Abstract
Objective: Anxiety is a commonly reported side-effect of psychostimulant treatment. Our goal was to quantify the risk of anxiety as a side effect of psychostimulant treatment for attention-deficit/hyperactivity disorder (ADHD).
Methods: We conducted a PubMed search to identify all double-blind, randomized, placebo-controlled trials examining the efficacy of psychostimulant medications in the treatment of children with ADHD. We used a fixed-effects meta-analysis to examine the risk ratio of anxiety reported as a side effect in children treated with psychostimulants compared with those treated with placebo. We used stratified subgroup analysis and meta-regression to examine the effects of stimulant type, dosage, duration of use, and trial design on the measured risk of anxiety.
Results: We identified 23 studies involving 2959 children with ADHD for inclusion in our meta-analysis. The risk of anxiety associated with psychostimulant treatment was significantly lower than that experienced with placebo (relative risk [RR] = 0.86 [95% CI: 0.77, 0.95], z = –2.90, p < 0.05). Higher doses of psychostimulants were associated with a reduced measured risk of anxiety of psychostimulants when compared with placebo (β = −0.0039 [95% CI: −0.00718, −0.00064], z = −2.34, p = 0.019).
Conclusions: Meta-analysis suggests that treatment with psychostimulants significantly reduced the risk of anxiety when compared with placebo. This finding does not rule out the possibility that some children experience increased anxiety when treated with psychostimulants, but suggests that those risks are outweighed by the number of children who experience improvement in anxiety symptoms (possibly as a secondary effect of improved control of ADHD symptoms). Clinicians should consider rechallenging children with ADHD who report new-onset or worsening anxiety with psychostimulants, as these symptoms are much more likely to be coincidental rather than caused by psychostimulants.
Introduction
Comorbid anxiety is common in children with attention-deficit/hyperactivity disorder (ADHD). Epidemiologic studies have demonstrated that 25–50% of children with ADHD also have a comorbid anxiety disorder (March et al. 2000; Sciberras et al. 2014). Anxiety has also been identified as a potential moderator of short-term treatment response in children with ADHD. Previous trial data have suggested that children with ADHD who have comorbid anxiety may exhibit a decreased response to methylphenidate (MPH) and experience more side effects than children with ADHD without anxiety (Buitelaar et al. 1995; Ter-Stepanian et al. 2010). The Multimodal Treatment Study of Children With Attention-Deficit/Hyperactivity Disorder (MTA) (1999)was unable to replicate this differential response to MPH in children with ADHD and comorbid anxiety, but was able to demonstrate an improved response to behavioral treatment in children with ADHD and comorbid anxiety compared with children with ADHD but no anxiety.
In addition to being a potential moderator of short-term treatments for ADHD, anxiety has also been reported to be a side-effect of pharmacological treatments for ADHD. Anxiety and nervousness are both reported to be “common” side-effects of both MPH and amphetamine-derived products that are experienced at least twice as frequently as with placebo in children with ADHD (NextWave Pharmaceuticals 2012; US 2013).
Given that psychostimulants are the most-effective short-term treatment for ADHD, and that anxiety is a common comorbidity in children with ADHD, accurately examining the likelihood of anxiety as a side-effect of psychostimulant medication is of clinical importance when managing the symptoms of children with ADHD (Feldman and Reiff 2014). The goal of this meta-analysis is to provide an evidence base for the care of children with ADHD who experience anxiety after starting psychostimulant medication. We will examine available data on anxiety as a side effect in randomized, placebo-controlled trials of psychostimulants in childhood ADHD in order to determine the risk of anxiety associated with psychostimulants. We will conduct secondary analyses to examine the effects of psychostimulant type (long vs. short-acting formulations, MPH vs. mixed amphetamine salt derivatives), and length and dose of psychostimulant treatment on the risk of anxiety with psychostimulant treatment.
Methods
Search strategy for identification of studies
Two reviewers (E.F. and J.M.M.) searched the electronic database of PubMed for relevant studies using the following search: (attention deficit disorder with hyperactivity OR ADHD OR ADDH OR hyperactiv* OR hyperkin* OR “attention deficit*” OR “brain dysfunction”) AND (methylphenidate OR Ritalin OR Metadate OR Equasym OR Daytrana OR Concerta OR Dextroamphetamine OR amphetamine OR Adderall OR Vyvanse OR Dexedrine OR Dextrostat). Search results were limited to randomized control trials. The references of appropriate articles on psychostimulant medications were searched for citations of further relevant published and unpublished research.
Selection of studies
Two reviewers (J.M.M. and E.F.) examined the titles and abstracts of the studies yielded in the search to determine inclusion in this meta-analysis. A final reviewer (M.H.B.) resolved any discrepancies between the two reviewers. Articles were eligible if they 1) compared psychostimulant medications to placebo in randomized, double-blind clinical trials (MPH or dextroamphetamine derivatives) and 2) included participants <18 years of age diagnosed with ADHD or hyperkinetic disorder by explicit criteria; that is, Diagnostic and Statistical Manual of Mental Disorders or International Classification of Diseases (ICD) criteria. Studies were excluded if 1) the study was not published in English, 2) the study population required that participants have an additional primary comorbidity (i.e., mental retardation, pervasive developmental disorder, oppositional defiant disorder, tics, or anxiety) in addition to ADHD, 3) psychostimulants were given for <7 days continuously, 4) there were <10 subjects (crossover design) or <20 subjects (parallel design), or 5) the primary goal of the trial was not treatment for ADHD (e.g., studies that were primarily concerned with neuroimaging or neuropsychological measures were excluded).
Meta-analytic procedures
Data were extracted by independent reviewers (Z.D.S., S.C.C., J.M.M., and C.G.C.) on specially designed Microsoft Excel spreadsheets. Our primary outcome measure was the proportion of children reporting anxiety as a side effect. When possible, clinician rated side effect measures were utilized as the main outcome measure. When this information was unavailable, participant-rated, parent-rated, or teacher-rated side effect measures were used. Additionally, reviewers gathered data on trial medication, trial design, maximum daily medication dose, number of participants, mean age of participants, duration of active treatment in trials, and other relevant attributes and results of the studies. Any disagreement among reviewers was mitigated through discussion and, when possible, study investigators were contacted to provide further information. If initial reviewers could not reach an agreement, the senior investigator (M.H.B.) resolved all disputes. When information about the proportion of participants experiencing anxiety was not available in the original articles, the corresponding author was contacted for further information. If contacting the corresponding author was ineffective, we additionally searched pharmaceutical company databases for data.
All statistical analysis was completed in Comprehensive Meta-Analysis Version 3. For our outcome measures of interest, the proportion of subjects experiencing anxiety was analyzed using pooled risk ratio. For all outcome measures, 95% confidence intervals (CIs) were conveyed. A fixed-effects model for meta-analysis was used, as well as a random-effects model in sensitivity analysis. Publication bias was assessed by plotting the effect size against standard error for each included trial (i.e., funnel plot). In addition, publication bias was statistically tested by the Egger's test (Egger et al. 1997).
For secondary analyses, several subgroup analyses and meta-regressions were performed. Stratified subgroup analyses were conducted based on 1) type of psychostimulant (MPH vs. mixed-amphetamine derivatives), 2) duration of action of medications (long-acting vs. short-acting psychostimulants), and 3) trial design (crossover vs. parallel group trials). We utilized the test for subgroup differences (between-group χ2 test for heterogeneity) in the fixed-effects model of Comprehensive Meta-Analysis to test for subgroup differences. Meta-regression analysis was used to examine the effect of 1) length of active psychostimulant treatment, and 2) maximum daily dose of psychostimulants utilized in trials on the risk of developing anxiety with psychostimulants compared with placebo. All daily doses of psychostimulants were converted into MPH equivalents using previously described methodology (Swenson, no date). We had initially intended to conduct a meta-regression examining the effects of mean age of participants in trials on the measured risk of anxiety with psychostimulant use. However, the vast majority of included trials had a mean age of between 8 and 10 years leading to a few trials with outlier ages dominating the analysis. Our threshold for statistical significance was p < 0.05 for the primary analysis, as well as for all stratified subgroup analyses and meta-regression.
Results
Included trials
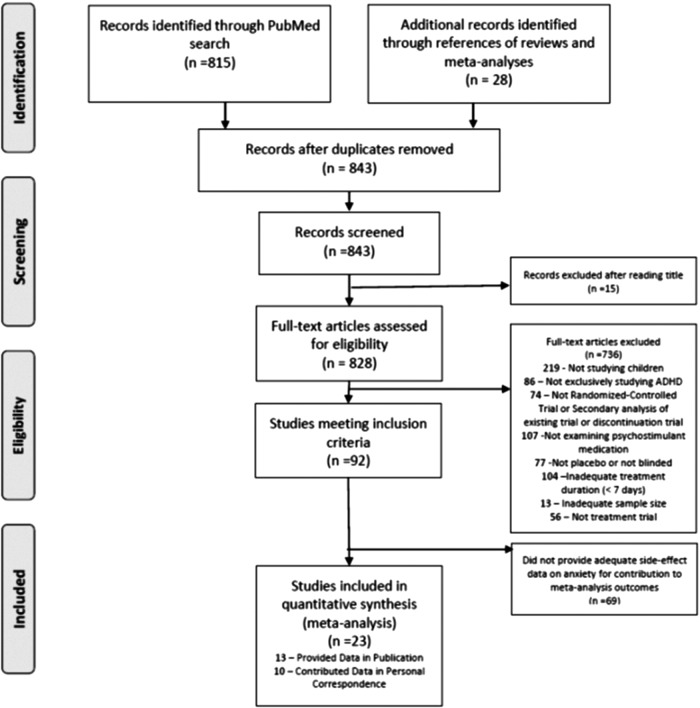
Figure 1 depicts the selection of trials for this meta-analysis (Liberati et al. 2009). A total of 815 references were identified in PubMed. A total of 92 trials were eligible for inclusion. Of these 92 trials, 13 trials published data on anxiety as a side effect of psychostimulant medication. Authors of 10 additional trials responded to email requests with unpublished data regarding the risks of anxiety in psychostimulant trials. Therefore, a total of 23 trials, involving 2959 subjects, were included in our meta-analysis (Gittelman-Klein et al. 1976; Conners and Taylor 1980; Werry et al. 1980; Rapport et al. 1985; Barkley et al. 1990; Ahmann et al. 1993; Buitelaar et al. 1996; Stein et al. 1996; Gillberg et al. 1997; Firestone et al. 1998; Pliszka et al. 2000; Biederman et al. 2002; Greenhill et al. 2002; McCracken et al. 2003; Stein et al. 2003; Wigal et al. 2004; Gorman et al. 2006; Spencer et al. 2006; Wigal et al. 2006; Newcorn et al. 2008; Childress et al. 2009; Solanto et al. 2009; Lee et al. 2011). The characteristics of included trials are depicted in Table 1.
FIG. 1.
Selection of studies.
Table 1.
Characteristics of Included Trials in the Meta-Analysis of the Risk of Anxiety with Psychostimulants
| Authors | Year | Medication | Stimulant class | Duration of action | Maximum dose | Design | Duration of active treatment | n | Mean age (years) |
|---|---|---|---|---|---|---|---|---|---|
| Gittelman-Klein et al. | 1976 | MPH IR | MPH | Short | 60 mg/day | Parallel | 4 weeks | 80 | 8.6 |
| Conners et al. | 1980 | MPH IR | MPH | Short | 60mg/day | Parallel | 8 weeks | 60 | 7.9 |
| Werry et al. | 1980 | MPH IR | MPH | Short | 0.4 mg/kg/day | Crossover | 3–4 weeks | 30 | 8.4 |
| Rapport et al. | 1985 | MPH IR | MPH | Short | 15 mg/day | Crossover | 1 week | 12 | 6–10 |
| Barkley et al. | 1990 | MPH IR | MPH | Short | 0.5 mg/kg b.i.d. | Crossover | 7–10 days | 82 | 8.2 |
| Ahmann et al. | 1993 | MPH IR | MPH | Short | 0.5mg/kg t.i.d. | Crossover | 7 days | 206 | 9.1 |
| Buitelaar et al. | 1996 | MPH IR | MPH | Short | 10 mg b.i.d. | Parallel | 4 weeks | 52 | 9.2 |
| Stein et al. | 1996 | MPH IR | MPH | Short | 20 mg t.i.d. | Crossover | 1 week | 25 | 8.0 |
| Gillberg et al. | 1997 | MAS IR | AMP | Short | 45 mg/day | Parallel | 3 months | 56 | 9 |
| Firestone et al. | 1998 | MPH IR | MPH | Short | 0.5 mg/kg b.i.d. | Crossover | 7–10 days | 32 | 4.8 |
| Pliszka et al. | 2000 | MPH IR | MPH | Short | 50 mg/day | Parallel | 3 weeks | 58 | 8.1 |
| MAS IR | AMP | Short | 30 mg/day | ||||||
| Biederman et al. | 2002 | LDX | AMP | Long | 70 mg/day | Parallel | 3 weeks | 509 | 8.6 |
| Greenhill et al. | 2002 | MPH MR | MPH | Long | 60 mg/day | Parallel | 3 weeks | 316 | 9 |
| McCracken et al. | 2003 | MAS XR | AMP | Long | 30 mg/day | Crossover | 1 week | 49 | 9.5 |
| MAS IR | AMP | Short | 10 mg/day | ||||||
| Stein et al. | 2003 | OROS® MPH | MPH | Long | 54 mg/day | Crossover | 1 week | 47 | 9 |
| Wigal et al. | 2004 | dMPH | MPH | Short | 10 mg b.i.d. | Parallel | 4 weeks | 132 | 9.8 |
| MPH IR | MPH | Short | 20 mg b.i.d. | ||||||
| Gorman et al. | 2006 | MPH IR | MPH | Short | 1 mg/kg divided daily | Crossover | 3 weeks | 41 | 9.1 |
| Spencer et al. | 2006 | AMP XR | AMP | Long | 40 mg/day | Parallel | 4 weeks | 287 | 14.2 |
| Wigal et al. | 2006 | MPH IR | MPH | Short | 22.5mg/day | Crossover | 7 days | 165 | 3–5 |
| Newcorn et al. | 2008 | OROS® MPH | MPH | Long | 54 mg/day | Parallel | 6 weeks | 293 | 10.2 |
| Childress et al. | 2009 | dMPH ER | MPH | Long | 30 mg/day | Parallel | 5 weeks | 245 | 9.0 |
| Solanto et al. | 2009 | MPH IR | MPH | Short | 50 mg/day | Crossover | 1 week | 25 | 8.8 |
| Lee et al. | 2011 | MPH IR | MPH | Short | 0.5 mg/kg/day | Crossover | 1 week | 157 | 9.0 |
MPH, methylphenidate; IR, immediate release; MAS, mixed amphetamine salts; AMP, amphetamine; LDX, lisdexamfetamine dimesylate; MR, modified release; ER or XR, extended release; OROS, osmotic controlled-release oral delivery system; dMPH, dexmethylphenidate.
Risk of anxiety with psychostimulants
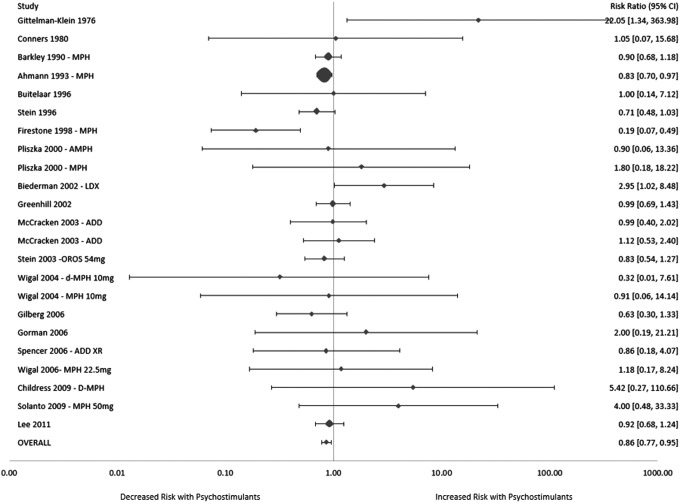
Meta-analysis demonstrated a significantly decreased risk of anxiety when comparing psychostimulant to placebo (relative risk [RR] = 0.86 [95% CI: 0.77, 0.95], z = −2.90, p = 0.004). There was no significant heterogeneity among trials (I2 = 22%, p = 0.17) or evidence of publication bias (Egger's test: p = 0.18). A random effects model produced a similar, although not statistically significant, reduction in risk ratio when examined in a sensitivity analysis (RR = 0.87 [95%CI: 0.75, 1.02], z = −1.71, p = 0.09)(Fig. 2).
FIG. 2.
Risk of anxiety in children with attention-deficit/hyperactivity disorder (ADHD) treated with psychostimulants compared with placebo. Meta-analysis demonstrated a significantly decreased risk of anxiety when comparing psychostimulant to placebo (relative risk [RR] = 0.86 [95% CI: 0.77, 0.95], z = −2.90, p = 0.004). There was no significant evidence of heterogeneity or publication bias.
MPH versus amphetamine derivatives
Stratified subgroup analysis demonstrated no significant difference in risk of anxiety (test for subgroup differences X2 = 0.76, p = 0.38) based on class of psychostimulants. However, MPH derivatives were associated with a significantly reduced risk of anxiety (RR = 0.85 [95% CI: 0.76, 0.94], k = 17, z = −3.02, p = 0.003), whereas amphetamine derivatives (RR = 1.02 [95% CI: 0.69, 1.50], k = 6, z = −2.90, p = 0.94) were associated with a similar risk of anxiety to that of placebo.
Long- versus short-acting psychostimulants
Stratified subgroup analysis demonstrated no significant difference in risk of anxiety (test for subgroup differences X2 = 1.46, p = 0.23) between short-acting and long-acting psychostimulants. However, short-acting psychostimulants were associated with a significant decreased risk of anxiety (RR = 0.83 [95% CI: 0.74, 0.93], z = −3.14, p = 0.002), whereas long-acting psychostimulants demonstrated a similar risk of anxiety to that of placebo (RR = 0.99 [95% CI: 0.77, 1.27], z = −0.10, p = 0.92).
Psychostimulant dose
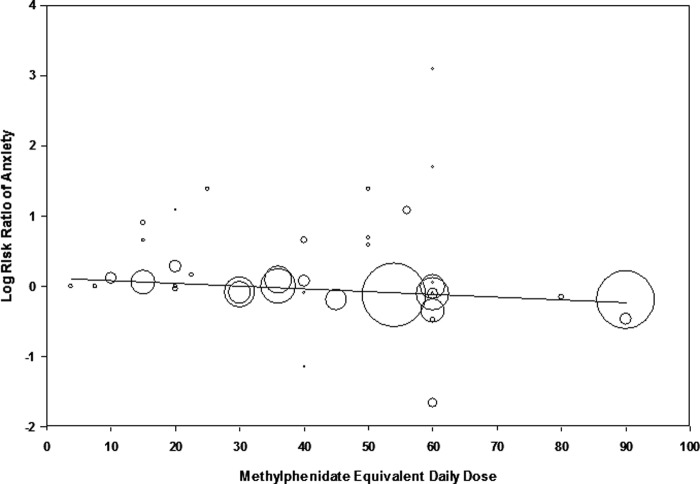
Meta-regression demonstrated a significant negative association between dosage of psychostimulants and the risk of anxiety (β = −0.0039 [95% CI: −0.00718, −0.00064], z = −2.34, p = 0.019). Higher doses of psychostimulants were associated with a decreased risk of anxiety relative to placebo. Figure 3 depicts a scatterplot demonstrating the negative association between psychostimulant dose and risk of anxiety. When analysis was restricted to MPH derivatives (β = −0.0034 [95% CI: −0.0069, 0.0000], z = −1.94, p = 0.05) and amphetamine derivatives (β = −0.0068 [95% CI: −0.0178, 0.0041], z = −1.22, p = 0.22), both stimulant classes displayed a similar negative relationship between risk of anxiety and dose of psychostimulants.
FIG. 3.
Meta-regression: Psychostimulant dose versus risk of anxiety. Meta-regression demonstrated a significant negative association between dosage of psychostimulants (x axis) and the risk of anxiety (y axis: β = −0.0039 [95% CI: −0.00718, −0.00064], z = −2.34, p = 0.019). Higher doses of psychostimulants were associated with a decreased risk of anxiety relative to placebo. Daily doses of psychostimulants are expressed in methylphenidate equivalents.
Duration of active treatment
Meta-regression demonstrated no significant association between duration of active treatment and the risk of anxiety associated with psychostimulant medication (β = −0.0016 [95% CI: −0.0111, 0.0079], z = −0.34, p = 0.74).
Trial design
Crossover studies reported no statistically significant difference in association of anxiety with psychostimulants compared with parallel-group studies (test for subgroup differences X2 = 2.08, p = 0.15). Crossover trials (RR = 0.83 [95% CI: 0.75, 0.93], z = −3.22, p = 0.001) report a significant decreased risk of anxiety, whereas parallel-group studies (RR = 1.05 [95% CI: 0.78, 1.41], z = 0.32, p = 0.75) reported no significant association between anxiety and psychostimulant use.
Discussion
Meta-analysis demonstrated a significantly reduced risk of anxiety in children with ADHD given psychostimulant treatment as compared with placebo. Meta-regression also suggested a dosing effect, with higher doses of psychostimulants being associated with lower rates of anxiety in children with ADHD. These findings run contrary to conventional wisdom and United States Food and Drug Administration (FDA) drug labeling, which suggest that anxiety is a common side-effect of psychostimulant treatment (Weiss and Hechtman 1993).
Although anxiety has been hypothesized to be a predictor of poor treatment response to MPH and as a moderator suggesting improved outcome with behavioral therapy compared with medications in ADHD, it seems that, on average, psychostimulants do not worsen anxiety and may actually improve it slightly on average. There are two potential explanations for this observed reduction in anxiety with psychostimulant treatment of children with ADHD: 1) That psychostimulants have a direct effect reducing anxiety or 2) that psychostimulants indirectly reduce anxiety symptoms by improving ADHD symptoms. The first hypothesis seems unlikely, as there is no evidence that psychostimulants are an effective treatment for anxiety disorders in animals or in humans (Young and Johnson 1991; Bilkei-Gorzo et al. 1998). We believe that a more likely explanation is that psychostimulants reduce measured anxiety symptoms indirectly by improving ADHD symptoms in children with ADHD. Improving ADHD symptoms in children with ADHD, likely decreases the number of anxiogenic situations they experience; children with ADHD successfully treated with psychostimulants experience fewer academic problems and peer and parental conflict that may cause them anxiety. Children with ADHD and comorbid social phobia (Golubchik et al. 2014) and general anxiety disorder have experienced a reduction in both ADHD symptoms and anxiety symptoms when treated with a psychostimulant in randomized, placebo-controlled trials. In placebo-controlled trials, children with ADHD and comorbid anxiety have even experienced greater reduction in their anxiety symptoms with psychostimulants than with traditional antianxiety medications (Abikoff et al. 2005). Psychostimulants are believed to decrease state anxiety in adults with ADHD but not in adults without ADHD (Bloch et al. 2013). Therefore, the improvement in anxiety symptoms associated with psychostimulant treatment is likely mediated through improvement in ADHD symptoms.
Given the significant findings in this meta-analysis, it is important to be transparent regarding several limitations of the current study. First, many of the potentially eligible randomized, placebo-controlled trials eligible for this meta-analysis did not report side-effect data on anxiety. This suggests the possibility of publication bias toward anxiety as a side effect outcome. Many researchers may have selectively reported anxiety as a side effect based on whether there was a significant difference between placebo and psychostimulants. This might lead to inflated association between anxiety and psychostimulants. We attempted to reduce this possible bias by contacting authors of psychostimulant trials to inquire about the presence of anxiety as a side effect, but most authors could or did not provide data upon our request. Further arguing against a significant publication bias, there was no evidence of publication bias on qualitative or quantitative analysis. Additionally, there were significant differences among trials in both in the frequency of anxiety reported as a side effect and the RR of anxiety with psychostimulants. The measurement of anxiety as a side effect differed among trials, from the informant (parent vs. researcher), to the assessment of anxiety (qualitative vs. quantitative), to the degree of impairment. As there was no consistent measurement among studies, it is difficult to truly determine the presence and severity of anxiety as a side effect.
Conclusions
We demonstrated in meta-analysis a reduced risk of anxiety in children with ADHD treated with psychostimulants as compared with those treated with placebo. We further demonstrated a negative association between dose of psychostimulants and risk of anxiety. These results do not rule out the possibility that some children experience increased anxiety when treated with psychostimulants, but suggests that those risks are outweighed by children who experience improvement in anxiety symptoms (possibly as a secondary effect of improved control of ADHD symptoms). As reports of worsening anxiety were common in placebo arms of randomized controlled trials in children with ADHD (24% in this meta-analysis), clinicians should further consider rechallenging children with ADHD who report new-onset or worsening anxiety with psychostimulants but significant improvement in ADHD symptoms, as worsening anxiety symptoms are much more likely to be coincidental rather than caused by psychostimulants. Further research examining comorbid anxiety in psychostimulant trials would benefit from a standardized and consistent format for reporting across trials as well as utilizing rating scales to evaluate anxiety symptoms.
Clinical Significance
Comorbid anxiety is common in children with ADHD. Furthermore, anxiety may be both a potential moderator of short-term treatment response in children with ADHD and a side effect of stimulant medication. As such, the presence of anxiety is tremendously important for clinicians managing the symptoms of children with ADHD. We seek to provide an evidence base for the care of children with ADHD who experience anxiety after starting psychostimulant medication.
Disclosures
No competing financial interests exist.
References
- Abikoff H, McGough J, Vitiello B, McCracken J, Davies M, Walkup J, Riddle M, Oatis M, Greenhill L, Skrobala A, March J, Gammon P, Robinson J, Lazell R, McMahon DJ, Ritz L, Rupp ADHD Anxiety Study Group: Sequential pharmacotherapy for children with comorbid attention-deficit/hyperactivity and anxiety disorders. J Am Acad Child Adolesc Psychiatry 44:418–427, 2005 [DOI] [PubMed] [Google Scholar]
- Ahmann PA, Waltonen SJ, Olson KA, Theye FW, Van Erem AJ, LaPlant RJ: Placebo-controlled evaluation of Ritalin side effects. Pediatrics 91:1101–1106, 1993 [PubMed] [Google Scholar]
- Barkley RA, McMurray MB, Edelbrock CS, Robbins K: Side effects of methylphenidate in children with attention deficit hyperactivity disorder: A systemic, placebo-controlled evaluation. Pediatrics 86:184–192, 1990 [PubMed] [Google Scholar]
- Biederman J, Lopez FA, Boellner SW, Chandler MC: A randomized, double-blind, placebo-controlled, parallel-group study of SLI381 (Adderall XR) in children with attention-deficit/hyperactivity disorder. Pediatrics 110:258–266, 2002 [DOI] [PubMed] [Google Scholar]
- Bilkei–Gorzo A, Gyertyan I, Levay G: mCPP-induced anxiety in the light-dark box in rats–a new method for screening anxiolytic activity. Psychopharmacology 136:291–298, 1998 [DOI] [PubMed] [Google Scholar]
- Bloch Y, Aviram S, Segev A, Nitzan U, Levkovitz Y, Braw Y, Mimouni Bloch A: Methylphenidate reduces state anxiety during a continuous performance test that distinguishes adult ADHD patients from controls. J Atten Disord 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, van der Gaag RJ, Swaab–Barneveld H, Kuiper M: Pindolol and methylphenidate in children with attention-deficit hyperactivity disorder. Clinical efficacy and side-effects. J Child Psychol Psychiatry 37:587–595, 1996 [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Van der Gaag RJ, Swaab–Barneveld H, Kuiper M: Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34:1025–1032, 1995 [DOI] [PubMed] [Google Scholar]
- Childress AC, Spencer T, Lopez F, Gerstner O, Thulasiraman A, Muniz R, Post A: Efficacy and safety of dexmethylphenidate extended-release capsules administered once daily to children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:351–361, 2009 [DOI] [PubMed] [Google Scholar]
- Conners CK, Taylor E: Pemoline, methylphenidate, and placebo in children with minimal brain dysfunction. Arch Gen Psychiatry 37:922–930, 1980 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman HM, Reiff MI: Clinical practice. Attention deficit-hyperactivity disorder in children and adolescents. N Engl J Med 370:838–846, 2014 [DOI] [PubMed] [Google Scholar]
- Firestone P, Musten LM, Pisterman S, Mercer J, Bennett S: Short-term side effects of stimulant medication are increased in preschool children with attention-deficit/hyperactivity disorder: A double-blind placebo-controlled study. J Child Adolesc Psychopharmacol 8:13–25, 1998 [DOI] [PubMed] [Google Scholar]
- Gillberg C, Melander H, von Knorring AL, Janols LO, Thernlund G, Hagglof B, Eidevall–Wallin L, Gustafsson P, Kopp S: Long-term stimulant treatment of children with attention-deficit hyperactivity disorder symptoms. A randomized, double-blind, placebo-controlled trial. Arch Gen Psychiatry 54:857–864, 1997 [DOI] [PubMed] [Google Scholar]
- Gittelman–Klein R, Klein DF, Katz S, Saraf K, Pollack E: Comparative effects of methylphenidate and thioridazine in hyperkinetic children. I. Clinical results. Arch Gen Psychiatry 33:1217–1231, 1976 [DOI] [PubMed] [Google Scholar]
- Golubchik P, Sever J, Weizman A: Methylphenidate treatment in children with attention deficit hyperactivity disorder and comorbid social phobia. Int Clin Psychopharmacol 29:212–215, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman EB, Klorman R, Thatcher JE, Borgstedt AD: Effects of methylphenidate on subtypes of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 45:808–816, 2006 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Findling RL, Swanson JM, ADHD Study Group: A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 109:E39, 2002 [DOI] [PubMed] [Google Scholar]
- Lee J, Grizenko N, Bhat V, Sengupta S, Polotskaia A, Joober R: Relation between therapeutic response and side effects induced by methylphenidate as observed by parents and teachers of children with ADHD. BMC Psychiatry 11:70, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D: The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann Inter Med 151:W65–94, 2009 [DOI] [PubMed] [Google Scholar]
- March JS, Swanson JM, Arnold LE, Hoza B, Conners CK, Hinshaw SP, Hechtman L, Kraemer HC, Greenhill LL, Abikoff HB, Elliott LG, Jensen PS, Newcorn JH, Vitiello B, Severe J, Wells KC, Pelham WE: Anxiety as a predictor and outcome variable in the multimodal treatment study of children with ADHD (MTA). J Abnorm Child Psychol 28:527–541, 2000 [DOI] [PubMed] [Google Scholar]
- McCracken JT, Biederman J, Greenhill LL, Swanson JM, McGough JJ, Spencer TJ, Posner K, Wigal S, Pataki C, Zhang Y, Tulloch S: Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolescent Psychiatry 42:673–683, 2003 [DOI] [PubMed] [Google Scholar]
- Moderators and mediators of treatment response for children with attention-deficit/hyperactivity disorder: The Multimodal Treatment Study of children with Attention-Deficit/Hyperactivity Disorder. Arch Gen Psychiatry 56:1088–1096, 1999 [DOI] [PubMed] [Google Scholar]
- Newcorn JH, Kratochvil CJ, Allen AJ, Casat CD, Ruff DD, Moore RJ, Michelson D, Atomoxetine/Methylphenidate Comparative Study Group: Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: Acute comparison and differential response. Am J Psychiatry 165:721–730, 2008 [DOI] [PubMed] [Google Scholar]
- NextWave Pharmaceuticals: Product Information: QUILLIVANT(TM) XR oral extended release suspension, methylphenidate HCl oral extended release suspension. Cupertino, CA; 2012 [Google Scholar]
- Pliszka SR, Browne RG, Olvera RL, Wynne SK: A double-blind, placebo-controlled study of Adderall and methylphenidate in the treatment of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 39:619–626, 2000 [DOI] [PubMed] [Google Scholar]
- Rapport MD, Stoner G, DuPaul GJ, Birmingham BK, Tucker S: Methylphenidate in hyperactive children: Differential effects of dose on academic, learning, and social behavior. J Abnorm Child Psychol 13:227–243, 1985 [DOI] [PubMed] [Google Scholar]
- Sciberras E, Lycett K, Efron D, Mensah F, Gerner B, Hiscock H: Anxiety in children with attention-deficit/hyperactivity disorder. Pediatrics 133:801–808, 2014 [DOI] [PubMed] [Google Scholar]
- Solanto M, Newcorn J, Vail L, Gilbert S, Ivanov I, Lara R: Stimulant drug response in the predominantly inattentive and combined subtypes of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 19:663–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Wilens TE, Biederman J, Weisler RH, Read SC, Pratt R: Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of attention-deficit/hyperactivity disorder in adolescent patients: A 4-week, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 28:266–279, 2006 [DOI] [PubMed] [Google Scholar]
- Stein MA, Blondis TA, Schnitzler ER, O'Brien T, Fishkin J, Blackwell B, Szumowski E, Roizen NJ: Methylphenidate dosing: Twice daily versus three times daily. Pediatrics 98:748–756, 1996 [PubMed] [Google Scholar]
- Stein MA, Sarampote CS, Waldman ID, Robb AS, Conlon C, Pearl PL, Black DO, Seymour KE, Newcorn JH: A dose-response study of OROS methylphenidate in children with attention-deficit/hyperactivity disorder. Pediatrics 112:e404, 2003 [DOI] [PubMed] [Google Scholar]
- Swenson M: Stimulant Equivalency Table. Utah Academy of Child and Adolescent Psychiatry. www.uacap.org/uploads/3/2/5/0/3250432/stimulant_equivalency.pdf
- Ter-Stepanian M, Grizenko N, Zappitelli M, Joober R: Clinical response to methylphenidate in children diagnosed with attention-deficit hyperactivity disorder and comorbid psychiatric disorders. Can J Psychiatry 55:305–312, 2010 [DOI] [PubMed] [Google Scholar]
- United States Product Information: ADDERALL XR(R) oral capsules, dextroamphetamine sulfate dextroamphetamine saccharate amphetamine aspartate monohydrate amphetamine sulfate oral capsules. Wayne, PA; 2013 [Google Scholar]
- Weiss G, Hechtman L: Hyperactive Children Grown Up. New York: Guilford Press, 1993 [Google Scholar]
- Werry JS, Aman MG, Diamond E: Imipramine and methylphenidate in hyperactive children. J Child Psychol Psychiatry 21:27–35, 1980 [DOI] [PubMed] [Google Scholar]
- Wigal S, Swanson JM, Feifel D, Sangal RB, Elia J, Casat CD, Zeldis JB, Conners CK: A double-blind, placebo-controlled trial of dexmethylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 43:1406–1414, 2004 [DOI] [PubMed] [Google Scholar]
- Wigal T, Greenhill L, Chuang S, McGough J, Vitiello B, Skrobala A, Swanson J, Wigal S, Abikoff H, Kollins S, McCracken J, Riddle M, Posner K, Ghuman J, Davies M, Thorp B, Stehli A: Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry 45:1294–1303, 2006 [DOI] [PubMed] [Google Scholar]
- Young R, Johnson DN: A fully automated light/dark apparatus useful for comparing anxiolytic agents. Pharmacol Biochem Behav 40:739–743, 1991 [DOI] [PubMed] [Google Scholar]