Abstract
Purpose of review
The bioactive lysophospholipids, lysophosphatidic acid (LPA) and sphingosine 1 phosphate (S1P) have potent effects on blood and vascular cells. This review focuses their potential contributions to the development of atherosclerosis, acute complications, such as acute myocardial infarction, and chronic ischemic cardiac damage.
Recent findings
Exciting recent developments have provided insight into the molecular underpinnings of LPA and S1P receptor signaling. New lines of evidence suggest roles for these pathways in the development of atherosclerosis. In experimental animal models, the production, signaling and metabolism of LPA may be influenced by environmental factors in the diet that synergize to promote the progression of atherosclerotic vascular disease. This is supported by observations of human polymorphisms in the lysophospholipid metabolizing enzyme, PPAP2B, that are associated with risk of coronary artery disease and myocardial infarction. S1P signaling protects from myocardial damage that follows acute and chronic ischemia both by direct effects on cardiomyocytes and through stem cell recruitment to ischemic tissue.
Summary
This review will suggest novel strategies to prevent the complications of coronary artery disease by targeting LPA production and signaling. Additionally, ways in which S1P signaling pathways may be harnessed to attenuate ischemia-induced cardiac dysfunction will be explored.
Keywords: Lysophosphatidic acid, sphingosine 1 phosphate, autotaxin, sphingosine kinase
Introduction
Acute myocardial infarction (AMI) and resulting ischemic heart disease (IHD) are the single most prevalent cause of morbidity and mortality in the western world. Recent lines of evidence suggest that bioactive lipids may mediate interactions between genetic and environmental factors that increase susceptibility to atherosclerosis and its complications, such as AMI and stroke. Lysophospholipids are derivatives of glycero- or sphingo- phospholipids lacking one of the radyl hydrocarbon chains. Two major bioactive lysophospholipids with wide-ranging, cell surface receptor-mediated effects on blood and vascular cell function are lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P). In this review, we summarize ways in which components of the LPA signaling nexus may contribute to the development and progression of atherosclerosis and discuss how S1P-mediated effects may be harnessed to attenuate the consequences of chronic ischemic heart disease.
The prototypic lysophospholipid receptors were originally identified as members of the Endothelial Differentiation Gene (Edg) family of G-protein coupled receptors. Receptors for these lipids have been re-named to reflect their selectivity for LPA and S1P. Currently six LPA-selective and five S1P-selective receptors have been described (Figure 1). Structural analysis of the S1P1 receptor suggests that S1P laterally diffuses through the membrane to gain access to the ligand binding pocket [1]. In contrast, the structure of the LPA1 receptor suggests that LPA may enter the ligand binding pocket directly from the extracellular space [2]. These differences may reflect differences in pathways for production and delivery of these ligands to their respective receptors.
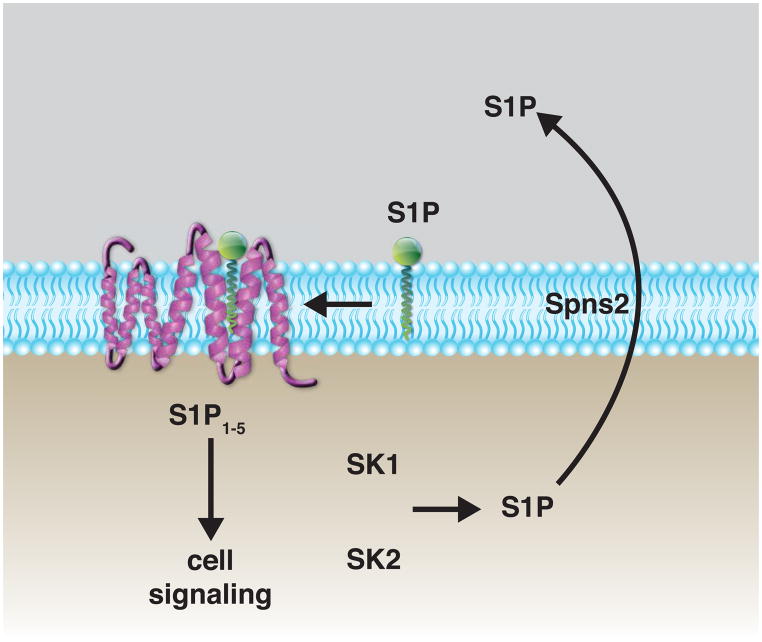
Figure 1. LPA and S1P receptor signaling systems.
(A) LPA, generated by autotaxin-mediated hydrolysis of LPC, acts on cell-surface receptors to elicit a wide variety of cellular responses. Autotaxin binds to activated integrins on the cell surface, which may localize LPA generation in the extracellular space in proximity to LPA receptors. (B) S1P is generated intracellularly by sphingosine kinases (SK1 and SK2). Transporters, such as spinster (Spns2), are responsible for movement of S1P to the extracellular space. S1P within the membrane diffuses to its receptor to elicit intracellular signaling events.
The LPA signaling nexus and the development of atherosclerosis
The primary pathway for production of LPA in blood involves hydrolysis of lysophospholipids, predominantly lysophosphatidylcholine (LPC) by autotaxin, a member of the ectonucleotide pyrophosphatase/phosphodiesterase (Enpp) family with a unique lysophospholipase D activity. Lack of autotaxin (Enpp2−/−) or expression of a catalytically inactive autotaxin in mice is lethal embryonically [3–5]. Enpp2+/− mice and adipose-specific Enpp2 knockout mice have reduced circulating levels of autotaxin and LPA [3, 4, 6, 7]. Emerging evidence indicates that systemic levels of LPA are also influenced by dietary intake [8, 9] and that intestinally-derived LPA may influence the development of atherosclerosis in mice [9, 10].
LPA is also present in the lipid rich core of human atheroma, where it has been suggested to play a role in platelet activation after plaque rupture [11]. The accumulation of LPA during experimental atherosclerosis has also been examined [12, 13] and LPA within atheroma has been directly visualized by time-of-flight secondary ion mass spectrometry [14]. The buildup of plaque-associated LPA is paralleled by changes in expression of LPA receptors and genes encoding enzymes that could play roles in de novo LPA synthesis pathways, although, at present the precise pathway(s) for LPA generation in atherosclerotic lesions, and, in particular, the relative contributions of LPA generated in situ versus accumulation of LPA from circulating sources, is not known. Exogenous administration of LPA promotes monocyte adhesion to the endothelium, stimulates perivascular macrophage accumulation, and heightens atherosclerotic plaque burden in Apoe−/− mice in an LPA1- and LPA3- receptor dependent manner [13]. Additionally, perivascular administration of LPA promotes the recruitment of inflammatory cells, in particular mast cells, to atherosclerotic plaques in carotid arteries and results in intraplaque hemorrhage [14]. Together, this evidence points to a potential role for autotaxin and LPA in the development of experimental atherosclerosis in animals.
Investigation of the genetics of coronary artery disease suggests that these findings in mice may be translatable to humans. A genome-wide association study suggested a link between a loci in the PPAP2B gene and coronary artery disease (CAD) susceptibility [15]. PPAP2B encodes the lipid phosphate phosphatase 3, (LPP3) an enzyme that dephosphorylates phosphatidic acid, LPA, and S1P. One function of LPP3, and other related LPP enzymes, is to negatively regulate LPA and S1P signaling by enzymatic dephosphorylation of the lysophospholipids. A risk allele in PPAP2B localizes to a CEBP/β binding site in the final intron of the gene, a region that appears to be involved in enhancing LPP3 expression. The protective allele is recognized by CEBP/β and, in macrophages, is associated with LPP3 upregulation following exposure to oxidized LDL. In contrast, the risk variant lacks CEBP/β, macrophages isolated from individuals with the risk polymorphism have lower LPP3 expression in response to oxidized LDL [16]. The PPAP2B risk allele was also associated with lower expression by Erbigin et al using expression quantitative locus (eQTL) mapping [17]. A causal link between lower LPP3 expression and atherosclerosis remains to be established. Lack of LPP3 is embryonically lethal in mice [18], and attenuated LPP3 expression promotes inflammation, enhances smooth muscle cell migration, and disrupts endothelial barrier formation in an LPA-dependent manner [19, 20]. Endothelial PPAP2B expression is reduced by flow patterns that promote the development of atherosclerosis, and lower LPP3 levels are accompanied by the development of an LPA1 receptor-dependent inflammatory phenotype [21]. Ppap2b expression in mice is upregulated in atherosclerotic lesions and endothelial cells isolated from atherosclerotic arteries [17]. Importantly, global deletion of Ppap2b expression in adult Ldlr−/− mice increases Western diet-promoted atherosclerosis (Mueller P, Morris AJ, Smyth SS, unpublished data: 2015 Circulation 2015 Abstract 18875). Taken together, these results suggest the hypothesis that increases in LPP3 expression may be a key element in suppressing the development and/or complications of atherosclerosis in humans.
Sphingolipids in chronic ischemic heart disease
Unlike biologically active LPA, which is thought to be the product of a single enzyme autotaxin, S1P can be generated by two distinct sphingosine kinases (SK1 and SK2). Intracellular S1P is trafficked to the extracellular space by the by Spinster homologue 2 (Spns2) transporter. Membrane- associated S1P signals through G-protein coupled seven transmembrane span receptors [22]. As with LPA, S1P-signaling can be terminated by LPP-mediated dephosphorylation. Additionally, S1P is degraded by S1P lyase and S1P specific phosphatases (SPP1 and SPP2) [23]. In plasma, S1P is partly associated with high-density lipoprotein (HDL) cholesterol particles by binding to apolipoprotein M and may contribute to the atheroprotective effects of HDL [24]. S1P signaling may attenuate the development of atherosclerosis in experimental models. In hyperlipidemic mice, development of atherosclerosis appears to be inversely proportional to S1P levels. For example, reducing S1P production by SK2 inhibition promotes the development of atherosclerosis [25]; whereas, an inhibitor of S1P lyase, which increases S1P, attenuates the development of atherosclerosis [26]. The S1P1 receptor may be responsible for these anti-atherosclerotic effects because S1P1 agonism reduces atherosclerosis [27]. In contrast, S1P2 and S1P3 receptors appear to have a pro-atherosclerotic role in that deficiency of either reduces the number of monocytes and macrophages within the atherosclerotic lesion in mice [28–30], and lack of S1P2 reduces the development of atherosclerosis [29, 30].
The complications of atherosclerotic coronary artery disease include AMI and chronic ischemic heart disease (IHD). Recent data from animal and human studies have demonstrate the capability of adults to renew their cardiac cells through mechanisms that involve both resident and recruited stem cells. Growing evidence indicates S1P signaling may be harnessed to improve the response to ischemia and recovery after myocardial injury. S1P increases at sites of myocardial ischemic injury and may have protective effects in pre- and post-conditioning to reduce infarct size [31]. In cell culture systems, S1P prevents hypoxic injury of neonatal rat cardiomyocytes [32] and adult cells [33] via S1P1 and S1P3 receptors. In vivo, S1P protects against ischemia reperfusion injury [34, 35], and increasing S1P by S1P lyase inhibition reduces cardiomyocyte ischemic damage through preconditioning [36].
Over the last decade, the dogma that the heart is a post-mitotic organ has been successfully challenged with growing evidence that cardiomyocytes undergo chimerism [37–39]. The mechanisms responsible for cardiomyocyte chimerism are poorly understood. Recent evidence suggests that adult cardiomyocytes retain limited ability to proliferate, and this phenomenon is more evident following injury [40]. Furthermore, the adult heart contains a pool of stem cells that could contribute to cardiomyocyte renewal and may have therapeutic potential based on work done in both animal models and with human tissue [41–43].
Myocardial ischemia elicits the mobilization of bone marrow-derived stem/progenitor cells (BMSPC) to peripheral blood of animal and humans in an S1P and S1P receptor dependent manner [44–47]. Early after the onset of AMI, plasma S1P levels are significantly elevated and correlate with higher numbers of circulating BMSPCs, suggesting a potential role for the bioactive lipid in BMSPC mobilization post-MI [48]. Numbers of circulating stem cells are highest in patients with ST-elevation myocardial infarction (STEMI), as compared to those observed in individuals with lesser degrees of ischemia such as non-STEMI (NSTEMI) or those with chronic IHD [44]. The degree of BMSPC mobilization correlates with cardiac recovery [45, 49]. The source of elevated plasma S1P in AMI is not known. Activated platelets release S1P, likely from intracellular granule stores [50]. Additionally, S1P may be released from blood cells by the effect of the membrane attack complex (MAC), which is generated by activation of the complement cascade during AMI [51]. MAC increases the release of S1P from RBCs and platelets and, simultaneously, stimulates granulocytes in the BM to release proteolytic enzymes that disrupt the BMSPCs-osteoclast interaction in BM niches, thus, liberating stem cells [52].
Stimuli responsible for the mobilization and homing of BMSPCs in the setting of myocardial ischemia are poorly understood but may include S1P. S1P has a dose-dependent chemotactic effect on human stem cells in a modified Boyden chamber assay [22]. Plasma, obtained from AMI patients at times that correlate with both peak S1P levels and mobilized stem cells, stimulates BMSPC migration in an S1P1 receptor-dependent manner [48]. Moreover, the inhibition of S1P lyase augments S1P-mediated mobilization of BMSPCs after MI and enhances angiogenesis, cardiomyocyte proliferation and cardiac functional recovery in animal models [53]. Taken together these findings highlight the importance of bioactive lipids and complement cascade in the mobilization and homing of BMSPCs to the ischemic myocardium.
Conclusions
Bioactive lysophospholipids are emerging as important mediators with roles in cardiovascular health and disease. Both the LPA and S1P signaling systems may contribute to the development and progression of atherosclerosis by regulating inflammatory events, smooth muscle cell and extracellular matrix biology, and thrombosis. Additionally, S1P may play an important role in stem cell mobilization and homing to the myocardium following ischemic injury. Therapeutic strategies that target the production, metabolism, or signaling pathways for LPA and S1P may therefore prove to be attractive means to prevent or attenuate the complications of ischemic vascular disease.
KEY POINTS.
Lysophosphatidic acid (LPA) and sphingosine 1 phosphate (S1P) receptor signaling pathways contribute to the development of atherosclerosis by regulating inflammatory events, smooth muscle cell and extracellular matrix biology, and thrombosis
Lipid phosphate phosphatase 3, an enzyme that dephosphorylates phosphatidic acid and several lysophospholipids, including LPA and S1P, may be critical to suppress the development and/or complications of atherosclerosis in humans
S1P signaling protects from myocardial damage that follows acute and chronic ischemia both by direct effects on cardiomyocytes and through stem cell recruitment to ischemic tissue
Targeting the production, metabolism, or signaling pathways for LPA and S1P may prevent or attenuate the complications of ischemic vascular disease
Acknowledgments
This project was supported by grants from the Heart Lung and Blood Institute (R01HL091812 and R56HL124266), the National Center for Research Resources (P20RR021954), an IDeA award from the National Institute of General Medical Sciences (P20GM103527), and the National Center for Advancing Translational Sciences through Grant UL1TR000117 of the National Institutes of Health. This material is the result of work supported in part with resources and the use of facilities at the Lexington VA Medical Center (through awards BX002769 and BX001014).
Footnotes
There are no conflicts of interest to report.
Disclosure of Funding
This project was supported by grants from the Heart Lung and Blood Institute (R01HL091812 and R56HL124266), the National Center for Research Resources (P20RR021954), an IDeA award from the National Institute of General Medical Sciences (P20GM103527), and the National Center for Advancing Translational Sciences through Grant UL1TR000117 of the National Institutes of Health. This material is the result of work supported in part with resources and the use of facilities at the Lexington VA Medical Center (through awards BX002769 and BX001014).
References
- 1.Hanson MA, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335(6070):851–5. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **2.Chrencik JE, et al. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell. 2015;161(7):1633–43. doi: 10.1016/j.cell.2015.06.002. This article provides structural insight into the basis for LPA binding to the LPA1 receptor and identify access to the binding pocket from the extracellular space. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281(35):25822–30. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- 4.van Meeteren LA, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26(13):5015–22. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferry G, et al. Functional invalidation of the autotaxin gene by a single amino acid mutation in mouse is lethal. FEBS Lett. 2007;581(18):3572–8. doi: 10.1016/j.febslet.2007.06.064. [DOI] [PubMed] [Google Scholar]
- 6.Dusaulcy R, et al. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J Lipid Res. 2011;52(6):1247–55. doi: 10.1194/jlr.M014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Nishimura S, et al. ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes. 2014;63(12):4154–64. doi: 10.2337/db13-1694. This publication demonstrated a link between adipose autotaxin, adiposity and insulin resistance. [DOI] [PubMed] [Google Scholar]
- 8.Tokumura A, et al. Increased formation of lysophosphatidic acids by lysophospholipase D in serum of hypercholesterolemic rabbits. J Lipid Res. 2002;43(2):307–15. [PubMed] [Google Scholar]
- **9.Navab M, et al. Source and role of intestinally derived lysophosphatidic acid in dyslipidemia and atherosclerosis. J Lipid Res. 2015;56(4):871–87. doi: 10.1194/jlr.M056614. This publication describes an autotaxin mediated pathway for production of unsaturated LPA from dietary LPC and its role in promoting experimental atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Navab M, et al. Transgenic 6F tomatoes act on the small intestine to prevent systemic inflammation and dyslipidemia caused by Western diet and intestinally derived lysophosphatidic acid. J Lipid Res. 2013;54(12):3403–18. doi: 10.1194/jlr.M042051. In this report, it was established that dietary LPA promotes the development of atherosclerosis in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siess W, et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96(12):6931–6. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bot M, et al. Atherosclerotic lesion progression changes lysophosphatidic acid homeostasis to favor its accumulation. Am J Pathol. 2010;176(6):3073–84. doi: 10.2353/ajpath.2010.090009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011;13(5):592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- *14.Bot M, et al. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J Lipid Res. 2013;54(5):1265–74. doi: 10.1194/jlr.M032862. Analysis of atherosclerotic plaque LPA content was performed by imaging mass spectromety and a role for LPA in destablizing mast cells in plaque was presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–8. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Reschen ME, et al. Lipid-induced epigenomic changes in human macrophages identify a coronary artery disease-associated variant that regulates PPAP2B Expression through Altered C/EBP-beta binding. PLoS Genet. 2015;11(4):e1005061. doi: 10.1371/journal.pgen.1005061. Using functional and structural chromatin mapping approaches, this work linked polymorphisms in PPAP2B with upregulation of LPP3 expression by oxidized-LDL in macrophages and suggested that the risk allele may alter a C/EBP beta binding site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erbilgin A, et al. Identification of CAD candidate genes in GWAS loci and their expression in vascular cells. J Lipid Res. 2013;54(7):1894–905. doi: 10.1194/jlr.M037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escalante-Alcalde D, et al. The lipid phosphatase LPP3 regulates extra-embryonic vasculogenesis and axis patterning. Development. 2003;130(19):4623–37. doi: 10.1242/dev.00635. [DOI] [PubMed] [Google Scholar]
- 19.Panchatcharam M, et al. Lipid phosphate phosphatase 3 negatively regulates smooth muscle cell phenotypic modulation to limit intimal hyperplasia. Arterioscler Thromb Vasc Biol. 2013;33(1):52–9. doi: 10.1161/ATVBAHA.112.300527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panchatcharam M, et al. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler Thromb Vasc Biol. 2014;34(4):837–45. doi: 10.1161/ATVBAHA.113.302335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Wu C, et al. Mechano-Sensitive PPAP2B Regulates Endothelial Responses to Athero-Relevant Hemodynamic Forces. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.117.306457. This study demonstrates that a lack of PPAP2B in arterial regions exposed to disturbed flow, mediates the anti-inflammatory and anti-permeable endothelial phenotype associated with athero-protective flow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seitz G, et al. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci. 2005;1044:84–9. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 23.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 24.Poti F, Simoni M, Nofer JR. Atheroprotective role of high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P) Cardiovasc Res. 2014;103(3):395–404. doi: 10.1093/cvr/cvu136. [DOI] [PubMed] [Google Scholar]
- 25.Poti F, et al. SKI-II--a sphingosine kinase 1 inhibitor--exacerbates atherosclerosis in low-density lipoprotein receptor-deficient (LDL-R−/−) mice on high cholesterol diet. Atherosclerosis. 2015;240(1):212–5. doi: 10.1016/j.atherosclerosis.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Bot M, et al. Hematopoietic sphingosine 1-phosphate lyase deficiency decreases atherosclerotic lesion development in LDL-receptor deficient mice. PLoS One. 2013;8(5):e63360. doi: 10.1371/journal.pone.0063360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poti F, et al. KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R−/− mice. Arterioscler Thromb Vasc Biol. 2013;33(7):1505–12. doi: 10.1161/ATVBAHA.113.301347. [DOI] [PubMed] [Google Scholar]
- 28.Keul P, et al. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res. 2011;108(3):314–23. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- 29.Skoura A, et al. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(1):81–5. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, et al. Sphingosine-1-phosphate receptor-2 deficiency leads to inhibition of macrophage proinflammatory activities and atherosclerosis in apoE-deficient mice. J Clin Invest. 2010;120(11):3979–95. doi: 10.1172/JCI42315. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Vessey DA, et al. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297(4):H1429–35. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karliner JS, et al. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33(9):1713–7. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, et al. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293(5):H3150–8. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 34.Vessey DA, et al. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375(3):425–9. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Means CK, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292(6):H2944–51. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 36.Bandhuvula P, et al. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011;300(5):H1753–61. doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Weger RA, et al. Stem cell-derived cardiomyocytes after bone marrow and heart transplantation. Bone Marrow Transplant. 2008;41(6):563–9. doi: 10.1038/sj.bmt.1705939. [DOI] [PubMed] [Google Scholar]
- 38.Deb A, et al. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107(9):1247–9. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- *39.Quaini F, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346(1):5–15. doi: 10.1056/NEJMoa012081. This is the first report to demonstrate the chimerism of adult cardiomyocytes. [DOI] [PubMed] [Google Scholar]
- 40.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–6. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malliaras K, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Mol Med. 2013;5(2):191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malliaras K, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63(2):110–22. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li TS, et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59(10):942–53. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdel-Latif A, et al. Evidence of mobilization of pluripotent stem cells into peripheral blood of patients with myocardial ischemia. Exp Hematol. 2010;83(12):1131–1142. doi: 10.1016/j.exphem.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leone A, et al. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26(12):1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 46.Massa M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 47.Wojakowski W, et al. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27(3):283–289. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]
- **48.Karapetyan AV, et al. Bioactive Lipids and Cationic Antimicrobial Peptides As New Potential Regulators for Trafficking of Bone Marrow Derived Stem Cell In Patients With Acute Myocardial Infarction. Stem cells Dev. 2013 doi: 10.1089/scd.2012.0488. p. Epub ahead of print. This is the first report to establish the role of sphingosine-1 phosphate in mediating the mobilization of bone marrow-derived stem cells in myocardial ischemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyderka R, et al. Mobilization of CD34+CXCR4+ stem/progenitor cells and the parameters of left ventricular function and remodeling in 1-year follow-up of patients with acute myocardial infarction. Mediators Inflamm. 2012;2012:564027. doi: 10.1155/2012/564027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jonnalagadda D, et al. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim Biophys Acta. 2014;1841(11):1581–9. doi: 10.1016/j.bbalip.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arumugam TV, et al. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21(5):401–9. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Ratajczak MZ, et al. A novel perspective on stem cell homing and mobilization: review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia. 2012;26(1):63–72. doi: 10.1038/leu.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **53.Klyachkin YM, et al. Pharmacological elevation of circulating bioactive phosphosphingolipids enhances myocardial recovery after acute infarction. Stem Cells Transl Med. 2015 doi: 10.5966/sctm.2014-0273. In Press. This is the first report to describe the therapeutic role of inhibiting sphingosine-1 phosphate lyase in enhancing stem cell mobilization, homing and cardiac recovery following myocardial infarction. [DOI] [PMC free article] [PubMed] [Google Scholar]