Abstract
The thromboxane synthase converts prostaglandin H2 to thromboxane A2 and malondialdehyde (MDA) in approximately equimolar amounts. A reactive dicarbonyl, MDA forms covalent adducts of amino groups, including the ε-amine of lysine, but the importance of this reaction in platelets was unknown. Utilizing a novel LC/MS/MS method for analysis of one of the MDA adducts, the dilysyl-MDA cross-link, we demonstrated that dilysyl-MDA cross-links in human platelets are formed following platelet activation via the cyclooxygenase (COX)-1/thromboxane synthase pathway. Salicylamine and analogs of salicylamine were shown to react with MDA preferentially, thereby preventing formation of lysine adducts. Dilysyl-MDA cross-links were measured in two diseases known to be associated with increased platelet activation. Levels of platelet dilysyl-MDA cross-links were increased by 2-fold in metabolic syndrome relative to healthy subjects, and by 1.9-fold in sickle cell disease (SCD). In patients with SCD, the reduction of platelet dilysyl-MDA cross-links following administration of nonsteroidal anti-inflammatory drug provided evidence that MDA modifications of platelet proteins in this disease are derived from the COX pathway. In summary, MDA adducts of platelet proteins that cross-link lysines are formed on platelet activation and are increased in diseases associated with platelet activation. These protein modifications can be prevented by salicylamine-related scavengers.
Keywords: mass spectrometry, thromboxane synthase, cross-link, metabolic syndrome, sickle cell disease, nonsteroidal anti-inflammatory drug, cyclooxygenase, salicylamine
Activation of platelets signals cytosolic phospholipase A2α activation and an explosive release of arachidonic acid, which is metabolized by cyclooxygenase (COX)-1 to prostaglandin H2 (PGH2). In platelets, the thromboxane synthase enzyme catalyzes conversion of PGH2 to both thromboxane A2 and malondialdehyde (MDA) in approximately equimolar amounts (1) (supplementary Fig. 1).
MDA is an electrophile that reacts with amino groups, including the ε-amine of protein lysines. Reaction of MDA with lysine in vitro leads to formation of adducts with several structures (2, 3), including one that results from the reaction of this dicarbonyl with two lysines to produce intra- and intermolecular cross-links of macromolecules. Such cross-links have been demonstrated when MDA is added to purified apoA-1 in vitro (2).
This evidence that MDA is a major product of the thromboxane synthase and can modify protein structure in vitro suggests a hypothesis that platelet activation could lead to modification of platelet proteins by MDA. However, there previously has been no evidence that this occurs.
The investigations reported here demonstrate that activation of platelets ex vivo leads to thromboxane synthase-dependent MDA modification of platelet proteins. A stable isotope dilution method for analysis for the dilysyl-MDA cross-link utilizing LC/MS/MS has been developed, making it possible to demonstrate increased levels of MDA adducts of platelet proteins in diseases that are associated with increased platelet activation.
METHODS
Chemicals and reagents
Ozagrel was from Cayman chemical Co. (Ann Arbor, MI). Tetramethoxypropane (TMP; precursor of MDA), arachidonic acid, sodium citrate, citric acid, lysine, and aspirin (acetylsalicylic acid) were from Sigma-Aldrich (St. Louis, MO). OasisTM HLB 1 cc cartridges containing 30 mg of poly(divinylbenzene-co-N-vinylpyrrolidone) copolymer, were obtained from Waters Corp. (Milford, MA). Lysine-[3H, 99%] was from American Radiolabeled Chemicals Inc. (St. Louis, MO), and lysine-[13C6, 99%] was from Cambridge Isotope Laboratories Inc. Salicylamine acetate (SA), 3-methoxysalicylamine acetate (3-MoSA), 5-ethylsalicylamine (EtSA), 5-methylsalicylamine (MeSA), and 4-hydroxybenzylamine (4-HoBA) were synthesized as previously described (4). PBS (10×) was from Fisher BioReagents-Fisher Scientific (Fair Lawn, NJ). Spin X centrifuge filters were purchased from Costar (Corning, NY). The conjugated monoclonal antibodies anti-CD62p (P-selectin) and R-phycoerythrin (PE)-conjugated anti-CD-41 were available from BD-Pharmingen (Franklin Lakes, NJ). All solvents were HPLC grade.
Equipment
A scintillation counter (liquid scintillation analyzer) Tri-Carb 1900TR was from PerkinElmer. HPLC was performed using a Perkin Elmer Series 200 system (Perkin Elmer Shelton, CT) consisting of a system controller, an LC pump, an autosampler, a column oven, a degasser, and a UV-vis detector. The analytical Aquasil C18 reverse phase column [250 × 4.6 mm inner diameter (ID)], from Thermo scientific Chromatography (Pittsburgh, PA), or a Phenomenex Kinetex 2.6 µM XB-C18 100A (75 × 2.1 mm ID), from Phenomenex (Torrance CA), was used. An LC system was connected to a Finnigan TSQ Quantum Triple Quadrupole mass spectrometer (Thermo Electron Inc., San Jose, CA) equipped with an electrospray source. LC/MS/MS Xcalibur software (version 1.3; ThermoFinnigan) was used to operate the instrument and to process the data. Aliquots of the samples were analyzed in the positive ion mode using an YMCTM-ODS-AQ (250 × 2.0 mm, 5 μm particle size) column from YMC Co. Ltd. (Allentown, PA).
Dilysyl-MDA cross-link adducts
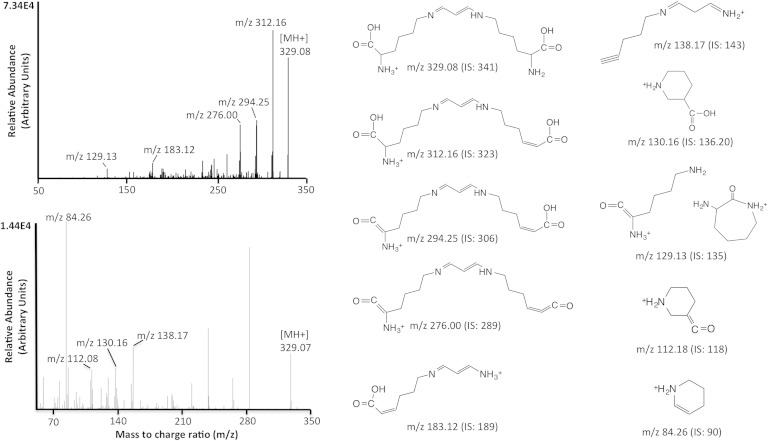
For the characterization of dilysyl-MDA cross-links, a mixture of [12C]lysine or [13C6]lysine and TMP (ratio 3:2) was incubated in 1 N HCl for 2 h at 37°C. The reaction was then neutralized and allowed to stand overnight at room temperature. The dilysyl-MDA cross-links were purified initially with solid phase extraction (SPE) columns (Oasis HLB cartridge), preconditioned with 2 ml of methanol and 2 ml of water. The samples were loaded and then washed twice with 2 ml of water. The dilysyl-MDA cross-links were eluted with methanol-ethyl acetate (1:1). The cartridge recovery was 99.83 ± 16.58%. Under nitrogen, the residual product was concentrated down to 100 μl under nitrogen stream and analyzed by LC/MS/MS in the positive ion mode as described below. For determination of product ions, collision-induced dissociation of molecular ions of putative dilysyl-MDA cross-link was performed from −10 to −45 eV and scanning product ions from m/z 50 to 350. Spectra shown were obtained at −15 eV and −28 eV (Fig. 1).
Fig. 1.
LC/ESI/MS/MS spectra and selected ion monitoring of dilysyl-MDA cross-link, and structure of product ions. The [MH]+ ions of dilysyl-MDA cross-link (m/z 329) were subjected to collision-induced dissociation (CID). The product ions were scanned from m/z 50 to 350. The spectra recorded at −15 (upper spectrum) and −28 eV (lower spectrum) are shown with the proposed structures of the major fragment ions. The corresponding m/z values for the internal standard (IS) are presented in parentheses.
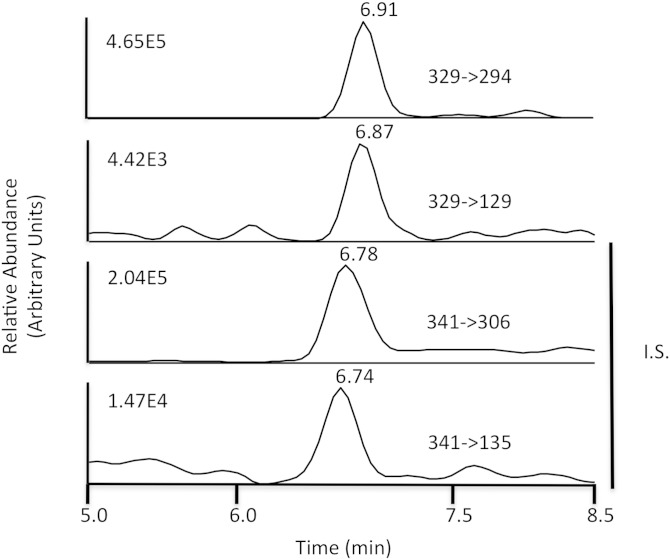
An IS of dilysyl-MDA cross-link was prepared as described above by reaction of TMP in the presence of [13C6]lysine and [3H]lysine. The dilysyl-MDA cross-links were extracted as described previously and purified by HPLC. Fractions were collected every 0.5 min, and the presence of radioactivity was determined using a scintillation counter. The fractions containing radioactive dilysyl-MDA cross-links ([3H]lysine tracer in IS) were combined and analyzed by LC/MS/MS. To achieve a higher peak intensity and better peak shape, optimization was done using a gradient of acetonitrile-water with an addition of 0.1% formic acid to the mobile phase. The development of the chromatographic system was focused on shortening retention times while preserving the chromatographic separation of the analytes from the matrix contaminants. This was achieved by using a flow rate of 0.2 ml/min with the gradient described in Methods. These ions had an acceptable retention time (6.9 min and 6.7 min, respectively; Fig. 2). The overall analysis time was 18 min including reequilibration time for the column with the initial mobile phase.
Fig. 2.
Typical selected reaction monitoring (SRM) chromatograms of the dilysyl-MDA cross-link. Dilysyl-MDA cross-links were purified by Oasis cartridge and HPLC, after being digested to single amino acid by step digestion with proteases. The fractions containing the radioactivity were pooled, concentrated, and analyzed by LC/MS/MS. SRM of specific transition ions for the precursor ions at m/z 329 (upper panels) or m/z 341 (IS, lower panels) are shown. The IS was prepared from reaction of MDA with lysine and is assumed to be cross-linked to the α-amine, thus yielding a shorter retention time.
The radioactivity of the solution was counted using a scintillation counter, and the concentration was calculated from the specific activity of the [3H]lysine. The dilysyl-MDA cross-link is stable: 99.23% was recovered after storage up to 9 months at −80°C.
Method validation procedure
Quality control (QC) samples were prepared from washed platelets obtained from aphorized platelet units obtained from the Vanderbilt Blood Bank. Aliquots of 3 mg of protein and all stock of dilysyl-MDA cross-link standard were stored at −80°C until used. The stability of dilysyl-MDA cross-links was assessed by analyzing samples stored at −80°C for up to 9 months. Carryover was evaluated by placing vials of blank solvent at several locations in the analysis set. Specificity and selectivity of the assay were assessed by comparing unactivated washed platelet samples with washed platelet samples activated with 5 μM arachidonic acid for 2 h.
To determine the overall recovery of the cartridge, digested QC sample was spiked with IS before or after SPE. The samples were purified and analyzed. The peak areas obtained in neat solution standards as A, the corresponding peak areas for postextraction spiked samples as B, and the peak areas for extracted samples as C were used to calculated the IIS (ionization suppression) and AE (absolute recovery) values as follows: IIS (%) = B/A × 100%; AE (%) = C/B × 100% (n = 3). The variability of the digestion was assessed by comparing the amount of dilysyl-MDA cross-links in samples split before or after digestion, and it was determined according to the following equation: % digestion = (amount of cross-links in sample digested after split/amount of cross-links in sample digested before split) × 100.
In order to assess the intra- and interbatch precision and accuracy of the assay, QC samples at 1.5, 2.1, 5.8, and 12.5 mg were analyzed. The intrabatch precision (repeatability) of the assay was evaluated by the relative standard deviation (% RSD) of three replicates, and the interbatch precision (reproducibility) was evaluated by the analysis of standard curve samples in three batches (in duplicate): % RSD = (SD/mean) × 100. The assay was considered to be acceptable if % RSD <15%.
To determine the linearity of the assay, we prepared 1.8 mM stock solution of dilysyl-MDA cross-link in water containing 0.1% formic acid. A dose-response curve ranging from 0.008 pg to 7.5 pg was prepared by weighing different amounts of synthetic dilysyl-MDA cross-link, and the area under the curve (AUC) was recorded by LC/MS/MS as described in Methods. Increasing amounts of dilysyl-MDA cross-links gave a linear response with y = 3.19e+006x + 442297, where y is the measured AUC of dilysyl-MDA cross-link and x is the weighed dilysyl-MDA cross-link in picograms. The 1/slope and the correlation coefficient (r2) of the calibration plot were 3.12e-007 and 0.93, respectively.
The limit of quantification (LOQ) and limit of detection (LOD) were calculated directly from the calibration plot. The calibration sample was prepared with [12C]lysine, trace of [3H]lysine, and TMP. The reaction mixture was extracted by SPE as previously described. The responses AUC of the selected fragment relative to the corresponding amount of adduct (in picograms) were used to construct standard curves by least square linear regression analysis. LOQ and LOD were calculated as 10σ/S and 3.3σ/S, respectively, where σ is the standard deviation of y-intercept of regression equation and S is the slope of the calibration plot (5).
Sample collection
Written informed consent was obtained from study participants, and human blood was obtained following a protocol approved by the Institutional Review Board of Vanderbilt University. Samples were obtained from three different populations: healthy subjects who had no component of metabolic syndrome (MetSyn) or sickle cell disease (SCD), patients with MetSyn, and patients with SCD. Patients with MetSyn were 35 to 75 years old and fit the MetSyn criteria in accord with the American Heart Association/National Heart, Lung, and Blood Institute criteria (6). Patients with SCD were 25 to 50 years old and were homozygous sickle (SS) or compound heterozygote with β-thalassemia (Sβ-thal) phenotype. All recruited patients were queried about their nonsteroidal anti-inflammatory drug (NSAID) use and enrolled only if they could confirm lack of NSAID use for at least 7 days prior to sample collection.
Preparation of washed platelets
Washed platelets were prepared following two different protocols depending on whether they were used for the in vitro studies in the mechanism by which MDA adducts are generated or for the in vivo measurements of protease-derived lysyl-MDA-lysyl cross-link (dilysyl-MDA cross-link) levels as a marker of platelet activation. The two protocols are described below.
In vitro platelet activation.
Blood obtained from healthy volunteers was collected into vacuum tubes containing citrate, from a peripheral vein using a 21-gauge needle, and washed platelets were prepared as previously described (7). The platelets were counted with a Coulter counter and diluted with resuspension buffer to a final concentration of 300,000 platelets/μl and aliquoted in volumes corresponding to 500 µl. The aliquots were preincubated for 40 min at room temperature with vehicle, the thromboxane synthase inhibitor ozagrel (100 µM), aspirin (100 µM), or the MDA scavengers SA (1.5 mM) or 3-MoSA (1.5 mM). Next, arachidonic acid (5 µM) was added, and the platelets were incubated for an additional 2 h at room temperature. The reactions were stopped by adding indomethacin (100 µM) and the aldehyde scavenger pyridoxamine (1 mM) for 30 min at 4°C to inhibit all residual COX-1 activity and to prevent formation of MDA adducts on proteins during the purification process, respectively. An aliquot (5 µl) of sample was used for protein estimations using a BCA assay.
In vivo platelet activation: MetSyn and SCD patients.
Formation of MDA adducts on platelet proteins during platelet activation in vitro suggested the hypothesis that MDA-protein adducts in platelets would be elevated in states of chronic platelet activation in vivo. Because most proteins in the anuclear platelet exhibit very little turnover, covalent adducts of these proteins would be expected to accumulate in states of ongoing platelet activation. Accordingly, the levels of dilysyl-MDA cross-links in platelets were measured in two conditions in which there is evidence of in vivo platelet activation, MetSyn (8, 9) and SCD (8, 9), and compared with the levels in platelets from healthy subjects isolated from blood collected in the same conditions.
Blood was collected into a 5 ml syringe containing 0.4% sodium citrate, aspirin (100 µM), apyrase (2 U), carbaprostacyclin (1 µM), and SQ 29,548 (10 nM) final concentration (to prevent ex vivo platelet activation). The blood was incubated at room temperature for 40 min prior to preparation of washed platelets and protein quantification, which was performed as described above. In all instances, two aliquots of washed platelets from the QC were processed in parallel. Indomethacin (100 µM) and pyridoxamine (1 mM) were added prior protein digestion as described above.
Preparation of QC samples from washed platelets
Human platelet concentrate obtained from the Blood Bank at Vanderbilt University Hospital was acidified to pH 6.4 with 0.15 M citric acid. Pyridoxamine (1 mM final concentration) and indomethacin (100 µM final concentration) were added to the concentrate and incubated for 30 min at room temperature. Platelets were then centrifuged at 1,000 g for 10 min. The pellet was resuspended in suspension buffer (8.3 mM sodium phosphate, pH 6.5, 0.109 M NaCl, and 5.5 mM glucose) at final concentration of 600,000 cells/µl, and 100 µl aliquots (2.5 mg of protein) were stored at −80°C until used.
Analysis of dilysyl-MDA cross-links in washed platelets by LC/ESI/MS/MS
Samples (∼3 mg of protein) were digested with proteases as previously described for lysyl-lactam adducts (7). In summary, the samples were subsequently heated at 95°C and allowed to cool at room temperature before step digestion with pronase (1 mg) at 37°C for 24 h. Pronase was inactivated by heating samples at 95°C for 10 min and then cooled to room temperature. Then, 1 µl of 500 mM aminopeptidase was added for every 1 mg of sample protein and incubated at 37°C for 24 h. Five nanograms of 13C6-dilysyl-MDA cross-link standard was added to each platelet sample, and dilysyl-MDA cross-links were purified using Oasis cartridge as described above. The eluate was dried under nitrogen flow to a volume of 100 µl, diluted up to 1 ml with a 0.1% formic acid aqueous solution, and filtered using a 0.22 µm nylon Spin-x centrifuge tube spun at 6,000 rpm for 5 min. Samples were purified by HPLC through the Thermo Scientific Aquasil C18 column. The fractions containing the radioactivity were pooled, concentrated in Oasis HLB cartridge, and then dried under nitrogen to a final volume of 40 µl.
Pooled HPLC fractions concentrated using Oasis were analyzed by LC/MS/MS using an YMC OD-AQ column. Solvent A (99.9% water/0.1% formic acid) and solvent B (99.9% acetonitrile (ACN)/0.1% formic acid) were filtered through a 0.45 μm filter prior to mixing and ultrasonically degassed after mixing. The gradient was as follows: 0–5 min 100–0% A, 5–7 min 0% A, 7–9 min 0–100% A, 9–15 min 100% A, with a flow rate of 0.2 ml/min. The mass spectrometer was operated in the positive ion mode, and the electrospray needle was maintained at 4,000 V. Nitrogen was used for both the sheath and auxiliary gas at pressures of 30 and 5 arbitrary units, respectively. The optimizing skimmer offset was set at 10, capillary temperature was 300°C, and the tube lens voltage was 195 V. SRM of specific transition ions for the precursor ions at m/z 329 → 294 (dilysyl-MDA cross-link) or m/z 341 → 306 (IS) at −15 eV was used. These settings were used during all experiments, including the validation procedure.
An SRM chromatogram of dilysyl-MDA cross-links from a representative experiment evaluating the effect of platelet activation on formation of the cross-links is depicted in Fig. 2, and this demonstrates that no interfering peaks from endogenous compounds were observed at the retention times for either the dilysyl-MDA cross-link or IS, demonstrating that our purification method removes potentially interfering endogenous compounds and that the observed signals are specific for the analytes.
Synthesis of scavenger-MDA adducts
A mixture of SA or 3-MoSA and TMP (0.1 mmol each) was incubated in 1 N HCl (1 ml) for 2 h, at 37°C. The reaction was then neutralized with 10 N NaOH and incubated overnight at room temperature. The scavenger-MDA adducts were extracted three times with 500 µl of ethyl acetate. The extracts were pooled and dried down under nitrogen stream, resuspended in 100 µl of ACN-water (1:1, v/v), vortexed, and filtered through a 0.22 μm spin X column. The scavenger-MDA adducts were analyzed by direct infusion on the TSQ Quantum triple quadrupole mass spectrometer equipped with a standard ESI source.
On a larger scale, TMP (0.84 ml, 5 mmol) was stirred with 1 N HCl (10 ml) at room temperature and diluted with water (90 ml). It was neutralized with K2HPO4 (7 g) and treated with SA (0.9 g, 5 mmol) or MoSA (1.06 g, 5 mmol) for 4 h. The adduct was extracted with ethyl acetate (4 × 20 ml). After removal of solvents, the adduct was purified on a column of silica with ethyl acetate as eluent, yield 30%.
Determination of the rate of reactivity of the scavengers with MDA in vitro
Nα-Acetyllysine with or without SA, 3-MoSA, or 4-HoBA was incubated at 37°C in the presence of MDA (ratio 1:1) in 0.1 M phosphate, pH 7.4. After 4 h, 10 μl aliquots was diluted to 200 μl with solvent A and analyzed by HPLC. Solvent A consisted to 20% methanol/0.2% formic acid and solvent B was 99.8% methanol/0.2% formic acid. The gradient was as follows: 0–1 min 100% A, 1–7 min 100 –0% A, 7–14 min 0% B. A Phenomenex Kinetex column at a flow rate of 0.2 ml/min and the absorbance at 280 nm were used.
LC/MS/MS quantification of scavenger-MDA adducts from washed platelets
Washed platelets were activated as described above. The scavenger-MDA adducts were extracted three times with 500 µl of ethyl acetate from 50 µl of the reaction mixture, as described above. The extract was dried down, resuspended in 100 µl of ACN-water (1:1, v/v), vortexed, and filtered through a 0.22 μm spin X column. The reactions were analyzed by LC/MS/MS using the column and gradient as described above. The mass spectrometer was operated in the positive ion mode, and the spray voltage was maintained at 5,000 V. Nitrogen was used for the sheath gas and auxiliary gas at pressures of 30 and 5 arbitrary units, respectively. The optimized skimmer offset was set at 10, capillary temperature was 300°C, and the tube lens voltage for both compounds was set to 49. SRM of specific transition ions for the precursor ions at m/z 178 → 106 (SA-MDA adduct) or m/z 208 → 136 (3-MoSA-MDA adduct) at −15 eV was used.
Quantification of thromboxane B2
Serum thromboxane B2 (sTxB2) was measured as an indicator of inhibition of platelet COX activity from blood drawn into noncitrated vacuum tubes. Serum from patients was prepared by incubating the blood at 37°C for 45 min and then centrifuged at 3,200 g for 15 min. Serum was separated and stored at −80°C until analysis. For the ex vivo experiment, 50 µl of washed platelets was used. sTxB2 was assayed by stable isotope dilution GC/MS with selective ion monitoring as described previously (10).
Analysis of P-selectin expression
An aliquot of citrated blood (10 μl) was added to 980 μl of PBS containing PE-conjugated 10 μl anti-CD-41a antibody and 10 μl allophycocyanin-conjugated anti-P-selectin antibody (CD62P), then incubated for 30 min at room temperature in the dark. At this time, platelets were fixed by adding 100 μl of 2% formaldehyde in PBS for 45 min and analyzed by flow cytometry as described below.
Analysis of reticulated platelets
Reticulated platelets were characterized by measuring platelets positive for staining with thiazole orange following a method described by Koike et al. (11) and modified as follows. A PE-conjugated anti-CD-41 an antibody (BD-Pharmingen) (10 μl) was added to 980 μl of PBS containing thiazole orange at 100 ng/ml. An aliquot of whole blood (10 μl) was added to this tube, incubated for 30 min at room temperature in the dark, and platelets were fixed by adding 100 μl of 2% formaldehyde in PBS, for 45 min. The platelets were then analyzed by flow cytometry as described below.
Flow cytometry analysis
Expression of markers of platelet activation was performed by flow cytometry analysis as described by Faull et al. (12). Single color staining controls were included in each assay to facilitate proper fluorescence compensation. Samples were analyzed on a FACS Canto II (BD Biosciences) (13). Increase in mean fluorescence intensity and % positive cells were recorded. Each time point and dose were compared with an unstimulated control, and data were expressed as the fold increases over unstimulated conditions or as the absolute values according to the experimental design.
Statistical methods
Statistical analysis was performed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA). Data are expressed as mean ± SEM, unless specified otherwise. The statistical significance was determined using one-way ANOVA (Tukey’s multiple comparisons test) or t-test (two-tailed) with Welch’s correction.
RESULTS
Formation and characterization of dilysyl-MDA cross-links
We examined the different adducts formed by the reaction of the amino acid lysine with MDA derived from TMP, an MDA precursor, using full-scan MS. Three major covalent adducts were identified: i) Nε-propenal-lysine (m/z 201), ii) dihydropyridine (DHP)-lysine (m/z 281), and iii) the N,N’-disubstituted 1-amino-3-iminopropenal cross-link formed by reaction of MDA with two lysines (dilysyl-MDA cross-link; m/z 329) (supplementary Fig. 2). Because both the Nε-propenal-lysine and the DHP-lysine adducts still contain aldehyde moieties, they can further react with free amines during sample processing. We analyzed the DHP lysyl adduct by MS before and after incubation with the aldehyde scavenger pyridoxamine. The data presented in supplementary Fig. 3 demonstrate that the DHP lysyl adduct still reacts with free amines, causing its disappearance and the concomitant appearance of the dipyridoxamine adduct, making them unsuitable for quantitation. In contrast, the dilysyl-MDA cross-link is no longer reactive and thus was chosen for quantification.
The full mass spectra for the dilysyl-MDA cross-link and its IS adduct revealed predominant peaks at m/z 329 and m/z 341, respectively, as protonated molecular ions ([M+H]+) in LC/MS/MS. These precursor ions were fragmented, and the product ion spectra were obtained. The corresponding fragments in the CID spectra obtained at −15 eV and −28 eV are shown in Fig. 1. The probable cleavage reactions of dilysyl-MDA cross-link (m/z 329) are presented in Fig. 1. The ion fragment at m/z 312, corresponding to the deaminated dilysyl-MDA cross-link, is seen at relatively low voltages, followed by dehydration to produce m/z 294. A second dehydration from m/z 294 will produce the product ion m/z 276. The ion m/z 183 corresponds to the loss of one lysine from m/z 294, while m/z 129 is the cyclization of the lysine fragment (Fig. 1). The fragmentation of the IS (at m/z 341) is indicated in parentheses (Fig. 1).
To ascertain that the fragmentation pattern observed for the dilysyl-MDA cross-link is different from the other MDA adduct structures, we purified both the Nε-propenal-lysine and the DHP-lysine adducts and analyzed them by LC/ESI/MS/MS. The corresponding fragments in the CID spectra obtained at −15 eV for the Nε-propenal-lysine adduct and at −21 eV and −28 eV for the DHP-lysine adduct are shown in supplementary Figs. 4 and 5, respectively.
SRM chromatograms for the transitions of m/z 329 to m/z 294 and 129 (dilysyl-MDA cross-links), and the transitions of m/z 341 to m/z 306 and 135 ([13C12]dilysyl-MDA cross-links IS), are shown in Fig. 2. The fragment ions of the [13C12]dilysyl-MDA cross-link IS elute at a similar but slightly earlier time than the unlabeled dilysyl-MDA cross-link. The use of an initial SPE cartridge followed by HPLC purification minimized impurities in the sample extracts that could produce ion suppression of the target analytes. The use of SRM provided high selectivity, sensitivity, and intensity for both dilysyl-MDA cross-link and IS.
Validation of LC/MS/MS method to measure dilysyl-MDA cross-link
Assays were validated according to US Food and Drug Administration guidance on bioanalytical method validation (5). The measured area under the SRM curve for the dilysyl-MDA cross-link was linear with the amount of weighed dilysyl-MDA cross-link added to the assay. The LOD and LOQ were 2.1 pg and 7.1 pg, respectively (signal-to-noise ratio >3). These low values are indicative of the high sensitivity of the method. The overall variability of the digestion steps was 4.0%. The intra- and interday precisions were acceptable with relative standard deviation of 6.7% and 6.6%, respectively, suggesting that the method is reproducible for quantification of dilysyl-MDA cross-link in washed platelets.
Platelet activation leads to modification of platelet proteins by MDA
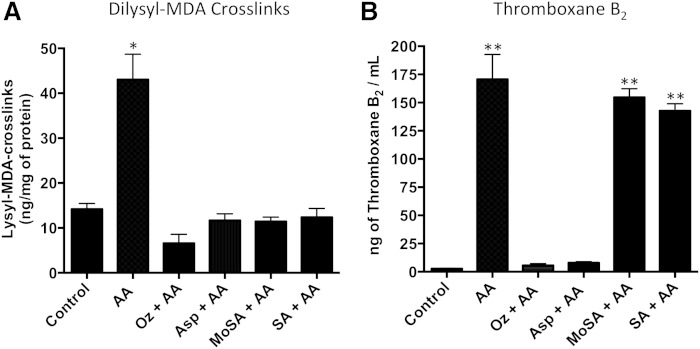
To determine whether platelet activation leads to modification of platelet proteins by MDA, we compared the level of protease-derived dilysyl-MDA cross-links in unactivated washed platelets with washed platelets activated with 5 μM arachidonic acid for 2 h. Dilysyl-MDA cross-links increased from a mean of 14.2 (SE ± 1.2) to 43.1 (SE ± 5.6) following platelet activation (P < 0.0001) (Fig. 3). To assess whether the cross-links come from proteins or from the pool of lysine available in platelets, we compared the levels of dilysyl-MDA cross-links from samples digested to single amino acids with those of samples in which the last protease (aminopeptidase) was omitted. Our data showed that 91% of the cross-links are derived from adducted proteins (supplementary Fig. 6).
Fig. 3.
Dilysyl-MDA cross-links are formed in activated human washed platelets in a thromboxane synthase-dependent way. Human washed platelets were preincubated with vehicle (subject) or either with 100 µM ozagrel (Oz), 100 µM aspirin (Asp), or 1.5 mM SA or 3-MoSA for 40 min. After incubation, 5 µM arachidonic acid (AA) was added for 2 h. A: Dilysyl-MDA cross-links were purified and quantified by isotopic dilution using LC/MS/MS. The product ions at m/z 294 for dilysyl-MDA cross-link and m/z 306 for the IS were monitored. * P < 0.0001 versus all other conditions. B: As a measure of COX activity, TxB2 was determined by GC/MS from 50 µl of washed platelet. ** P < 0.0001 versus subject, Oz or Asp. One-way ANOVA followed by Tukey’s multiple comparisons test was used (n > 6).
Inhibitors of thromboxane synthesis and scavengers of reactive dicarbonyls protect proteins from adduction by MDA in activated platelets
MDA can be synthesized in platelets by sequential metabolism of arachidonic via COX-1 followed by the thromboxane synthase (supplementary Fig. 1) (1). To ascertain that dilysyl-MDA cross-links were formed during ex vivo platelet activation via the COX-1-initiated thromboxane pathway, human washed platelets were preincubated with vehicle, the COX-1 inhibitor aspirin, or the thromboxane synthase inhibitor ozagrel prior addition of 5 µM arachidonic. Both aspirin and ozagrel reduced levels of the dilysysl-MDA cross-link to baseline levels (Fig. 3A). Together, these data demonstrate that MDA is generated in human platelets in a COX-1/thromboxane synthase-dependent fashion and forms covalent adducts with proteins.
We previously have developed small molecules that react with the γ-ketoaldehyde levuglandins three orders of magnitude faster than with lysine and have shown that these “scavengers” protect proteins from covalent adduction by these 1,4-dicarbonyls (4). We now find that these scavengers also protect proteins from covalent modifications by the 1,3-dicarbonyl MDA. Human washed platelets were preincubated with the scavengers SA or 3-MoSA, prior to activation with arachidonic acid. The data show that SA and 3-MoSA decreased levels of dilysysl-MDA cross-links to baseline levels (Fig. 3A), demonstrating that the scavengers are efficient in protecting proteins from adduction by MDA. We further evaluated the efficacy of other SA analogs to protect protein against adduction by MDA and found that EtSA and MeSA were as effective as scavengers as was SA (supplementary Fig. 7).
To exclude the possibility that the scavengers reduced MDA adducts by inhibiting COX-1, we measured the level of thromboxane B2 (TxB2) following platelet incubation with scavengers. In contrast to aspirin or ozagrel, which both inhibited TxB2 levels by 96%, neither SA nor 3-MoSA significantly affected TxB2 levels (Fig. 3B), demonstrating that the reduced formation of protein adducts by these compounds is due to direct scavenging of MDA and not by inhibiting COX-1.
Characterization of the product of reaction of scavengers with MDA
It was hypothesized that the scavengers prevent formation of MDA-lysine adducts by preferential formation of stable MDA-scavenger adducts, and that the greater reactivity of the scavengers with MDA results from the presence of 2-hydroxyl in SA and its analogs. The products of the reaction of SA and 3-MoSA with MDA were characterized by LC/MS/MS as the N-propenal adducts of the scavengers (supplementary Fig. 8). The structures of N-propenal-3-MoSA and N-propenal-SA adduct were confirmed by NMR (supplementary Fig. 9). The data show that the propenal adducts are stabilized by a hydrogen bond between the carbonyl of MDA and the 2-hydroxyl group on the ring.
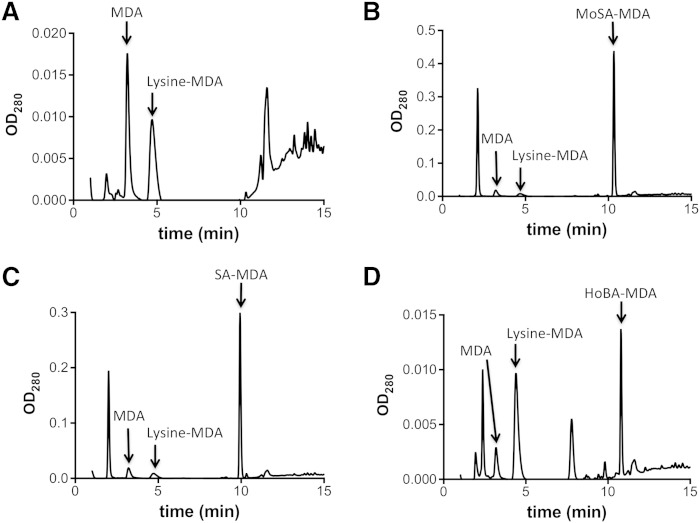
The formation of MDA adducts of the scavengers was compared with that of MDA adducts of lysine, using analysis of the products on HPLC (Fig. 4). Formation of the MDA adduct of 3-MoSA was 15.1-fold greater than that of the MDA-lysine adduct, and the formation of the MDA adduct of SA was 9.2-fold greater than that of the lysine adduct, indicating the strong preferential reactivity of MDA with these 2-hydroxybenzylamine scavengers. By contrast, 4-HoBA formed an MDA adduct in an amount even less than that of lysine (0.6-fold).
Fig. 4.
Lysine-MDA adducts formation is inhibited by SA or MoSA. Lysine and MDA were incubated at 37°C for 4 h in presence of vehicle (A), MoSA (B), SA (C), or 4-HoBA (D). The profiles were determined by HPLC as described in Methods.
The hydrogen bonding of MDA with the 2-hydroxyl of the scavengers likely facilitates the interaction of MDA with the amino group that initiates formation of the adduct, accounting for the preferential reactivity of MDA with the scavengers relative to reaction with the amino group of lysine. By contrast, the 4-hydroxyl of 4-HoBA is unable to participate in H bonding with MDA, likely due to its remoteness from the amine (supplementary Fig. 8).
Levels of scavenger-MDA adducts in human washed platelets
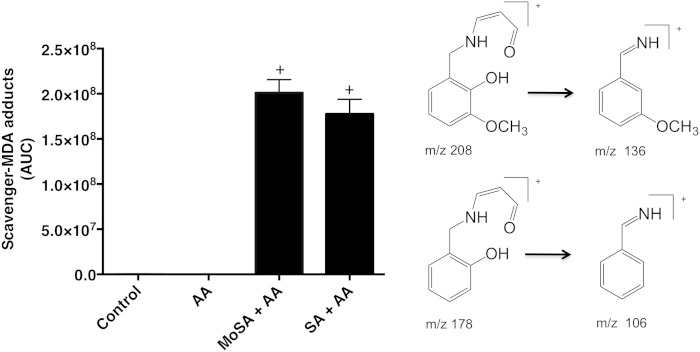
The formation of MDA adducts of SA and 3-MoSA in activated platelets was analyzed. The adducts were extracted from the platelet supernatant and analyzed by LC/MS/MS by monitoring the transitions of m/z = 208 to m/z = 136 and m/z = 178 to m/z = 106 for 3-MoSA-MDA and SA-MDA, respectively (Fig. 5). We find that inhibition of dilysyl-MDA cross-links on platelet proteins is associated with the concomitant appearance of the equivalent scavenger-MDA adduct (Fig. 5). These results demonstrate that the protective effect of the scavengers is due to their reaction with MDA and formation of a stable nonreactive adduct.
Fig. 5.
Scavenger-MDA adducts are formed when human washed platelets are activated in presence of scavengers. Human washed platelets were preincubated with MoSA or SA (1.5 mM final concentration) for 40 min, followed by arachidonic acid (AA; at 5 µM final concentration) for an additional 2 h. Scavenger-MDA adducts were extracted with ethyl acetate and analyzed by LC/MS/MS by monitoring the specific SRM transitions of m/z 208→136 (SA-MDA) and 178→106 (MoSA-MDA). + P < 0.0001 versus subject or AA. One-way ANOVA followed by Tukey’s multiple comparisons test was used (n > 7).
Increased formation of MDA adducts of platelet proteins in vivo in patients with MetSyn
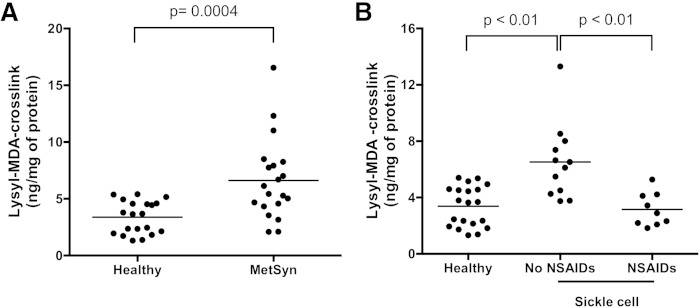
Analysis of dilysyl-MDA cross-links of platelet proteins provided significant discrimination between the group of patients with MetSyn and healthy subjects. Levels of dilysyl-MDA cross-links in the platelets of patients with MetSyn were increased 2-fold in comparison with those of healthy subjects (MetSyn, 6.63 ± 3.56 ng/mg protein; healthy subjects, 3.38 ± 1.43 ng/mg protein; P = 0.0002) (Fig. 6A). Fifty-five percent of the patients had values elevated above the normal range. The capacity of platelets to produce thromboxane A2 ex vivo was not different between patients with MetSyn and healthy subjects (sTxB2 was 265.2 ± 74.6 ng/ml in patients with MetSyn and 278.1 ± 114.2 ng/ml in healthy subjects).
Fig. 6.
Dilysyl-MDA cross-links isolated from washed platelets of patients with MetSyn or SCD. The dilysyl-MDA cross-link was purified and analyzed by LC/MS/MS. A: Levels of dilysyl-MDA cross-links were 2-fold higher in unactivated platelets from MetSyn patients (n = 25) than healthy volunteers [n = 20; P = 0.0002 by t-test (two-tailed) with Welch’s correction]. B: In platelets from SCD patients with no NSAIDs (n = 12), levels of dilysyl-MDA cross-links were 2-fold higher than from healthy volunteers (n = 20; P < 0.0001, SCD no NSAIDs vs. healthy patients or vs. SCD NSAIDs patients). One-way ANOVA followed by Tukey’s multiple comparisons test was used. The bars represent the mean for each category. No statistical significance was found between healthy volunteers and SCD NSAIDs (n = 9) patients.
The increases in dilysyl-MDA cross-links were compared with those of single platelet P-selectin expression, a marker of in vivo platelet activation. Single platelet levels of P-selectin were increased significantly but to a lesser extent (1.3-fold); levels in MetSyn were 4.73 ± 2.06% and in healthy subjects 3.66 ± 0.89% (P = 0.0002), with values above the upper limit of normal in 37.5% of patients (supplementary Fig. 10). Levels of reticulated platelets, indicators of platelet turnover, were increased only 1.2-fold in MetSyn (2.18 ± 1.55%) when compared with healthy subjects (1.85 ± 1.18%), and the difference was not significant (supplementary Fig. 10).
Increased formation of MDA adducts of platelet proteins in vivo in patients with SCD
The levels of dilysyl-MDA cross-links in protein from platelets of patients with SCD (6.52 ± 2.67 ng/mg of protein) were 2-fold higher than those of healthy subjects (3.38 ± 1.43 ng/mg of protein; P < 0.0001) (Fig. 6B). When patients were treated with the nonselective COX inhibitor ketorolac, during admission for vaso-occlusive crisis, the levels of platelet dilysyl-MDA cross-links were significantly lower (3.15 ± 1.18 ng/mg of protein), consistent with inhibition of the biosynthesis of MDA that is derived from COX-1. Verifying the inhibition of platelet COX-1 by ketorolac, levels of sTxB2 during ketorolac treatment were reduced from 219.7 ± 183.1 ng/ml to 68.9 ± 127.7 ng/ml.
DISCUSSION
We have demonstrated that platelet activation leads to formation of MDA adducts of platelet proteins via the COX-1/thromboxane synthase pathway. Metabolism of PGH2 by the thromboxane synthase yields MDA in amounts approximately equivalent to thromboxane A2 (1). As a reactive 1,3-dicarbonyl, MDA forms adducts of proteins, such as the Nε-propenal-lysine, that can further react with an additional amino group to form intra- and intermolecular cross-links.
Evidence that MDA adducts of platelet proteins are formed during activation of platelets ex vivo engendered the hypothesis that these modifications of platelet proteins also would occur in diseases in which platelet activation occurs in vivo. Development of an LC/MS/MS method for analysis of one of these adducts (the dilysyl-MDA cross-link) has made it possible to address this hypothesis.
Platelet function is disordered in MetSyn. Platelets from these patients are hyperactive when studied ex vivo (14–16), and there is evidence for in vivo platelet activation (14, 15, 17–19). We found that dilysyl-MDA cross-links were elevated in MetSyn to a mean level 2-fold greater than normal (P = 0.0002), and 52% of the patients had values greater than the upper limit of the normal range. Platelet activation is also a feature of SCD, and levels of dilysyl-MDA cross-links also are increased in platelets from patients with this disease (P < 0.0001). Thus, increased levels of MDA adducts of platelet proteins are correlated with diseases that produce platelet activation, recapitulating the formation of these adducts during ex vivo platelet activation. These findings raise the possibility that analysis of dilysyl-MDA cross-links could provide a biomarker for in vivo platelet dysfunction.
The origin of the MDA adducts from the platelet COX-1/thromboxane synthase pathway is clearly demonstrated in the experiments with normal platelets activated ex vivo, in which aspirin and the thromboxane synthase inhibitor ozagrel reduce the dilysyl-MDA cross-links to the levels in nonactivated platelets. A major contribution of the COX-1 pathway to the increased formation of dilysyl-MDA cross-links SCD is also suggested by the finding that therapeutic use of the COX inhibitor ketorolac in these patients was associated with a reduction of dilysyl-MDA cross-links to the level found in normal platelets; this occurred in conjunction with a 69% reduction in sTxB2. However, MDA is also a product of radical catalyzed lipid peroxidation, and reactive oxygen species are generated by platelet activation (20). Whereas normal platelets have robust antioxidant defenses and virtually all of MDA adduct formation is COX-1 derived, oxidant stress is evident in platelets in MetSyn. We have found an increase in F2-isoprostanes esterified to platelet lipids in MetSyn, and ascorbate levels are decreased (21, 22). Thus, it is possible that in diseases such as MetSyn, platelet activation could generate MDA via radical catalyzed lipid peroxidation in addition to that generated via COX-1. If that were the case in MetSyn, the in vivo formation of MDA-protein adducts as a marker of platelet dysfunction would be amplified.
These findings provide a basis for considering the dilysyl-MDA cross-link as a biomarker of in vivo platelet dysfunction in MetSyn. The alternative approach to assessing platelet activation-induced metabolism of arachidonic acid via the COX-1/thromboxane synthase pathway is based on our discovery that 11-dehydrothromboxane B2 (TxM) is a major metabolite of TxB2 in humans (23). An average of 70–80% of urinary TxM is derived from platelets (24), but the amount from nonplatelet sources is highly variable (10, 24, 25). Thus, the urinary excretion of TxM has been a useful indicator of large increases in platelet activation such as occurs in acute coronary syndrome (26) and antiphospholipid antibody syndrome (27, 28). However, assessing individual changes in urinary TxM closer to the normal range is problematic because the amounts derived from nonplatelet sources is variable; for example, nonplatelet production of TxB2 is increased in smokers (25). By contrast, formation of MDA specifically in the platelets is the likely source of virtually all of the dilysyl-MDA cross-links in platelets.
SA and its analogs 3-MoSA, EtSA, and MeSA prevent formation of MDA adducts of platelet proteins. Demonstration that MDA reacts preferentially with these scavengers to form covalent adducts of the scavengers provides a mechanism for their ability to protect proteins from the attack of MDA. In addition to the COX-1-dependent formation of the 1,3-dicarbonyl MDA upon platelet activation, platelet COX-1 is also the source of highly reactive 1,4-dicarbonyls, the levuglandins, which are formed from rearrangement of PGH2. We have shown previously that SA also reacts with the levuglandins to prevent formation of levuglandinyl adducts of platelet proteins (4). Thus, these 2-hydroxybenzylamines are highly reactive with both 1,3- and 1,4-dicarbonyls, acting to scavenge these and similar dicarbonyls to prevent them from reacting with amino groups of cellular molecules. The efficacy of these scavengers to prevent protein modification by MDA and levuglandins in the platelets is likely to be extended to other cells and to lipoproteins.
The discovery of MDA adducts of platelet proteins is not per se a basis for concluding that they lead to functional consequences. However, demonstration that platelet activation produces MDA-protein adducts and cross-linking now provides a hypothesis for research to determine whether there is a functional consequence of these protein modifications. MDA has been shown to modify the functions of several proteins. ApoA-1-induced cholesterol efflux is inhibited by MDA (2). Paraoxonase-1 catalytic activity is blocked by treatment of HDL with MDA (29), MDA inhibits nuclear translocation of aldehyde dehydrogenase (30), and it inhibits endothelial NO production (31). Exploration of the contribution of MDA and its protein adducts to platelet function will be enhanced by the availability of SA and its analogs that scavenge MDA but do not inhibit the COXs (4).
Supplementary Material
Footnotes
Abbreviations:
- ACN
- acetonitrile
- AUC
- area under the curve
- CID
- collision-induced dissociation
- COX
- cyclooxygenase
- DHP
- dihydropyridine
- EtSA
- 5-ethylsalicylamine
- 4-HoBA
- 4-hydroxybenzylamine
- IS
- internal standard
- LOD
- limit of detection
- LOQ
- limit of quantification
- MDA
- malondialdehyde
- MeSA
- 5-methylsalicylamine
- MetSyn
- metabolic syndrome
- 3-MoSA
- 3-methoxysalicylamine acetate
- NSAID
- nonsteroidal anti-inflammatory drug
- PE
- R-phycoerythrin
- PGH2
- prostaglandin H2
- QC
- quality control
- RSD
- relative standard deviation
- SA
- salicylamine acetate
- SCD
- sickle cell disease
- SPE
- solid phase extraction
- SRM
- selected reaction monitoring
- sTxB2
- serum thromboxane B2
- TMP
- tetramethoxypropane
- TxB2
- thromboxane B2
- TxM
- 11-dehydrothromboxane B2
This work was supported in part by the Specialized Centers of Clinically Oriented Research in Hemostatic and Thrombotic Diseases (P50HL81009 and RO1 GM087603). R. Sosa received support from National Institutes of Health Training Grant T32 GM007569-36 and American Heart Association Award 13FTF17400011. J. A. Oates holds the Thomas F. Frist, Sr. Chair in Medicine.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Hecker M., Haurand M., Ullrich V., Diczfalusy U., Hammarstrom S. 1987. Products, kinetics, and substrate specificity of homogeneous thromboxane synthase from human platelets: development of a novel enzyme assay. Arch. Biochem. Biophys. 254: 124–135. [DOI] [PubMed] [Google Scholar]
- 2.Shao B., Pennathur S., Pagani I., Oda M. N., Witztum J. L., Oram J. F., Heinecke J. W. 2010. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J. Biol. Chem. 285: 18473–18484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida K. 2000. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 28: 1685–1696. [DOI] [PubMed] [Google Scholar]
- 4.Zagol-Ikapitte I., Amarnath V., Bala M., Roberts L. J., II, Oates J. A., Boutaud O. 2010. Characterization of scavengers of gamma-ketoaldehydes that do not inhibit prostaglandin biosynthesis. Chem. Res. Toxicol. 23: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, and Center for Veterinary Medicine. 2001. Guidance for industry: bioanalytical method validation. Accessed September 22, 2015, at http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070107.pdf.
- 6.Grundy S. M., Cleeman J. I., Daniels S. R., Donato K. A., Eckel R. H., Franklin B. A., Gordon D. J., Krauss R. M., Savage P. J., Smith S. C., Jr, et al. 2005. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 7.Boutaud O., Li J., Zagol I., Shipp E. A., Davies S. S., Roberts L. J., II, Oates J. A. 2003. Levuglandinyl adducts of proteins are formed via a prostaglandin H2 synthase-dependent pathway after platelet activation. J. Biol. Chem. 278: 16926–16928. [DOI] [PubMed] [Google Scholar]
- 8.Sugimori H., Tomoda F., Koike T., Kinuno H., Kurosaki H., Masutani T., Inoue H. 2012. Blood rheology and platelet function in untreated early-stage essential hypertensives complicated with metabolic syndrome. Int. J. Hypertens. 2012: 109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santilli F., Vazzana N., Liani R., Guagnano M. T., Davi G. 2012. Platelet activation in obesity and metabolic syndrome. Obes. Rev. 13: 27–42. [DOI] [PubMed] [Google Scholar]
- 10.Smith J. P., Haddad E. V., Taylor M. B., Oram D., Blakemore D., Chen Q., Boutaud O., Oates J. A. 2012. Suboptimal inhibition of platelet cyclooxygenase-1 by aspirin in metabolic syndrome. Hypertension. 59: 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koike Y., Yoneyama A., Shirai J., Ishida T., Shoda E., Miyazaki K., Sunaga S., Horie R., Aoki K., Koike K., et al. 1998. Evaluation of thrombopoiesis in thrombocytopenic disorders by simultaneous measurement of reticulated platelets of whole blood and serum thrombopoietin concentrations. Thromb. Haemost. 79: 1106–1110. [PubMed] [Google Scholar]
- 12.Faull R. J., Du X., Ginsberg M. H. 1994. Receptors on platelets. Methods Enzymol. 245: 183–194. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D., Benjamin E. J., Go A. S., Arnett D. K., Blaha M. J., Cushman M., de Ferranti S., Després J. P., Fullerton H. J., Howard V. J., et al. 2015. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131: e29–322. [DOI] [PubMed] [Google Scholar]
- 14.Serebruany V. L., Malinin A., Ong S., Atar D. 2008. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. J. Thromb. Thrombolysis. 25: 207–213. [DOI] [PubMed] [Google Scholar]
- 15.Vaduganathan M., Alviar C. L., Arikan M. E., Tellez A., Guthikonda S., DeLao T., Granada J. F., Kleiman N. S., Ballantyne C. M., Lev E. I. 2008. Platelet reactivity and response to aspirin in subjects with the metabolic syndrome. Am. Heart J. 156: 1002.e1–1002.e7. [DOI] [PubMed] [Google Scholar]
- 16.Vaidya D., Yanek L. R., Faraday N., Moy T. F., Becker L. C., Becker D. M. 2009. Native platelet aggregation and response to aspirin in persons with the metabolic syndrome and its components. Metab. Syndr. Relat. Disord. 7: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arteaga R. B., Chirinos J. A., Soriano A. O., Jy W., Horstman L., Jimenez J. J., Mendez A., Ferreira A., de Marchena E., Ahn Y. S. 2006. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am. J. Cardiol. 98: 70–74. [DOI] [PubMed] [Google Scholar]
- 18.Gokulakrishnan K., Deepa R., Mohan V., Gross M. D. 2006. Soluble P-selectin and CD40L levels in subjects with prediabetes, diabetes mellitus, and metabolic syndrome—the Chennai Urban Rural Epidemiology Study. Metabolism. 55: 237–242. [DOI] [PubMed] [Google Scholar]
- 19.Natal C., Restituto P., Inigo C., Colina I., Diez J., Varo N. 2008. The proinflammatory mediator CD40 ligand is increased in the metabolic syndrome and modulated by adiponectin. J. Clin. Endocrinol. Metab. 93: 2319–2327. [DOI] [PubMed] [Google Scholar]
- 20.Pignatelli P., Sanguigni V., Lenti L., Ferro D., Finocchi A., Rossi P., Violi F. 2004. gp91phox-dependent expression of platelet CD40 ligand. Circulation. 110: 1326–1329. [DOI] [PubMed] [Google Scholar]
- 21.Ford E. S., Mokdad A. H., Giles W. H., Brown D. W. 2003. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 52: 2346–2352. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri V. O., Grattagliano I., Portincasa P., Palasciano G. 2006. Systemic oxidative alterations are associated with visceral adiposity and liver steatosis in patients with metabolic syndrome. J. Nutr. 136: 3022–3026. [DOI] [PubMed] [Google Scholar]
- 23.Roberts L. J., II, Sweetman B. J., Payne N. A., Oates J. A. 1977. Metabolism of thromboxane B2 in man. Identification of the major urinary metabolite. J. Biol. Chem. 252: 7415–7417. [PubMed] [Google Scholar]
- 24.Catella F., FitzGerald G. A. 1987. Paired analysis of urinary thromboxane B2 metabolites in humans. Thromb. Res. 47: 647–656. [DOI] [PubMed] [Google Scholar]
- 25.McAdam B. F., Byrne D., Morrow J. D., Oates J. A. 2005. Contribution of cyclooxygenase-2 to elevated biosynthesis of thromboxane A2 and prostacyclin in cigarette smokers. Circulation. 112: 1024–1029. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald D. J., Roy L., Catella F., FitzGerald G. A. 1986. Platelet activation in unstable coronary disease. N. Engl. J. Med. 315: 983–989. [DOI] [PubMed] [Google Scholar]
- 27.Lellouche F., Martinuzzo M., Said P., Maclouf J., Carreras L. O. 1991. Imbalance of thromboxane/prostacyclin biosynthesis in patients with lupus anticoagulant. Blood. 78: 2894–2899. [PubMed] [Google Scholar]
- 28.Martinuzzo M. E., Forastiero R. R., Kordich L., Carreras L. O. 2001. Increased lipid peroxidation correlates with platelet activation but not with markers of endothelial cell and blood coagulation activation in patients with antiphospholipid antibodies. Br. J. Haematol. 114: 845–851. [DOI] [PubMed] [Google Scholar]
- 29.Blache D., Gautier T., Tietge U. J., Lagrost L. 2012. Activated platelets contribute to oxidized low-density lipoproteins and dysfunctional high-density lipoproteins through a phospholipase A2-dependent mechanism. FASEB J. 26: 927–937. [DOI] [PubMed] [Google Scholar]
- 30.Choi J. W., Kim J. H., Cho S. C., Ha M. K., Song K. Y., Youn H. D., Park S. C. 2011. Malondialdehyde inhibits an AMPK-mediated nuclear translocation and repression activity of ALDH2 in transcription. Biochem. Biophys. Res. Commun. 404: 400–406. [DOI] [PubMed] [Google Scholar]
- 31.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D. M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. 2011. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121: 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.