Abstract
Tocopherols and tocotrienols are metabolized via hydroxylation and oxidation of their hydrophobic side chain to generate 13′-hydroxychromanols (13′-OHs) and various carboxychromanols, which can be further metabolized by conjugation including sulfation. Recent studies indicate that long-chain carboxychromanols, especially 13′-carboxychromanol (13′-COOH), appear to be more bioactive than tocopherols in anti-inflammatory and anticancer actions. To understand the potential contribution of metabolites to vitamin E-mediated effects, an accurate assay is needed to evaluate bioavailability of these metabolites. Here we describe an LC/MS/MS assay for quantifying vitamin E metabolites using negative polarity ESI. This assay includes a reliable sample extraction procedure with efficacy of ≥ 89% and interday/intraday variation of 3–11% for major metabolites. To ensure accurate quantification, short-chain, long-chain, and sulfated carboxychromanols are included as external/internal standards. Using this assay, we observed that sulfated carboxychromanols are the primary metabolites in the plasma of rodents fed with γ-tocopherol or δ-tocopherol. Although plasma levels of 13′-COOHs and 13′-OHs are low, high concentrations of these compounds are found in feces. Our study demonstrates an LC/MS/MS assay for quantitation of sulfated and unconjugated vitamin E metabolites, and this assay will be useful for evaluating the role of these metabolites in vivo.
Keywords: tocopherol, tocotrienol, metabolism, liquid chromatography tandem mass spectrometry, sulfation
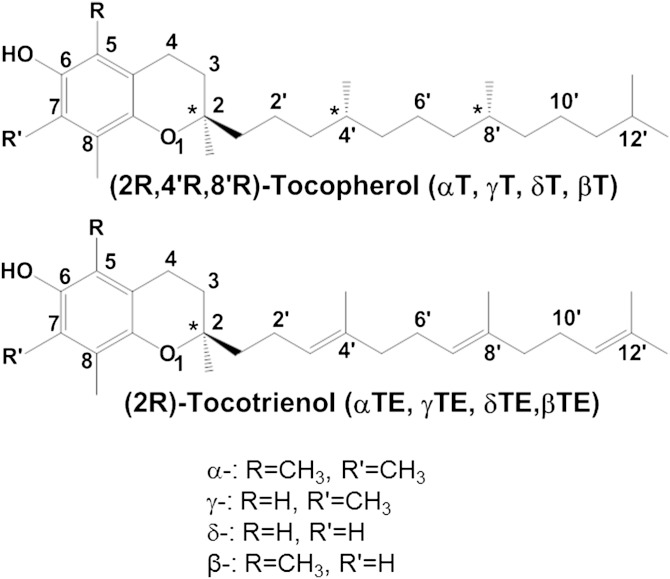
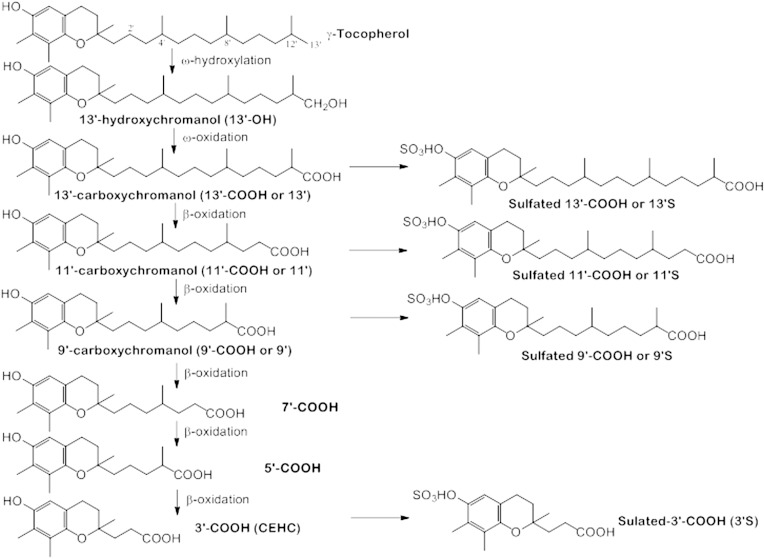
The vitamin E family comprises eight lipophilic antioxidants, α-, β-, γ- and δ-tocopherol (αT, βT, γT, and δT) and the corresponding tocotrienols (αTE, βTE, γTE, and δTE) (Fig. 1). Among the members of the vitamin E family, αT is the predominant form in tissues and is preferentially bound to a tocopherol transport protein that prevents αT from being extensively metabolized (1). In contrast, other vitamin E forms are largely metabolized in the liver via ω-hydroxylation and oxidation to generate 13′-hydroxychromanol (13′-OH) and 13′-carboxychromanol (13′-COOH), the latter of which is subsequently metabolized via β-oxidation to various shorter-chain carboxychromanols including the terminal metabolite observed in urine, 3′-COOH [or 2-(β-carboxyethyl)-6-hydroxychroman (CEHC)] (Fig. 2) (1–3). In parallel with β-oxidation, long-chain carboxychromanols and CEHCs appear to be conjugated via sulfation to form conjugated carboxychromanols (Fig. 2) (4, 5). The terminal metabolite CEHCs and their conjugated forms are found in the urine (6–11). On the other hand, unconjugated short-, mid-, and long-chain carboxychromanols have recently been detected in feces after supplementation of γT or δT (12–14).
Fig. 1.
Structures of vitamin E forms.
Fig. 2.
Vitamin E (γT) metabolism and metabolites. Vitamin E forms are metabolized via ω-hydroxylation and oxidation to generate 13′-OH and 13′-COOH, which is subsequently metabolized via β-oxidation to form 11′-, 9′-, 7′-, 5′-, and 3′-COOH. Sulfation of intermediate carboxychromanols appears to take place in parallel with β-oxidation in response to supplementation of vitamin E forms.
Although research on vitamin E has predominantly focused on tocopherols and tocotrienols, their metabolites have been shown to have biological activities that are stronger than or differ from the unmetabolized vitamins. For instance, γ-CEHC has been reported to have natriuratic activity in vitro and in animals (15). Recent studies including ours have demonstrated that long-chain carboxychromanols appear to have unique anti-inflammatory effects by inhibition of cyclooxygenases and 5-lipoxygenase and induced apoptosis in cancer cells (16–18). For these activities, long-chain carboxychromanols are much stronger than tocopherols or shorter-chain carboxychromanols. Despite these interesting findings of vitamin E metabolites, their role in vitamin E-mediated beneficial effects remains unclear. One of the reasons is that there is limited information regarding the bioavailability of these metabolites in tissues. To this end, a reliable and accurate analytical method is needed to evaluate whether these metabolites can be generated at sufficient amounts in the body.
Although we have previously developed an HPLC-fluorescent assay for analyzing various vitamin E metabolites (4), this method is not suitable for simultaneously quantifying metabolites as a result of supplementation of a mixture of vitamin E forms. Previously published LC/MS assays are not ideal because they do not directly measure sulfated carboxychromanols and did not have suitable standards for extraction and quantification (19, 20). Furthermore, an assay using GC/MS, despite being highly sensitive, is not capable of directly detecting conjugated metabolites (9). In this study, we describe a sensitive LC/MS/MS assay that simultaneously analyzes hydroxychromanols, carboxychromanols, and sulfated metabolites. To ensure accurate quantitation, our assay includes an improved sample processing procedure, and uses various carboxychromanols and a sulfated carboxychromanol as internal and external standards. We then applied this assay to quantify bioavailability of vitamin E metabolites in plasma, urine, and fecal samples from rodents supplemented with γT or δT.
MATERIALS AND METHODS
Materials
αT (99%), γT (97%–99%), and δT (97%) were purchased from Sigma (St. Louis, MO). γ-CEHC (≥98%), α-CEHC, and (±)-αT-5′-COOH (α-CMBHC) were from Cayman Chemicals (Ann Arbor, MI). δT-13′-COOH and δTE-13′-COOH, which are metabolites from δT and δTE, respectively, were synthesized according to a published procedure (21). Tissue culture reagents were from Invitrogen (Rockville, MD). All other chemicals were purchased from Sigma.
Cell culture
The human alveolar epithelial A549 cells from American Type Culture Collection (Manassas, VA) were maintained routinely in RPMI-1640 with 10% FBS. For obtaining conditioned media, confluent cells (8–10 × 105 cells per well in 6-well plates) were incubated with DMEM containing 1% FBS and γTE at 20 µM for 48 or 72 h (4). Media were collected, frozen immediately, and stored in −20°C until use. These conditioned media containing long-chain carboxychromanols and sulfated metabolites (4) were used for optimizing LC/MS/MS conditions.
Extraction of metabolites from plasma or cell culture media
Plasma samples or cell culture media were extracted by a solvent mixture containing 6 vol of working methanol (containing 0.2 mg/ml ascorbic acid) and 12 vol of hexane via vigorous vortexing for 1 min. δTE-13′-COOH (100 pmol) was added as an internal standard (IS). After centrifugation at >12,000 rpm for 2 min, the upper hexane layer was collected in a new tube with preadded butylated hydroxytoluene (BHT; 0.1 mg). The methanol layer (90–95%) was transferred into a clean tube, and the residual pellet was extracted one more time with 4 vol of working methanol. After vortexing and centrifugation, the combined methanol layers were dried under nitrogen. Both dried methanol- and hexane-extracted samples were resuspended in working methanol before being analyzed by HPLC or LC/MS/MS. During the extraction procedure, samples were protected from light. Before LC/MS/MS analysis, α-CMBHC (1 or 5 μM) was added as an additional IS.
Extraction of vitamin E metabolites from feces and urine
Approximately 30 mg of fecal samples was homogenized in 2 ml of methanol with ascorbate (0.2 mg/ml). After centrifugation, 1.5 ml of methanol layer was dried and resuspended in 200 μl methanol, which was then diluted 10 times with addition of synthesized δTE-13′-COOH (1 µM) as IS before being analyzed by LC/MS/MS. One hundred microliters of urine samples was added with δTE-13′-COOH (1 μM) and extracted by 500 μl of working methanol (0.2 mg/ml ascorbic acid). The extraction was repeated one more time with 200 μl of working methanol. The combined methanol was dried under N2. This procedure yielded >95% recovery of δTE-13′-COOH.
Analysis of vitamin E metabolites and vitamin E forms by HPLC with fluorescent detection
As previously described (4), vitamin E metabolites, tocopherols, and tocotrienols were separated on a 150 × 4.6 mm, 5 μm Supelcosil™ LC-18-DB column using HPLC and detected by a Shimadzu RF-10AXL spectrofluorometric detector (Columbia, MD) with the excitation and emission wavelength at 292 nm and 327 nm, respectively. This method was used in experiments in studying the extraction efficacy and accuracy of metabolites and vitamin E forms.
LC/MS/MS
The LC/MS/MS analysis was done with an Agilent 1200 LC system coupled to an Agilent 6460 QQQ mass spectrometer equipped with a jet stream ESI source (Santa Clara, CA). The chromatography utilized an Atlantis dC18 column (2.1 × 150 mm, 3 µm) from Waters Corporation (Milford, MA). Buffer A consisted of acetonitrile-ethanol-water (165:135:700, v/v/v), and buffer B was acetonitrile-ethanol-water (539:441:20, v/v/v), both of which contained 10 mM ammonium acetate with acetic acid to adjust pH to 4–4.3. The LC gradient was as follows: time 0 min, 0% B; time 1 min, 0% B; time 30 min, 99% B; time 40 min, 99% B; time 43 min, 0% B; time 48 min, 0% B. The flow rate was 0.3 ml/min with a total run time of 48 min. Multiple reaction monitoring was used to analyze each compound. Negative polarity ESI was used with the following source conditions: gas temperature, 325°C; gas flow, 10 liters per min; nebulizer pressure, 30 psi; sheath gas temperature, 250°C; sheath gas flow, 7 liters per min; capillary voltage, 4,000 V; nozzle voltage, 1,500 V; and an electron multiplier voltage of −300 V. All data were evaluated with Agilent MassHunter Qualitative Analysis software, version B.06.00.
Quantification of sulfated γTE-9′-COOH
Sulfated γTE-9′-COOH (γTE-9′S) was purified by HPLC from conditioned media generated by incubation of γTE (20 µM) with A549 cells for 72 h as previously described (4). To quantify the concentration of γTE-9′S, sulfatase (Helix pomatia H-1, at 30 U/ml) was used to convert γTE-9′S to 9′-COOH (22), which was then quantified using HPLC with UV detection. γTE-9′S is used as an external standard for quantifying sulfated long-chain metabolites.
Animal studies
All the animal studies were approved by Purdue Animal Care and Use Committee. In the study of metabolite formation in response to a single gavage of γT or δT, male Wistar rats (230–250 g) were purchased from Charles River (San Diego, CA). Rats were housed in Purdue Life Science Animal Facility for a week for adaptation before experiments and then randomly grouped by body weight match. Rats were administered with γT or δT at 100 mg/kg body weight by gavage using tocopherol-stripped corn oil (0.5 ml) as the vehicle (n = 3 in each group). Control animals received 0.5 ml of tocopherol-stripped corn oil. Six hours later, animals were euthanized, and plasma, liver, and other tissues were collected. In another study, rats were given γT at 50 mg/kg by gavage, and urine was collected for 7 h. The urine samples were then aliquoted and frozen at −80°C until use.
In the study of metabolite formation in response to γT- or δT-supplemented diets, male Balb/c mice (5–6 weeks) were obtained from Harlan (Indianapolis, IN) and single-housed under controlled temperature with unrestricted access to diets and water. After a week of acclimatization, mice were randomly divided into control (AIN-93G diet) and γT- or δT-supplemented (0.1% diet) group. These mice were subjected to induction of colon tumorigenesis by azoxymethane/dextran sodium sulfate (AOM/DSS) as previously described (12). When the study was terminated, mice were on supplemented diets for more than 150 days (12). During euthanasia, plasma and feces were collected.
Statistical analysis
In the study of extraction efficacy, ANOVA was used to calculate intraday and interday variances. Student’s t-test was used in the statistical analyses for comparison of controls with tocopherol-supplemented groups. All results are expressed as mean ± SD.
RESULTS
Optimization of sample processing procedure
In our previously published HPLC method, vitamin E metabolites were extracted into ethylacetate after sample solutions were acidified to pH <4. This protocol has been shown to effectively extract short- or medium-length carboxychromanols such as CEHC and 9′-COOH (4). However, extraction efficiency for 13′-COOHs by this procedure was not optimal, especially for plasma or serum samples (Q. Jiang, unpublished observations). Another weakness is that vitamin E forms and their metabolites need to be extracted separately by hexane/methanol, which requires additional samples. For these reasons, we decided to modify and optimize the extraction procedure aiming to effectively extract vitamin E forms and all the metabolites simultaneously from one (30–50 µl) biological sample.
To optimize and evaluate extraction efficacy, we used FBS spiked with vitamin E forms and metabolites including γT, δT, γ-CEHC, α-CEHC, α-CMBHC, δTE-13′-COOH, and δT-13′-COOH. The optimized extraction procedure included an extraction step with a solvent mixture containing 6 vol of methanol and 12 vol of hexane. After centrifugation, we collected both layers and performed an additional extraction of the pellet with 4 vol methanol (details in Materials and Methods). The hexane layer contains tocopherols and tocotrienols, and the combined methanol layer contains carboxychromanols and hydroxychromanols. This procedure yielded high extraction efficiency and accuracy, as indicated in Table 1 based on HPLC-fluorescent analyses.
TABLE 1.
Extraction efficacy and variance during sample processing
| γ-CEHC | α-CEHC | α-CMBHC | δTE-13′ | δT-13′ | γT | δT | |
| Efficacy (%) | 97 | 89 | 96 | 98 | 95 | 107 | 101 |
| Intraday CV% | 9 | 4 | 7 | 4 | 3 | 7 | 6 |
| Interday CV% | 3 | 11 | 6 | 10 | 5 | 11 | 8 |
CV, coefficient of variation. Tocopherols and metabolites were spiked into FBS to yield γ-CEHC (2 µM), α-CEHC (10 µM), α-CMBHC (2 µM), δTE-13′-COOH (1 µM), δT-13′-COOH (0.4 µM), γT (0.4 µM), and δT (0.4 µM). One hundred microliters of spiked FBS was extracted by 600 μl working methanol (with 0.2 mg/ml ascorbic acid) and 1.2 ml hexane via vortexing vigorously for 1 min. After centrifugation for 2 min at 10,000 rpm, the upper hexane layer was transferred into a tube with preadded 10 µl BHT (10 mg/ml). The methanol layer was transferred into a clean tube, and the pellet was extracted with 400 µl working methanol for the second time. The two methanol extractions were combined and dried under N2. The dried methanol and hexane-extracted samples were resuspended in 100 µl working methanol before being analyzed by HPLC with fluorescent detection. Data are expressed as mean ± SD (based on >4 independent experiments).
Optimization of LC/MS/MS conditions
At the early stage of method development, we compared relative ionization efficacy of carboxychromanols among different MS ionization approaches including ESI and atmospheric pressure chemical ionization and photoionization interfaces. In these studies, we used δT-13′-COOH and γ-CEHC as the model compounds and found that ESI in the negative mode gave much stronger signals than other ionization methods.
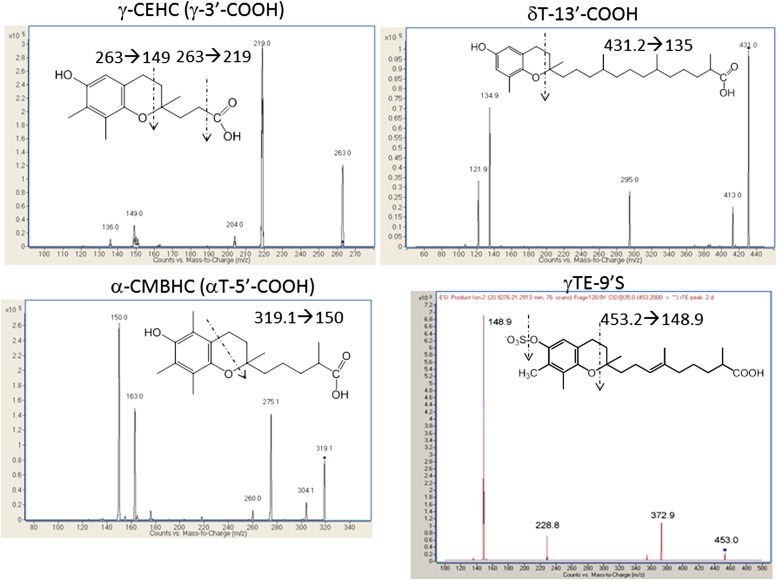
We then optimized the ESI conditions by varying fragmentation transitions and collision energies for γ-CEHC, α-CEHC, 5′-COOH, δTE-13′-COOH, and δT-13′-COOH. To optimize ionization conditions of other metabolites including sulfated counterparts, we used conditioned media that contain sulfated carboxychromanols produced by incubation of A549 cells with γ-tocotrienol (4, 5). Typical fragmentations of carboxychromanols are shown in Fig. 3. Major fragmentations of various vitamin E metabolites were caused by loss of the side chain along with two carbons in the nonaromatic part of the chromanol (Fig. 3). An additional loss of -CH2 at the 4-position was observed for α-CMBHC. Sulfated metabolites were similarly fragmented with a signature loss of the sulfate group (Fig. 3). To maximize signals, we chose the transition showing the highest intensity under varied collision energies for each analyte for quantifying carboxychromanols. The parameters for each carboxychromanols and vitamin E forms are summarized in Table 2.
Fig. 3.
LC/MS/MS fragmentation of vitamin E metabolites.
TABLE 2.
MS conditions for vitamin E metabolites
| Compound Name | Precursor Ion | Product Ion | Retention Time (min) | Fragmentor | Collision Energy |
| α-CEHC (α-3′-COOH) | 277.2 | 233 | 12.4 | 120 | 10 |
| α-5′-COOH | 319.2 | 150 | 18.4 | 180 | 20 |
| α-7′-COOH | 347.2 | 163 | — | 180 | 20 |
| α-9′-COOH | 389.3 | 163 | — | 120 | 35 |
| α-11′-COOH | 417.3 | 163 | — | 120 | 35 |
| α-13′-COOH | 459.4 | 163 | 30.1 | 120 | 35 |
| α-13′-OH | 445.4 | 163 | 32.5 | 120 | 35 |
| α-Tocopherol | 429.4 | 163 | 36.8 | 120 | 20 |
| δ-CEHC (δ-3′-COOH) | 249.1 | 205 | 9.8 | 120 | 10 |
| δ-SO3-CEHC | 329.1 | 135 | 5.3 | 120 | 45 |
| δ-5′-COOH | 291.2 | 135 | — | 180 | 20 |
| δ-7′-COOH | 319.2 | 135 | 19.1 | 180 | 20 |
| δ-9′-COOH | 361.3 | 135 | — | 120 | 35 |
| δ-11′-COOH | 389.3 | 135 | 27.0 | 120 | 35 |
| δ-13′-OH | 417.3 | 135 | 31.3 | 120 | 35 |
| δ-13′-COOH | 431.3 | 135 | 30.3 | 120 | 35 |
| δ-SO3-9′-COOH | 441.2 | 135 | 20.6 | 120 | 45 |
| δ-SO3-11′-COOH | 469.2 | 135 | 23.2 | 120 | 45 |
| δ-SO3-13′-COOH | 511.3 | 135 | 26.7 | 120 | 45 |
| δ-Tocopherol | 401.4 | 135 | 34.6 | 120 | 35 |
| δTE-5′-COOH (one double bond) | 289.2 | 135 | — | 120 | 35 |
| δTE-7′-COOH (one double bond) | 317.2 | 135 | — | 120 | 35 |
| δTE-9′-COOH (one double bond) | 359.2 | 135 | — | 120 | 35 |
| δTE-9′-COOH (two double bonds) | 357.2 | 135 | — | 120 | 35 |
| δTE-11′-COOH (two double bonds) | 385.3 | 135 | — | 120 | 35 |
| δTE-11′-COOH (one double bond) | 387.3 | 135 | — | 120 | 35 |
| δTE-13′-OH | 411.3 | 135 | — | 120 | 35 |
| δTE-13′-COOH (three double bonds) | 425.3 | 135 | 27.7 | 120 | 30 |
| δTE-13′-COOH (two double bonds) | 427.3 | 135 | — | 120 | 30 |
| δTE-SO3-9′-COOH (one double bond) | 439.2 | 135 | — | 120 | 45 |
| δTE-SO3-9′-COOH (two double bonds) | 437.2 | 135 | — | 120 | 45 |
| δTE-SO3-11′-COOH (two double bonds) | 465.2 | 135 | — | 120 | 45 |
| δTE-SO3-11′-COOH (one double bond) | 467.3 | 135 | — | 120 | 45 |
| δTE-SO3-13′-COOH (three double bonds) | 505.2 | 135 | — | 120 | 45 |
| δTE-SO3-13′-COOH (two double bonds) | 507.3 | 135 | — | 120 | 45 |
| δ-Tocotrienol | 395.3 | 135 | — | 120 | 35 |
| γ-CEHC (γ-3′-COOH) | 263.1 | 219 | 10.9 | 120 | 5 |
| γ-5′-COOH | 305.2 | 149 | 18.3 | 180 | 20 |
| γ-7′-COOH | 333.2 | 149 | — | 180 | 20 |
| γ-9′-COOH | 375.3 | 149 | 25.2 | 120 | 40 |
| γ-11′-COOH | 403.3 | 149 | 27.6 | 120 | 40 |
| γ-13′-OH | 431.4 | 149 | 31.2 | 120 | 40 |
| γ-13′-COOH | 445.3 | 149 | 30.6 | 120 | 40 |
| γ-SO3-CEHC | 343.1 | 149 | 6.2 | 120 | 30 |
| γ-SO3-9′-COOH | 455.2 | 149 | 20.7 | 120 | 45 |
| γ-SO3-11′-COOH | 483.3 | 149 | 23.2 | 120 | 45 |
| γ-SO3-13′-COOH | 525.3 | 149 | 26.7 | 120 | 45 |
| γ-Tocopherol | 415.4 | 149 | 36.1 | 120 | 40 |
| γTE-5′-COOH | 303.2 | 149 | — | 120 | 25 |
| γTE-7′-COOH (one double bond) | 331.2 | 149 | — | 120 | 25 |
| γTE-9′-COOH (two double bonds) | 371.2 | 149 | — | 120 | 25 |
| γTE-9′-COOH (one double bond) | 373.3 | 149 | 23.7 | 120 | 25 |
| γTE-11′-COOH (two double bonds) | 399.3 | 149 | 25.0 | 120 | 25 |
| γTE-11′-COOH (one double bond) | 401.3 | 149 | — | 120 | 25 |
| γTE-13′-OH (three double bonds) | 425.3 | 149 | 28.9 | 120 | 35 |
| γTE-13′-COOH (three double bonds) | 439.3 | 149 | 27.8 | 120 | 35 |
| γTE-13′-COOH (two double bonds) | 441.3 | 149 | 27.8 | 120 | 35 |
| γTE-SO3-9′-COOH (two double bonds) | 451.2 | 149 | — | 120 | 45 |
| γTE-SO3-9′-COOH (one double bond) | 453.2 | 149 | 19.2 | 120 | 45 |
| γTE-SO3-11′-COOH (two double bonds) | 479.2 | 149 | 20.6 | 120 | 45 |
| γTE-SO3-11′-COOH (one double bond) | 481.2 | 149 | 20.6 | 120 | 45 |
| γTE-SO3-13′-COOH (three double bonds) | 519.3 | 149 | 23.7 | 120 | 45 |
| γTE-SO3-13′-COOH (two double bonds) | 521.3 | 149 | 23.7 | 120 | 45 |
| γ-Tocotrienol | 409.3 | 149 | 33.1 | 120 | 35 |
All the parameters are set using ESI negative mode. The transitions with retention time have been confirmed by the experimental data.
It should be mentioned that besides sulfated metabolites, we attempted to directly detect glucuronidated carboxychromanols using full scan mode on a time-of-flight instrument or monitoring M+176 ions and fragments by product ion scanning, precursor loss scanning, and multiple reaction monitor. However, we did not observe significant formation of glucuronidated metabolites in the plasma of rats gavaged with tocopherols.
Quantification of sulfated carboxychromanols
Conjugated carboxychromanols have often been quantified after being converted to unconjugated counterparts by sulfatase/glucuronidase (22). However, this approach could not reveal the nature of conjugation and may be inappropriate when sample size is small. Further, our unpublished data indicate that many sulfatase or glucuronidases were not able to effectively deconjugate long-chain carboxychromanols such as sulfated-13′-carboxychromanol (13′S; unpublished observations). In order to directly quantify sulfated metabolites, we purified γTE-9′S from conditioned media from cells incubated with γTE (16) (Materials and Methods). The concentration of stock γTE-9′S was then determined by HPLC with UV-visible detection after being converted to γTE- 9′-COOH with sulfatase digestion. We have subsequently used γTE-9′S as an external standard to quantify sulfated long-chain carboxychromanols.
Quantification of short- and long-chain metabolites in biological samples
We observed a broad range of linearity between LC/MS/MS response and analyte concentrations (from 10 nM to 5 µM) for short- and long-chain metabolites including γ-CEHC, α-CEHC, α-CMBHC, and 13′-COOHs. Under optimized ionization conditions, the detection limits for carboxychromanols were ∼0.1–0.4 pmol on column with a signal-to-noise ratio of >8. To test ionization efficacy in extraction matrix, known amounts of γ-CEHC, α-CMBHC, γTE-9′S, and δT-13′-COOH were added to the reconstituted solution of FBS or plasma that were extracted by hexane/methanol with δTE-13′-COOH as an IS. These samples were then analyzed under the optimized LC/MS conditions. We found that these compounds spiked in the plasma or FBS extracts showed similar ionization efficacy/intensity to those prepared in methanol, indicating that the extraction matrix does not have significant impact on ionization. These data, therefore, justify quantitation of metabolites using external standards combined with ISs. Because relative ionization efficacy among different carboxychromanols can vary from time to time, it is necessary to quantify these compounds by internal and external standards that are analyzed along with biological samples. Specifically, in the subsequent analysis of biological samples, we used δT-13′-COOH and δTE-13′-COOH to quantify 13′-OHs and long-chain carboxychromanols, γTE-9′S for sulfated long-chain metabolites, and γ-CEHC or α-CEHC for short-chain carboxychromanols. In these studies, δTE-13′-COOH and α-CMBHC were used as IS and the rest as external standards.
We noticed that γTE-derived long-chain metabolites bearing different numbers of double bonds were coeluted under the current LC conditions. For instance, γTE-11′S containing one (481.2 → 149) and two (479.2 → 149) double bonds have the same retention time (Table 2). Because these coeluted metabolites differ in one double bond, 13C isotopic correction will be necessary for proper quantification of these compounds (23).
Vitamin E metabolites detected in the plasma and feces of mice fed γT- or δT-supplemented diets
Using the established LC/MS/MS assay, we found that mice fed a diet supplemented with γT or δT had low but detectable amounts of CEHC (3′-COOH) and sulfated long-chain carboxychromanols as well as 13′-COOH in the plasma, whereas none of these metabolites were detectable in mice fed the control AIN93G diet (Table 3). Interestingly, in contrast to low concentrations of metabolites in the plasma, we found relatively high levels of unconjugated short-, mid-, and long-carboxychromanols in feces. Among fecal excretion metabolites, 13′-COOH and 13′-OH were the predominant vitamin E metabolites (Table 4).
TABLE 3.
Metabolites in the plasma of mice fed γT- or δT-supplemented diet
| Metabolites (μM) | Control | γT diet (0.1%) | δT diet (0.1%) |
| α-CEHC | 0.034 ± 0.11 | 0.026 ± 0.01 | 0.029 ± 0.01 |
| γ-CEHC | Low | 0.085 ± 0.031* | 0.012 ± 0.004 |
| 9′S | Low | γT-9′S: 0.04 ± 0.011* | δT-9′S: 0.032 ± 0.006* |
| 11′S | Low | γT-11′S: 0.032 ± 0.016* | δT-11′S: 0.045 ± 0.01* |
| 13′S | Low | γT-13′S: 0.0085 ± 0.0042 | δT-13′S: 0.032 ± 0.007* |
| 13′ | Low | γT-13′: 0.021 ± 0.0021* | δT-13′: 0.01 ± 0.005 |
Balb/c mice were fed AIN93G control diet or γT- or δT-supplemented diet (0.1% w/w) for 3 months, and these mice were subjected to AOM/DSS treatment (see Materials and Methods for details). Plasma samples were collected and extracted for LC/MS/MS analysis. “Low” indicates below or at the detection limit. Data are expressed as mean ± SD (n = 3–5 per group). * P < 0.05: difference between control and supplemented diets.
TABLE 4.
Metabolites detected in fecal samples from mice fed diet supplemented with γT or δT (0.1% diet)
| Metabolites (µmol/g) | Control | γT diet (0.1%) | δT diet (0.1%) |
| γ-CEHC | Low | 0.082 ± 0.036* | Low |
| 5′-COOH | Low | γT-5′: 0.04 ± 0.011* | δT-5′: 0.029 ± 0.03* |
| 7′-COOH | Low | γT-7′: 0.024 ± 0.017* | δT-7′: 0.012 ± 0.003* |
| 9′-COOH | Low | γT-9′: 0.094 ± 0.047* | δT-9′: 0.031 ± 0.014* |
| 11′-COOH | Low | γT-11′: 0.15 ± 0.05* | δT-11′: 0.12 ± 0.06* |
| αT-13′-COOH | 0.015 ± 0.004 | 0.029 ± 0.01 | 0.039 ± 0.01 |
| γT-13′-COOH | 0.03 ± 0.023 | 1.04 ± 0.24** | 0.18 ± 0.06* |
| δT-13′-COOH | 0.015 ± 0.01 | 0.078 ± 0.06 | 0.96 ± 0.47** |
| αT-13′-OH | 0.013 ± 0.01 | 0.022 ± 0.01 | 0.025 ± 0.01 |
| γT-13′-OH | 0.03 ± 0.021 | 0.34 ± 0.08** | 0.064 ± 0.03 |
| δT-13′-OH | 0.025 ± 0.01 | 0.043 ± 0.02 | 0.67 ± 0.33** |
Fecal samples were obtained from the same animal study described in Table 3. “Low” indicates below or at detection limit. Data are expressed as mean ± SD (n = 3–5 per group). * P < 0.05, ** P < 0.01: difference between control and supplemented diets.
Metabolites detected in the plasma and urine after a single gavage of γT and δT
To evaluate the bioavailability of metabolites as a result of a high supplement dose of tocopherols, we gave rats a single gavage of γT or δT at 100 mg/kg body weight and collected plasma samples 6 h later. We found that sulfated CEHCs (SO3-CEHC), 9′S, and 11′S were among the major metabolites in the plasma of tocopherol-supplemented rats (Table 5). Unlike analyses by HPLC with fluorescent detection (4, 5), the LC/MS/MS method allowed direct measurement of sulfated CEHCs. Compared with feeding on tocopherol-supplemented diet, oral gavage resulted in much elevated conjugated carboxychromanols in the rats’ plasma at the time of sample collection (cf. Tables 3 and 5).
TABLE 5.
Metabolites detected in the plasma of rats at 6 h after given a single gavage of γT or δT (100 mg/kg body weight)
| Metabolites (μM) | Control | γT at 100 mg/kg | δT at 100 mg/kg |
| SO3-γ-CEHC | 0.061 ± 0.02 | 2.03 ± 0.85* | 0.079 ± 0.024 |
| SO3-δ-CEHC | Low | Low | 1.69 ± 0.74** |
| α-CEHC | 0.13 ± 0.059 | 0.14 ± 0.035 | 0.13 ± 0.03 |
| γ-CEHC | 0.097 ± 0.024 | 0.25 ± 0.033* | 0.085 ± 0.04 |
| δ-CEHC | Low | Low | 0.085 ± 0.033* |
| 7′-COOH | Low | Low | δT-: 0.078 ± 0.016 |
| 9′S | Low | γT-: 0.51 ± 0.11** | δT-: 0.17 ± 0.056* |
| 11′S | γT-: 0.013 ± 0.0029 | γT-: 0.66 ± 0.26** | δT-: 0.079 ± 0.03 |
| 13′S | Low | γT-: 0.094 ± 0.046* | δT-: 0.079 ± 0.024 |
| 13′ | Low | γT-: 0.015 ± 0.012* | δT-: 0.038 ± 0.023* |
| 13′-OH | Low | γT-: 0.035 ± 0.02* | δT-: 0.044 ± 0.019* |
Wistar rats were given a single gavage of γT or δT in corn oil or corn oil alone (control). Six hours later, animals were euthanized, and plasma samples were obtained and stored at −80°C. Detailed information on animal studies, sample processing, and the LC/MS/MS method are described in Materials and Methods. “Low” indicates below or at the detection limit. Data are expressed as mean ± SD (n = 3 per group). * P < 0.05, ** P < 0.01: difference between control and tocopherol supplementation.
In a separate animal study, we collected urine samples within 7 h after rats were gavaged with γT at 50 mg/kg or corn oil (controls). We observed high excretion of sulfated γ-CEHC even in control rats (i.e., 16.4 ± 2.4 μM) but could not detect γ-CEHC. Supplementation of γT led to increase of sulfated γ-CEHC and unconjugated form to 78.59 ± 35.9 and 0.014 + 0.003 μM, respectively.
DISCUSSION
We have developed a highly sensitive and specific LC/MS/MS assay for simultaneously analyzing hydroxychromanols, carboxychromanols, and sulfated vitamin E metabolites. Our method is strengthened by optimized ionization of major metabolites and an improved extraction procedure that results in high sensitivity and excellent reproducibility. We have used multiple carboxychromanols as external standards and ISs to ensure accurate quantification. In addition, our method has a 9′S as a standard, which allows direct quantification of sulfated metabolites without the need for enzyme digestion. As a result, our study, for the first time, documented the level of sulfated CEHCs in the plasma.
The ability to directly quantify conjugated metabolites including sulfated derivatives allows simpler and more accurate quantitation of these compounds than traditional procedures with enzyme digestion to remove the sulfate and/or glucuronide group. Our unpublished data indicate that enzyme digestion with sulfatase/glucuronidase could not effectively remove the sulfate group from sulfated 13′-COOH, although conjugated CEHC and 9′-COOH can be effectively converted to their unconjugated counterparts via sulfatase/glucuronidase digestion (22). This is probably because sulfated long-chain metabolites are poor substrates for the enzymes tested (unpublished observations). As a result, the approach with enzyme digestion can only accurately quantify conjugated short-chain carboxychromanols, but not the long-chain analogs. Our present assay enables accurate and direct quantitation of 9′S, 11′S, and 13′S using γTE-9′S as a standard. Because sulfated and glucuronidated metabolites may have different bioactivities, this method offers an opportunity to measure different types of conjugates and may therefore be useful for further evaluation of their roles in vivo. On the other hand, despite our effort to detect glucuronidated carboxychromanols (176+M), we did not observe any significant amount of these metabolites in the plasma of rats gavaged with γT. It remains to be determined whether glucuronidated carboxychromanols can be seen in other tissue or human samples.
Using the developed LC/MS/MS assay, we found some interesting aspects of vitamin E metabolite formation in response to supplementation of γT or δT. First, administration of γT or δT via a single gavage appeared to achieve much higher plasma concentrations of sulfated carboxychromanols than the steady concentrations achieved via chronic feeding with tocopherol-supplemented diets. This observation can be explained by the fact that animals consume a much smaller amount of γT or δT from supplemented diet at any given time than a single gavage by which tocopherols were given as a large boost. That relatively high plasma levels of sulfated metabolites were detected after gavage indicates that sulfation of long-chain or intermediate carboxychromanols takes place in parallel with β-oxidation when a relatively large quantity of tocopherols is consumed. Second, large amounts of unconjugated metabolites, especially 13′-COOH and 13′-OH, which are very low in the plasma, are detected in feces. This observation is consistent with previous reports (12–14). The high level of carboxychromanols in feces suggests that most long-chain metabolites are not transported to the circulation after being generated in the liver, but rather are excreted via the bile. It has recently been estimated that up to 70% of vitamin E metabolites may be excreted to feces (13, 14). Alternatively, it is also possible that high levels of these metabolites in feces are produced by gut flora, an intriguing possibility warranting further investigation. Regardless, our current results together with previous reports confirm that 13′-COOH and 13′-OH are the major fecal excretion metabolites of vitamin E. In addition, although conjugated CEHCs have been reported in the urine (6–11), here we quantified sulfated γ-CEHC. Interestingly, there are substantial amounts of SO3-γ-CEHC even in the urine of control rats that were fed standard chow containing ∼9 mg/kg of γT.
It has been demonstrated that specific vitamin E metabolites are more bioactive or have different activities compared with unmetabolized vitamins. γ-CEHC, but not tocopherols, has natriuretic activity (15) and is thought to be responsible for enhanced sodium excretion in response to supplementation of γTE in rodents (24). We have demonstrated that 13′-COOHs are competitive inhibitors of cyclooxygenases, whereas none of the tocopherols or sulfated long-chain metabolites directly inhibit the cyclooxygenase activity (16). 13′-COOH derived from δT inhibits 5-lipoxygenase activity and leukotriene formation in stimulated neutrophils. On the other hand, tocopherols have no effect on 5-lipoxygenase activity, and their suppression of leukotriene in white blood cells varies with specific stimuli (17). In addition, 13′-COOHs induce apoptosis in liver hepatoma HpG2 cells more effectively than tocoherols (18). 13′-OH and 13′-COOH derived from αT are found to have antiatherogenic activities (25). Given these bioactivities of long-chain metabolites, it is reasonable to assume that these compounds may play a role in vitamin E-mediated beneficial effects in vivo (1), which warrants further investigation.
In summary, we have described an LC/MS/MS assay that simultaneously quantifies hydroxychromanols, carboxychromanols, and sulfated vitamin E metabolites. This method should be useful for further evaluation of pharmacokinetics of vitamin E metabolite formation and their bioavailability in tissues and excretion, as well as for investigation of potential contributions of metabolites to vitamin E-mediated beneficial effects in animals and humans.
Footnotes
Abbreviations:
- 3′-, 5′-, 7′-, 9′-, 11′-, and 13′-COOH
- 3′-, 5′-, 7′-, 9′-, 11′-, and 13′-carboxychromanol
- 9′S, 11′S, and 13′S
- sulfated 9′-, 11′-, and 13′-carboxychromanol
- 13′-OH
- 13′-hydroxychromanol
- α-CMBHC
- αT-5′-COOH
- αT, βT, γT, or δT
- α-, β-, γ-, or δ-tocopherol
- αTE, βTE, γTE, or δTE
- α-, β-, γ-, or δ-tocotrienol
- γTE-9′S
- sulfated γTE-9′-COOH
- CEHC
- 2-(β-carboxyethyl)-6- hydroxychroman, also called 3′-COOH
- IS
- internal standard
This work was supported in part by National Institutes of Health Grants R01AT006882 and R01ES023349. This project was also partially supported by National Institutes of Health Grant P30CA023168 and the Indiana Clinical and Translational Sciences Institute, funded in part by Grant UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
REFERENCES
- 1.Jiang Q. 2014. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 72: 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birringer M., Pfluger P., Kluth D., Landes N., Brigelius-Flohe R. 2002. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 132: 3113–3118. [DOI] [PubMed] [Google Scholar]
- 3.Sontag T. J., Parker R. S. 2002. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 277: 25290–25296. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Q., Freiser H., Wood K. V., Yin X. 2007. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J. Lipid Res. 48: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freiser H., Jiang Q. 2009. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. J. Nutr. 139: 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiku S., Hamamura K., Nakamura T. 1984. Novel urinary metabolite of d-delta-tocopherol in rats. J. Lipid Res. 25: 40–48. [PubMed] [Google Scholar]
- 7.Schultz M., Leist M., Petrzika M., Gassmann B., Brigelius-Flohe R. 1995. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 62: 1527S–1534S. [DOI] [PubMed] [Google Scholar]
- 8.Traber M. G., Elsner A., Brigelius-Flohe R. 1998. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 437: 145–148. [DOI] [PubMed] [Google Scholar]
- 9.Swanson J. E., Ben R. N., Burton G. W., Parker R. S. 1999. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J. Lipid Res. 40: 665–671. [PubMed] [Google Scholar]
- 10.Stahl W., Graf P., Brigelius-Flohe R., Wechter W., Sies H. 1999. Quantification of the alpha- and gamma-tocopherol metabolites 2,5,7, 8-tetramethyl-2-(2′-carboxyethyl)-6-hydroxychroman and 2,7, 8-trimethyl-2-(2′-carboxyethyl)-6-hydroxychroman in human serum. Anal. Biochem. 275: 254–259. [DOI] [PubMed] [Google Scholar]
- 11.Pope S. A., Burtin G. E., Clayton P. T., Madge D. J., Muller D. P. 2002. Synthesis and analysis of conjugates of the major vitamin E metabolite, alpha-CEHC. Free Radic. Biol. Med. 33: 807–817. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q., Jiang Z., Hall Y. J., Jang Y., Snyder P. W., Bain C., Huang J., Jannasch A., Cooper B., Wang Y., et al. 2013. Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice. Free Radic. Biol. Med. 65: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bardowell S. A., Ding X., Parker R. S. 2012. Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism. J. Lipid Res. 53: 2667–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardowell S. A., Duan F., Manor D., Swanson J. E., Parker R. S. 2012. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. J. Biol. Chem. 287: 26077–26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechter W. J., Kantoci D., Murray E. D., Jr., D’Amico D. C., Jung M. E., Wang W. H. 1996. A new endogenous natriuretic factor: LLU-α. Proc. Natl. Acad. Sci. USA. 93: 6002–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q., Yin X., Lill M. A., Danielson M. L., Freiser H., Huang J. 2008. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA. 105: 20464–20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Z., Yin X., Jiang Q. 2011. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 186: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birringer M., Lington D., Vertuani S., Manfredini S., Scharlau D., Glei M., Ristow M. 2010. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic. Biol. Med. 49: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 19.Leonard S. W., Gumpricht E., Devereaux M. W., Sokol R. J., Traber M. G. 2005. Quantitation of rat liver vitamin E metabolites by LC-MS during high-dose vitamin E administration. J. Lipid Res. 46: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y., Lee M. J., Cheung C., Ju J. H., Chen Y. K., Liu B., Hu L. Q., Yang C. S. 2010. Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. J. Agric. Food Chem. 58: 4844–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maloney D. J., Hecht S. M. 2005. A stereocontrolled synthesis of delta-trans-tocotrienoloic acid. Org. Lett. 7: 4297–4300. [DOI] [PubMed] [Google Scholar]
- 22.Freiser H., Jiang Q. 2009. Optimization of the enzymatic hydrolysis and analysis of plasma conjugated gamma-CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E. Anal. Biochem. 388: 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang K., Han X. 2011. Accurate quantification of lipid species by electrospray ionization mass spectrometry - Meet a key challenge in lipidomics. Metabolites. 1: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito H., Kiyose C., Yoshimura H., Ueda T., Kondo K., Igarashi O. 2003. Gamma-tocotrienol, a vitamin E homolog, is a natriuretic hormone precursor. J. Lipid Res. 44: 1530–1535. [DOI] [PubMed] [Google Scholar]
- 25.Wallert M., Schmolz L., Koeberle A., Krauth V., Glei M., Galli F., Werz O., Birringer M., Lorkowski S. 2015. alpha-Tocopherol long-chain metabolite alpha-13′-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW264.7 macrophages. Mol. Nutr. Food Res. 59: 1524–1534. [DOI] [PubMed] [Google Scholar]