 33.McCleverty CJ, Koesema E, Patapoutian A, Lesley SA, Kreusch A. Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Sci. 2006;15(9):2201–2206. doi: 10.1110/ps.062357206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson RP, Hartman DA, Tomchek LA, Walter TL, Lugay JR, Calhoun W, Sehgal SN, Chang JY. Rapamycin, a potential disease-modifying antiarthritic drug. J Pharmacol Exp Ther. 1993;266(2):1125–1138. [PubMed] [Google Scholar]
- 35.Mohan K, Issekutz TB. Blockade of chemokine receptor CXCR3 inhibits T cell recruitment to inflamed joints and decreases the severity of adjuvant arthritis. J Immunol. 2007;179(12):8463–8469. doi: 10.4049/jimmunol.179.12.8463. [DOI] [PubMed] [Google Scholar]
- 36.Cejka D, Hayer S, Niederreiter B, Sieghart W, Fuereder T, Zwerina J, Schett G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010;62(8):2294–2302. doi: 10.1002/art.27504. [DOI] [PubMed] [Google Scholar]
- 37.Bruyn GA, Tate G, Caeiro F, Maldonado-Cocco J, Westhovens R, Tannenbaum H, Bell M, Forre O, Bjorneboe O, Tak PP, et al. Everolimus in patients with rheumatoid arthritis receiving concomitant methotrexate: a 3-month, double-blind, randomised, placebo-controlled, parallel-group, proof-of-concept study. Ann Rheum Dis. 2008;67(8):1090–1095. doi: 10.1136/ard.2007.078808. [DOI] [PubMed] [Google Scholar]
- 38.Yellin M, Paliienko I, Balanescu A, Ter-Vartanian S, Tseluyko V, Xu LA, Tao X, Cardarelli PM, Leblanc H, Nichol G, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64(6):1730–1739. doi: 10.1002/art.34330. [DOI] [PubMed] [Google Scholar]
- 39.Tobar N, Villar V, Santibanez JF. ROS-NFkappaB mediates TGF-beta1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol Cell Biochem. 2010;340(1–2):195–202. doi: 10.1007/s11010-010-0418-5. [DOI] [PubMed] [Google Scholar]
- 40.Miagkov AV, Kovalenko DV, Brown CE, Didsbury JR, Cogswell JP, Stimpson SA, Baldwin AS, Makarov SS. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci U S A. 1998;95 (23):13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin MT, Lin BR, Chang CC, Chu CY, Su HJ, Chen ST, Jeng YM, Kuo ML. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer. 2007;120(12):2600–2608. doi: 10.1002/ijc.22599. [DOI] [PubMed] [Google Scholar]
- 42.Li H, Huang C, Huang K, Wu W, Jiang T, Cao J, Feng Z, Qiu Z. STAT3 knockdown reduces pancreatic cancer cell invasiveness and matrix metalloproteinase-7 expression in nude mice. PLoS One. 2011;6(10):e25941. doi: 10.1371/journal.pone.0025941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price LS, Collard JG. Regulation of the cytoskeleton by Rho-family GTPases: implications for tumour cell invasion. Semin Cancer Biol. 2001;11(2):167–173. doi: 10.1006/scbi.2000.0367. [DOI] [PubMed] [Google Scholar]
- 44.Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23(9):1415–1423. doi: 10.1016/j.cellsig.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikuchi K, Li X, Zheng Y, Takano Y. Invasion of breast cancer cells into collagen matrix requires TGF-alpha and Cdc42 signaling. FEBS Lett. 2011;585(2):286–290. doi: 10.1016/j.febslet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 47.Leivonen SK, Kahari VM. Transforming growth factor-beta signaling in cancer invasion and metastasis. Int J Cancer. 2007;121(10):2119–2124. doi: 10.1002/ijc.23113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.