Abstract
Peptide vaccines are capable of eliciting immune responses targeting tumor-associated antigens such as the Wilms’ Tumor 1 (WT1) antigen, often overexpressed in myeloid malignancies. Here, we assessed the safety, tolerability, and immunogenicity of a polyvalent WT1 peptide vaccine. Individuals with WT1-positive acute myeloid leukemia (AML) in first (CR1) or second (CR2) remission or with higher-risk myelodysplastic syndrome (MDS) following at least 1 prior line of therapy were vaccinated with a mixture of peptides derived from the WT1 protein, with sargramostim injections before vaccination to amplify immunogenicity. Six vaccinations were delivered biweekly, continuing then monthly until patients received 12 vaccinations or showed disease relapse or progression. Therapeutic efficacy was evaluated by progression-free and overall survival. Immune responses were evaluated by delayed-type hypersensitivity testing and T-cell IFNγ ELISPOT at specified intervals. In 16 patients who received at least one vaccination, 10 completed the planned course of six vaccinations and six continued for up to six additional monthly vaccinations. Vaccinations were well tolerated, with no patients discontinuing due to toxicity. One of two patients with high-risk MDS experienced a prolonged decrease in transfusion dependence. Two of 14 AML patients demonstrated relapse-free survival >1 year. Both patients were in CR2 at time of vaccination, with duration of their remission exceeding duration of their first remission, suggesting a potential benefit. Our WT1 vaccine was well-tolerated. The clinical benefit that we observed in several patients suggests engagement of a protective immune response, indicating a need for further trials.
Introduction
Vaccine-based immunotherapies represent an intriguing approach to cancer therapy. In the context of acute myeloid leukemia (AML), chemotherapy remains the frontline treatment option, with allogeneic hematopoietic stem cell transplant offering the best opportunity for consolidation and cure in high-risk disease. The curative potential of allogeneic hematopoietic stem cell transplant is now understood to be, at least in part, due to the recruitment of an immune-based graft-versus-leukemia response, thus establishing the potential role of immune-targeted therapies in AML, and similarly by extrapolation, with myelodysplastic syndrome (MDS).
In recent years, Wilms’ Tumor 1 (WT1) has emerged as an encouraging vaccine target in AML [1–4]. Known for its association with Wilm’s tumor, WT1 is a zinc finger transcription factor essential in urogenital embryogenesis. More recently, its overexpression has been detected in AML and particularly in leukemic blasts [5,6], portending a worse overall survival [5], but also hypothesized as a means to detect minimal residual disease [6,7]. Furthermore, WT1 mutations, detected in ~10% of normal karyotype AML cases [8,9] and less frequently in MDS [10], are also predictive of poor outcome. Exploitation of WT1 as a vaccine target has emerged from observations that cytotoxic T-cell responses could be elicited against HLA-restricted epitopes derived from the native WT1 sequence [1,11–14].
Early vaccine developmental strategies emphasized the utilization and optimization of class I determinants, modifying native epitopes to enhance human leukocyte antigen (HLA) binding and presentation [3,15]. Consequently, early pilot studies clearly showed a peptide-specific expansion of CD8+ cytotoxic lymphocytes (CTLs) in vaccinated patients [2,4], often associated with measurable clinical responses. Unfortunately, high-avidity responses were short-lived, yielding to more durable responses against predominantly against low-avidity cryptic epitopes [16]. Viewed from an alternate perspective, perhaps the failure to generate a sustained or protective anti-leukemic response lay not in the hierarchical dominance of the target epitope, but rather in the lack of appropriate or optimal activation, as CTL memory and persistence are well-established to be dependent on CD4 help [17].
Here, we revisited the efficacy of a WT1-directed peptide vaccination in a phase I pilot study in heavily pretreated AML and MDS patients, attempting to further optimize immunogenicity. We hypothesized that limited clinical responses, despite consistently measurable CTL reactivity (as reported in prior studies), may result from inadequate T-cell help. Therefore, our approach combined four peptides comprising a mixture of both native and heteroclitic WT1-derived class I peptides, as well as extended peptides containing putative class II epitopes intended to promote an associated helper response.
Methods
Trial design and patient inclusion/exclusion criteria
In this phase I pilot study (ClinicalTrials.gov #NCT00665002), we enrolled patients with high-risk MDS or AML in remission with documented WT1-positive disease. Inclusion criteria included a confirmed diagnosis of AML or MDS with an International Prognostic Scoring System (IPSS) score of intermediate-2 or greater, age ≥18 years, Karnofsky performance status ≥70%, and detectable expression of WT1 by RT-PCR from pre-treatment bone marrow biopsies or peripheral blood samples. AML patients were required to be in CR1 or CR2, with completion of all chemotherapy at least 4 weeks before initiation of the protocol. MDS patients were required to have relapsed after, or progressed through, at least one approved therapy (e.g., hypomethylating agent or lenalidomide). Additionally, patients were required to meet hematologic and biochemical parameters including an absolute neutrophil count (ANC) ≥1000/μL (≥500/μL for MDS patients), platelet count ≥50,000 (≥25,000 for MDS), total bilirubin ≤2 mg/dL, AST and ALT ≤2.5x the upper limit of normal, and creatinine ≤2.0 mg/dL. This protocol was approved by our local IRB, and written informed consent was obtained from all patients prior to starting the protocol.
Vaccination protocol
The vaccine contained a mixture of four peptides, one of which contained a heteroclitic WT1-derived class I epitope to stimulate CD8 T-cell responses, two contained class II peptide sequences designed to stimulate CD4 T-cell responses [18], and the last contained both class I and class II epitopes (see Supporting Information Table S1). Each vaccine contained 200 μg of each peptide, for a total of 800 μg delivered in each injection, mixed 1:1 with an emulsion of Montanide ISA 51 VG adjuvant in phosphate-buffered saline, with a total volume of 1 mL. Peptides were synthesized by American Peptide Company (Vista, CA) in their GMP plant, and the vaccine was manufactured under GMP conditions by the University of Iowa, Division of Pharmaceutical Services. Vaccinations were delivered according to a schedule of biweekly injections over a 10-week period, followed by monthly injections extended out to 6 months, or until the patient demonstrated clinical or diagnostic evidence of relapse or intolerance. On days -2 and 0 before each vaccination, patients were administered 70 μg sargramostim (Berlex Pharmaceuticals) subcutaneously for the purpose of further immunogenic enhancement.
Immune response assessment
Each patient underwent immunologic assessment prior to initial vaccination, with reassessment at 6- and 12-week time points. An additional 100 mL of peripheral blood were collected in heparinized tubes at these time points for ex vivo analyses, which included antigen-specific IFN-γ release by ELISPOT and CTL expansion as determined by tetramer analysis in flow cytometric analysis. Clinically, this was correlated to delayed-type hypersensitivity (DTH) responses measured at these time points as well.
CD4 and CD8 T-cell ELISPOT assay
Antigen-specific T-cell activation was detected by IFN-γ ELISPOT as described previously [3]. In brief, IFN-γ ELISPOT plates were prepared by conditioning Multiscreen plates (Millipore) with mouse anti-human IFN-γ capture antibody (5 μg/mL, clone 1-D1K, Mabtech) overnight at 4 °C, followed by blocking with RPMI-1640-10% AP human serum for 2 hr at 37 °C. PBMC-derived lymphocytes (105 cells/well) were cocultured for 20 hr at 37 °C with irradiated (10,000 cGy) T2 cells (104 cells/well) used as antigen-presenting cells, pulsed with native class I WT1 peptide, RMFPNAPYL, or individual class II peptides, RSDELVRHHNMHQRNMTKL (WT1-427long) or PGCNKRYFKLSHLQM HSRKHTG (WT1-331long) at a concentration of 10 μg/mL. β2-Microglobulin (10 μg/mL) served as a nonspecific control, while stimulation with 25 μg/mL phytohemagglutinin served as a positive control. IFN-γ production was detected using anti-human IFN-γ detection antibody (2 μg/mL, clone 7-B6-1, Mabtech), incubated at room temperature for 2 hr, followed by colorimetric development using avidin-peroxidase (1:1000 dilution, incubated at room temperature for 1 hr) and AEC substrate. After the development, reaction was stopped and membranes were dried overnight, plates were analyzed and counted on an AID spot reader.
Analysis of WT1-specific CTL activation
In patients expressing the HLA-A*0201 antigen, expansion of WT1-specific CD8 T cells isolated from peripheral blood samples collected at baseline and at 6 and 12 weeks was assayed using tetramer analysis by flow cytometry.
Flow cytometry reagents
All flow cytometry data were acquired using an LSR II flow cytometer (BD Biosciences, San Jose, CA), with data analysis performed using FlowJo software (Tree Star, Ashland, OR). Fluorochrome-conjugated antibodies used in these experiments included PerCP-Cy5.5-CD4, PE-IL-17, and AlexaFluor 647-FoxP3, APC-CD8, FITC-CD45RA, PE-WT1-tetramer.
DTH responses
Before treatment was started, as well as before the week 6 and 12 vaccinations, DTH responses were assessed. A mixture of 15 μg of each of the 4 WT1-derived peptides was prepared in phosphate-buffered saline. A volume of 70 μL of this preparation was injected intradermally in the forearm, and the injection site was marked. A separate site was injected intradermally with a preparation of candida (Allermed Laboratories), used as a reference positive control. We reassessed the injection sites after 48–72 hr and considered these positive if erythema or induration of >0.5 cm was observed.
In vitro lymphocyte restimulation
Patient peripheral blood samples were collected at specified time points and resuspended in RPMI-5% AP at 37 °C at 1.5 × 107 cells/well in six-well culture plates for 2 hr allowing for adherence. Nonadherent cells were removed, with adherent cells subsequently harvested with cold EDTA buffer for use as APCs. A negative pan-T-cell selection (Miltenyi Biotech) was performed on nonadherent cells to separate CD3-positive from CD3-negative cells. CD3-negative cells were stored at −80 °C for future use as APCs. T cells were restimulated twice prior to functional assessment. Lymphocytes were first seeded at 106 cells/well at a 10:1 ratio with adherent APCs pulsed with 20 μg/mL of each of the 4 WT1-derived peptides (WT1-A1, WT1-122A1, WT1-427, and WT1-331) in RPMI-5% AP-β2-microglobulin (1 μg/mL), supplemented with IL-2 at 20 U/mL on days 2, 4, and 6. In the second restimulation, peptide-loaded dendritic cells (DCs) generated as described below were used as APCs. Bulk restimulation cultures combined lymphocytes with DCs at a ratio of 30:1 in RPMI-5% AP-β2-microglobulin (1 μg/mL) in six-well plates, incubated for 7 days at 37 °C with supplementation of 20 U/mL IL-2 on days 2, 4, and 6. On completion of the second restimulation, T cells were harvested for measurement of CD4 or CD8 function by IFN-γ ELISPOT and WT1-tetramer analysis (for CD8 T cells only).
DC APC generation
Adherent cells isolated as described above, or similarly isolated from bone marrow aspirate samples from each patient, were plated in RPMI-5% AP supplemented with 1000 U/mL GM-CSF and 500 U/mL IL-4. Each well received 20 μg/mL of each of the 4 WT1 peptides. On days 2 and 4, half the media in each well were removed and replaced with fresh RPMI-5% AP supplemented with 1000 U/mL GM-CSF and 500 U/mL IL-4. On day 5, an additional 20 μg/mL of each of the four WT1 peptides were added. On day 6, DC maturation was induced with the addition of 500 U/mL IL-4, 1000 U/mL GM-CSF, 10 ng/mL TNF-α, and 1 μg/mL PGE-2. DCs were then harvested on day 7, to be used directly as APCs in the second round of T-cell restimulation, or frozen for storage, to be used as APCs later.
WT1 expression by RT-PCR
Total RNA was prepared from 106 cells isolated from patient samples (RNeasy mini-columns and RNase-free DNase; Qiagen, Valencia, CA). cDNA was prepared using iScript cDNA Synthesis Kit (Bio-Rad), and qRT-PCR reactions were conducted using the SYBR green two-step qRT-PCR (Bio-Rad) with transcript-specific primers (supplied upon request) and cDNA from either peripheral blood monocytes or bone marrow aspirate cells as templates. qRT-PCR amplification reactions were resolved on CFX iCycler (Bio-Rad), and fold changes were quantified (2−ΔΔCt) according to established standardization protocols [19].
Assessment of clinical outcome
Patients were monitored clinically for evidence of disease progression. We evaluated bone marrow before vaccination and after vaccination (on weeks 6 and 12). Clinical response was evaluated by percentage of blast counts on BM biopsies performed before and after vaccination Therapeutic responses in MDS patients were assessed according to criteria set forth in IWG 2006 [20]. AML patients were monitored for relapse as per IWG 2003 criteria [21].
As an additional measure of clinical performance, AML patients enrolled after achieving CR2 were compared retrospectively to a matched group of AML patients previously treated and followed at our institution. The comparator population included patients diagnosed and treated after 2001 who were ineligible for transplant due to age or inability to identify a matched donor.
Statistical analysis
With regard to patient survival comparisons, overall survival was compared according to the Kaplan-Meier nonparametric testing. Survival differences between the two groups were assessed statistically according to the log-rank nonparametric method.
Toxicity assessment
Toxicities were tabulated according to the NCI Common Toxicity (version 3.0) by grade and category.
Results
Patient characteristics
A total of 41 patients were screened for WT1 expression, identifying 24 patients overexpressing the WT1 protein. Sixteen patients, two with refractory MDS and 14 with relapsed AML in CR1 or CR2, were enrolled between February 2009 and September 2012 (median age was 74 years). All patients demonstrated measurable WT1 transcript by PCR at the time of enrollment. Nine of the 14 AML patients and both of the MDS patients exhibited a normal karyotype on cytogenetic analysis of the bone marrow. Of the five AML patients with abnormal karyotype at baseline, three had complex karyotype, one contained a t(15;17) translocation not involving the classic PML-RARA translocation, and one contained a CBFB-MY H11 (inversion 16) translocation. Five patients with AML had a prior diagnosis of MDS or myeloproliferative neoplasm. All patients were heavily pre-treated, having received, at minimum two and as many as four prior lines of therapy (median of two). None had undergone transplant. At time of enrollment, all patients with AML had achieved remission on prior therapy according to standard criteria, with bone marrow involvement reported as <5% blasts. Both MDS patients had each received three prior lines of therapy, including a hypomethylating agent, and had remained transfusion-dependent. By IPSS Criteria, one patient had intermediate-2 risk MDS while the other had high-risk MDS. Patient demographics are summarized in Table I.
TABLE I.
Patient Characteristics
| ID | Age/sex | Disease | Prior disease | Karyotype | CR# | Number of prior treatments | %Blasts in BM | Months to relapse or progression |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 72/F | MDS | 46,XX | N/A | 3 | 11% | 3·2 | |
| Patient 2 | 76/F | MDS | 46,XX | N/A | 3 | 8% | 22·4 | |
| Patient 3 | 73/M | AML | 46,XY, FLT3+, NPM1+ | 2 | 3 | <5% | 10 | |
| Patient 4 | 68/F | AML | ET | Complex | 1 | 2 | 3% | 3·7 |
| Patient 5 | 74/M | AML | Complex | 2 | 3 | 2% | 14·8 | |
| Patient 6 | 69/M | AML | 46,XY, FLT3+, NPM1+ | 2 | 2 | 6% | 3·9 | |
| Patient 7 | 70/M | AML | 46,XY t(15;17) | 2 | 3 | 3% | 6·1 | |
| Patient 8 | 75/F | AML | RAEB-2 | 46,XX | 2 | 2 | 4% | 2·8 |
| Patient 9 | 72/M | AML | 46,XY | 2 | 2 | 0% | 11·2 | |
| Patient 10 | 82/M | AML | Inv(16), complex | 2 | 4 | 2.5% | 7·2 | |
| Patient 11 | 80/F | AML | RAEB-2 | 46,XX | 1 | 3 | 4% | 11·7 |
| Patient 12 | 77/M | AML | RAEB-2 | 46,XY | 1 | 2 | 0% | 7·6 |
| Patient 13 | 71/M | AML | 46,XY, inv(16) | 2 | 2 | <5% | 12·7 | |
| Patient 14 | 75/M | AML | 46,XY | 1 | 2 | 0% | 9·6 | |
| Patient 15 | 65/M | AML | 46,XY | 2 | 2 | 4–5% | 10·1 | |
| Patient 16 | 75/F | AML | 46,XX | 2 | 2 | 4% | 11·4 |
Protocol administration and toxicity
Patients received six biweekly vaccinations over a 10-week period, with continuation with up to 6 additional monthly injections for patients without evidence of leukemic relapse or progression of MDS. Nine of 16 patients (one with MDS and eight with AML) completed the planned six biweekly vaccine regimen. The mean number of vaccinations was 7.7, ranging from 2 to 13. Four of the 14 patients with AML completed an additional six vaccines, while the remaining 10 participants discontinued treatment upon evidence of disease progression. One of two patients with MDS completed the biweekly vaccinations through 10 weeks, continuing on to receive a total of 10 vaccinations (see Table I). In this case, one vaccine was missed and one was held due to a transient elevation in total bilirubin, but the patient continued upon resolution of hyperbilirubinemia to complete the remainder of the vaccine schedule. The other MDS patient received 4 vaccinations before discontinuation due to progression of disease with transformation to AML.
Overall, the vaccine was safe and well tolerated. All reported toxicities were grade 1/2 except for a single incident of grade 3/4 neutropenia (ANC range 250–930/mm3). Six patients developed localized injection site reactions (grade 1/2). One patient developed large, painful dermal nodules on week 10 of the vaccination protocol; a fine needle aspirate biopsy report described an inflammatory infiltrate consistent with localized reaction to the vaccine. Notably, two individuals developed localized induration and erythema several months after completion of the vaccination protocol. Interestingly, the rash appeared at the time of disease relapse in one of these patients (patient 10). Furthermore, both of these patients developed positive DTH responses detected with the immunologic screening at week 6. Treatment-related toxicities are detailed in Table II.
TABLE II.
Adverse Events Attributable to Vaccine
| Toxicity | Grade 1/2 | Grade 3/4 |
|---|---|---|
| Fatigue | 3 (18·75%) | 0 |
| Generalized weakness | 1 (6·67%) | 0 |
| Anorexia | 1 (6·67%) | 0 |
| Flushing/hot flashes | 1 (6·67%) | 0 |
| Pain | 2 (12·5%) | 0 |
| Leukopenia | 2 (12·5%) | 1 (6·67%) |
| Anemia | 1 (6·67%) | 0 |
| Thrombocytopenia | 1 (6·67%) | 0 |
| Hematoma/abnormal bleeding | 2 (12·5%) | 0 |
| Nausea | 2 (12·5%) | 0 |
| Emesis | 1 (6·67%) | 0 |
| Diarrhea | 1 (6·67%) | 0 |
| Bloating | 1 (6·67%) | 0 |
| Transaminitis | 1 (6·67%) | 0 |
| Hyperbilirubinemia | 1 (6·67%) | 0 |
| Cough | 1 (6·67%) | 0 |
| Rash | 1 (6·67%) | 0 |
| Local injection site reaction | 6 (37·5%) | 0 |
| Pruritis | 1 (6·67%) | 0 |
Clinical outcomes
The secondary objective of our pilot study was to evaluate clinical response/efficacy, assessed in terms of leukemic blast involvement in the marrow. The clinical details of the 16 patients who participated in this study are summarized in Table I.
The two MDS patients included in this study exhibited high-risk features at the time of enrollment and remained transfusion-dependent despite prior therapy. One patient experienced a 50% reduction in transfusion requirements over the course of five vaccinations delivered, when compared to the 4 months prior to enrollment. Interestingly, the bone marrow biopsy collected at the 6-week interval showed a 35–40% reduction in myeloblast percentage (decreasing from 11 to 7%). Unfortunately, this patient subsequently experienced disease evolution to AML ~2 months later, with a diagnostic bone marrow biopsy revealing 25% myeloblast involvement. The second MDS patient had no response, despite receiving 10 injections in total. Intriguingly, however, this patient became transfusion-independent shortly after completion of the protocol, ongoing for over 14 months.
By study design, AML patients were in remission (CR1 or CR2) at the time of initiation of the vaccine protocol. The mean interval between documentation of complete remission status and first vaccination was 2.7 months (range, 0.6–8.7 months). Clinical efficacy was therefore evaluated as a measure of time to recurrence from the time of documented CR after the previous chemotherapy. The calculated mean time to recurrence seen in these 14 individuals was 244 days (range, 30–445 days), while the mean overall survival from time of remission was 608 days (range, 201–1071 days) (see Fig. 1). At the time of analysis, all participating patients had exhibited evidence of disease progression; three AML patients remain alive. Of note, four of the 14 (28%) AML patients experienced sustained responses with durations either equaling or exceeding the duration of first remission. One was HLA-A*0201 positive and exhibited evidence of cognate immune responses in vitro.
Figure 1.
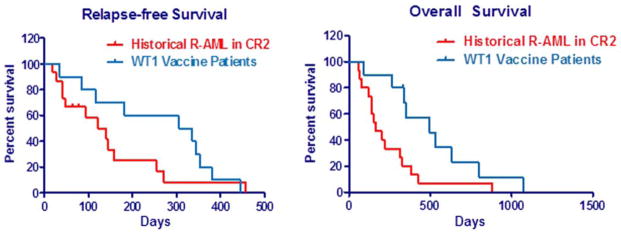
Relapse-free survival and overall survival in acute myeloid leukemia (AML) patients receiving WT1 vaccine. Ten AML patients receiving ≥ two vaccinations were compared to a similar group of 15 relapsed AML (R-AML) patients in second remission (CR2) previously treated at our institution. A: Time to relapse. B: Overall survival from date of CR2.
In an unplanned analysis, AML patients enrolled in the trial who had achieved CR2 were compared in terms of disease-free and overall survival against a similar cohort of AML patients previously treated at our institution. Patients in the historical cohort were identified based on documentation of attained CR2 after second-line chemotherapy and ineligible for allogeneic transplant either due to age or lack of a matched donor marrow. Curiously, although there was no statistical relevance in disease-free survival results (319 days vs. 131 days, p = 0.19 by log-rank analysis), there was a comparative and statistically significant improvement in overall survival observed in the WT1-treated individuals compared with the historical cohort (495 days versus 165 days, p = 0.0175 by log-rank analysis), represented in Fig. 1.
Immunologic response and correlation to disease status
DTH response results, assessed at baseline and at 6 and 12 weeks, are shown in Table III. One of the 16 patients demonstrated reactivity to WT1 at baseline. Neither MDS patient developed a DTH response to vaccination. Two of 14 AML patients developed a positive DTH response at 6 weeks; however, this was a transient response, with further DTH testing at 12 weeks negative in both patients. A third AML patient developed a positive DTH response at 12 weeks. A fourth patient developed a slight DTH reaction not meeting criteria to be considered positive, but interestingly developed a cutaneous erythema and induration at the initial vaccination site noted upon disease relapse ~352 days after achieving CR2. The significance of this cutaneous reaction is uncertain but raises speculation as to whether this reflects a WT1-specific recall immune response.
TABLE III.
Characterization of Immune Responses
| Patient No. | HLA-A | HLA.DRβ | DTH | ELISPOT CD4/CD8 | Tetramer |
|---|---|---|---|---|---|
| 1 | 0201/2902 | 0701/– | Negative | Pos/Neg | Negative |
| 2 | 0201/6801 | 0301/0401 | Negative | Pos/Pos | Positive |
| 3 | 1101/2902 | 0102/0801 | Negative | Neg/Neg | Negative |
| 4 | 0201/6801 | 0401/0802 | Negative | Neg/Neg | Positive |
| 5 | 0201/1101 | 0404/1501 | Negative | Neg/Pos | Positive |
| 6 | Unknown | Unknown | Negative | Neg/Neg | Negative |
| 7 | 0101/– | 0301/0701 | Negative | Neg/Neg | Negative |
| 8 | 0101/– | 0301/0701 | Negative | Neg/Neg | Negative |
| 9 | 1101/2402 | Unknown | Positive | Neg/Neg | Negative |
| 10 | 0101/3101 | Unknown | Negative | Neg/Neg | Negative |
| 11 | 0301/6801 | 0901/1104 | Positive | Neg/Neg | Negative |
| 12 | 0101/1101 | Unknown | Negative | Neg/Neg | Negative |
| 13 | 2902/3301 | Unknown | Negative | Pos/Pos | Negative |
| 14 | 2402/6802 | 1101/1303 | Negative | Neg/Neg | Negative |
| 15 | 0201/2402 | Unknown | Negative | ND/ND | ND |
| 16 | Unknown | Unknown | Negative | Neg/Neg | Negative |
ND: Not done.
At pre-specified time points, peripheral blood lymphocytes were collected to assess development of WT1-specific immunity. Four patients demonstrated a measurable IFN-γ response by CD4 T cells, with three of these patients also demonstrating CD8 T-cell-mediated IFN-γ responses (Table III, Supporting Information Fig. s1). We further characterized CD8 T-cell responses using flow cytometric analyses of restimulated lymphocytes, quantitating WT1-specific CD8 T cells from patients whose HLA phenotype contained the HLA-A*0201 antigen. In our 16 study patients, total, five harbored the HLA-A*0201 phenotype. Three of the four patients with measurable IFN-γ response exhibited a slight expansion of WT1-specific CD8 T cells. Due to low yields of tetramer+ CD8+ T cells even after two rounds of bulk restimulation, further analysis of the WT1-reactive CTL population for expression of memory and activation markers was not feasible. Similarly, we were unable to explore alterations in TH17:TReg polarization in the CD4 T-cell compartment because of low responder T-cell yields.
When clinical and immunologic parameters were considered collectively, several interesting observations emerged. In our two trial patients with high-risk MDS, both transfusion-dependent requiring blood products approximately every 2 weeks, we observed clinical changes, suggesting they may have derived some clinical benefit from the vaccine. Of note, both patients expressed the HLA-A*0201 allele, thus increasing the likelihood for a CTL response to the vaccine. One patient on therapy appeared to manifest a subtle measurable response with the initiation of the protocol, demonstrating a notable 50% decrease in transfusion frequency that was associated with a 35–40% reduction in marrow myeloblasts from 11 to 7% by the sixth week. However, this patient was removed from the trial after the fifth vaccination, when clinical suspicion prompted a repeat bone marrow biopsy that confirmed evolution to AML. Interestingly, DTH testing at week 12 was also marginally positive for WT1 but not the candida antigen control, while T-cell proliferation and ELISPOT assays detected cognate T-cell responses manifesting by week 12 at the time that the patient’s MDS was evolving into AML. The clinical significance of these findings remains uncertain but could represent an escalation in antigen-driven immune activation associated with increasing exposure to the WT1 antigen. In line with this hypothesis, WT1 mRNA transcript copy number demonstrated an upward trend, easily doubling over the same interval that WT1-specific lymphocytes became detectable (Supporting Information Fig. s2). In the second MDS patient, clinical disease parameters remained stable throughout the entire vaccine course; however, after the final vaccination, hematologic parameters stabilized and the patient reverted to a state of minimal intermittent transfusion dependence lasting ~14 months. WT1 mRNA transcript copy numbers remained relatively stable and detectable throughout the initial vaccine course, but decreased by ~75% when remeasured at 1 year. This reduction in WT1 mRNA levels coincides chronologically with the patient’s reduced transfusional needs. Additionally, tetramer analysis detected WT1-specific CTL clones in peripheral blood samples obtained at the 1-year time point, suggestive of a possible underlying immunologic mechanism involved in the patient’s clinical improvement. Unfortunately, the few available immunologic data time points limited further insight into whether the delayed response correlated to an emergent WT1 immune response.
The evaluation of immunologic responses to WT1 antigen in the AML patients was substantially limited by the fact that only 4 patients were confirmed to express the HLA-A*0201 antigen. Furthermore, only one of these four patients mounted a detectable response by both tetramer and IFN-γ ELISPOT analysis. A second HLA-A*0201+ patient demonstrated positive tetramer staining but failed to mount a detectable IFN-γ response. The clinical relevance of these responses are uncertain, but the patient with a WT1 response detectable by both means remained in remission for 14.8 months, which exceeded the duration of his prior remission (13 months) following first-line chemotherapy. The second patient demonstrating only an early subtly detectable response by tetramer analysis relapsed quickly after only 3.7 months, but overall survival extended to 23.7 months despite rapid recurrence. Both patients were in CR2 at time of enrollment. Perhaps more interesting, IFN-γ responses against both the CD4- and CD8-derived epitopes were detected in one AML patient who did not express the HLA-A*0201 allele; the HLA-DRβ was not reported in this patient. While the significance of this is uncertain, it may reflect an unpredicted binding compatibility between the class I peptide and a non-HLA-A*0201 allele in this patient. Both patients with detectable IFN-γ production against class I peptide demonstrated similar responsiveness against the class II WT1 epitopes. Two additional AML patients, neither displaying the HLA-A*0201 allele, developed DTH responses. This could be consistent with engagement of a CD4-mediated response, although neither patient manifested evidence for a CD4 T-cell response according to IFN-γ ELISPOT assays directed against the class II peptides. DTH positivity did not appear to be associated with any clear evidence of clinical benefit in terms of progression-free or overall survival.
Discussion
In this pilot study, we explored the impact of T cell help on peptide vaccine strategies emerging as potentially viable cancer therapies. The primary objective of this pilot study was to address safety, tolerability, and immunogenicity of an oligopeptide vaccine designed to elicit or augment native WT1-specific immunity through the incorporation of a combination of class I and II peptide epitopes. Consistent with prior WT1 vaccine studies, this vaccine was well-tolerated. There were no protocol interruptions or discontinuations resulting from adverse reactions to the vaccine in this study.
Results from immunologic assessments, and more specifically the lack of consistent detection of a measurable WT1-specific T-cell response, were unexpected. In a similar study in AML patients achieving complete responses after initial induction therapy, this vaccine strategy produced consistent measurable WT1-specific T-cell responses in AML patients in complete remission [22]. Unlike this prior study, we failed to detect consistent WT1-specific immune responses, although several patients with detectable WT1 reactivity also demonstrated subtle evidence of clinical improvement. However, retrospectively, there are several potential contributors that could independently or collectively account for these findings.
Importantly, our peptide cocktail included class I epitopes designed to prime CTLs in the context of HLA-A*0201 presentation, yet HLA-A*0201 allelic specificity was not specified as an inclusion parameter. Of the 16 patients enrolled, only five were HLA-A*0201. WT1 vaccine studies to date have largely been limited to a subset of patients expressing either HLA-A*0201 or HLA-A*2401. While important in modeling and characterizing potentiated immune reactivity, this limits broad therapeutic utility of peptide vaccines. The inclusion of 2 class II epitopes with increased promiscuity for multiple HLA-DRβ molecules is intended to accomplish 2 goals; (1) to begin to assess the importance of CD4 helper responses in the optimization of HLA-A*0201-directed CTL responses, but also (2) to begin to evaluate a vaccine strategy that will target a much more inclusive patient population even in the absence of specific CD8 T cell stimulation. Furthermore, while the class II epitopes are believed to be more promiscuous [18] in binding to HLA-DR.β1*0101, *0301, *0401, *0701, *1101, *1501, patients were not selected or typed for HLA-DR.β1 allelic variation. Considering the massive heterogeneity inherent in the HLA alleles, the probability for incompatibility could explain these results.
Also notable, Rezvani et al. demonstrated that high avidity WT1-specific T-cell responses detected 2 weeks after initial peptide vaccination subsequently disappeared with repetitive immunizations, leaving predominantly low avidity clones with diminished responsiveness corresponding to a transient but significant loss of FoxP3+CD25+CD4+ T cells upon initial vaccination [16]. In another small study of WT1 vaccination in AML patients, Uttenthal et al. similarly reported the detection of cognate CTL responses by ELISPOT and tetramer analysis, which lacked capacity for secondary expansion [23]. In contrast to this, we were able to detect peptide-specific T-cell responses to both class I and II epitopes at later time points rather than early on. Unfortunately, differences in methods of detection prevented more direct comparisons, but the progressive loss of high-avidity CTL responsiveness may reflect inadequate helper T-cell responses necessary for CD8 T-cell persistence [17,24–26]. Nonetheless, their data raise the distinct possibility that the lack of peptide-specific immunity in our study may reflect suboptimal timing with respect to assessment of elicited immune responses.
Inevitably, one must also consider that inherent differences in patient populations may contribute to the contrasting trial results. Unlike prior studies, we selected for a heavily pretreated population, potentially resulting in a depleted capacity to mount any cognate immune response. Nonetheless, the decreased immunologic response rate in these patients is inconsistent with prior WT1 vaccine trials, identifying a need to probe more precisely into the regulatory mechanisms shaping these vaccine responses to better understand the dichotomy of the resulting immune responses in the comparative studies.
In conclusion, an expanded vaccination protocol to incorporate a combination of heteroclitic peptides including epitopes predicted to elicit both CD4 and CD8 T-cell responses can elicit a detectable immune response in ~20% of the vaccinated patients; however, the protective value of any elicited immunity remains to be clearly determined. Our data point to a potential signature of clinical benefit based on survival curves comparing vaccine-treated patients to an unvaccinated control population. However, the lack of a robust immune response adequate for further characterization prevented any correlation between immunologic assessments and potential clinical benefit. Furthermore, the suggestion that there may be a dissociation between progression-free and overall survival would not be unprecedented, as sipuleucel-T, a DC-based vaccine therapy active in prostate cancer, demonstrated a benefit in overall survival despite no improvement in clinical response [27]. Inevitably, one must consider that the limited responses seen with this peptide vaccine reflects the fact that peptide-directed therapeutics intend to prime a protective response but the durability of any elicited response will likely be dependent on adequate amplification in the expansion phase of the primed response. Thus, coupling a vaccine approach with sequential checkpoint inhibition, such as CTLA-4 or PD1 blockade, may be required to ultimately achieve a measureable therapeutic benefit. Therefore, recognizing that isolated peptide vaccinations may be insufficient to generate long-term protective immunity, our data can be viewed as encouraging as a potential component of a more complex immunotherapeutic approach worthy of further study.
Supplementary Material
Acknowledgments
Contract grant sponsor: Contract grant sponsor: American Society of Clinical Oncology (Career Development Award); Contract grant sponsor: Lauri Strauss Leukemia Foundation..
The authors thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Footnotes
Author Contributions: This study was designed by JPI. Data was collected by JEL, AL, LB, and RK who contributed equally. Data interpretation and analysis was accomplished by JPI, JB, JEL, and JP. The manuscript was drafted by JB with editorial commentary and discussion from JPI and JEL.
Conflict of Interest: JPI owns patent rights for the WT1 peptide vaccine and has previously received royalties in association with this proprietary relationship. There are no other conflicts of interest amongst the remaining authors.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a cd8(+) cytotoxic T-lymphocyte clone specific for wt1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- 2.Oka Y, Tsuboi A, Taguchi T, et al. Induction of wt1 (wilms’ tumor gene)-specific cytotoxic T lymphocytes by wt1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci USA. 2004;101:13885–13890. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pinilla-Ibarz J, May RJ, Korontsvit T, et al. Improved human T-cell responses against synthetic HLA-0201 analog peptides derived from the wt1 oncoprotein. Leukemia. 2006;20:2025–2033. doi: 10.1038/sj.leu.2404380. [DOI] [PubMed] [Google Scholar]
- 4.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined pr1 and wt1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miwa H, Beran M, Saunders GF. Expression of the wilms’ tumor gene (wt1) in human leukemias. Leukemia. 1992;6:405–409. [PubMed] [Google Scholar]
- 6.Bergmann L, Miething C, Maurer U, et al. High levels of wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217–1225. [PubMed] [Google Scholar]
- 7.Kim SC, Yoo NC, Hahn JS, et al. Monitoring of WT-1 gene expression in peripheral blood of patients with acute leukemia by semiquantitative RT-PCR; possible marker for detection of minimal residual leukemia. Yonsei Med J. 1997;38:212–219. doi: 10.3349/ymj.1997.38.4.212. [DOI] [PubMed] [Google Scholar]
- 8.Virappane P, Gale R, Hills R, et al. Mutation of the wilms’ tumor 1 gene is a poor prognostic factor associated with chemotherapy resistance in normal karyotype acute myeloid leukemia: The United Kingdom medical research council adult leukaemia working party. J Clin Oncol. 2008;26:5429–5435. doi: 10.1200/JCO.2008.16.0333. [DOI] [PubMed] [Google Scholar]
- 9.Paschka P, Marcucci G, Ruppert AS, et al. Wilms’ tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: A cancer and leukemia group B study. J Clin Oncol. 2008;26:4595–4602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst T, Chase A, Zoi K, et al. Transcription factor mutations in myelodysplastic/myeloproliferative neoplasms. Haematologica. 2010;95:1473–1480. doi: 10.3324/haematol.2010.021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oka Y, Elisseeva OA, Tsuboi A, et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type wilms’ tumor gene (wt1) product. Immunogenetics. 2000;51:99–107. doi: 10.1007/s002510050018. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Bellantuono I, Elsasser A, et al. Selective elimination of leukemic cd34(+) progenitor cells by cytotoxic T lymphocytes specific for wt1. Blood. 2000;95:2198–2203. [PubMed] [Google Scholar]
- 13.Doubrovina E, Carpenter T, Pankov D, et al. Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias. Blood. 2012;120:1633–1646. doi: 10.1182/blood-2011-11-394619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellantuono I, Gao L, Parry S, et al. Two distinct HLA-A0201-presented epitopes of the wilms tumor antigen 1 can function as targets for leukemia-reactive CTL. Blood. 2002;100:3835–3837. doi: 10.1182/blood.V100.10.3835. [DOI] [PubMed] [Google Scholar]
- 15.Tsuboi A, Oka Y, Udaka K, et al. Enhanced induction of human WT1-specific cytotoxic T lymphocytes with a 9-mer wt1 peptide modified at HLA-a*2402-binding residues. Cancer Immunol Immunother. 2002;51:614–620. doi: 10.1007/s00262-002-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezvani K, Yong AS, Mielke S, et al. Repeated pr1 and wt1 peptide vaccination in Montanide-adjuvant fails to induce sustained high-avidity, epitope-specific cd8+ T cells in myeloid malignancies. Haematologica. 2011;96:432–440. doi: 10.3324/haematol.2010.031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janssen EM, Lemmens EE, Wolfe T, et al. CD4+ T cells are required for secondary expansion and memory in cd8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 18.May RJ, Dao T, Pinilla-Ibarz J, et al. Peptide epitopes from the wilms’ tumor 1 oncoprotein stimulate cd4+ and cd8+ T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res. 2007;13:4547–4555. doi: 10.1158/1078-0432.CCR-07-0708. [DOI] [PubMed] [Google Scholar]
- 19.Willasch AM, Gruhn B, Coliva T, et al. Standardization of wt1 mRNA quantitation for minimal residual disease monitoring in childhood AML and implications of wt1 gene mutations: A European multicenter study. Leukemia. 2009;23:1472–1479. doi: 10.1038/leu.2009.51. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the international working group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Maslak PG, Dao T, Krug LM, et al. Vaccination with synthetic analog peptides derived from wt1 oncoprotein induces T-cell responses in patients with complete remission from acute myeloid leukemia. Blood. 2010;116:171–179. doi: 10.1182/blood-2009-10-250993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uttenthal B, Martinez-Davila I, Ivey A, et al. Wilms’ tumour 1 (wt1) peptide vaccination in patients with acute myeloid leukaemia induces short-lived WT1-specific immune responses. Br J Haematol. 2014;164:366–375. doi: 10.1111/bjh.12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shedlock DJ, Shen H. Requirement for cd4 T cell help in generating functional cd8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 25.Bevan MJ. Helping the cd8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 26.Fuller MJ, Hildeman DA, Sabbaj S, et al. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 27.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


