Abstract
Despite undisputable benefits, conventional pacemaker therapy is associated with specific complications related to the subcutaneous device and the transvenous leads. Recently, two miniaturized leadless pacemakers, Nanostim™ (St. Jude Medical) and Micra™ (Medtronic), which can be completely implanted inside the right ventricle using steerable delivery systems, entered clinical application. The WiCS™-cardiac resynchronisation therapy (CRT) system (wireless cardiac stimulation for CRT, EBR Systems) delivers leadless left ventricular endocardial stimulation for cardiac resynchronization. In addition to obvious cosmetic benefits, leadless pacing systems may have the potential to overcome some complications of conventional pacing. However, acute and long-term complications still remains to be determined, as well as the feasibility of device explantation years after device placement.
Keywords: Pacemaker, Leadless pacing, Catheter delivery
Introduction
Since the first report on the successful use of an external cardiac pacemaker system by Alber S. Hyman in 1932, the first complete implantation of an epicardial pacing system by Rune Elmquist and Åke Senning in 1958, and the implantation of a transvenous temporary pacing lead the same year by Seymour Furman, pacemaker therapy has evolved considerably. Advances in battery and circuit technology have led to programmable multichamber pacemakers with a lifespan of 7–10 years. Conventional subcutaneous pacemaker implantation and transvenous lead placement requires a surgical procedure. Despite the technological strides made over the last decades, conventional pacemaker treatment continues to be associated with complications. Immediate post-operative adverse events occur in ∼10% of patients and are either related to the device (haematoma, skin erosion, pocket infection) or result from transvenous lead placement (pneumothorax, cardiac perforation, lead dislodgement).1
The leads are the most vulnerable component of the system, because they can fracture, develop insulation defects, connector issues, or become infected. Due to steadily increasing numbers of implantations and the higher life expectancy of patients, it is to be expected that the total number of long-term complications will markedly increase.
As early as 1970, miniaturized batteries and electronic circuits led to the development of concepts of pacemakers that could be completely implanted inside the right ventricle (RV).2,3 Size, battery longevity, and secure fixation were the key issues. Various shapes and battery types, including nuclear batteries, were tested. Despite the remarkable technological advances at that time, intracardiac pacemakers did not make it into clinical practice. Subsequently, various concepts aiming to eliminate the need for pacemaker leads were evaluated. Gene and stem-cell therapy for treating cardiac rhythm and conduction disorders have been studied in the experimental setting. However, these biological pacemakers are still in their early phases of development and have not yet been tested in humans.4 Leadless devices were usually based on external wireless energy transmission to an intracardiac receiver, i.e. they could not be completely implanted in the heart.5–7
A completely implantable system into the RV) became reality in 2012 when the Nanostim™ Leadless Pacemaker System (St. Jude Medical, Sylmar, CA, USA) first became available. One year later, the Micra™ Transcatheter Pacing System (Medtronic, Minneapolis, MN, USA) was introduced. The currently available completely intracardiac pacemaker systems are suitable for patients who have an indication for VVIR pacing. In addition to the cosmetic advantage, the leadless design and lack of a surgically created pocket eliminate the main sources of complications associated with conventional pacemaker implantation.
A different concept of leadless pacing is pursued by the ultrasound-based WiCS™ system (Wireless Cardiac Stimulation; EBR Systems, Sunnyvale, CA, USA). Cardiac resynchronisation therapy (CRT) is still associated with severe limitations, primarily a persistent 30–40% non-responder rate and a rate of up to 10% of chronic left ventricular (LV) lead failure.8–12 Targeting of optimal LV pacing sites is limited by the anatomy of the coronary sinus tributaries. Left ventricular endocardial pacing is another approach, aiming for better clinical results with CRT by improving hemodynamics by optimizing the LV pacing sites.13–15 Historically, LV endocardial pacing has been accomplished via an atrial transseptal approach16,17 and more recently via puncture of the interventricular septum.18 Both techniques are limited by procedural issues and the long-term risk of thromboembolic events related to the lead permanently residing inside the LV. The WiCS™ system is intended to provide an alternative by pacing LV endocardially for CRT with a device receiving its energy from a subcutaneous ultrasound transmitter.19 This system is potentially suitable for patients requiring CRT especially if conventional LV lead placement failed.
In this review, we summarize and discuss technological and clinical aspects of the three systems mentioned above.
Technological aspects of leadless pacing
Leadless systems for right ventricle pacing
There are two leadless pacing systems currently available, which can be completely implanted in the right ventricle: the Nanostim™ leadless cardiac pacemaker (LCP) (Figure 1) and the Micra™ transcatheter pacing system (TPS) (Figure 2).
Figure 1.
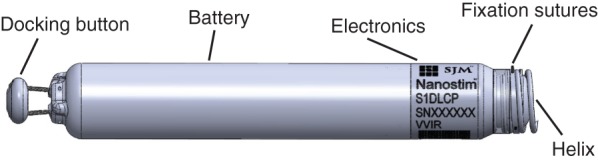
The St. Jude Medical Nanostim™ leadless pacemaker (reproduced with permission from St. Jude Medical Inc.).
Figure 2.

The Medtronic Micra™ Transcatheter Pacing System (reproduced with permission from Medtronic Inc.).
Characteristics of both systems are compared in Table 1.
Table 1.
Specifications of completely implantable cardiac pacemakers
| Specificationms | Nanostim™ leadless cardiac pacemaker | Micra™ transcatheter pacing system |
|---|---|---|
| Volume (cm3) | 1 | 0.8 |
| Length (mm) | 41.4 | 25.9 |
| Weight (g) | 2 | 2 |
| Introducer size (French) | 18 | 23 |
| Primary fixation mechanism | Screw-in helix | Self-expanding nitinol tines |
| Secondary fixation mechanism | Nylon tines | |
| Pacing mode | VVI/VVIR | VVI/VVIR |
| Rate response sensor | Temperature | Accelerometer |
| Energy supply | Integrated battery | Integrated battery |
| Battery | Lithium carbon-monofluoride | Lithium silver vanadium oxide/carbon monoflouride |
| Battery longevity (years) | 9.8 100%/2.5 V/0.4 ms/60b.p.m. |
10 100%/1.5 V/0.24 ms/60 b.p.m. |
| Device retrieval option | Yes | Yes |
| Telemetry | Conductive | Radio frequency |
Both systems offer pacing features similar to conventional VVIR pacemakers including rate response algorithms. Moreover, a steroid-eluting tip is incorporated to reduce inflammation. Although these devices are significantly smaller than conventional pacing systems, the predicted longevities are ∼10 years. This is comparable to the longevity of a standard pacemaker and is made possible by a design optimizing both energy consumption and the electrode/tissue interface. The proximal end of the devices incorporate a mechanism for recapturing the systems if repositioning becomes necessary after implantation or if devices need to be retrieved. To date no data exist for the removal of chronic implanted systems. In the event of battery depletion, removal of the leadless pacemaker may be unnecessary, as an option may be to simply implant an additional device. How this will affect cardiac function, and how many additional devices may be implanted, remains however to be determined.
Main differences between the systems are related to the fixation and programming of the devices.
The primary fixation mechanism of the LCP device is a screw-in helix with a maximum penetration depth in tissue of 1.3 mm. Three nylon tines provide a secondary fixation mechanism by avoiding unscrewing of the helix. The tip electrode is a steroid-eluting disc located at the centre of the fixating helix. The ring electrode is the uncoated part of the titanium pacemaker case. A dedicated programmer connected to the Merlin™ patient care system communicates with the device using conducted communication and displays the intracardiac electrogram and the status of the implanted device; it is also used for programming the parameter settings. Signal transmission from the programmer to the implanted LCP is accomplished by applying subliminal 250 kHz pulses to skin leads. The programmer automatically selects the optimal pair of skin leads for communication with the LCP. Except for this special type of signal transmission, the programmer uses the same operating principles as conventional pacemaker programmers.
The TPS is currently the smallest standalone system available. The fixation mechanism comprises four flexible self-expanding nitinol tines, which hook into the myocardium and anchor the device inside the right ventricle. The tines are electrically inactive. A steroid-eluting cathode located at the distal end of the device delivers the pacing current, a titanium nitride band on the casing acts as the anode. Thresholds are measured automatically on a daily basis, with hourly checks of the capture safety margin. These checks do not require loss of capture. Rate–response is provided by an accelerometer that filters out cardiac motion with a bandpass filter. Monitoring and diagnostic features are available, including rate histograms, pacing counters, evolution of sensing amplitudes, capture thresholds, and pacing impedances. Follow-up and programming are performed using the standard Medtronic Model 2090 Programmer, similar to standard Medtronic pacemakers.
Leadless system for left ventricle pacing
The leadless ultrasound-based technology used by the WiCS™ (Wireless Cardiac Stimulation; EBR Systems) system is designed to achieve LV endocardial pacing. Leadless ultrasound pacing is accomplished by transmitting ultrasound energy from a subcutaneous transmitter unit to an endocardial receiver unit. Fixation to the endocardium is achieved by three self-expanding nitinol tines mounted at the distal end of the device. The whole CRT system comprises (i) the LV endocardial unit, which receives ultrasound energy and converts it to electrical energy for LV pacing, (ii) the subcutaneously implanted pulse generator and (iii) a conventional pacing device. The subcutaneously implanted pulse generator comprises the battery and the transmitter, connected by a cable. The transmitter includes an ultrasound transducer array, the energy of which can be focused for effective transmission to the LV endocardial unit. The system detects RV stimulation provided by a co-implanted pacemaker, CRT or implantable cardiac defibrillator (ICD) device, and applies a synchronized LV stimulus after a 3 ms delay.
Implantation of leadless pacemakers
Nanostim™ leadless cardiac pacemaker and Micra™ transcatheter pacing system implantation procedure
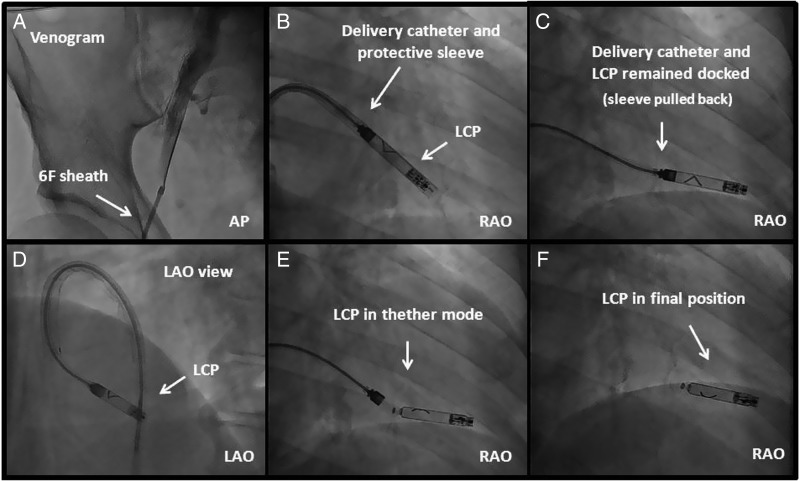
For both systems, the intervention can be performed in the cathlab using a fluoroscopic imaging system and local anaesthesia. After puncture of the femoral vein the delivery system with the pacemaker mounted on a deflectable tip is advanced under fluoroscopic guidance through the inferior vena cava to the right atrium. From there the system is advanced to the RV via the tricuspid valve using the deflectable delivery system. The LCP is implanted into the endocardium in the apico-septal area by rotating the screw-in helix with 1.25 turns. For fixation of the TPS at the endocardium of the target region, the tines are deployed by retracting a protective outer sheath. After fixation, the pacemaker is undocked from the delivery system but still connected via tethers. This allows the measurement of pacing threshold, Sensing amplitude and impedance without applying force on the device. In the case of inappropriate electrical values, the pacemaker can be repositioned. Adequate fixation is confirmed for both systems by performing the so called tug test via the delivery system under fluoroscopic visualization. If electrical performance and stability of the device is confirmed, it may be finally released. Figure 3 shows as an example the implant steps for the LCP.
Figure 3.
Leadless cardiac pacemaker implantation steps. (A) A venogram may optionally be performed; (B) The LCP is positioned into the RV by deflecting the catheter and placed ∼0.5–1 cm from the RV apex; (C and D) Protective cover is pulled back to expose the flexible part of the catheter; (E) The pacemaker is undocked from the delivery catheter while a tethered connection is maintained. In case the position is suboptimal, the LCP can be reengaged, unscrewed, and repositioned. (F) The LCP is released by rotating the release knob of the catheter.
WiCS™ implant procedure
Individual assessment of potential acoustic windows is performed preoperatively by standard echocardiography to determine the best subcutaneous position of the transmitter. An optimal acoustic path should be free of lung and bone tissue and provide an unobstructed pathway from the transducer to the heart. The best acoustic window, which is known to vary with patient movement and respiration, is usually found in the 4th, 5th, and/or 6th intercostal space lateral of the left parasternal border.20 The receiver/pulse generator is delivered retrogradely across the aortic valve to the desired endocardial pacing site of the left ventricle. Fixation to the endocardium is achieved with three self-expanding nitinol tines mounted at the distal end of the device.
Scientific data
Data for Nanostim™ leadless cardiac pacemaker
The LEADLESS trial21 was a prospective, multicentre, non-randomized trial conducted at three European centres. Patients were enrolled from December 2012 to April 2013. The main inclusion criteria were age >18 years and a clinical indication for single-chamber VVIR pacing. Patients were excluded if they were pacemaker dependent, had a mechanical tricuspid valve prosthesis, pulmonary hypertension, previously implanted pacemaker/defibrillator leads, or an inferior vena cava filter. After implantation of a LCP system, follow-up was performed pre-discharge and at 2, 6, and 12 weeks post-implant. The primary endpoint was freedom from serious adverse device events (SADE) at 90 days. Secondary endpoints included implant success rate and pacemaker performance characteristics (e.g. pacing thresholds, battery voltage, R-wave amplitude). A total of 33 patients (mean age 77 ± 8 years, 67% male) received an LCP system. Implant success rate was 97% (n = 32), and the majority of patients (n = 23, 70%) did not require repositioning of the LCP after its initial deployment. The overall complication-free rate was 94% (31/33). There were two SADE. A 70-year-old male experienced cardiac tamponade with hemodynamic collapse during LCP implantation. Although emergency surgery was successful and the patient was recovering, he suffered a middle cerebral artery ischaemic stroke on post-procedure Day 5 and expired on post-procedure day eighteen. The second SADE was due to inadvertent placement of the LCP in the left ventricle through a persistent foramen ovale. The device was retrieved and a second device was successfully placed in the right ventricle during the same procedure. Three patients (9%) were re-hospitalized; one patient because of an elevated international normalized ratio, one for acute exacerbation of chronic obstructive lung disease, and one patient for syncope due to monomorphic ventricular tachycardia originating from a small scar in the left ventricle. In the latter patient, the LCP was retrieved and a transvenous ICD was placed. The mean R-wave amplitude, pacing threshold and impedance were 8.3 mV, 0.80 V/0.4 ms, 773 Ohms at implantation, which improved to 9.7 mV, 0.41 V/0.4 ms, 719 Ohms at hospital discharge and remained stable until 12 weeks (10.7 mV, 046 V/0.4 ms, 627 Ohms). There were no reports of early battery depletion, or of any issues with sensing or capture.
These results support the use of the LCP as an alternative to conventional pacemaker systems. Continued evaluation is however warranted to further characterize this system and follow-up of the study cohort is ongoing. A limited market release is currently underway, and resumed after temporary interruption and repeated training of the implanting physicians, following two deaths due to tamponade.
Current data on chronic LCP device retrieval are limited to results from animal experiments.22 In this study, all devices implanted in 10 sheep could be explanted after 5 months without difficulties.
Data for Micra™ transcatheter pacing system
An initial feasibility study was performed in 16 sheep implanted with a wired capsule similar in design to the current device. The capsules were placed in the apex of the right ventricle via a transvenous route.23 Wires connected the capsules to standard pacemakers used for measuring thresholds. After 24 weeks, the average pacing threshold was 0.7 ± 0.3 V/ 0.2 ms. No dislodgments or other complications occurred. The good electrical parameters of the pacing system were confirmed in a similar study in a sheep model with a 6-week follow-up.24 In another report, the TPS was implanted in the right ventricular apex of 10 mini-pigs.25 Capture thresholds were good at the time of implantation and at the 12 week follow-up (0.58 ± 0.17 V/0.24 ms and 0.94 ± 0.46/ 0.24 ms, respectively). Extraction of the device was assessed in four sheep,26 each implanted with a TPS prototype equipped with a retrieval/extraction system at the proximal end. Prototype extraction tools (steerable sheaths and snares) were developed by Cook Medical (Bloomington, IN, USA). After 18 months, all four devices were successfully retrieved and had remained relatively free from encapsulation at their proximal ends. The flexible nitinol tines permitted extraction by simple traction. Histological analyses demonstrated only minimal damage to cardiac tissues. However, longer-term data are still required to determine whether the device can be safely removed several years after implantation.
Currently, the Micra clinical trial is enrolling patients, and the first human implantation was performed in December 2013. This single-arm multicentre global clinical trial will enrol up to 780 patients at ∼50 centres. The primary endpoints after 6 months will be safety (major complication-free rate) and efficacy (capture thresholds).
As part of the CE mark trial, the first four Micra™ TPS were successfully implanted at the RV apex of four patients (ages 74–83 years, 2 females, 2 males) whose left ventricular ejection fraction was preserved and who had an indication for VVIR pacing.27 All procedures were successful. The mean total procedure time was 47 min (range 37–73 min). The mean R-wave amplitude, pacing threshold and impedance at implantation were 11.98 mV, 0.41 V/0.24 ms, and 713 Ohms respectively. During the first month after implantation, no major complications occurred. Electrical parameters assessed at the 1 month follow-up remained stable or improved to a mean R-wave amplitude of 17.85 mV, a pacing threshold of 0.38 V/ 0.24 ms and an impedance of 770 Ohms, respectively.
Data for WiCS™
The WiSE-CRT study19 was a multicenter, prospective, non-comparative first-in-man study in heart failure patients with an indication for CRT. Primary objectives were the evaluation of safety (device-related complications at 24 h and at 1-month follow-up) and performance (effective biventricular pacing capture at 1 month) of the WiCS™ device. Secondary objectives included a 6-month analysis with preliminary efficacy endpoints. The study enrolled 17 patients (14 males, 12 with ischaemic cardiomyopathy), including seven after unsuccessful CS lead implantation, two who failed to benefit from conventional CRT, and eight with an indication for CRT who already had a pacemaker or an ICD. Mean baseline LV ejection fraction was 26%, mean QRS duration 176 ms, and mean NYHA class 3.1. Patients were found to have 1–3 (mean 2.4 ± 0.7) intercostal spaces with adequate acoustic windows. The 5th intercostal space was suitable in all patients.
The WiCS™ system was successfully implanted in 13 patients (76%). In four patients, no system was implanted due to procedure and/or device-related pericardial effusions in three patients and because of difficult system manoeuverability in one patient. Based on recommendations given by the independent Clinical Events Committee, the sponsor discontinued the study on 16 March 2012.
The primary 1-month performance endpoint of effective biventricular pacing was documented in 10 of the 12 evaluable patients (83%). The secondary 6-months performance endpoint of effective biventricular pacing was achieved in 11 of the 12 evaluable patients (92%). Satisfactory performance meant statistically significant reduction of the QRS duration and increased LV ejection fraction in conjunction with clinical improvements including an improved NYHA functional class, better self-assessment, and a higher clinical composite score. These efficacy results were also observable in the subgroup of patients who had failed to benefit from conventional CRT.
The WiSE-CRT study successfully documented the feasibility of delivering LV endocardial-based CRT using wireless ultrasound energy transfer. Preliminary results showed clinical benefits similar to those observed during conventional atrio-biventricular pacing, even in patients in whom conventional CRT had failed or could not be initiated. Although this new technology remains very promising, the delivery system requires improvements to reduce the incidence of pericardial effusions. At a later time, further studies should re-evaluate the safety and performance of this novel approach to LV endocardial CRT.
Leadless pacing: current status and future perspectives
Despite all the enthusiasm generated by these novel and remarkable devices, it has to be stressed that the periprocedural risks associated with implantation require attention. For two out of the three systems, clinical trials have either been terminated or put temporarily on hold because of severe complications, including death. Cardiac perforation and tamponade seem to be issues that need to be addressed. Device design, especially as regards the fixation mechanism, should be carefully evaluated and if necessary adapted. A safe implantation technique that is applicable to a broad clinical setting is desirable. This will also require proper education and training of implanting physicians. Evaluation of these devices in clinical studies and registries will be necessary to judge the benefit-to-risk profile, especially in the long-term.
Future steps that may be taken are the development of leadless multichamber devices that communicate with each other. Such new devices would make leadless pacing suitable for a much larger population. Boston Scientific is currently developing a leadless pacemaker system, which may in the future be complementary to their subcutaneous ICD (e.g. for delivering antitachycardia or antibradycardia pacing, or for enhancing arrhythmia diagnosis). Research is currently underway for harvesting kinetic energy from cardiac motion to fuel pacemaker function (similarly to automatic watches). Intracardiac pacemakers may one day profit from such technology, thereby dispensing with the need for device replacement. Intravascular defibrillators are another next step, and initial results of an investigational device have recently been published.28
Conclusions
The miniaturization of pacemaker components has allowed the development of leadless pacemakers, and has marked a new era in device therapy. Many of the problems inherent to conventional pacing, such as pocket issues and lead dysfunction, may thus be avoided. In addition to the cosmetic advantage, the implantation procedure may be simplified, especially in the case of difficult venous access via the thoracic veins. Currently, the devices are limited to patients with an indication for single-chamber ventricular pacing. However, it is likely that wireless communication between several implanted devices (e.g. atrial and ventricular leadless pacemakers, or with a subcutaneous ICD), will open up new perspectives in this therapy. Nevertheless, further data need to be gathered regarding safety, long-term performance and extractability of these devices, in order to ensure that the therapy lives up to its promises.
Funding
Funding to pay the Open Access publication charges for this article was provided by St. Jude Medical.
Acknowledgements
This review was prepared by a writing group which was invited on behalf of the European Heart Rhythm Association Education Committee. This document was supported by a grant from St Jude Medical; however, the content has not been influenced in any way by its sponsor.
Conflict of interest: none declared.
References
- 1.Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J 2014;35:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spickler JW, Rasor NS, Kezdi P, Misra SN, Robins KE, LeBoeuf C. Totally self-contained intracardiac pacemaker. J Electrocardiol 1970;3:325–31. [DOI] [PubMed] [Google Scholar]
- 3.Vardas PE, Politopoulos E, Manios E, Parthenakis F, Tsagarakis C. A miniature pacemaker introduced intravenously and implanted endocardially. Preliminary findings form an experimental study. Eur J CPE 1991;1:27–30. [Google Scholar]
- 4.Chauveau S, Brink PR, Cohen IS. Stem cell-based biological pacemakers from proof of principle to therapy: a review. Cytotherapy 2014;16:873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Echt DS, Cowan MW, Riley RE, Brisken AF. Feasibility and safety of a novel technology for pacing without leads. Heart Rhythm 2006;3:1202–6. [DOI] [PubMed] [Google Scholar]
- 6.Lee KL, Lau CP, Tse HF, Echt DS, Heaven D, Smith W, et al. First human demonstration of cardiac stimulation with transcutaneous ultrasound energy delivery: implications for wireless pacing with implantable devices. J Am Coll Cardiol 2007;50:877–83. [DOI] [PubMed] [Google Scholar]
- 7.Wieneke H, Konorza T, Erbel R, Kisker E. Leadless pacing of the heart using induction technology: a feasibility study. Pacing Clin Electrophysiol 2009;32:177–83. [DOI] [PubMed] [Google Scholar]
- 8.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539. [DOI] [PubMed] [Google Scholar]
- 10.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 11.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385–95. [DOI] [PubMed] [Google Scholar]
- 12.Gras D, Böcker D, Lunati M, Wellens HJ, Calvert M, Freemantle N, et al. Implantation of cardiac resynchronization therapy systems in the CARE-HF trial: procedural success rate and safety. Europace 2007;9:516–22. [DOI] [PubMed] [Google Scholar]
- 13.Garrigue S, Jaïs P, Espil G, Labeque JN, Hocini M, Shah DC, et al. Comparison of chronic biventricular pacing between epicardial and endocardial left ventricular stimulation using Doppler tissue imaging in patients with heart failure. Am J Cardiol 2001;88:858–62. [DOI] [PubMed] [Google Scholar]
- 14.Spragg DD, Dong J, Fetics BJ, Helm R, Marine JE, Cheng A, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol 2010;56:774–81. [DOI] [PubMed] [Google Scholar]
- 15.Bordachar P, Grenz N, Jais P, Ritter P, Leclercq C, Morgan JM, et al. Left ventricular endocardial or triventricular pacing to optimize cardiac resynchronization therapy in a chronic canine model of ischemic heart failure. Am J Physiol Heart Circ Physiol 2012;303:H207–15. [DOI] [PubMed] [Google Scholar]
- 16.Bordachar P, Derval N, Ploux S, Garrigue S, Ritter P, Haissaguerre M, et al. Left ventricular endocardial stimulation for severe heart failure. J Am Coll Cardiol 2010;56:747–53. [DOI] [PubMed] [Google Scholar]
- 17.van Gelder BM, Scheffer MG, Meijer A, Bracke FA. Transseptal endocardial left ventricular pacing: an alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm 2007;4:454–60. [DOI] [PubMed] [Google Scholar]
- 18.Betts TR, Gamble JH, Khiani R, Bashir Y, Rajappan K. Development of a technique for left ventricular endocardial pacing via puncture of the interventricular septum. Circ Arrhythm Electrophysiol 2014;7:17–22. [DOI] [PubMed] [Google Scholar]
- 19.Auricchio A, Delnoy PP, Butter C, Brachmann J, Van Erven L, Spitzer S, et al. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the Wireless Stimulation Endocardially for CRT (WiSE-CRT) study. Europace 2014;16:681–8. [DOI] [PubMed] [Google Scholar]
- 20.DeFaria Yeh D, Lonergan KL, Fu D, Yeh RW, Echt DS, Foster E. Clinical factors and echocardiographic techniques related to the presence, size, and location of acoustic windows for leadless cardiac pacing. Europace 2011;13:1760–5. [DOI] [PubMed] [Google Scholar]
- 21.Reddy VY, Knops RE, Sperzel J, Miller MA, Petru J, Simon J, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 2014;129:1466–71. [DOI] [PubMed] [Google Scholar]
- 22.Sperzel J, Khairkhahan A, Ligon D, Zaltsberg S. Feasibility, efficacy and safety of percutaneous retrieval of a leadless cardiac pacemaker in an in vivo bovine model. Europace 2013;15(Suppl 2):859. [Google Scholar]
- 23.Bonner M, Eggen M. Chronic Animal Study of Leadless Pacer Design (abstract). Heart Rhythm 2011;8:S1. [Google Scholar]
- 24.Bonner M, Eggen M, Depalo J, Sheldon T, Williams E. Assessment of leadless pacemaker performance (abstract). Eur Heart J 2013;34:347. [Google Scholar]
- 25.Bonner M, Eggen M, Hilpisch K, Sheldon T, Williams E. Performance of the Medtronic Micra transcatheter pacemaker in a GLP study (abstract). Heart Rhythm 2014;11:S19. [Google Scholar]
- 26.Bonner M, Neafus N, Byrd C, Schaerf R, Goode L. Extraction of the Micra transcatheter pacemaker system (abstract). Heart Rhythm 2014;11:S342. [Google Scholar]
- 27.Steinwender C, Hönig S, Lambert T, Saleh K, Kammler J, Blessberger H, et al. First-in-man experience with a minimally invasive transcatheter pacemaker (abstract). Presented at: Innovation Abstract Posters, Heart Rhythm 2014. [Google Scholar]
- 28.Neuzil P, Reddy VY, Merkely B, Geller L, Molnar L, Bednarek J, et al. Implantable intravascular defibrillator: defibrillation thresholds of an intravascular cardioverter-defibrillator compared with those of a conventional ICD in humans. Heart Rhythm 2014;11:210–5. [DOI] [PubMed] [Google Scholar]