Introduction and History
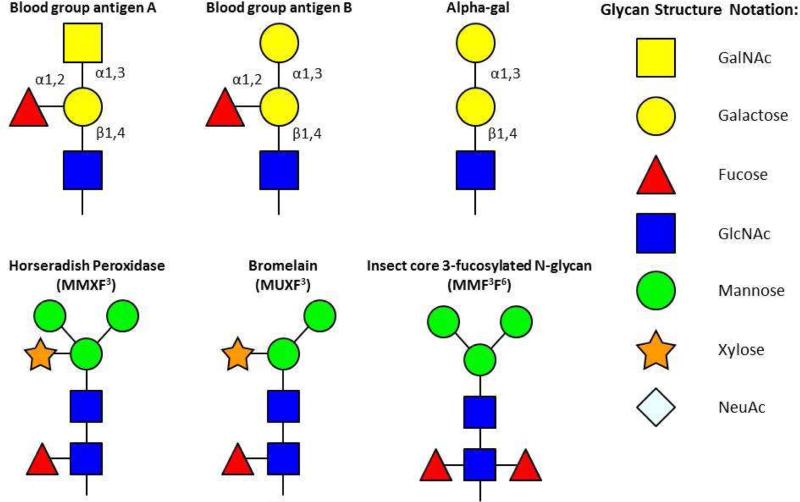
When Karl Landsteiner first defined the ABO system, he also recognized a “B like” substance on mammalian cells [1]. At that time he reported that all immunocompetent individuals had agglutinating antibodies against this substance. Interestingly, adsorption of these antibodies against rabbit red cells was part of the proof that rabbit cells can activate the alternate pathway of human complement [2]. This “B like” substance is now known to be galactose-alpha-1, 3-galactose (alpha-gal), which is structurally similar to the B blood group (Fig 1) [3, 4]. This carbohydrate is a well-recognized immunologic barrier in xenotransplantation. Xenoreactive natural antibodies directed against the alpha-gal moieties decorating non-primate mammalian tissue are often implicated in acute organ rejection. One way to circumvent this obstacle may be to raise transgenic organs in pig knockouts lacking the gene expressing alpha-(1, 3)-galactosyl transferase, an enzyme inactivated in all primates [5]. It has also been suggested that the antibody response to alpha-gal is as much as 1% of the total immunoglobulin; however, more recent assays in our group suggest a relatively low quantity of IgG antibodies [6, 7].
Fig 1.
Glycan structure of blood group antigens and alpha-gal contrasted with those of plant- or insect-related cross-reactive carbohydrate determinants (CCD). Adapted from Commins SP, Platts-Mills TAE. Anaphylaxis syndromes related to a new mammalian cross-reactive carbohydrate determinant. J Allergy Clin Immunol 2009; 124(4): 652–57.
The story of IgE to alpha-gal starts in 2006 with two anaphylactic reactions to cetuximab in Bentonville, Arkansas, one of which was fatal. At this same time, there were four observations that were interesting but unexplained:
The monoclonal antibody (mAb) cetuximab was causing severe anaphylactic or urticarial reactions in up to 15% of patients treated in Tennessee and North Carolina but not in New York or Boston [8].
A patient age 43 who reported four episodes of anaphylaxis, each of which started four hours after eating a hamburger (Box 1).
60% of the school children in a Kenyan village had serum IgE specific for cat extract although there were no cats in the village and no relevant allergic symptoms [9].
The increasing deer population in rural and suburban Virginia had reached epidemic numbers.
At that point, there was no reason to connect these observations and none of them made sense. Investigation of the specificity of the IgE present in the sera of patients prior to their first cetuximab treatment necessitated the development of an assay for IgE to cetuximab. This assay was simplified because a technique in which the target antigen was biotinylated and then bound to streptavidin ImmunoCAP (Phadia, Portage, Michigan) had been established in the previous year [10]. At this point, we were approached by Bristol Myers Squibb (BMS) to become involved with the investigation of the reactions to cetuximab.
Investigation of the Reactions to Cetuximab: establishing that the antigen on that molecule is galactose alpha-1, 3-galactose
The involvement with BMS brought with it connections to ImClone who manufacture cetuximab as well as to Dr. Chung and the oncology group at Vanderbilt who had pre-treatment sera from patients who had been treated with cetuximab. Many of the sera from Tennessee had IgE antibodies to cetuximab; however, an equal percentage of sera of controls from Tennessee had IgE specific for cetuximab. Thus, it was clear that the presence of these antibodies had everything to do with rural Tennessee and nothing to do with cancer. As an additional control, sera were assayed from a cohort of mothers in Boston (n = 341). In that group, only one serum had detectable IgE to cetuximab. Thus in keeping with the known geographic distribution of reactions to cetuximab, there was good evidence that these antibodies were common in some southern states but rare in northern cities. Among the treated patients, the presence of IgE antibodies to cetuximab of ≥0.35 IU/ml was very strongly associated with severe reactions during the first infusion (odds ratio 35) [11].
The next problem was to identify the specificity of the antibodies to this mAb remembering that they were pre-existing. Initially, a wide variety of known allergens including weeds, fungi, and also parasites were considered. Given that other mAbs did not give positive results with these sera it seemed possible that the epitope for these antibodies could be a post-translational modification of the molecule related to the mouse cell line SP2.0 in which it is expressed. The solution of specificity became easier, because of two contributions from ImClone. They provided the same amino acid sequence as cetuximab expressed in Chinese Hamster Ovary (CHO) cells, a cell line that is not used for the commercial production of cetuximab and is known to produce different glycosylation. In addition, they published the details of the glycosylation of the commercially available cetuximab which is expressed in a mouse cell line SP2.0 [12]. These studies established that the sera with IgE antibodies to cetuximab did not bind to the same antibody expressed in CHO cells, and then that the binding was specific for the Fab portion of the molecule. It was actually Dr. Beloo Mirakhur at BMS who first suggested that the target had to be alpha-gal. Full evidence for the specificity was obtained both from direct binding studies and inhibition studies [11]. The target of these IgE antibodies has now been confirmed from studies in Stockholm, Germany and the Netherlands [13-15]. Today, there is no doubt that most of the severe reactions to cetuximab are a direct consequence of the presence of IgE to alpha-gal prior to the first infusion [11, 16].
Further developments related to Cetuximab and other monoclonal antibodies
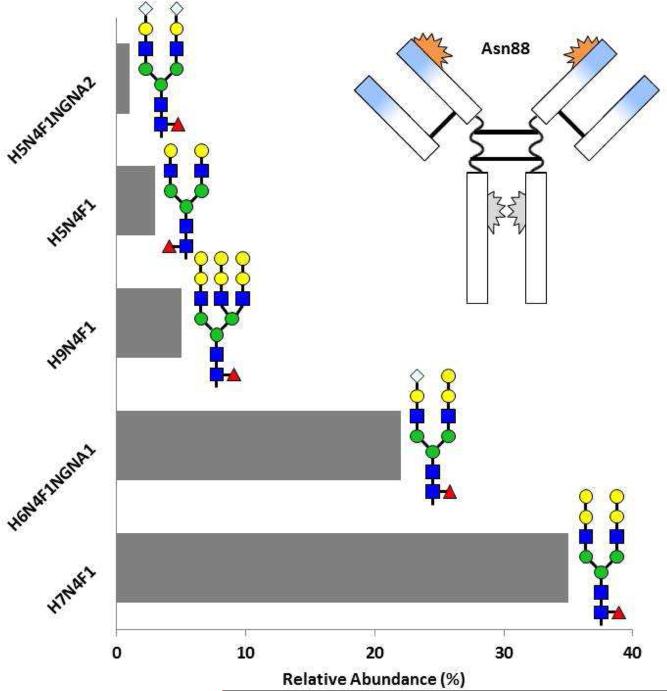
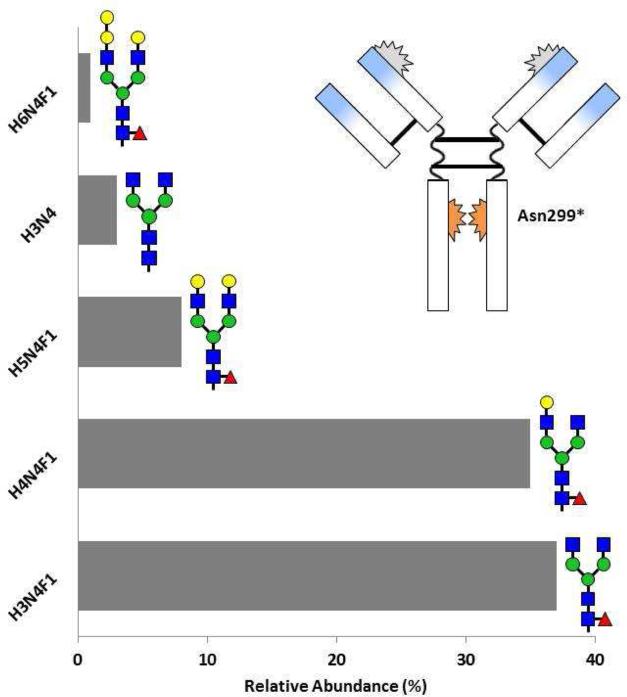
In our initial publication, there was a simplistic image of the structure of cetuximab showing the glycosylation sites [11]. Over the next two years it became clear that this was not completely accurate. First, the sugars are complex including diantennary- and triantennary-complex oligosaccharides with different expression of alpha-gal (Fig 2) [17]. Second, there can be alpha-gal on the Fc portion of the molecule, and third, in the normal structure of the Fc portion of IgG the glycosylation at Asn299 does not face outwards (Fig 2) [17, 18]. Dr. Paul Parren and his group at Genmab in Utrecht used sera from Virginia to investigate the presence of relevant sugars on the Fc portion of cetuximab and infliximab. Those results confirmed our main finding that the alpha-gal, which could be identified on the whole molecule, was on the Fab portion of the heavy chain at Asn88. However, when the two chains of the Fc were separated using proteinase K there was detectable alpha-gal on the heavy chain [15].
Fig 2.
Relative abundance of glycosylation patterns on cetuximab at sites Asn88 (A) and Asn299 (B) as resolved by mass spectroscopy The blue sections on the Fab arms indicate the murine segments of the antibody. See Figure 1 for glycan structure notation. Adapted from Ayoub D, Jabs W, Resemann A, et al: Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. Landes Bioscience 2013, 5(5):699-710.
The combination of these results with other findings made it clear that the glycosylation site on the Fab at Asn88 is a problem, although this glycosylation is not thought to have a significant role in the activity of the molecule. Consequently, the glycosylation site on the Fab at Asn88 has been engineered out of the structure of most new mAbs. Thus, this specific problem is unlikely to arise again. The glycosylation on the Fc portion of IgG molecules plays an important role in defining the binding of IgG to different Fc receptors. Indeed it is now possible to engineer the expression of oligosaccharides on these antibodies so that the structure allows binding to different Fc receptors [18].
Delayed Anaphylaxis to Red Meat (DARM)
Once the assay for IgE to alpha-gal was established, sera from several different kinds of patients were screened. This included patients with histories of asthma, chronic urticaria, atopic dermatitis and anaphylaxis. The most common history that correlated with IgE to alpha-gal was of urticarial or anaphylactic reactions occurring a few hours after eating red meat, beef, pork or lamb (Box 1). This included the original case and also two members of our group. In 2009, we reported 24 cases, all of whom had a typical history of delayed urticaria or anaphylaxis and had IgE specific for alpha-gal [19]. In that same year, it was confirmed that the oligosaccharide that Dr. Van Hage and her colleagues in Sweden had previously identified as an IgE binding epitope on cat IgA was also alpha-gal [20, 21].
By 2011, we knew of about 400 cases of delayed urticaria or anaphylaxis to red meat and we evaluated symptoms, lung function, exhaled NO, and serum IgE on ~200 cases. The resulting evidence demonstrated that IgE to alpha-gal had no association with asthma [22]. Thus even patients with high titer IgE to alpha-gal living in a house with a cat had no increase in their risk of asthma. This was surprising since cats like all mammals have alpha-gal on many of their proteins and lipids. On the other hand, using an assay for alpha-gal, we were not able to detect this antigen airborne in homes with a cat even in the presence of high Fel d 1 levels [22, 23]. The studies at this time also examined sera from the Kenyan village and established that the IgE to cat we had observed earlier was explained by IgE to alpha-gal [9, 22]. Interestingly, a recent report on patients in Zimbabwe found that IgE antibodies to alpha-gal were common among patients being evaluated in an allergy clinic [24]. On the other hand, the results from Africa have not so far identified cases of meat allergy.
Anaphylactic or urticarial reactions to red meat are well recognized in Australia [25]. This came to light from the original observations of Dr. Sherril Van Nunen. In 2006, she had reported to the New South Wales Allergy Society that individuals who had experienced tick bites in the bush were at risk of serious reactions following ingestion of red meat. She published her results in 2009 and related those results to the published evidence about IgE to alpha-gal [11, 19, 25]. Subsequently, with Dr. Mullins we confirmed that the patients with delayed reactions to red meat in Australia had IgE antibodies specific for alpha-gal [26]. Cases of reactions to red meat have now been identified in France, Sweden, Germany, Japan, and Australia as well as in the United States (Table 1 and Fig 3A) [27-34].
Table 1.
Countries where tick bites are associated with IgE responses to alpha-gal
| Country | Tick Species | Cases | Food Reaction | Reference |
|---|---|---|---|---|
| Australia | I. holocyclus | +400 | Beef, lamb, pork, kangaroo, goat, venison, rabbit Beef, lamb, pork, horse, kangaroo, gelatin | [25, 26] |
| USA | A. americanum | >1000 | Beef, lamb, pork, venison, cow's milk, squirrel/road kill | [19, 22] |
| France | 20 | Beef, veal, lamb, pork kidney, cow's milk rabbit, horse | [27,28] | |
| Spain | I. ricinus | 5 | Beef, lamb, pork, rabbit | [29] |
| Japan | 29 | Beef, pork | [30] | |
| Germany | I. ricinus | 60 | Beef, lamb, pork kidney, gelatin | [14, 31, 32] |
| Sweden | I. ricinus | 40 | Beef, pork, cow's milk, moose | [33] |
| Central America | A. cajennerise | 4 | Mammalian meat | [34] |
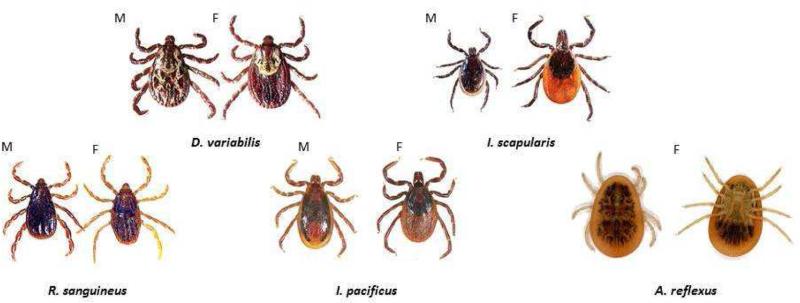
Fig 3.
A, Tick species in which bites are associated with IgE responses to alpha-gal. B, Tick species associated with common tick-borne diseases or tick bite anaphylaxis. (M = male; F = female; L = larval; N = nymph). Pictures of tick species from the Americas (A. americanum, I. scapularis, D. variabilis, etc.) are from http://www.tickencounter.org/; with permission.
The evidence that ticks play an important role and are possibly the only significant cause of IgE responses to alpha-gal in the United States
The speed with which new cases of DARM have been diagnosed in the United States strongly suggests that the condition has increased over the last ten years. Although it is difficult to prove that a disease that was not previously recognized has increased, the reintroduction of deer to the East Coast and the subsequent dramatic rise in deer population provides a possible explanation for the rise in cases. The white-tailed, or Virginia, deer (Odocoileus virginianus), which nearly became extinct in large areas of the East Coast in 1930, is a major host for ticks. These deer were reintroduced to the Blue Ridge Mountains around 1950, and their numbers have been increasing rapidly since then. Thus it is plausible that the dramatic increase in the deer population, particularly close to residential areas, has played a major role in increasing exposure to the lone star tick and its larvae[35].
The initial reason for suspecting tick bites in the United States was that the areas of the two diseases associated with IgE to alpha-gal appeared to be similar to the area with maximum incidence of Rocky Mountain spotted fever (RMSF) [36-38]. That disease is transmitted by two species of ticks: Dermacentor variabilis (the brown dog tick) and Amblyomma americanum (the lone star tick) (Fig 3B; Table 2). The lone star tick has been spreading rapidly in the United States and is being followed by the CDC because it is the major vector for Ehrlichiosis [36, 37].
Table 2.
Causal organisms and tick species involved with the transmission of common tick-borne diseases or the less common tick bite anaphylaxis
| Tickborne Disease | Causal Organism | Implicated Tick |
|---|---|---|
| Lyme Disease |
Borrelia burgdorferi (USA) Borrelia afzelii & Borrelia garinii (Europe) |
Ixodes scapularis Other Ixodes spp. |
| Rocky Mountain Spotted Fever | Rickettsia rickettsii |
Dermacentor variabilis
Rbipicepbalus sanguineus Amblyomma americanum |
| Human Monocytic Ehrlichiosis | Ehrlichia chaffeensis | Amblyomma americanum |
| Human Granulocytic Anaplasmosis | Anaplasma pbagocytophilum | Ixodes spp. |
| Anaphylaxis Caused by Tick Bites | [Tick saliva] |
Argas reflexus (Europe) Ixodes holocyclus (Australia) Rhipicephalus sanguineus (Brazil) Ixodes pacificus (USA) |
The evidence for tick bites as a major cause of IgE to alpha-gal comes from several observations (Box 2). First we have documented increases in IgE antibodies after tick bites in four subjects. Second, there is a significant correlation between reports of prolonged itching after tick bites and the presence of IgE antibodies to alpha-gal in the serum. Bites of larval lone star ticks, like adult ticks, can be intensely pruritic. Interestingly, bites from the deer tick Ixodes scapularis, which transmits Lyme disease, do not generally induce a pruritic skin response. In fact, itching after tick bites has been associated with a decrease in the risk of developing positive Lyme serology [39]. Third, there is an excellent correlation between IgE to alpha-gal and IgE to extract of the lone star tick (r=0.75; p<0.001) [38]. In addition, evidence from the group in Stockholm demonstrating the presence of alpha-gal in the gut of the tick and a similar correlation with tick bites in southern Sweden [33] has strengthened this correlation. Finally, subjects living in areas void of ticks do not have IgE to alpha-gal [11, 22, 33]. The lone star tick is the only tick in the USA whose larvae bite humans, and in several cases we have found high titer IgE antibodies to alpha-gal following bites from larval ticks. These larval ticks are often known as “seed ticks” or “chiggers”. Given the evidence that bites of the lone star tick can give rise to this IgE response the question becomes why does this give rise to such dramatic IgE responses and why are they directed at this oligosaccharide? The problem is in understanding whether this response has more in common with other responses to oligosaccharides (which are usually IgM) or with IgE responses to proteins. There is increasing evidence that the skin can be an important route for IgE antibody responses to proteins such as peanut and wheat [40, 41]. However, with those antigens neither the route through which it enters the skin nor the time frame from exposure to antibody response is known.
Monitored Challenges using Mammalian Meat in patients with delayed anaphylaxis or urticaria to red meat (DARM)
The nature of delayed anaphylaxis creates problems for any investigational or diagnostic challenge procedure. Essentially, it is not feasible to carry out either graded challenges or placebo-controlled challenges to investigate reactions that can take up to 6 hours. Our protocol involves a single dose of meat product and most of these studies were carried out on patients who reported delayed episodes of urticaria. The potential risk of more severe reactions, i.e., anaphylaxis, presents an additional layer of difficulty to diagnostic challenges because the majority of patients are males in an age range in which creating a risk of anaphylaxis or the need for epinephrine would not be appropriate. Accordingly, the studies were not carried out on patients who reported previous episodes of anaphylaxis and were restricted to subjects under age 50 in order to reduce the risk of the reactions or the use of epinephrine [32, 42].
Monitored challenges have now been reported from France, Germany, and the United States [28, 32, 42]. Our challenge results involved 12 cases and 13 controls (Table 3). In ten cases, there was a significant clinical response involving the skin and in one of these cases, severe intestinal spasms as well. Three of the cases had an elevation of serum tryptase at the time of the skin reaction, i.e., 2.5 to 5 hours after eating meat. All the patients who developed urticaria received anti-histamines and in two cases the symptoms required epinephrine [42]. None of the controls had any symptoms and none of them received any treatment.
Table 3.
Red meat challenges in patients with or without IgE specific for galactose-alpha-1, 3-galactose
| Group | Time to Symptoms | Urticaria* | Treatment |
Tryptase Elevation | ||
|---|---|---|---|---|---|---|
| Antihistamine | Epinephrine | Prednisone | ||||
| Allergic (n=12] | 2.45-5.00 hr | 10/12‡ | 9/12 | 2/12 | 4/12 | 3/11# |
| Controls (n=13) | NR | 0/13$ | 0/13 | 0/13 | 0/13 | 0/13 |
Urticaria was characterized by flushing
Flushing with intestinal spasms was observed in only one of these subjects
Tryptase rises: i) 4.7 to 10.7 ng/mL, ii) 4.2 to 20,1 ng/mL, iii) 6.2 to 183 ng/mL
p<0.001
Data from Commins SP, James H, Stevens W, et al: Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1, 3-galactose. JACI 2014, 134:108-115.
To investigate the mechanism of the delayed reaction, we also monitored patients’ basophils during the challenge to investigate the activation of these cells as judged by upregulation of CD63. For the basophil studies the cells were fixed ex vivo without further stimulation. Ten of the alpha-gal IgE positive subjects had significant upregulation of CD63 starting at 3 hours or later, which broadly correlated with the timing of the skin reactions. Surprisingly, 5 of the 13 controls demonstrated significant upregulation of CD63 ex vivo at the same time, i.e., 3-5 hours after eating meat [42]. We assumed that this upregulation reflects the time at which significant alpha-gal enters the circulation. However, there is published evidence that basophils have a receptor for VLDL and LDL that can trigger histamine release [43, 44]. Our current hypothesis is that the delay in the clinical response is best explained by the time needed for absorption of lipids and glycolipids as chylomicrons and the subsequent processing to VLDL and LDL.
In the Alsace region of France and the North Eastern region of Germany, two separate groups have described cases of DARM. [27, 28, 31, 32]. In both areas they have identified pork kidney as an important cause of the naturally occurring reactions as well as an excellent agent for inducing reactions in the context of diagnostic challenges. In each case, the reactions were more severe and also more rapid with the kidney challenge. However, even with pork kidney none of the patients reported “immediate,” i.e., within 20 minutes, oral symptoms.
A large proportion of the patients report that their responses are not only delayed, but that they are also inconsistent. Some patients report that responses occur more consistently if they take alcohol at the same time as the meat. Recently, the Biedermann group in Germany have also reported that the response to pork kidney can be enhanced with exercise, aspirin and/or alcohol [32]. The important question at this point, is to establish what form of the glycolipid enters the circulation 2-5 hours after eating mammalian meat or organs, such as kidney, heart, stomach or liver from non-primate mammals.
Conclusions
Two completely different and novel forms of anaphylaxis have now been associated with IgE to alpha-gal. Reactions to cetuximab continue to occur, including a fatal reaction that occurred during the first infusion in a 40-year-old patient with colon cancer, in August of 2014 [11, 16]. In addition, severe delayed anaphylactic reactions to red meat continue to appear in patients who were not aware of being allergic, or had actually been dismissed by a physician because the physician did not believe that the reactions could take 4 hours. In 2014, we reported a case of severe anaphylaxis to pork in a previously non atopic 73 year old lady [45].
The characteristic features of the syndrome of delayed anaphylaxis to red meat are late onset of urticarial or anaphylactic episodes, together with no immediate cause, a history of pruritic tick bites and exposure to red meat three to five hours prior to the onset of symptoms. However, many cases are less obvious with longer delays, no clear history of tick bites and symptoms such as gastrointestinal spasm alone, or just facial swelling. In some areas of the United States it is normal practice to test cases of otherwise unexplained cases of anaphylaxis. Indeed in central Virginia today IgE to alpha-gal is the most common “cause” of anaphylaxis in adults. Among city dwellers the syndrome is rare, but nonetheless the syndrome needs to be on the list of possible causes of otherwise unexplained anaphylaxis.
The evidence about ticks as a cause of the IgE response is good but certainly does not exclude a role for other parasitic exposures. Interestingly, ticks in other countries are not the same. In Australia the relevant tick is Ixodes holocyclus, while in Europe, the relevant tick is Ixodes ricinus (Fig 3). The group of investigators in Stockholm has demonstrated that Ixodes ricinus has alpha-gal in its gastrointestinal tract [13]. In our experience, alpha-gal IgE titers appear to diminish over time with avoidance of ticks, though there is neither a regular time course for this loss of sensitivity nor a guaranteed reduction in symptom severity among all patients. On the other hand, continued exposure to ticks appears to augment the already existing IgE antibody response. The important question is how these ticks induce an IgE responses and why it is directed against this specific oligosaccharide.
One of the striking things about the syndrome is that the route of exposure to the antigen is via the skin while the symptoms follow oral exposure. This adds to an increasing list of allergic diseases in which the route of initial exposure is not the same as the site of the symptoms (Box 3). In addition, the delay in reaction after eating meat adds to the group of conditions where oral exposure appears to be causal and IgE antibodies are involved but the patient cannot identify the relevant food or food groups via more immediate, oral symptoms, i.e., pruritus or swelling [41, 46, 47]. Interestingly, none of these conditions can be evaluated using double blind placebo controlled challenges.
The evolution in our understanding of DARM has important implications in our understanding and treating IgE mediated hypersensitivity. No feature of the disease was obvious to allergists in practice, including to those of us who now study it. Most adult patients who reported reactions occurring 4 or more hours after eating meat, and were found to have negative or very small prick test responses to the relevant meat were informed that this could not be IgE mediated hypersensitivity to the food. Furthermore, histories that included previous tick bites were thought to be irrelevant! Finally, the role of the increasing deer population in suburban areas of the East Coast focuses attention on the many ways in which changes in human behavior have impacted allergic disease.
Key Points.
In 2007, the monoclonal antibody cetuximab was causing severe hypersensitivity reactions during the first infusion in a region of the southeastern United States.
Investigation of pre-treatment sera established that they contained IgE against the oligosaccharide alpha-gal present on the Fab of cetuximab.
Alpha-gal (correctly galactose alpha-1, 3-galactose) is a blood group substance of non-primate mammals.
These IgE antibodies are also associated with delayed anaphylaxis to red meat, i.e., to meat or organs of those animals that carry this oligosaccharide.
There is now extensive evidence that the primary cause of these IgE antibodies is bites from the tick Amblyomma americanum or its larvae.
Box 1.
Delayed, or Late Night, Anaphylaxis to Red Meat: the presentation and the clinical problem
| I. For several years prior to 2008, patients were presenting to general medicine or allergy clinics for investigation of anaphylaxis or recurrent episodes of urticaria where the cause was not obvious |
| II. The histories were striking for i) onset as an adult, ii) presentation late at night and/or iii) no apparent acute cause; i.e., no bites or stings; no medicines and no foods within 3 hours of the episode |
| III. The majority of cases had no history of conventional symptoms of food allergy; however, a significant proportion of the cases had made an association with eating beef, pork or lamb earlier in the day |
| IV. When the subjects were tested using a prick test with commercial food extract, including beef, pork or lamb, they were found to have negative or very small responses (i.e., 2-3mm wheals) to the meats |
| V. Because of the adult onset, long interval after eating, and “negative” prick skin tests, many or most of the patient were told they did not have food allergy; nonetheless, they had specific IgE for alpha-gal as well as beef, pork and lamb |
Box 2.
Evidence that tick bites are a or the major cause of IgE responses to alpha-gal
| I. The area of the USA where the IgE antibodies are common, coincides with the distribution of the lone star tick |
| II. A large proportion of patients with these antibodies report prolonged itching after tick bites |
| III. In four cases we have followed increases in IgE antibodies to alpha-gal after tick bites |
| IV. IgE to alpha-gal correlates strongly with IgE to extract of the lone star tick |
| V. In other countries bites of ticks of other species have been associated with IgE to alpha-gal |
| VI. Among populations living in large cities of the USA or the extreme north of Sweden where ticks are not present, IgE antibodies to alpha-gal are absent |
IgE antibodies to alpha-gal can be measured with cetuximab on a streptavidin CAP (see Ref 10). The assay is also available commercially from IBT/Viracor (Lee's Summit, MO).
Box 3.
Allergic diseases in which the route of sensitization is not the same as the route of exposure giving symptoms
| Allergic Syndrome | Presentation | Route of Sensitization | Mechanism |
|---|---|---|---|
| Oral Allergy Syndrome | Oral symptoms to apple, cherries, hazelnut, etc. | Sensitization to inhaled pollen, e.g. birch | Cross-reactivity between Bet v 1 and major allergens, e.g. Mal d 1, Pru av 1, Cor a 1, etc. |
| Pork/Cat Syndrome | Oral symptoms and anaphylaxis to pork | Sensitization to cat albumin (Fel d 2) | Cross-reactivity between albumins of cat and pork |
| Peanut Allergy | Anaphylactic reactions to oral peanut | Sensitization through the skin in patients with a filaggrin defect | Sensitization to Ara h 1 and Ara h 2 |
| Delayed Anaphylaxis to Red Meat | Delayed urticarial and anaphylactic reactions to red meat | Sensitization to alpha-gal from tick bites | Sensitization to mammalian oligosaccharide galactose-alpha-1,3-galactose |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose
References
- 1.Landsteiner K. Specificity of Serological Reactions. Charles C. Thomas; Baltimore: 1936. [Google Scholar]
- 2.Platts-Mills TA, Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974;113(1):348–358. [PubMed] [Google Scholar]
- 3.Milland J, Sandrin MS. ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens. 2006;68:459–466. doi: 10.1111/j.1399-0039.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- 4.Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolutionary and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, Goodman M. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci U S A. 2007;104:559–564. doi: 10.1073/pnas.0610012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83(6):674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 7.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One. 2013;8(2):e55566. doi: 10.1371/journal.pone.0055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 9.Perzanowski MS, Ng'ang'a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, Chapman MD, Kennedy MW, Platts-Mills TA. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140(5):582–588. doi: 10.1067/mpd.2002.122937. [DOI] [PubMed] [Google Scholar]
- 10.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, Wickens K, Platts-Mills TA. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115:1029–1035. doi: 10.1016/j.jaci.2004.12.1131. [DOI] [PubMed] [Google Scholar]
- 11.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q. Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem. 2007;364:8–18. doi: 10.1016/j.ab.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, Sallberg M, Gronlund H, van Hage M. Identification of galactose-alpha-1,3- galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68(4):549–552. doi: 10.1111/all.12128. [DOI] [PubMed] [Google Scholar]
- 14.Jappe U. [Update on meat allergy. alpha-Gal: a new epitope, a new entity?]. Hautarzt. 2012;63(4):299–306. doi: 10.1007/s00105-011-2266-y. [DOI] [PubMed] [Google Scholar]
- 15.Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, James H, van Berkel PH, van de Winkel JG, Platts-Mills TA, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nature biotechnology. 2011;29(7):574–576. doi: 10.1038/nbt.1912. [DOI] [PubMed] [Google Scholar]
- 16.Maier S, Chung C, Morse M, Platts-Mills T, Townes L, Mukhopadhyay P, Bhagavatheeswaran P, Racenberg J, Trifan O. A retrospective analysis of cross-racting cetuximab IgE antibody and its association with severe infusion reactions. Cancer Med. 2014;9(10):333. doi: 10.1002/cam4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayoub D, Jabs W, Resemann A, Evers W, Evans C, Main L, Baessmann C, Wagner-Rousset E, Suckau D, Beck A. Correct primary structure assessment and extensive glyco-profiling of cetuximab by a combination of intact, middle-up, middle-down and bottom-up ESI and MALDI mass spectrometry techniques. Landes Bioscience. 2013;5(5):699–710. doi: 10.4161/mabs.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes J, Cosgave E, Struwe W, Wormald M, Davey G, Jefferis R, Rudd P. Glycosylation and Fc Receptors. Current Topics in Microbiology and Immunology. 2014;382:165–199. doi: 10.1007/978-3-319-07911-0_8. [DOI] [PubMed] [Google Scholar]
- 19.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, Woodfolk JA, Platts-Mills TA. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123:426–433. doi: 10.1016/j.jaci.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adedoyin J, Gronlund H, Oman H, Johansson SG, van Hage M. Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol. 2007;119(3):640–645. doi: 10.1016/j.jaci.2006.11.637. [DOI] [PubMed] [Google Scholar]
- 21.Gronlund H, Adedoyin J, Commins SP, Platts-Mills TA, van Hage M. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol. 2009;123(5):1189–1191. doi: 10.1016/j.jaci.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Commins SP, Kelly LA, Ronmark E, James HR, Pochan SL, Peters EJ, Lundback B, Nganga LW, Cooper PJ, Hoskins JM, et al. Galactose-alpha-1,3- Galactose-Specific IgE Is Associated with Anaphylaxis but Not Asthma. Am J Respir Crit Care Med. 2012;185:723–730. doi: 10.1164/rccm.201111-2017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platts-Mills JA, Custis NJ, Woodfolk JA, Platts-Mills TA. Airborne endotoxin in homes with domestic animals: implications for cat-specific tolerance. J Allergy Clin Immunol. 2005;116(2):384–389. doi: 10.1016/j.jaci.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Arkestal K, Sibanda E, Thors C, Troye-Blomberg M, Mduluza T, Valenta R, Gronlund H, van Hage M. Impaired allergy diagnostics among parasite- infected patients caused by IgE antibodies to the carbohydrate epitope galactose-alpha 1,3-galactose. J Allergy Clin Immunol. 2011;127(4):1024–1028. doi: 10.1016/j.jaci.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–511. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 26.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3- galactose. J Allergy Clin Immunol. 2012;129:1334–1342. e1331. doi: 10.1016/j.jaci.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacquenet S, Moneret-Vautrin DA, Bihain BE. Mammalian meat-induced anaphylaxis: clinical relevance of anti-galactose-alpha-1,3-galactose IgE confirmed by means of skin tests to cetuximab. J Allergy Clin Immunol. 2009;124(3):603–605. doi: 10.1016/j.jaci.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, Cordebar V, Morel-Codreanu F, Petit N, Moneret-Vautrin DA, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67:699–704. doi: 10.1111/j.1398-9995.2012.02799.x. [DOI] [PubMed] [Google Scholar]
- 29.Nunez R, Carballada F, Gonzalez-Quintela A, Gomez-Rial J, Boquete M, Vidal C. Delayed mammalian meat-induced anaphylaxis due to galactose-alpha- 1,3-galactose in 5 European patients. J Allergy Clin Immunol. 2011;128(5):1122–1124. e1121. doi: 10.1016/j.jaci.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Chinuki Y, Tanaka A, Morita E. Laminin y-1 and collagen x-1 (VI) chain are galactose-a-1, 3-galactose-bound allergens in beef. Allergy. 2013;69:199–207. doi: 10.1111/all.12302. [DOI] [PubMed] [Google Scholar]
- 31.Caponetto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-α-1,3-galactose. J Allergy Clin Immunol: In Practice. 2013;1(3):302–303. doi: 10.1016/j.jaip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. The Journal of allergy and clinical immunology. 2014;133(3):755–759. e751. doi: 10.1016/j.jaci.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 33.Hamsten C, Tran T, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, Van Hage M. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. JACI. 2013;132(6):1431–1434. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wickner P, Commins S. The First 4 Central American Cases of delayed meat allergy with galactose-Alpha-1, 3-galactose positivity clustered among field biologists in Panama. JACI AB212 Abstracts. 2014;(736) [Google Scholar]
- 35.Pound J, Lohmeyer K, Davey R, Miller J, George J. Efficacy of amitraz impregnated collars on white-tailed deer (Artiodactyla: Cervidae) in reducing free-living populations of lone star ticks (Acari: Ixodidae). J Econ Entomol. 2012;105(6):2207–2212. doi: 10.1603/ec12219. [DOI] [PubMed] [Google Scholar]
- 36.Stein K, Waterman M, Waldon J. The effects of vegetation density and habitat disturbance on the spatial distribution of ixodid ticks (Acari: Ixodidae). Geospatial Health. 2008;2(2):241–252. doi: 10.4081/gh.2008.247. [DOI] [PubMed] [Google Scholar]
- 37.Romero C. Reportable disease surveillance in Virginia. Virginia Department of Health; Richmond, VA: 2012. 2012. [Google Scholar]
- 38.Commins S, James H, Kelly E, Pochan S, Workman L, Perzanowski M, Kocan K, Fahy J, Nganga L, Ronmark E, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose- α-1,3-galactose. J Allergy Clin Immunol. 2011;127:1286–1293. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burke G, Wikel SK, Spielman A, Telford SR, McKay K, Krause PJ. Tick-borne Infection Study G: Hypersensitivity to ticks and Lyme disease risk. Emerg Infect Dis. 2005;11:36–41. doi: 10.3201/eid1101.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brough H, Simpson A, Makinson K, Hankinson J, Brown S, Dourir A, Belgrave D, Penagos M, Stephens A, McLean W, et al. Peanut allergy: Effect on environmental peanut exposure in children with filaggrin loss-of-function mutations. The Journal of allergy and clinical immunology. 2014;1344:867–875. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokooji T, Kurihara S, Murakami T, Chinuki Y, Takahashi H, Morita E, Harada S, Ishii K, Haragun M, Hide M, et al. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol Int. 2013;64(4):435–445. doi: 10.2332/allergolint.13-OA-0561. [DOI] [PubMed] [Google Scholar]
- 42.Commins SP, James H, Stevens W, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1, 3-galactose. JACI. 2014;134:108–115. doi: 10.1016/j.jaci.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonen B, O'Donnell P, Post T, Quinn T, Schulman E. Very low density lipoproteins (VLDL) trigger the release of histamine from human basophils. Biochimica et biophysica acta. 1987;917(3):418–424. doi: 10.1016/0005-2760(87)90121-4. [DOI] [PubMed] [Google Scholar]
- 44.Virgolini I, Li S, Yang Q, Koller E, Sperr W, Leimer M, Angelberger P, Nimpf J, Schneider W, Valent P. Characterization of LDL and VLDL binding sites on human basophils and mast cells. Arterioscler Thromb Vasc Biol. 1995;15(1):17–26. doi: 10.1161/01.atv.15.1.17. [DOI] [PubMed] [Google Scholar]
- 45.Tripathi A, Commins SP, Heymann P, Platts-Mills TA. Delayed anaphylaxis to red meat masquerading as idiopathic anaphylaxis. JACI in Practice. 2014 May-Jun;(3):259–265. doi: 10.1016/j.jaip.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Ann Allergy Asthma Immunol. 2010;104(6):496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brockow K, Kneissl D, Valentini L, Zelger O, Grosber M, Kugler C, Werich M, Darsow U, Matsuo H, Morita E, et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. The Journal of allergy and clinical immunology. 2014;14:01197–X. doi: 10.1016/j.jaci.2014.08.024. [DOI] [PubMed] [Google Scholar]