Abstract
Glucagon and insulin have opposing action in governing glucose homeostasis. In type 2 diabetes mellitus (T2DM), plasma glucagon is characteristically elevated, contributing to increased gluconeogenesis and hyperglycemia. Therefore, glucagon receptor (GCGR) antagonism has been proposed as a pharmacologic approach to treat T2DM. In support of this concept, a potent small-molecule GCGR antagonist (GRA), MK-0893, demonstrated dose-dependent efficacy to reduce hyperglycemia, with an HbA1c reduction of 1.5% at the 80 mg dose for 12 weeks in T2DM. However, GRA treatment was associated with dose-dependent elevation of plasma LDL-cholesterol (LDL-c). The current studies investigated the cause for increased LDL-c. We report findings that link MK-0893 with increased glucagon-like peptide 2 and cholesterol absorption. There was not, however, a GRA-related modulation of cholesterol synthesis. These findings were replicated using structurally diverse GRAs. To examine potential pharmacologic mitigation, coadministration of ezetimibe (a potent inhibitor of cholesterol absorption) in mice abrogated the GRA-associated increase of LDL-c. Although the molecular mechanism is unknown, our results provide a novel finding by which glucagon and, hence, GCGR antagonism govern cholesterol metabolism.
Keywords: diabetes, glucagon receptor antagonist, cholesterol/absorption, hypercholesterolemia, glucagon-like peptide 2, bile acids
It is through mostly opposing actions that the pancreatic islet hormones, insulin and glucagon, interact in the governance of hepatic glucose production and its uptake. In type 2 diabetes mellitus (T2DM), as well as in type 1 diabetes mellitus, fasting plasma glucagon is generally elevated, inappropriate to prevailing hyperglycemia, and there is less suppression during prandial metabolism (1). This imbalance in secretion of islet hormones is considered to be a key aspect of the pathophysiology causing hyperglycemia (1, 2). Glucagon receptor (GCGR) antagonism has accordingly drawn considerable interest as a novel pharmacological approach for treating T2DM. Several GCGR antagonists (GRAs) have advanced into human clinical trials in patients with T2DM. MK-0893 (3), MK-3577 (4), LY2409021 (5, 6), and an anti-sense oligo targeting the GCGR (ISIS-GCGRrx) (7) have each demonstrated efficacy in lowering fasting and postprandial hyperglycemia, leading to substantial reductions of HbA1c, thereby providing clinical proof of concept for the efficacy of GRAs.
In a 12 week placebo-controlled dose-ranging clinical study in T2DM using the GRA, MK-0893, dose-response improvement of hyperglycemia was observed, with a reduction of HbA1c of 1.5% at 80 mg per day, the top dose examined (supplementary Fig. 1) (3). This efficacy is substantial and, arguably, as or more effective than contemporary standard-of-care oral agents for treatment of T2DM. Yet, in association with the dose-responsive improvements in hyperglycemia, a dose-dependent increase in plasma LDL-cholesterol (LDL-c) was observed. At the 80 mg dose, plasma LDL-c increased by 16.7% relative to baseline, significantly greater than under placebo or metformin treatment arms (−3.1 and 2.2% changes, respectively) (supplementary Fig. 1) (3). LDL-c and T2DM are recognized to adversely influence risk for cardiovascular disease, and increased LDL-c in the setting of T2DM is a cause for concern, as this could potentiate the risk for cardiovascular disease (8–10).
The current studies were undertaken using a preclinical rodent model and cholesterol isotopic flux determinations, as well as further exploration of archived plasma samples from the clinical trial with MK-0893, to elucidate the principal mechanism underlying increased plasma LDL-c. A fundamental related question is whether the findings are unique to a specific GRA compound or, instead, represent a mechanism-based response. To address this, several structurally distinct GRAs were investigated for effects on cholesterol homeostasis. The findings yield novel insights into glucagon physiology, as well as GRA pharmacology, and indicate a substantial effect in the regulation of cholesterol absorption.
MATERIALS AND METHODS
Animal studies
All mice used in the studies were purchased from Taconic (Germantown, NY) at 10–12 weeks of age. Animals were maintained in a 12 h/12 h light-dark cycle with free access to food and water in an environment with temperature maintained at 22°C. Four mice were housed in a regular cage. Male humanized GCGR (hGCGR) mice were generated on a B6.129S6 background and had been backcrossed to C57BL/6 for more than 13 generations. This strain of mice showed no metabolic phenotypes in glucose and cholesterol (11). All tests were performed in the same strain of mice and comparisons were made between the compound treatments and vehicle groups. Animals were maintained on regular rodent chow diet 7012 (5% dietary fat; 3.75 kcal/g) (Teklad, Madison, WI) for 2 weeks before receiving compound treatments. Compounds were dissolved in 0.5% methylcellulose and oral gavage (po) dosing volume was 10 ml/kg body weight.
A glucagon challenge assay was performed in mice under ad libitum feeding, as previously described (12). Briefly, at 1 h post compound administration via po, glucagon dissolved in PBS was injected at 15 μg/kg ip followed by glucose measurements using a glucometer (Life Scan) via tail bleeding at 0, 12, 24, and 48 min post injection. For cholesterol absorption and synthesis studies, stable isotope-labeled cholesterol was prepared as described previously (13). Briefly, 2,2,3,4,4,6-D6-cholesterol (Cambridge Isotope Laboratory, 92543-08-3) and 3,4-13C2-cholesterol (Sigma, 662291) were dissolved in US Pharmacopoeia grade ethanol at 20 mg/ml and filtered through a 0.2 μm solvent-resistant filter (Sterlitech). The solutions were warmed to 37°C for 5 min and added dropwise over 1 min to 2 volumes of 20% Intralipid (Sigma, I141) with gentle mixing. After incubation at 37°C for 5 min, the solutions were cooled down to room temperature for 15 min, and then passed through a 1.2 micron filter (Sigma). Solutions were stored at 4°C for 1 day before administration.
Clinical samples
Human serum samples used in this study were obtained from a randomized double-blind placebo-controlled crossover trial comprising a 1 week screening period and a 6 week washout of previous anti-hyperglycemic agents followed by a 2 week placebo run-in period and then 12 week treatment periods (clinical trial NCT00479466). Available serum samples for placebo (n = 8) and monotherapy of MK-0893 60 mg (n = 46) and MK-0893 80 mg (n = 16) were assayed for glucose, total cholesterol, LDL-c, campesterol, sitosterol, and bile acid profiling. For glucagon-like peptide (GLP)-1 measurement, six samples in the MK-0893 60 mg group had insufficient serum left, thus, sample numbers were placebo (n = 8), MK-0893 60 mg (n = 40), and MK-0893 80 mg (n = 16).
cAMP production assay
Cryopreserved human primary hepatocytes were purchased from CellzDirect (presently Life Technologies, Hu8080). One vial of frozen primary hepatocytes (approximately five million cells in total) was quickly thawed to 37°C in a water bath and washed in cryopreserved hepatocyte recovery medium (Life Technologies, CM7000) and resuspended in buffer containing HBSS (Life Technologies, 14025), 0.1% BSA (Sigma, A9205), and 1.2 mM 3-isobutyl-1-methylxanthine (IBMX) (Sigma, I-5879). To assess antagonist activity, 4,000 cells per well were preincubated with compounds or 0.1% DMSO for 30 min and stimulated with glucagon (5 nM) (Sigma, G2044) for an additional 30 min at room temperature. The assay was terminated with the addition of Cisbio Dynamic 2 (62AM4PEC) detection reagents, as per the manufacturer’s instructions (Cisbio). cAMP was detected by a decrease in time-resolved fluorescence energy transfer using an EnVision plate reader (PerkinElmer). The IC50 values were calculated using nonlinear regression curve fit analysis in Prism (GraphPad).
Measurement of plasma or serum GLP-1 and GLP-2
Whole blood of mice was collected in EDTA-coated tubes and plasma was separated by centrifugation at 8,500 rpm at 4°C and stored at −80°C until assayed. Human serum was collected following a standard blood collection procedure after overnight fasting. Plasma or serum levels of GLP-1 and GLP-2 were measured using a total GLP-1 assay kit (Meso Scale Discovery) and mouse/human GLP-2 kit (Alpco).
Analysis of plasma lipid, apolipoprotein, PCSK9, and fecal cholesterol
A commercial enzymatic colorimetric kit was used for the determination of plasma total cholesterol (Wako cholesterol E kit) according to manufacturer’s instructions (WakoUSA). The plasma level of proprotein convertase subtilisin/kexin type 9 (PCSK9) was determined by PCSK9 dissociation-enhanced lanthanide fluorescence immunoassay, as described elsewhere (14). The plasma or serum lipoprotein profile was assayed by fast-protein LC, as described previously (15). Fecal cholesterol was measured by extracting lipids using the Folch method (16), whereby fecal samples were homogenized with 5 ml of chloroform:methanol (2:1, v:v). The homogenate was then filtered and washed with 2 ml of 0.9% saline, followed by centrifugation and drying of the lower phase under nitrogen gas. The extract was reconstituted with 10% Triton X-100 in isopropanol and analyzed using a commercial cholesterol kit (WakoUSA).
2H-labeling of body water and analysis of 2H-labeling of total plasma cholesterol and apoprotein
The 2H-labeling of body water was determined using headspace analyses following exchange with acetone, as described by Shah et al. (17). Briefly, 20 μl of sample (or standard) was reacted with 2 μl of 10 N NaOH and 4 μl of a 5% (v/v) solution of acetone in acetonitrile for 4 h at room temperature. The instrument was programmed to inject 5 μl of headspace gas from the GC vial in a splitless mode. Samples were analyzed using a 2.0 min isothermal run [Agilent 5973 mass spectrometer coupled to a 6890 GC oven fitted with an Agilent DB-5MS column (30 m × 250 μm × 0.15 μm); the oven was set at 170°C and helium carrier flow was set at 1.0 ml/min−1], acetone elutes at ∼1.4 min; the mass spectrometer was set to perform selected ion monitoring of m/z 58 and 59 (10 ms dwell time per ion) in the electron impact ionization mode.
The isotopic labeling of total cholesterol was determined using GC-MS (18). Lipids were saponified by heating plasma (50 μl) with 1 N KOH in 80% methanol (200 μl) at 65°C for 1 h. Samples were acidified with 25 μl 6 N HCl and then extracted in 125 μl chloroform followed by vigorous vortexing for 20 s. The samples were centrifuged at 3,000 rpm for 5 min and 100 μl of chloroform (lower layer) was collected and evaporated to dryness under N2. Samples were derivatized by reacting with 100 μl of pyridine:acetic anhydride (1:2, v:v) at 65°C for 1 h. Excess reagent was evaporated to dryness under N2 and the acetylated derivative was reconstituted in 50 μl ethyl acetate for analysis by GC-MS. All analyses were performed using an Agilent 5973 mass spectrometer coupled to a 6890 GC oven fitted with an Agilent DB-5MS column (30 m × 250 μm × 0.15 μm). The instrument was programmed to inject 1 μl of sample using a 10:1 split (helium carrier flow was set at 1.0 ml/min−1). The oven temperature was started at 150°C, raised at 20°C/min−1 to 310°C, and held for 6 min; cholesterol elutes at ∼9 min. The mass spectrometer was set to perform selected ion monitoring of m/z 368 and 369 (10 ms dwell time per ion) in the electron impact ionization mode.
Calculations and statistical analysis
To quantify the contribution of cholesterol synthesis to blood cholesterol level, the data was fit (using a precursor:product labeling ratio) to the general equation (18): newly made cholesterol = [product labeling/(precursor labeling × n)] × concentration, where n is the number of exchangeable hydrogens (assumed to equal 26 for cholesterol) (2).The change in the ratio of m/z 369:368 (i.e., M+1/M0) was used to model the product labeling, whereas the precursor labeling was assumed to equal plasma water. The concentration of total circulating cholesterol was determined via enzymatic assay (19, 20). The plasma level and flux of ApoB were quantified by the LC-MS/MS method, as described previously (21).
Analysis of campesterol and sitosterol in plasma or serum samples of mice and humans.
Five microliters of plasma or serum were mixed with 25 μl of internal standard mix (1 μg/ml of D6-campesterol and D7-sitosterol prepared in ethanol) and 100 μl of 1 N KOH in glass inserts placed on a deep 96-well polypropylene plate. The mixture was sealed and heated at 80°C with shaking at 600 rpm for 1 h on an R-shaker (Eppendorf). Samples were evaporated to dryness under nitrogen. Derivatization reagent [150 μl (1,000 mg of 2-methyl 6-nitro benzoic anhydride, 300 mg of 4-dimethyl pyridine, and 800 mg of picolinic acid dissolved in 2 ml of triethylamine and 12 ml of pyridine] was added to each tube and the plate was incubated at 80°C for 1 h. After incubation, 500 μl of hexane was added to each tube, vortexed, and centrifuged at 4,000 rpm at room temperature for 10 min. Supernatant (400 μl) was transferred to a new glass microtube, evaporated to dryness under a constant flow of nitrogen at 45°C, and reconstituted in 80 μl of loading solution (80% acetonitrile, 20% water, and 0.1% formic acid). Samples were then loaded for LC-MS analysis. Contents of campesterol and sitosterol were normalized to the internal controls in each sample. For each assay, five pooled plasma samples from multiple subjects were used as quality controls and each quality control sample was injected in triplicate for LC-MS assay. Quality controls with a variation of ±15% coefficient were deemed as acceptable.
Bile acid and intermediate analysis
Serum (150 μl) was transferred into a deep 2 ml 96-well plate followed by the addition of 585 μl ice-cold acetonitrile containing 0.1% formic acid solution and 5 μl 60 ng/ml internal standard mixture made of d6-7α,12α-dihydroxy-4-cholesten-3-one and d7-7α-hydroxy-4-cholesten-3-one. The plate was sealed and vortexed for 1 min followed by centrifugation at 4,000 rpm for 20 min at room temperature. After centrifugation, 600 μl of supernatant was passed (under positive pressure) through a protein precipitation plate, which retained phospholipids but eluted the bile acid intermediates (Ostro plate; Waters Corp., Milford, MA). The eluent was collected and evaporated under a constant flow of N2 at 45°C. The samples were then reconstituted in 100 μl of 80% acetonitrile and 0.1% formic acid/20% water. The resultant extract (10 μl) was injected onto an LC-MS/MS system operated in positive ion mode electrospray (UPLC/TQS mass spectrometer; Waters Corp.). Isotopic dilution quantitation was conducted to obtain concentrations of 7α,12α dihydroxy-4-cholesten-3-one and 7α-hydroxy-4-cholesten-3-one.
Western blotting
One piece of liver (∼100 mg) was homogenized in 500 μl of RIPA buffer by using FastPrep™-24 (MP Biomedicals). After incubation on ice for 30 min, homogenate was centrifuged at 14,000 rpm at 4°C for 30 min. The protein concentration of the supernatant was determined by BCA protein assay kit (Pierce) and the final concentration was calibrated to 1 mg/ml with RIPA buffer. After mixing with 2× loading buffer and heating at 70°C for 5 min, samples were loaded at 20 μg per well to a 4–10% SDS-PAGE gel for electrophoresis. After transferring the protein to polyvinylidene difluoride membrane, LDL receptor (LDLR) was blotted by using a rabbit monoclonal antibody (Abcam, ab52818). Loading control was blotted by using β-actin polyclonal antibody (Cell Signaling Technology).
Real-time quantitative PCR analysis and gene profiling
Liver samples isolated from mice treated with vehicle and GRA compound(s) were kept in RNAlater solution (Qiagen) until processing. Tissues were homogenized and total RNA was isolated by using an RNA Easy kit and QIACube instrument (Qiagen). Total RNA (2 μg) from each sample was reverse transcribed with a cDNA kit (Life Technologies), and mRNA levels for the genes of interest were measured by RT-PCR with TaqMan Universal Master Mix reagents and TaqMan primer/probe sets (Life Technologies) or SYBR Green Master Mix reagents and a custom-designed PCR array developed in collaboration with SABiosciences-Qiagen (22). The relative amounts of specific target amplicons for each gene were estimated by a cycle threshold (CT) value and were normalized to the copy number of housekeeping genes, with all genes in a vehicle group arbitrarily set at one (22). The P values were determined by two-tailed equal variance Student’s t-test, comparing the 2−ΔCT values of the vehicle and GRA-treated groups.
Data analysis and statistics
All data are presented as mean ± SEM. For rodent results, statistical analysis was performed by using one-way ANOVA followed by an unpaired two-tailed Student’s t-test to compare mean values between treatment groups and the control group. For human results, due to an uneven number of human samples in different groups, the percent change of the measurements (parameters at 12 weeks vs. day −1) was calculated for each individual and then averaged. Statistical analysis was performed by using the Mann-Whitney test.
Declaration
For rodent studies, all testing protocols were reviewed and approved by the Merck Research Laboratories Institutional Animal Care and Use Committees in Rahway and Kenilworth, NJ. The Guide for the Care and Use of Laboratory Animals was followed in the conduct of the animal studies. Veterinary care was given to animals requiring medical attention. Finally, the ARRIVE guidelines (https://www.nc3rs.org.uk/arrive-guidelines), published by NC3Rs, were followed for reporting the in vivo experiments in animal research.
Clinical trial protocols of MK-0893 were reviewed and approved by an independent institutional review board or ethical review committee before being initiated. For each site, the institutional review board/ethical review committee and Merck’s consent form review department (US studies) or local medical director (non-US studies) approved the patient informed consent form. Written informed consent was received from participants prior to inclusion in the study. In all cases, Merck clinical studies were consistent with standards established by the Declaration of Helsinki and in compliance with all local and/or national regulations and directives.
RESULTS
Selection and characterization of GRA test compounds
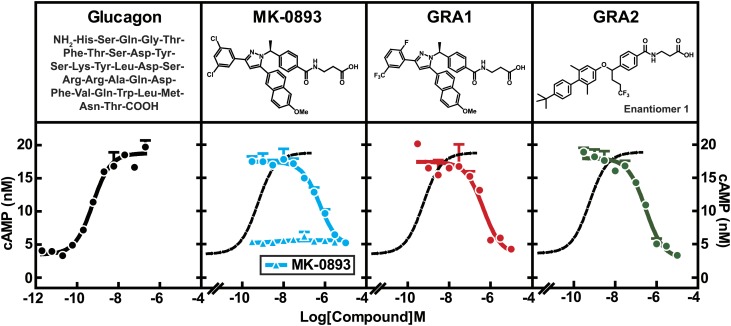
Three GRA compounds [MK-0893 (23), GRA1 (24), and GRA2 (25)] were demonstrated to potently block glucagon binding to the GCGR and were selected for use in the preclinical studies that comprise this investigation. MK-0893 and GRA1 were developed by Merck and GRA2 is under development by Eli Lilly. GRA2 possesses a biaryl benzyl ether core that is distinctly different from the tri-substituted diazole core present in GRA1 and MK-0893 (Fig. 1). To assess relative potencies, cryopreserved human hepatocytes were incubated with MK-0893, GRA1, or GRA2 in the presence of 5 nM glucagon followed by cAMP quantitation in the culture media. Glucagon induced cAMP production dose dependently, with an EC50 of 575 pM, which is comparable to physiological levels of glucagon in blood (2). MK-0893, GRA1, or GRA2 alone had no effect on cAMP production. In the presence of glucagon, each compound suppressed cAMP production, with IC50s of 563, 448, and 292 nM, respectively (Fig. 1). Comparing these results with values earlier obtained from CHO cells stably overexpressing human GCGR (hGCGR.CHO) (23, 24), IC50s of the compounds are right-shifted, likely reflecting overexpression of GCGR in engineered cells versus human primary hepatocytes. Nonetheless, these data indicate the three GRA compounds are comparably potent. To further characterize these compounds, the compounds were compared for effects on in vitro binding of glucagon to GCGR, β-arrestin recruitment, cellular level of Ca2+, and cAMP production in hGCGR.CHO cells (supplementary Table 1). No meaningful differences among the three selected GRAs were observed across these parameters, indicating good similarity of pharmacology of antagonizing GCGR-mediated signaling.
Fig. 1.
GRAs suppress glucagon-induced cAMP production in human primary hepatocytes. Human primary hepatocytes were thawed and washed with CHAMPS buffer followed by cAMP production assay. The assay was done in triplicate. The EC50 of glucagon on cAMP production is 575 pM. The IC50s of MK-0893, GRA1, and GRA2 are 563, 448, and 292 nM, respectively. All compound treatments were performed in the presence of 5 nM of glucagon. Dose titration of MK-0893 alone is shown in the panel of MK-0893. All treatments were done in duplicate. Dotted curve is a replication of the glucagon dose response curve in the panel of glucagon.
Effect of GRA on cholesterol synthesis and clearance in hGCGR mice
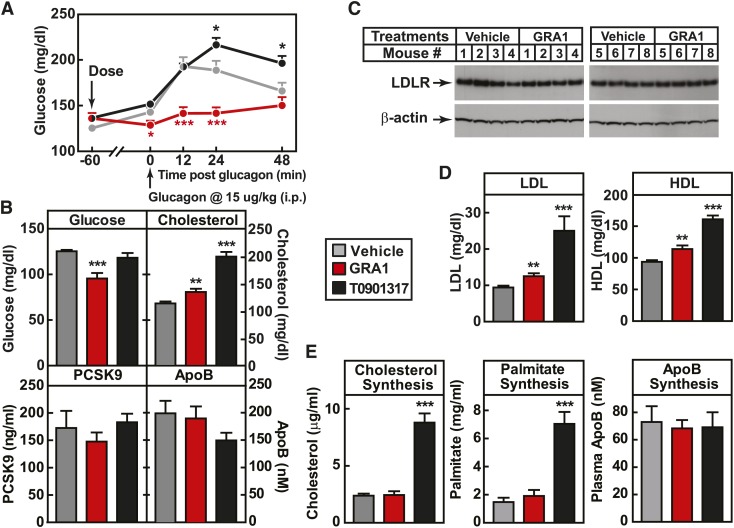
The next prerequisite was to identify a relevant preclinical model. For this purpose, the hGCGR mouse model was chosen (11). The selected GRAs have more potent and specific binding for human than mouse GCGR (24). hGCGR mice are lean, healthy, and without abnormal plasma glucose and lipids (11). To test for qualification of hGCGR mice for these investigations, the mice were maintained on chow diet and treated with GRA1 at 30 mpk once daily (QD) for 5 days. Glucagon-induced glucose excursion on day 1 after a single dose and ambient glucose on day 5 were measured as indicators of acute and subchronic GCGR blockade. Glucose excursion induced by glucagon after a single dose was suppressed (Fig. 2A), and ambient glucose decreased by 20% after 5 days (Fig. 2B), demonstrating effective target engagement. On chow diet under GRA1 treatment, plasma levels of cholesterol, LDL-c, and HDL-cholesterol (HDL-c) were increased by 19, 33, and 21%, respectively (compared with vehicle) (Fig. 2B, D; supplementary Fig. 3), an increase at least comparable to the rise in cholesterol observed clinically in T2DM (3, 26). Plasma TG level was not affected by GRA1 treatment (supplementary Fig. 3). Taken together, it was concluded that hGCGR mice were a suitable preclinical model for investigating the mechanism of action by which GRA induces a rise in plasma cholesterol, LDL-c, and HDL-c.
Fig. 2.
GRA1 increases plasma total cholesterol, LDL-c, and HDL-c without effect on cholesterol synthesis. Male hGCGR mice at 10–12 weeks of age were treated with vehicle, GRA1 (30 mpk), and T0901317 (40 mpk) QD for 5 days (n = 8). A: Glucagon-induced glucose excursion after single dose on day 1 in mice under ad libitum feeding. B: Plasma levels of glucose, cholesterol, PCSK9, and ApoB after 5 days of treatment in mice under ad libitum. C: Liver LDLR protein levels determined by Western blotting. LDLR is shown as a single band at a molecular mass between 100 and 150 kDa of protein markers. D: Lipoprotein profile determined by fast-protein LC on plasma samples obtained from hGCGR mice after 5 days of treatment. E: Synthesis of cholesterol, palmitate, and ApoB after 24 h incorporation of D2O prior to euthanasia of mice, followed by processing of plasma samples and measurement of stable isotope-labeled cholesterol by LC-MS. All data are shown as mean ± SEM. Statistical significance is calculated by unpaired two-tailed Student’s t-test. Asterisks denote statistical significance of the compound treatment groups compared with the vehicle group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
It had been reported that glucagon inhibits HMG-CoA reductase (HMGCR) and suppresses cholesterol synthesis (27). Therefore, we initially focused upon testing the hypothesis that GRA increases cholesterol synthesis. Isotope tracer methodology was used. hGCGR mice were administered D2O (20 ml/kg body weight ip) to label newly synthesized cholesterol, palmitate, and ApoB, and determine the rate of their synthesis (28). As a positive pharmacological comparator for the assays of palmitate and cholesterol synthesis, T0901317, an LXR α and β agonist, was used (29). T0901317 treatment induced increased plasma cholesterol, LDL-c, and HDL-c by 76, 166, and 21%, respectively, and markedly increased synthesis of cholesterol and palmitate (Fig. 2). GRA1 treatment, while replicating the increase in plasma cholesterol earlier described, did not have a significant effect on synthesis of cholesterol, palmitate, or ApoB (Fig. 2E). Liver cholesterol content was similar between vehicle and GRA1 treatment (supplementary Fig. 3). It was also examined in these studies whether GRA would alter key components governing LDL-c clearance. GRA1 treatment had no effect relative to vehicle treatment on plasma PCSK9 and plasma ApoB (Fig. 2B), and did not change hepatic LDLR protein level (Fig. 2C). Consistent with unchanged LDLR protein level, LDL-c clearance was not affected by treatments of MK-0893, GRA1, and GRA2 (supplementary Fig. 5).
Effects of GRA on cholesterol absorption in hGCGR mice
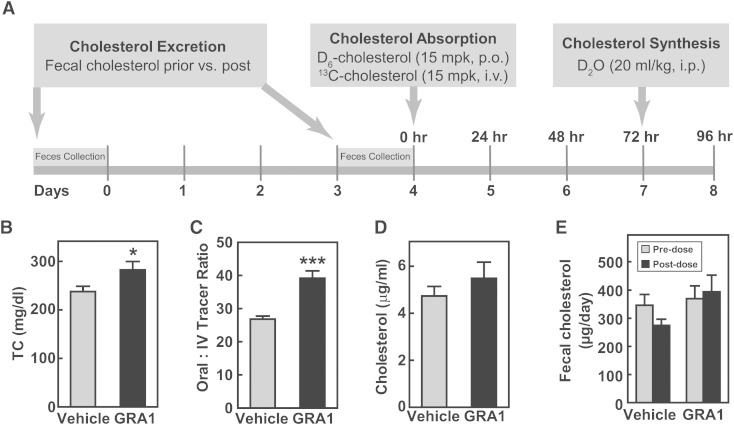
The next set of studies was undertaken to examine a potential effect of GRA1 on cholesterol absorption and its excretion. An in vivo study performed in hGCGR mice on chow diet was adapted from a study design previously published (28). Cholesterol absorption was measured using a stable isotope method and cholesterol excretion was monitored by measuring fecal cholesterol. Stable isotope-labeled cholesterols, 2,2,3,4,4,6-D6-cholesterol (15 mpk, po) and 3,4-13C2-cholesterol (15 mpk, iv), were administered on day 4 followed by blood collection at 24, 48, and 72 h post injection. Cholesterol absorption was calculated by the ratio of 2,2,3,4,4,6-D6-cholesterol to 3,4-13C2-cholesterol in plasma at different time points. At 24 h prior to euthanasia, mice were dosed with D2O (ip, 20 ml/kg) for measurement of cholesterol and palmitate synthesis (Fig. 3A). After 9 days of treatment, GRA1 increased plasma levels of total cholesterol by 18%, thus, a highly consistent response (Fig. 3B). Cholesterol absorption was found to be increased by 46% (Fig. 3C). A similar increase of cholesterol absorption was observed for MK-0893 and GRA2 (supplementary Fig. 5). It was again observed that there was not a significant change in the rate of cholesterol synthesis (Fig. 3D). Cholesterol excretion was not found to be significantly changed (Fig. 3E).
Fig. 3.
GRA1 increases plasma total cholesterol and cholesterol absorption in hGCGR mice. A: Experimental design for the effect of GRA treatment on cholesterol excretion, absorption, and synthesis. Male hGCGR mice at 10–12 weeks of age were treated with vehicle or GRA1 (30 mpk) QD for 9 days (n = 8). For cholesterol excretion measurement, feces were collected for 1 day before and for 1 day (days 3–4) following GRA1 treatment. Stable isotope-labeled cholesterol and water were administered as indicated, and blood samples were collected in mice under ad libitum for plasma preparation and analysis. B–E: Plasma total cholesterol (TC), cholesterol absorption calculated by the ratio of 2,2,3,4,4,6-D6-cholesterol to 3,4-13C2-cholesterol, cholesterol synthesis from D2O (24 h incorporation), and cholesterol excretion based on fecal total cholesterol measurement. All data are shown as mean ± SEM. Statistical significance is calculated by unpaired two-tailed Student’s t-test. Asterisks denote statistical significance of the GRA1 group compared with the vehicle group. *P ≤ 0.05, ***P ≤ 0.001.
GRA1 and GRA2 increase blood cholesterol and cholesterol absorption
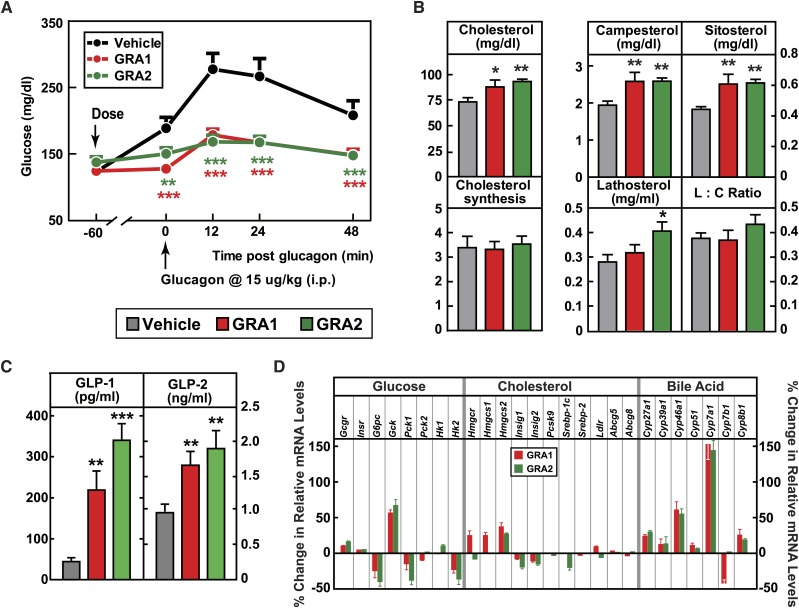
The key aspect of these studies was to address whether the finding of increased cholesterol absorption was unique for a specific GRA compound or instead could be mechanism based. The isotope study described above was repeated using the structurally distinct GRA2. A second aspect was to add measurements of phytosterols (plant sterols), the plasma levels of which are recognized as biomarkers of intestinal cholesterol absorption (30). GRA1 and GRA2 were dosed (30 mpk each) to achieve similar and robust target engagement, as assessed by blockade of a glucagon-induced glucose excursion (Fig. 4A). After 5 days of treatment, GRA1 and GRA2 increased plasma total cholesterol by 20 and 27.3%, respectively (Fig. 4B). Both compounds increased cholesterol absorption, as indicated by treatment-related increases in plasma phytosterols. Campesterol increased 33.1% for GRA1 and 33.3% for GRA2, and plasma sitosterol increased 37.2% for GRA1 and 38.9% for GRA2 (Fig. 4B). Based on D2O labeling, no significant change in the rate of cholesterol synthesis was found for either GRA1 or GRA2. GRA2 slightly increased the plasma level of lathosterol; however, there was not a significant difference in the lathosterol-to-cholesterol ratio on treatment with GRA1 or GRA2 (Fig. 4B), an indirect measurement of cholesterol synthesis (31).
Fig. 4.
Parallel comparisons of different GRA1s and GRA2s on cholesterol metabolism. Male hGCGR mice at 10–12 weeks of age were treated with vehicle, GRA1 (30 mpk), and GRA2 (30 mpk) QD for 5 days (n = 8). A: Glucagon-induced glucose excursion after single dose on day 1. B: Plasma levels of cholesterol, campesterol, sitosterol, lathosterol, lathosterol:cholesterol ratio (L:C), and cholesterol synthesis from D2O (micrograms per milliliter) (24 h incorporation) after 5 days of treatment. C: Plasma levels of total GLP-1 and GLP-2 after 5 days of treatment. All data are shown as mean ± SEM. Statistical significance is calculated by unpaired two-tailed Student’s t-test. Asterisks denote statistical significance of the compound treatment groups compared with the vehicle group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. D: Percent changes of hepatic genes related to metabolism of glucose, cholesterol, and bile acid in hGCGR mice treated with GRA1 (30 mpk) and GRA2 (30 mpk) over vehicle (n = 8 per group, data are shown as mean ± SEM). Insr, insulin receptor; G6pc, glucose-6-phosphatase catalytic subunit; Gck, glucokinase; Pck, phosphoenolpyruvate carboxykinase; Hk, hexokinase; Hmgcs, HMG-CoA synthase; Insig1(2), insulin induced gene 1(2);
It was reported that plasma levels of GLP-1 and GLP-2 were dramatically increased in GCGR−/− mice (32, 33). In hGCGR mice treated with GRA1 and GRA2, plasma levels of total GLP-1 were increased by 398 and 674%, respectively, and levels of GLP-2 were increased by 71 and 96%, respectively (Fig. 4C), consistent with the pattern found in clinical samples from the MK-0893 clinical trial (Fig. 5C). In a separate study in hGCGR mice, MK-0893 treatment increased the plasma level of total GLP-1 by 168% and of GLP-2 by 37% (supplementary Fig. 7).
Fig. 5.
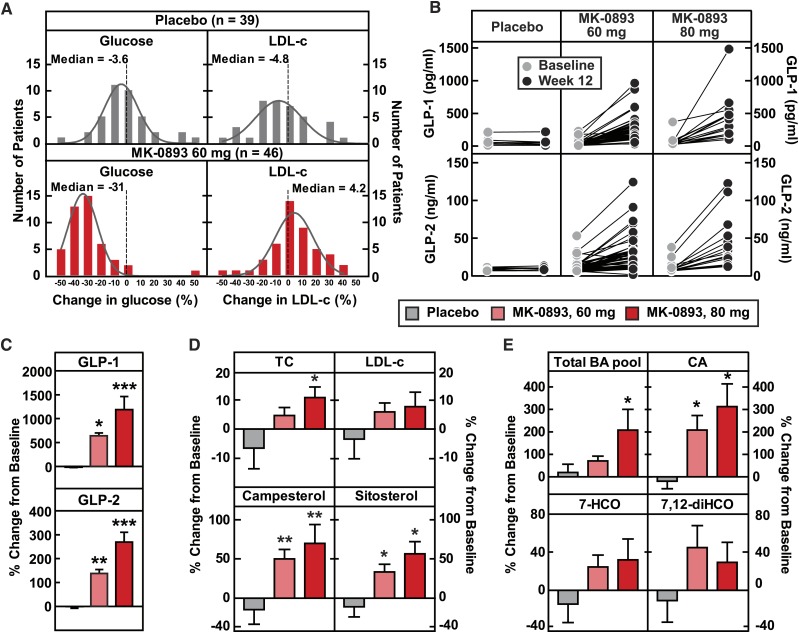
MK-0893 dose-dependently increases serum total cholesterol, LDL-c, GLP-1, GLP-2, phytosterols, and the bile acid pool in T2DM. Serum of T2DM patients on placebo or MK-0893 (60 mg and 80 mg QD) for 12 weeks were analyzed for serum levels of glucose, total cholesterol (TC), LDL-c, GLP-1, GLP-2, campesterol, sitosterol, bile acids, cholic acid, 7-HCO, and 7,12-diHCO at baseline (day −1) and week 12 post treatment. BA, bile acid; CA, cholic acid; 7,12-diHCO, 7,12-α-dihydroxycholestenone. A: The frequency distributions of percent change in glucose and LDL-c for placebo (n = 39) and MK-0893 60 mg (n = 46) after 12 week treatment. B: Plasma levels of GLP-1 and GLP-2 at baseline (day −1) and week 12 in T2DM patients treated with placebo (n = 8), MK-0893 60 mg (n = 40 for GLP-1, n = 46 for GLP-2), and MK-0893 80 mg (n = 16). C: Averaged percent changes of plasma GLP-1 and GLP-2 on week 12 from baseline (day −1) in (B). D, E: Percent change of plasma total cholesterol, LDL-c, campesterol, sitosterol, bile acids, cholic acid, 7-HCO, and 7,12-diHCO on week 12 from baseline (day −1) in T2DM patients treated with placebo (n = 8), MK-0893 60 mg (n = 46), and MK-0893 80 mg (n = 16). The data in (B–E) are shown as mean ± SEM. Statistical significance is calculated by nonparametric two-tailed Mann-Whitney test. Asterisks denote statistical significance of the MK-0893 group compared with the placebo group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
As a corollary experiment to determine whether the observed effects on cholesterol homeostasis are compound specific, we sought to exploit that MK-0893 achieves substantially more robust binding to human GCGR than mouse GCGR by examining the respective treatment effect upon cholesterol in WT versus hGCGR mice. We posited that if the increase in cholesterol absorption was an “off-target” effect, then resultant increases in plasma cholesterol would be similar in WT and hGCGR mice. Doses of 3 and 10 mpk were used for MK-0893 in WT and hGCGR mice, but robust target engagement (glucose response to glucagon challenge) was demonstrable at both doses only in hGCGR mice. MK-0893 had no significant effect on plasma cholesterol or phytosterols in WT mice, but demonstrated a significantly increased plasma level of campesterol at 10 mpk and a trend for dose-dependent increase in plasma sitosterols in hGCGR mice (supplementary Fig. 2). Taken together, these studies bolster the concept that blockade of GCGR induces an increase in cholesterol absorption and plasma cholesterol as a mechanism-based effect.
MK-0893 increases phytosterols, GLP-1, GLP-2, and bile acids in T2DM
Having generated a hypothesis based on pharmacologic interventions in hGCGR mice that GRA induces increased cholesterol absorption and, importantly, established utility of using phytosterols as relevant biomarkers of this process, we were then positioned for further examination of archived plasma samples obtained during the MK-0893 clinical study (ClinicalTrials.govidentifier: NCT00479466). In this trial, placebo, metformin, and MK-0893 at 20, 40, 60, and 80 mg were chosen to treat T2DM (3). Archived samples from the placebo and the MK-0893 60 mg and 80 mg treatment arms were assayed for glucose, total cholesterol, GLP-1, GLP-2, LDL-c, phytosterols, bile acids, and bile acid metabolites (Fig. 5, supplementary Fig. 4). The 60 mg treatment with MK-0893 decreased glucose by 31% (versus a 3.6% rise with placebo) and increased LDL-c by 4.2% (versus a 4.8% decline with placebo), as shown in Fig. 5A. Serum campesterol and sitosterol were significantly increased with MK-0893 treatment (Fig. 5D).
It is recognized that GRA or genetic knockout of GCGR evokes large compensatory increases in plasma glucagon, together with increased expression for other peptides derived from preproglucagon. It has been reported that GLP-2 enhances nutrient absorption and induces epithelial cell proliferation and regeneration (34–37). In the archived plasma samples, we observed a treatment-related increase of total GLP-1 (6-fold for 60 mg and 12-fold for 80 mg) and a less pronounced increase in plasma GLP-2 (1.4-fold for 60 mg and nearly 3-fold for 80 mg) (Fig. 5B, C). In the aqueous environment of the small intestine, bile acids have a critical role in facilitating absorption of highly hydrophobic cholesterol. Though an effect of GRA on bile acid metabolism has not previously been reported, the plasma samples were examined for total bile acid concentration, which was found to be significantly increased by MK-0893 (Fig. 5E). In particular, there was an increase of cholic acid and in 7-α-hydroxy-4-cholesten-3-one (7-HCO), a biochemical intermediate in the rate-limiting reaction converting cholesterol to bile acids. The 7α,12α-dihydroxy-4-cholesten-3-one, an intermediate in cholic acid synthesis, showed a trend for increase (Fig. 5E, supplementary Fig. 4E, F). These results augment the finding that GRA induces an increase in cholesterol absorption and suggest that GRA mediates an increase of bile acid synthesis, increases GLP-2, and modulates bile acid composition, as factors that could potentially contribute to the mechanism for increasing intestinal absorption of cholesterol.
Effect of GRA on hepatic gene expression in hGCGR mice
Based on these novel findings in clinical samples from T2DM patients who were treated with MK-0893, we investigated further effects of GRA on mRNA expression in hGCGR mice. As expected, GRA1 and GRA2 suppressed hepatic mRNA levels of glucose-6-phosphatase catalytic subunit (G6pc), phosphoenolpyruvate carboxykinase 1 (Pck1), and hexokinase 2 (Hk2), but increased glucokinase (Gck). However, we observed that the effects of GRA1 and GRA2 on gene expression in the pathway of cholesterol synthesis were marginal. Only HMG-CoA synthase 2 (Hmgcs2) was found to be slightly increased by both compounds (Fig. 4D). The lack of significant changes in mRNA levels for Pcsk9, sterol regulatory element-binding protein (Srebp)-1c, Srebp-2, and Ldlr is consistent with the limited impact of GRA1 and GRA2 on cholesterol synthesis (as ascertained isotopically in earlier aspects of this study) and a limited effect on components governing cholesterol clearance (Figs. 2E, 4B). On the other hand, the gene encoding Cyp7a1, which catalyzes the rate-limiting step of bile acid synthesis, was dramatically increased by GRA1 and GRA2 (Fig. 4D). Other genes controlling bile acid synthesis, Cyp27a1 and Cyp46a1, were also induced by GRA1 and GRA2, though to a lesser extent (Fig. 4D). In both acute and subchronic studies, GRAs had no effect on mRNA levels of intestinal Npc1l1 and Abcg5/g8, and they did not affect mRNA levels of liver Abcg5/g8 (Fig. 4D, supplementary Table 2).
GRA-induced increase in plasma cholesterol is abolished by ezetimibe
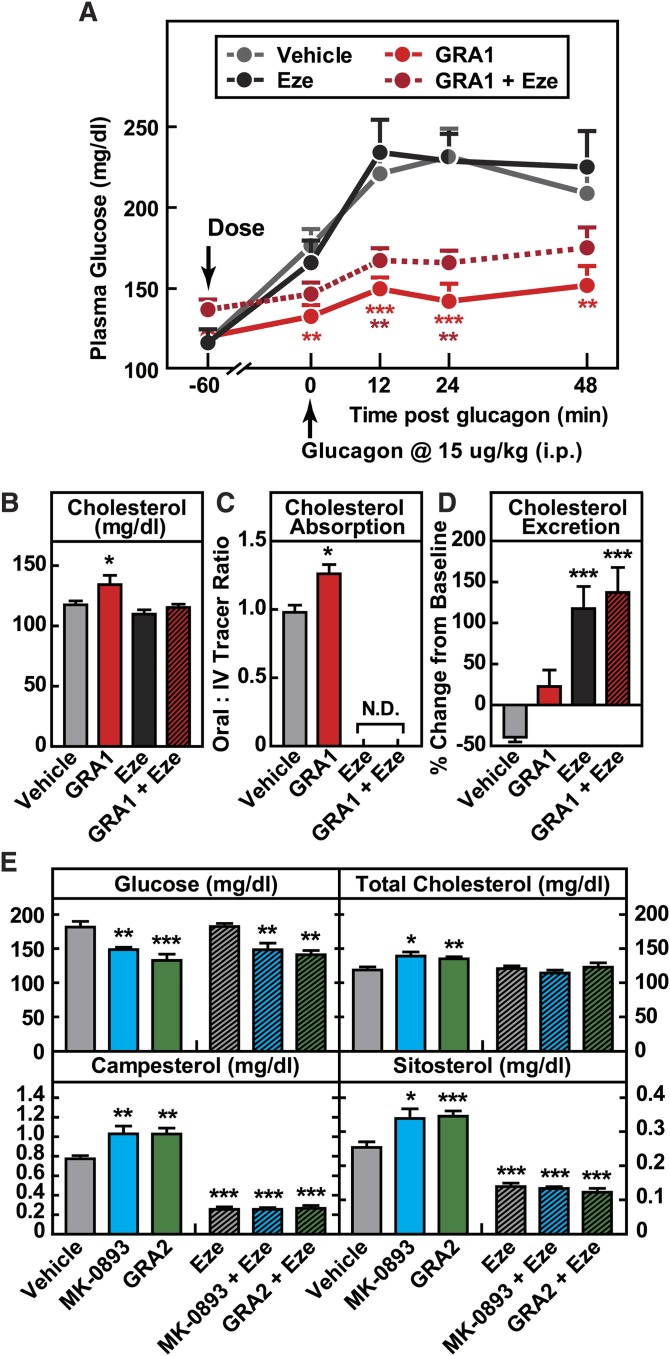
Based on the findings that GRA increases cholesterol absorption and that this is a mechanism-based effect, we next undertook a study of whether administration of ezetimibe, which acts to reduce cholesterol absorption, would effectively mitigate GRA-induced increases in LDL-c. hGCGR mice on chow diet were treated with vehicle and GRA1 in the absence or presence of ezetimibe for 9 days. Ezetimibe did not alter the target engagement or glucose-lowering efficacy of GRA1 (Fig. 6A). Ezetimibe alone had no effect on plasma cholesterol due to a large increase in the rate of cholesterol synthesis (supplementary Fig. 6), a compensatory effect previously demonstrated (38), and this was similar during coadministration with GRA. But its coadministration with GRA1 abrogated the 14% increase of plasma cholesterol observed in the GRA1 treatment arm (Fig. 6B). Ezetimibe, alone and combined with GRA1, strongly inhibited intestinal absorption of cholesterol as measured using orally administered D6-cholesterol (Fig. 6C), thus eliminating the increase of 29% that was observed under GRA1 treatment. In these studies, it was again observed that despite a clear effect of GRA1 treatment to increase cholesterol absorption, little net effect on fecal cholesterol excretion was detected; whereas ezetimibe, alone and in combination with GRA1, caused a marked increase in fecal cholesterol excretion (Fig. 6D).
Fig. 6.
Induction of GRAs on cholesterol is abolished by combined treatment with ezetimibe (Eze). Male hGCGR mice at 10–12 weeks of age were treated with vehicle, GRA1 (30 mpk), MK-0893 (30 mpk), and GRA2 (30 mpk) with or without ezetimibe (10 mpk) for 9 days (n = 8). A: Glucagon-induced glucose excursion after single dose on day 1. B: Plasma levels of total cholesterol. C: Cholesterol absorption calculated by the ratio of 2,2,3,4,4,6-D6-cholesterol to 3,4-13C2-cholesterol in plasma. N.D., nondetectable. D: Cholesterol excretion determined by the percent change in fecal total cholesterol on day 4 versus day 0. E: Plasma levels of glucose, total cholesterol, campesterol, and sitosterol after 9 day treatment. All data are shown as mean ± SEM. Statistical significance is calculated by unpaired two-tailed Student’s t-test. Asterisks denote statistical significance of the compound treatment groups compared with the vehicle group. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
This pattern of findings observed using GRA1 in combination with ezetimibe was then tested using both MK-0893 and GRA2, and highly similar results were obtained. MK-0893 and GRA2 increased plasma total cholesterol by 17 and 14%, respectively, together with significant increases in plasma campesterol and sitosterol. Ezetimibe alone and in combination with MK-0893 and GRA2 significantly and similarly reduced plasma campesterol and sitosterol, and in the coadministration arms, maintained plasma cholesterol at normal levels (Fig. 6E).
DISCUSSION
The impetus for these investigations of the effect of GRA on cholesterol homeostasis in hGCGR mice arose from clinical observations that dose-dependent increases in LDL-c occur in T2DM patients treated with the GRA, MK-0893. The 12 week clinical trial of MK-0893 achieved clear evidence that antagonizing endogenous glucagon can improve hyperglycemia in T2DM, attaining impressive efficacy on HbA1c and with minimal risk of hypoglycemia. Yet, the collateral finding of a dose-dependent statistically significant increase in LDL-c raises a concern of increased cardiovascular risk, despite substantial improvement in hyperglycemia.
This was therefore a “bedside-to-bench”-inspired translational study, undertaken to determine whether the rise in plasma LDL-c was restricted to MK-0893 or, instead, was more likely to be mechanism based. Because our data supported that the effect was mechanism based, we sought to determine among cholesterol synthesis, excretion, absorption, or plasma clearance of LDL-c, which mechanism was primarily responsible. The initial step was to identify an appropriate preclinical rodent model, a necessity because the GRA compounds had been optimized for potency against human GCGR and were much weaker against mouse GCGR, reflecting species differences in GCGR homology (11). The hGCGR mouse demonstrated elevation of plasma cholesterol with three structurally distinct GRA compounds and to an extent similar to the rise observed in clinical studies, establishing both that this was a suitable model and inferring that the rise in plasma LDL-c is mechanism based. Chiefly on the basis of isotopic flux determinations of endogenous cholesterol synthesis and separately of intestinal cholesterol absorption, it was determined that GRA raises LDL-c by increasing cholesterol absorption. Bolstering this interpretation is the observation that GRA significantly increases plasma phytosterols, recognized biomarkers of intestinal absorption of cholesterol, and comparable to the increase of cholesterol absorption detected by isotopic methodology. Furthermore, these findings on plasma phytosterols in the hGCGR mouse enabled our studies to pivot back to bench-to-bedside, and by using archived samples from the clinical study of MK-0893, significant increases of phytosterols were observed during treatment with this GRA.
The concept that glucagon influences cholesterol metabolism has been previously reported, though with uncertainty as to the responsible mechanism. Reports have appeared in the literature since the 1950s, describing an association between glucagon action and cholesterol. For example, destruction of α-cells increased plasma cholesterol in rabbits (39) and dogs (40), while infusing glucagon prevented hypercholesterolemia in rats fed with a high cholesterol diet (41). LDL-c and the rate of cholesterol synthesis in a patient with familial hypercholesterolemia were reduced by portacaval shunt surgery, which was associated with marked increases of plasma glucagon and bile acids (42). Furthermore, glucagon infusion into humans profoundly reduced plasma levels of cholesterol (43). GCGR−/− mice were reported to have a significantly higher plasma LDL-c than WT mice (32). Plasma cholesterol was reported to increase in diet-induced obese mice treated with a monoclonal antibody inhibiting GCGR (44). In preclinical studies with MK-0893, there was not a prominent signal of increased plasma cholesterol that was noted (unpublished observations), perhaps simply reflecting the lesser potency of this compound for rodent compared with human GCGR. Regardless, with the hindsight afforded by the clinical trial data with MK-0893, it is clear that the published literature does contain precedents for the observation that GRAs can influence cholesterol metabolism.
The mechanism underlying the prior findings was not rigorously addressed although the findings were attributed to various causes, prominent among which is the hypothesis that antagonism of glucagon can increase cholesterol synthesis. There is a rationale that blocking glucagon signaling and, hence, increasing cAMP would release inactivation of HMGCR (27). In addition, it has been reported that glucagon suppresses plasma PCSK9 (45) and increases hepatic LDLR protein levels (46), presumably as a consequence of effects on the Srebp-2 pathway (45). Knockdown of GCGR in db/db mice significantly increased LDL-c, which was attributed to elevated hepatic lipogenesis and cholesterol synthesis (47). Therefore, in the current studies, it was initially evaluated whether GRA mediated an increase in cholesterol synthesis or might decrease LDL-c clearance. Our findings do not support either hypothesis. LDLR, PCSK9, and rates of cholesterol synthesis were not significantly changed during treatment with multiple GRAs, despite unequivocal induction of increased plasma cholesterol.
Instead, our studies reveal the novel finding that there is a strong effect of GRAs to increase absorption of cholesterol. It appears that there are marked differences between GRA treatment and GCGR knockdown. While GRAs increased LDL-c and HDL-c, GCGR knockdown only increased LDL-c without any effect on HDL-c. Mechanistically, GCGR knockdown induced cholesterol synthesis (47), whereas GRAs induced cholesterol absorption without any effect on cholesterol synthesis. Though our studies did obtain some insights as to what may, in turn, contribute to increased absorption of cholesterol, it is acknowledged that there are a number of aspects of this novel hypothesis that will require further investigation. One factor might be the effect of GRAs to raise secretion of the gut hormone, GLP-2. It is known that glucagon, GLP-1, and GLP-2 are dramatically increased in GCGR−/− mice (33, 48). Plasma levels of total GLP-1 and GLP-2 were both significantly increased by subchronic treatment of GRA1, GRA2, and MK-0893 in hGCGR mice. Dramatic inductions of plasma total GLP-1 and GLP-2 were observed in T2DM patients chronically treated with MK-0893. GLP-2 has been reported to induce crypt cell proliferation and its effect on intestinal lipid absorption depends on nutritional status and presence of GLP-1 (34, 36, 37, 49). Although induction of GRAs on GLP-2 is predictable based on results from GCGR−/− mice, it appears that GRA does not mirror the effect of glucagon on cholesterol metabolism. This, however, does not negate the published findings of glucagon on the Srebp-2 pathway, including LDLR (46), PCSK9 (50), and cholesterol synthesis (29). The induction of GRA on GLP-2 is so dramatic that it might have masked the activation on the Srebp-2 pathway caused by blockade of GCGR, inasmuch as intestinal cholesterol absorption and hepatic cholesterol synthesis are reversely affected by each other (51, 52).
Another set of observations obtained in the present studies of GRA treatment that we posit to be fruitful for further examinations concerns bile acids. Bile acids play a critical role in facilitating intestinal absorption of hydrophobic lipids, notably including cholesterol and also, bile acids are derived from cholesterol and thus intimately associated with hepatic cholesterol metabolism. The archived plasma samples from the clinical study with MK-0893 were assayed for bile acid concentration and it was found that these levels were increased, including cholic acid and cholic acid precursors. Cholic acid supplementation enhances cholesterol absorption in humans (50). In GRA-treated hGCGR mice, a consistent finding is increased Cyp7a1 mRNA expression, the enzyme that is rate-limiting in bile acid synthesis. Consistent with an increased mRNA level of Cyp7a1 in hGCGR mice, its proximal product (7-HCO) was increased in T2DM during GRA treatment. It might also be considered that bile acids can stimulate secretion of GLP-1 and GLP-2 from L-cells via activating the bile acid receptor, TGR5 (53). There is resurgent interest in the role of bile acids in metabolic signaling, particularly in the context of diabetes mellitus (54), and the current findings indicate that glucagon agonism and antagonism can be influential in governing bile acid homeostasis.
Our studies indicate that the effect of MK-0893 to increase LDL-c derives mostly from a net increase in absorption of cholesterol, and that this is a mechanism-based rather than a compound-specific effect. This conclusion is based upon consistent findings in the hGCGR mice using three structurally diverse GRAs. Yet, while the data of our preclinical studies indicate a likely mechanism-based effect to increase plasma LDL-c via increased cholesterol absorption, we do recognize that the reported clinical findings, to date, with the effect of GRA on plasma LDL-c are inconsistent. In the clinical trials of LY2409021 (5, 6) and with ISIS-GCGRrx (7), no induction of LDL-c was reported. It remains uncertain why these findings may differ from what was observed in the clinical trial with MK-0893. Perhaps it is noteworthy that clinical trials of LY2409021 and ISIS-GCGRrx were not exclusively GRA monotherapy, as was the situation with the clinical dose-ranging study of MK-0893; instead, many of the T2DM participants in those studies were also receiving metformin treatment (5, 7). Metformin generally improves hypercholesterolemia in humans, although the mechanism is still not well-understood (55). Clinical studies using MK-0893 in combination with metformin treatment found it to have increased efficacy in reducing hyperglycemia and substantially mitigated the increase in LDL-c observed with GRA monotherapy in T2DM (26).
In keeping with a translational emphasis of the present studies, the effectiveness of ezetimibe to abrogate a GRA-induced increase in cholesterol absorption and plasma LDL-c was investigated. Ezetimibe, the molecular target for which is inhibition of NPC1L1, effectively abolished the increase in cholesterol absorption caused by GRA without interfering with efficacy in reducing hyperglycemia. It is possible that GRA-induced hypercholesterolemia could be mediated, in part, by an effect on Npc1l1 (56, 57). This remains to be fully characterized, but regardless, we describe that blocking NPC1L1 with ezetimibe in a rodent model effectively suppresses GRA-induced hypercholesterolemia. We postulate that coadministering GRAs and ezetimibe will work equally well, or better, in humans because, unlike the exclusive expression of Npc1l1 in intestine in mice, NPC1L1 is expressed abundantly in the liver and intestine in humans (58), and ezetimibe exerts its lipid-lowering effect by blocking NPC1L1 in both the liver and intestine in humans (59).
In summary, we used various pharmacological tools to study the major cause of GRA-induced hypercholesterolemia in mice and humans. Studies in mice demonstrated that increased plasma cholesterol was mainly attributable to cholesterol absorption. Consistent with this finding obtained in rodents, we report the novel finding that the increase of plasma LDL-c and cholesterol was associated with increases in plasma phytosterols in humans. With respect to potential mechanisms that mediate GRA-induced increase in cholesterol absorption, we observed that GRA-induced increases in GLP-2 and a trend of increase in bile acid concentrations in patients with T2DM and in the rodent model, together with mRNA expression of hepatic bile acid synthesis enzymes in mice. These are factors that can plausibly contribute to increased cholesterol absorption. Finally, in keeping with the translational emphasis of these studies, it was shown that ezetimibe effectively mitigated GRA-induced cholesterolemia in mice. Thus, combining GRA and ezetimibe may provide a feasible approach to mitigating MK-0893-induced elevation of cholesterol in patients with T2DM. Collectively, these results provide novel insights into the effect of glucagon and GCGR antagonism in governing cholesterol homeostasis.
Supplementary Material
Acknowledgments
The authors thank Taro Akiyama and Peter Stein for critically reading the manuscript and providing constructive suggestions. Doug Johns, Cai Li, Dan Kemp, Tom Roddy, Jing Li, Kristian Jensen, Juliann Ehrhart, Heather Zhou, Seongah Han, Eric Muise, Brad Sherborne, and Paul Carrington provided insightful suggestions to the study design and data interpretation.
Footnotes
Abbreviations:
- Cyp7a1
- Cytochrome P450 family 7 subfamily A polypeptide 1
- Cyp7b1
- Cytochrome P450 family 7 subfamily B polypeptide 1
- Cyp8b1
- Cytochrome P450 family 8 subfamily B polypeptide 1
- Cyp27a1
- Cytochrome P450 family 27 subfamily A polypeptide 1
- Cyp39a1
- Cytochrome P450 family 39 subfamily A polypeptide 1
- Cyp46a1
- Cytochrome P450 family 46 subfamily A polypeptide 1
- Cyp51
- Cytochrome P450 family 51
- GCGR
- glucagon receptor
- GLP
- glucagon-like peptide
- GRA
- glucagon receptor antagonist
- 7-HCO
- 7-α-hydroxy-4-cholesten-3-one
- HDL-c
- HDL cholesterol
- hGCGR
- humanized glucagon receptor
- HMGCR
- HMG-CoA reductase
- LDL-c
- LDL cholesterol
- LDLR
- LDL receptor
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- QD
- once daily
- Srebp
- sterol regulatory element-binding protein
- T2DM
- type 2 diabetes mellitus
This work was supported by Merck Research Laboratories, Merck & Co., Inc.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. 1970. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N. Engl. J. Med. 283: 109–115. [DOI] [PubMed] [Google Scholar]
- 2.Unger R. H., Cherrington A. D. 2012. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J. Clin. Invest. 122: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel S. S., Xu L., Andryuk P. J., Davies M. J., Amatruda J., Kaufman K., Goldstein B. J. 2011. Efficacy and tolerability of MK-0893, a glucagon receptor antagonist (GRA), in patients with type 2 diabetes (T2DM). Diabetes. 60(Suppl 1): A85. [Google Scholar]
- 4.Engel S. S., Reitman M., Xu L., Andryuk P. J., Davies M. J., Kaufman K., Goldstein B. J. 2012. Glycemic and lipid effects of the short-acting glucagon receptor antagonist MK-3577 in patients with type 2 diabetes. Diabetes. 61(Suppl 1): A266. [Google Scholar]
- 5.Kazda C. M., Headlee S. A., Ding Y., Kelly R. P., Garhyan P., Hardy T. A., Lewin A. J. 2013. The glucagon receptor antagonist LY2409021 significantly lowers HbA1c and is well tolerated in patients with type 2 diabetes mellitus: a 24-week phase 2 study. Diabetologia. 56(Suppl 1): S391. [Google Scholar]
- 6.Kelly R. P., Garhyan P., Raddad E., Fu H., Lim C. N., Prince M. J., Pinaire J. A., Loh M. T., Deeg M. A. 2015. Short-term administration of the glucagon receptor antagonist LY2409021 lowers blood glucose in healthy subjects and patients with type 2 diabetes. Diabetes Obes. Metab. 17: 414–422. [DOI] [PubMed] [Google Scholar]
- 7.Morgan E., Smith A., Watts L., Xia S., Cheng W., Geary R., Bhanot S. 2014. ISIS-GCGRRX, an antisense glucagon receptor antagonist, caused rapid, robust, and sustained improvements in glycemic control without changes in BW, BP, lipids, or hypoglycemia in T2DM patients on stable metformin therapy. Diabetes. 63(Suppl 1A): LB28. [Google Scholar]
- 8.Colhoun H. M., Betteridge D. J., Durrington P. N., Hitman G. A., Neil H. A., Livingstone S. J., Thomason M. J., Mackness M. I., Charlton-Menys V., Fuller J. H. 2004. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 364: 685–696. [DOI] [PubMed] [Google Scholar]
- 9.Betteridge J. 2005. Benefits of lipid-lowering therapy in patients with type 2 diabetes mellitus. Am. J. Med. 118(Suppl 12A): 10–15. [DOI] [PubMed] [Google Scholar]
- 10.Howard B. V., Robbins D. C., Sievers M. L., Lee E. T., Rhoades D., Devereux R. B., Cowan L. D., Gray R. S., Welty T. K., Go O. T., et al. 2000. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscler. Thromb. Vasc. Biol. 20: 830–835. [DOI] [PubMed] [Google Scholar]
- 11.Shiao L. L., Cascieri M. A., Trumbauer M., Chen H., Sullivan K. A. 1999. Generation of mice expressing the human glucagon receptor with a direct replacement vector. Transgenic Res. 8: 295–302. [DOI] [PubMed] [Google Scholar]
- 12.Dallas-Yang Q., Shen X., Strowski M., Brady E., Saperstein R., Gibson R. E., Szalkowski D., Qureshi S. A., Candelore M. R., Fenyk-Melody J. E., et al. 2004. Hepatic glucagon receptor binding and glucose-lowering in vivo by peptidyl and non-peptidyl glucagon receptor antagonists. Eur. J. Pharmacol. 501: 225–234. [DOI] [PubMed] [Google Scholar]
- 13.Bosner M. S., Lange L. G., Stenson W. F., Ostlund R. E., Jr 1999. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J. Lipid Res. 40: 302–308. [PubMed] [Google Scholar]
- 14.Ni Y. G., Di Marco S., Condra J. H., Peterson L. B., Wang W., Wang F., Pandit S., Hammond H. A., Rosa R., Cummings R. T., et al. 2011. A PCSK9-binding antibody that structurally mimics the EGF(A) domain of LDL-receptor reduces LDL cholesterol in vivo. J. Lipid Res. 52: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro-Perez J., Briand F., Gagen K., Wang S. P., Chen Y., McLaren D. G., Shah V., Vreeken R. J., Hankemeier T., Sulpice T., et al. 2011. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J. Lipid Res. 52: 1965–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 17.Shah V., Herath K., Previs S. F., Hubbard B. K., Roddy T. P. 2010. Headspace analyses of acetone: a rapid method for measuring the 2H-labeling of water. Anal. Biochem. 404: 235–237. [DOI] [PubMed] [Google Scholar]
- 18.Previs S. F., Mahsut A., Kulick A., Dunn K., Andrews-Kelly G., Johnson C., Bhat G., Herath K., Miller P. L., Wang S. P., et al. 2011. Quantifying cholesterol synthesis in vivo using (2)H(2)O: enabling back-to-back studies in the same subject. J. Lipid Res. 52: 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turley S. D., Herndon M. W., Dietschy J. M. 1994. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J. Lipid Res. 35: 328–339. [PubMed] [Google Scholar]
- 20.Zilversmit D. B., Hughes L. B. 1974. Validation of a dual-isotope plasma ratio method for measurement of cholesterol absorption in rats. J. Lipid Res. 15: 465–473. [PubMed] [Google Scholar]
- 21.Zhou H., Li W., Wang S. P., Mendoza V., Rosa R., Hubert J., Herath K., McLaughlin T., Rohm R. J., Lassman M. E., et al. 2012. Quantifying apoprotein synthesis in rodents: coupling LC-MS/MS analyses with the administration of labeled water. J. Lipid Res. 53: 1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen K. K., Previs S. F., Zhu L., Herath K., Wang S. P., Bhat G., Hu G., Miller P. L., McLaren D. G., Shin M. K., et al. 2012. Demonstration of diet-induced decoupling of fatty acid and cholesterol synthesis by combining gene expression array and 2H2O quantification. Am. J. Physiol. Endocrinol. Metab. 302: E209–E217. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Y., Guo J., Candelore M. R., Liang R., Miller C., Dallas-Yang Q., Jiang G., McCann P. E., Qureshi S. A., Tong X., et al. 2012. Discovery of a novel glucagon receptor antagonist N-[(4-{(1S)-1-[3-(3, 5-dichlorophenyl)-5-(6-methoxynaphthalen-2-yl)-1H-pyrazol-1-yl]ethyl}phenyl)carbo nyl]-β-alanine (MK-0893) for the treatment of type II diabetes. J. Med. Chem. 55: 6137–6148. [DOI] [PubMed] [Google Scholar]
- 24.Mu J., Qureshi S. A., Brady E. J., Muise E. S., Candelore M. R., Jiang G., Li Z., Wu M. S., Yang X., Dallas-Yang Q., et al. 2012. Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist. PLoS One. 7: e49572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conner S. E., Zhu G., inventors; Eli Lilly and Company, assignee. Glucagon receptor antagonists, preparation and therapeutic uses. United States patent US7816557. 2010 Oct 19. [Google Scholar]
- 26.Engel S. S., Teng R., Edwards R. J., Davies M. J., Kaufman K., Goldstein B. J. 2011. Efficacy and safety of the glucagon receptor antagonist, MK-0893, in combination with metformin or sitagliptin in patients with type 2 diabetes mellitus. Diabetologia. 54(Suppl 1): S86. [Google Scholar]
- 27.Edwards P. A., Lemongello D., Fogelman A. M. 1979. The effect of glucagon, norepinephrine, and dibutyryl cyclic AMP on cholesterol efflux and on the activity of 3-hydroxy-3-methylglutaryl CoA reductase in rat hepatocytes. J. Lipid Res. 20: 2–7. [PubMed] [Google Scholar]
- 28.Wang S. P., Daniels E., Chen Y., Castro-Perez J., Zhou H., Akinsanya K. O., Previs S. F., Roddy T. P., Johns D. G. 2013. In vivo effects of anacetrapib on prebeta HDL: improvement in HDL remodeling without effects on cholesterol absorption. J. Lipid Res. 54: 2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz J. R., Tu H., Luk A., Repa J. J., Medina J. C., Li L., Schwendner S., Wang S., Thoolen M., Mangelsdorf D. J., et al. 2000. Role of LXRs in control of lipogenesis. Genes Dev. 14: 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miettinen T. A., Tilvis R. S., Kesaniemi Y. A. 1989. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism. 38: 136–140. [DOI] [PubMed] [Google Scholar]
- 31.Lakoski S. G., Xu F., Vega G. L., Grundy S. M., Chandalia M., Lam C., Lowe R. S., Stepanavage M. E., Musliner T. A., Cohen J. C., et al. 2010. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. J. Clin. Endocrinol. Metab. 95: 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelling R. W., Du X. Q., Dichmann D. S., Romer J., Huang H., Cui L., Obici S., Tang B., Holst J. J., Fledelius C., et al. 2003. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc. Natl. Acad. Sci. USA. 100: 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali S., Lamont B. J., Charron M. J., Drucker D. J. 2011. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J. Clin. Invest. 121: 1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drucker D. J. 2001. Glucagon-like peptide 2. J. Clin. Endocrinol. Metab. 86: 1759–1764. [DOI] [PubMed] [Google Scholar]
- 35.Grigoryan M., Kedees M. H., Charron M. J., Guz Y., Teitelman G. 2012. Regulation of mouse intestinal L cell progenitors proliferation by the glucagon family of peptides. Endocrinology. 153: 3076–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hein G. J., Baker C., Hsieh J., Farr S., Adeli K. 2013. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes. 62: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drucker D. J., Erlich P., Asa S. L., Brubaker P. L. 1996. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA. 93: 7911–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudhop T., Reber M., Tribble D., Sapre A., Taggart W., Gibbons P., Musliner T., von Bergmann K., Lutjohann D. 2009. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J. Lipid Res. 50: 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caren R., Carbo L. 1956. Pancreatic alpha-cell function in relation to cholesterol metabolism. J. Clin. Endocrinol. Metab. 16: 507–516. [DOI] [PubMed] [Google Scholar]
- 40.Caren R., Corbo L. 1960. Glucagon and cholesterol metabolism. Metabolism. 9: 938–945. [PubMed] [Google Scholar]
- 41.Eaton R. P. 1973. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. J. Lipid Res. 14: 312–318. [PubMed] [Google Scholar]
- 42.Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. 1975. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J. Clin. Invest. 56: 1420–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aubry F., Marcel Y. L., Davignon J. 1974. Effects of glucagon on plasma lipids in different types of primary hyperlipoproteinemia. Metabolism. 23: 225–238. [DOI] [PubMed] [Google Scholar]
- 44.Gu W., Yan H., Winters K. A., Komorowski R., Vonderfecht S., Atangan L., Sivits G., Hill D., Yang J., Bi V., et al. 2009. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J. Pharmacol. Exp. Ther. 331: 871–881. [DOI] [PubMed] [Google Scholar]
- 45.Persson L., Galman C., Angelin B., Rudling M. 2009. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology. 150: 1140–1146. [DOI] [PubMed] [Google Scholar]
- 46.Rudling M., Angelin B. 1993. Stimulation of rat hepatic low density lipoprotein receptors by glucagon. Evidence of a novel regulatory mechanism in vivo. J. Clin. Invest. 91: 2796–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han S., Akiyama T. E., Previs S. F., Herath K., Roddy T. P., Jensen K. K., Guan H. P., Murphy B. A., McNamara L. A., Shen X., et al. 2013. Effects of small interfering RNA-mediated hepatic glucagon receptor inhibition on lipid metabolism in db/db mice. J. Lipid Res. 54: 2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuguin P. M., Charron M. J. 2011. Novel insight into glucagon receptor action: lessons from knockout and transgenic mouse models. Diabetes Obes. Metab. 13(Suppl 1): 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsieh J., Longuet C., Maida A., Bahrami J., Xu E., Baker C. L., Brubaker P. L., Drucker D. J., Adeli K. 2009. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 137: 997–1005. [DOI] [PubMed] [Google Scholar]
- 50.Woollett L. A., Buckley D. D., Yao L., Jones P. J., Granholm N. A., Tolley E. A., Tso P., Heubi J. E. 2004. Cholic acid supplementation enhances cholesterol absorption in humans. Gastroenterology. 126: 724–731. [DOI] [PubMed] [Google Scholar]
- 51.Tremblay A. J., Lamarche B., Lemelin V., Hoos L., Benjannet S., Seidah N. G., Davis H. R., Jr., Couture P. 2011. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J. Lipid Res. 52: 558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engelking L. J., McFarlane M. R., Li C. K., Liang G. 2012. Blockade of cholesterol absorption by ezetimibe reveals a complex homeostatic network in enterocytes. J. Lipid Res. 53: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker H. E., Wallis K., le Roux C. W., Wong K. Y., Reimann F., Gribble F. M. 2012. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 165: 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haeusler R. A., Astiarraga B., Camastra S., Accili D., Ferrannini E. 2013. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 62: 4184–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolen S., Feldman L., Vassy J., Wilson L., Yeh H. C., Marinopoulos S., Wiley C., Selvin E., Wilson R., Bass E. B., et al. 2007. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann. Intern. Med. 147: 386–399. [DOI] [PubMed] [Google Scholar]
- 56.Alrefai W. A., Annaba F., Sarwar Z., Dwivedi A., Saksena S., Singla A., Dudeja P. K., Gill R. K. 2007. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: Role of sterol regulatory element binding protein 2. Am. J. Physiol. Gastrointest. Liver Physiol. 292: G369–G376. [DOI] [PubMed] [Google Scholar]
- 57.Davis H. R., Jr., Zhu L. J., Hoos L. M., Tetzloff G., Maguire M., Liu J., Yao X., Iyer S. P., Lam M. H., Lund E. G., et al. 2004. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279: 33586–33592. [DOI] [PubMed] [Google Scholar]
- 58.Pramfalk C., Jiang Z. Y., Parini P. 2011. Hepatic Niemann-Pick C1-like 1. Curr. Opin. Lipidol. 22: 225–230. [DOI] [PubMed] [Google Scholar]
- 59.Davis H. R., Jr., Tershakovec A. M., Tomassini J. E., Musliner T. 2011. Intestinal sterol transporters and cholesterol absorption inhibition. Curr. Opin. Lipidol. 22: 467–478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.