Abstract
Angiotensin (Ang)-(1-7) is a potential vasoprotective peptide. In the present study, we investigated its counteractive effects to Ang II on vascular smooth muscle cells (VSMCs) and intracerebral hemorrhagic stroke (ICH) through inflammatory mechanism. In in vitro experiments, human brain VSMCs (HBVSMCs) were treated with vehicle, Ang II, Ang II + Ang-(1-7), Ang II + A-779 or Ang II + Ang-(1-7) + A-779 (Mas receptor antagonist). HBVSMC proliferation, migration and apoptosis were determined by methyl thiazolyltetrazolium, wounding healing assay and flow cytometry, respectively. In in vivo experiments,C57BL/6 mice were divided into vehicle, Ang II, Ang II + Ang-(1-7), Ang II + A-779 or Ang II + Ang-(1-7) + A-779 groups before they were subjected to collagenase-induced ICH or sham surgery. Hemorrhage volume and middle cerebral artery (MCA) remodeling were determined by histological analyses. Levels of NFκB, Inhibitor of κBα (IκBα), tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein 1 (MCP-1) and interleukin (IL-8) were measured by western blot or ELISA. We found that 1) Ang II increased HBVSMC migration, proliferation and apoptosis, and increased the blood pressure (BP), neurological deficit score, MCA remodeling and hemorrhage volume in ICH mice. 2) Ang-(1-7) counteracted these effects of Ang II, which was independent of BP, with the down-regulation of NFκB, up-regulation of IκBα, and decreased levels of TNF-α, MCP-1 and IL-8. 3) The beneficial effects of Ang-(1-7) could be abolished by A-779. In conclusion, Ang-(1-7) counteracts the effects of Ang II on ICH via modulating NFκB inflammation pathway in HBVSMCs and cerebral microvessels.
Keywords: Ang-(1-7), Ang II, VSMCs, hemorrhagic stroke, NFκB
Graphical Abstract
1. Introduction
Intracerebral hemorrhagic stroke (ICH) is a serious cerebrovascular complication related to arteriosclerosis and hypertension. The role of angiotensin (Ang) II/AT1 pathway of renin angiotensin system (RAS) has been well documented in the pathophysiology of hypertension and vascular diseases. Ang-(1-7)/Mas is a newly discovered pathway of the RAS, which counteracts the effects of Ang II/AT1 pathway [1] and have protective effects on stroke [2]. An earlier study showed intracerebral administration of Ang-(1-7) has beneficial effects on endothelin-1-induced ischemic stroke, which can be reversed by A-779, a Mas receptor antagonist [3]. Centrally administered Ang-(1-7) increases the survival of stroke-prone spontaneously hypertensive rats (SHRSP) [4]. Our recent study demonstrated that neuron angiotensin converting enzyme 2 (ACE2) over-expression, which catalyzes Ang II into Ang-(1-7), protects brain from ischemic stroke injury [5]. However, it is unknown whether Ang-(1-7) might provide vascular protective effects on ICH by counteracting the deleterious effects of Ang II and ultimately possess great potential for the prevention and treatment of ICH.
Vascular smooth muscle cells (VSMCs) are the main components of vascular parenchyma and play an important role in vascular pathology changes [6, 7]. Evolving evidence demonstrates that phenotypic changes of VSMCs have a significant impact on cerebral aneurysm formation and rupture [8]. Ang II has been shown to induce VSMC proliferation/migration and hypertrophic response [9]. Moreover, Ang II-induced vascular inflammation involves in vascular dysfunction/injury, cerebral aneurysm formation and rupture and subsequential ICH [10]. Tumor necrosis factor alpha (TNF-α) is a key proinflammatory factor that has been implicated in ICH pathophysiology. This may occur through TNF-α-mediated VSMC phenotypic modulation, release of chemotactic cytokines, and ultimately cellular apoptosis [11]. Ang II induces VSMC dysfunction/inflammation via TNF-α dependent nuclear factor-KappaB (NFκB) pathway [12]. Inhibition of the Ang II signaling could prevent VSMC inflammation related vascular remodeling [13, 14]. Therefore, investigating whether Ang-(1-7) protects VSMCs from Ang II-induced injury via regulating the NFκB inflammatory pathway will provide rationale for protective effects of Ang-(1-7) on vascular remodeling and ICH.
In this study, we determined the role of Ang-(1-7) in protecting human brain VSMCs (HBVSMCs) from Ang II-induced apoptosis and activation, and in attenuating middle cerebral artery (MCA) remodeling and brain injury in ICH by counteracting the effects of Ang II. The NFκB inflammatory pathways was investigated to explore the underlying mechanisms.
2. Methods
2.1. Cell culture and treatments
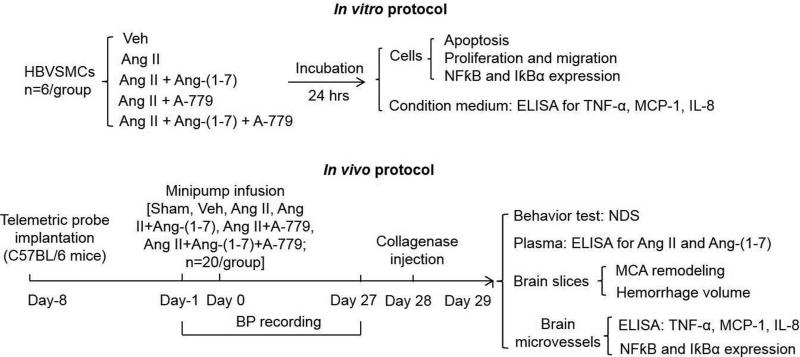
HBVSMCs (Sciencell Research Laboratories, Carlsbad, CA, USA) were cultured in 75-mm2flasks in SMC medium, supplemented with 2% fetal bovine serum, 100 U/ml penicillin and streptomycin, and 100 U/ml cell growth supplement (Sciencell Research Laboratories) in a 37°C incubator with humidified atmosphere of 5% CO2/95% air. After reaching confluence, cells were randomly subjected to different treatments for 24 hrs (Figure 1): vehicle, Ang II, Ang II + Ang-(1-7), Ang II + A-779, Ang II + Ang-(1-7) + A-779. The concentration of Ang II (10−6 M, Sigma-Aldrich, St. Louis, MO) was chosen according to our previous study [15]. The dose of Ang-(1-7) (10−7 M, Bachem AG) was determined based on the concentration-response study (Figure 2A). A-779 (Mas receptor antagonist, 1 μM, BachemAG, Torrance, CA, USA) was pre-incubated with cells for 30 min [16].
Fig. 1. Experimental procedures and time flow chart.
HBVSMCs: human brain smooth muscle cells; Veh: vehicle; Ang II: angiotensin II; NFκB: nuclear factor-KappaB; MCA: middle cerebral artery; ICH: intracerebral hemorrhage. TNF-α: tumor necrosis factor; MCP-1: monocyte chemoattractant protein 1; IL-8: interleukin-8; NDS: neurological deficit scores.
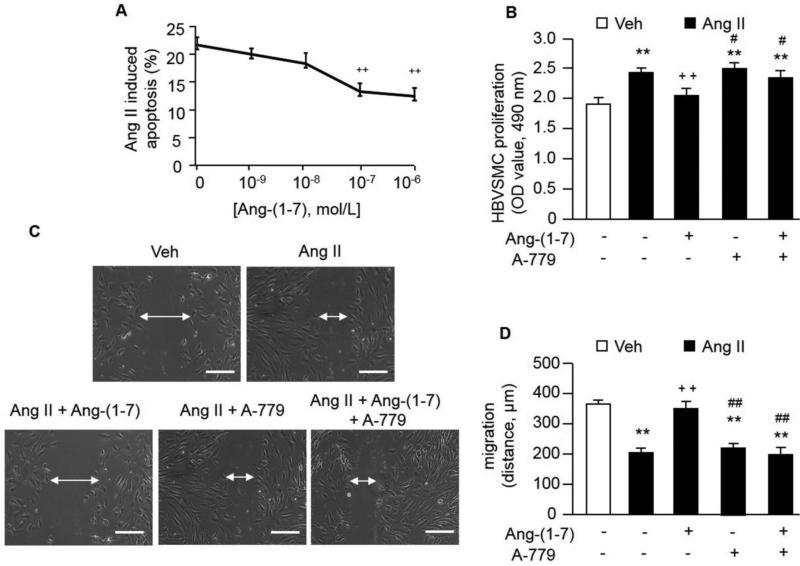
Fig. 2. The effects of Ang-(1-7) on HBVSMC apoptosis, proliferation and migration.
A, Ang-(1-7) protects HBVSMCs from Ang II-induced apoptosis. ++P< 0.01 vs. 0 M Ang (1-7), n=6/group. B, Ang-(1-7) decreased the Ang II-induced increased in HBVSMC proliferation. C, Representative images of migration abilities of HBVSMCs. Scale bars: 200 μm. D, Ang-(1-7) decreased the Ang II-induced increased in HBVSMC migration. **P< 0.01 vs. veh; ++P< 0.01 vs. Ang II, #P< 0.05, ##P< 0.01 vs. Ang II + Ang-(1-7), n=6/group.
2.2. Concentration-response study of Ang-(1-7) on Ang II-induced cell apoptosis
To determine the effective dose of Ang-(1-7) for protecting HBVSMCs from Ang II-induced apoptosis, cells were treated with Ang II (10−6 M) and different doses (0, 10−9, 10−8, 10−7 and 10−6 M) of Ang-(1-7). After 24 hrs, HBVSMCs were harvested for apoptosis analysis.
2.3. Flow cytometric analysis of cell apoptosis
The apoptosis of HBVSMCs was assessed by using apoptosis assay kits (Invitrogen, NY) as our previous study [15]. The HBVSMCs were suspended in 100 μl annexin-binding buffer, and incubated with 5 μl of annexin V-FITC and 5μl of propidium iodide (PI) at RT in the dark for 15 min. The apoptotic rate was measured by a flow cytometer (BD Accuri C6, Franklin Lakes, NJ, USA).
2.4. Cell proliferation assay
Methyl thiazolyltetrazolium (MTT) assay kit (Invitrogen, NY) was used to determine the cell proliferation [15]. The cell culture medium was replaced with 100 μl of fresh culture medium with 10 μl of 12 mM MTT solution and incubated at 37°C for 4 hrs. Then, 100 μl of DMSO was added to each well (96-well plate) and incubated at 37°C for 10 min. The absorbance of samples was measured at 490 nm (OD490) by using a multi-well plate reader (Synergy H1, BioTek Inc., Winooski, VT, USA).
2.5. Cell migration assay
The migration ability of HBVSMCs was measured by wound healing assays [17]. Cells were grown to confluence on 6-well culture plates. A straight scratch injury was made using a sterile 1mL pipette tip on 6-well plates. Cells were then subjected to different treatment groups as shown in Figure 1. Photographs of the scratch area were taken immediately (0 hr time point) and 6hrsafter making to check the invasion of cells into the wounded area.
2.6. Animals and in vivo experimental design
2.6.1. Animals
Total of 128 adult (8-10 weeks of age, weight ranges from 25 g to 32 g) C57BL/6J mice were used for this study. Mice were maintained in a 22°C room with a 12-hr light/dark cycle and fed with standard chow and drinking water ad libitum. 8 mice were died of surgery intervention, and the rest of 120 were used for the experiments. The group sizes was kept to 20/group by adding the corresponding number of animals that died of surgery in each group. All experimental procedures were approved by the Wright State University Laboratory Animal Care and Use Committee and were in accordance with the Guide of the Care and Use of Laboratory Animals issued by the National Institutes of Health (NIH).
2.6.2. Experimental design
As showing in Figure 1, mice were implanted with telemetric probe for recording arterial blood pressure (BP). After 7 days, mice were randomly divided into four treatment groups (n=12/group): sham, vehicle, Ang II, Ang II + Ang-(1-7), Ang II + A-779, Ang II + Ang-(1-7) + A-779. After 4 weeks, mice were subjected to induce ICH stroke by injecting collagenase into brain. After 24 hrs, mice were euthanized under an overdose of pentobarbital (150 mg/kg i.p). The doses used for Ang II (42 μg/kg/h), Ang-(1-7) (24 μg/kg/h) and A-779 (200 ng/kg/min) were based on previous studies [5, 18, 19].
2.7. BP recording
A radiotelemetry system (TA11PA-C10, Data Science International) was used for recording arterial BP under anesthesia by inhaling 2.5% isoflurane as we reported previously [5]. The telemetric probes were implanted in the left carotid artery. BP was recorded for 24 hours before minipump infusion and continued for 27 days after Ang II infusion. We recorded BP for 10 min (sample rate 500 hz) once a hour for 24 hrs to calculate the mean arterial pressure (MAP) for each day. The BP was started to record at the same time for each mouse.
2.8. Minipump implantation
The osmotic minipumps (Alzet, Model #1004, 4 weeks) were loaded with Ang II, Ang-(1-7) and/or A-779 according to the grouping and then placed in a sterile isotonic saline solution for 48 hrs at 37°C to initiate their operation at a constant pumping rate and to minimize the possibility of clot formation in the catheter. Intraperitoneally implantation of minipumps was performed in a stereotaxic apparatus under anesthesia (ketamine:xylazine mixture, 100:8 mg/kg, i.p).
2.9. Microinjection of collagenase to induce ICH model
The collagenase injection surgery was conducted using a mouse stereotaxic frame under anesthesia (Ketamine: Xylazine mixture, 100:8 mg/kg, 20-30μl, i.p). A 1.0-mm burr hole was made on the skull by using a dental drill-trephine. The bacterial collagenase (type VII-S; 0.075 U/0.5 μL) was injected into the caudate putamen by injection cannula (a glass micro-needle, 30 μm in diameter) at a rate of 0.5 μL/min[20]. The cannula was localized using stereotaxic coordinates related to bregma (0.5 mm anterior to bregma, 3.0 mm right lateral to the midline, 4.0 mm in depth)[21].
2.10. Behavior test
Animals began to exhibit neurological signs of an ICH within 60 minutes. At 24 hrs after ICH induction, neurological deficit scores (NDS) were determined by using a 24-point scoring system [22, 23], including body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling. Each test was graded from 0 to 4, establishing a maximum deficit score of 24. All behavioral tests were conducted in a quiet and low light room by observers who were blind to the treatment condition.
2.11. Histological analyses of MCA remodeling and hemorrhage volume
Brain tissues were fixed in 4% formaldehyde overnight, and in 4% formaldehyde plus 30% sucrose for three days. The brains were sequentially cut into coronal sections (20 μm for MCA remodeling or 1mm for hemorrhage volume).
The MCA was analyzed from both ipsilateral and contralateral sides. To keep the sections from the similar level in each animal, the brains sections (20 μm) were sequentially put into six separate wells of a 6-well plate containing 2 ml of phosphate-buffered saline as we reported before [5, 24]. In total, three slices (sequentially from each well) of each brain were used for HE staining to present the data of MCA remodeling for each brain.
MCA remodeling (wall-to-lumen ratio, W/L) and hemorrhage volume were quantified with Image J from NIH. W/L were calculated as follows: W/L= (De0Ca - Di0Ca)/2Di0Ca, where De0Ca and Di0Ca are the external and internal diameter of MCA [25]. Hemorrhage volume was estimated from the sum of all value calculated from the formula: [(area of hemorrhage region in each section)×1](mm3) [26]. The MCA remodeling and hemorrhage volume were calculated by an investigator who was blind to animal grouping.
2.12. Isolation of cerebral microvessels
The cerebral microvessels were isolated from the ipsilateral hemisphere of ICH mice after different treatments [5]. Briefly, the brains were removed free of meninges, dissected into small pieces and homogenized in 0.4 M sucrose solution containing 0.1 mM CaCl2, 0.4 mM MES buffer, 0.1 mM ATP, 1% BSA, and 20,000 IU sodium heparin at 4°C. The protease inhibitors (0.5 mg/ml aprotinin, 0.5 mg/ml leupeptin, and 0.7 mg/ml pepstatin) and 0.1 mM EDTA were added. The homogenate was subsequently passed through nylon mesh sieves (125 μm). After centrifuging at 200×g for 5 min, the pellet was resuspended in 18% Detran solution and centrifuged at 10,000×g for 10 min. The pellet was then resuspended in Ca2+-Mg2+ free Hank balance salt solution (HBSS) and passed through nylon mesh filters (75 μm). The vessels on the filter was obtained by rinsing the filter with HBSS. The microvessel fraction consisted predominantly of capillaries and venules with infrequent contamination from small arterioles, and no observable contamination by intact parenchymal cells, as determined by light microscopic examination.
2.13. ELISA assays
The plasma levels of Ang II and Ang-(1-7) were determined by commercial kits (Enzo Life Sciences, Farmingdale, NY) [5]. A volume of 0.5-1 ml of peripheral blood was taken from the left ventricle of heart into a syringe containing 1% volume of heparin (1,000 U/ml; Sigma, Fairfax, VA).The levels of TNF-α, monocyte chemoattractant protein 1 (MCP-1) and interleukin (IL)-8in the cell culture condition medium and cerebral microvessels were determined by commercial ELISA kits (R&D System, Minneapolis, MN).
2.14. Western blot analysis
Proteins from cells and cerebral microvessels were obtained with lysis buffer (Thermo Scientific, FL) containing protease inhibitors. The proteins were subjected to electrophoresis and transferred onto nitrocellulose membranes. The membranes were blocked by incubating with 5% dry milk for 1 hr, and then incubated with primary antibodies: against NFκB (1:1000; Cell signaling, Danvers, MA), Inhibitor of κBα (IκBα) (1:1000; Cell signaling, Danvers, MA), at 4°C overnight. After being washed thoroughly, membranes were incubated with horseradish peroxidase (HRP) conjugated IgG (1:40000; Jackson ImmunoResearch Labs, INC. PA) for 1 hr at RT. β-actin (1:4000; Sigma-Aldrich, St. Louis, MO) was used to normalize protein loading.
2.15. Statistical analysis
Appropriate sample size was determined by a statistical power analysis with the type I and type II error rates at 0.05 and 0.20, respectively. An estimate of the error variance was obtained from data in previous studies. The results of the power analysis indicated that a sample size of 10 animals per group is sufficient. All data, excepting neurologic deficit scores, are presented as mean ± SE. The neurologic deficit scores were expressed as median (range). The neurological deficit scores among different groups were compared by the Kruskal-Wallis test. When the Kruskal–Wallis test showed a significant difference, the Mann-Whitney U-tests were applied. For the rest measurements, comparisons between groups were analyzed by two-way ANOVA followed by a Turkey post-hoc test (SPSS version 16.0; SPSS, Chicago, IL, USA). For all tests, a P-value<0.05 was considered significant.
3. Results
3.1. Ang-(1-7) Protects HBVSMCs from Ang II-induced Apoptosis
In Figure 2A, we found that Ang-(1-7) significantly decreased Ang-II induced cell apoptosis at the dose of 10−7 M (22817 ± 523 and 13720 ± 586 cells, P<0.01, 10−7 M vs. 0 M) and 10−6 M (22817 ± 523 and 12285 ± 330 cells, P<0.01, 10−6 M vs. 0 M). Based on these data, we chose 10−7 M of Ang-(1-7) for the following in vitro experiments.
3.2. Ang-(1-7) Decreases Ang II-induced Proliferation and Migration of HBVSMCs
Ang II significantly increased proliferation and migration in HBVSMCs. Ang-(1-7) inhibited Ang II-induced HBVSMC proliferation by 20% (P<0.01; Figure 2B) and migration ability by about 50% (P<0.01; Figure 2C and D). These effects of Ang-(1-7) were abolished by Ang-(1-7) antagonist (A-779) (P<0.05 for proliferation, P<0.01 for migration). The effects of endogenous Ang-(1-7) were investigated by incubating cells with Ang II and A-779. Results showed that there were no significant differences between Ang II and Ang II + A-779 groups in HBVSMC proliferation and migration.
3.3. Ang-(1-7) Inhibits Ang II-induced Activation of NFκB Pathway in HBVSMCs
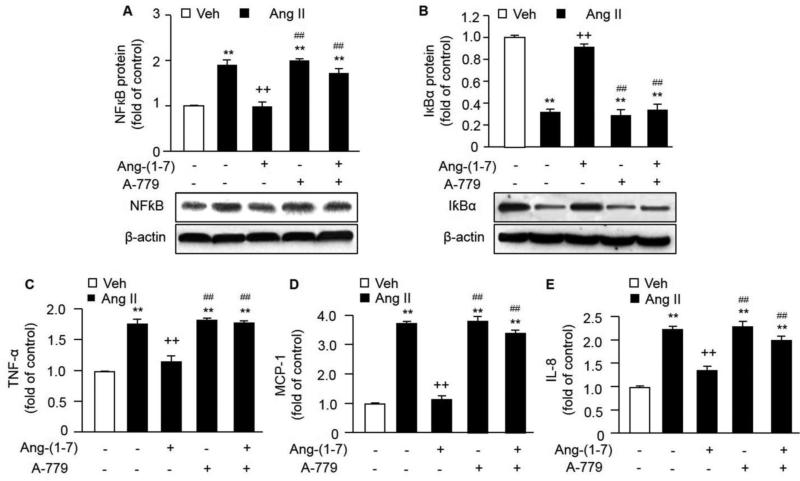
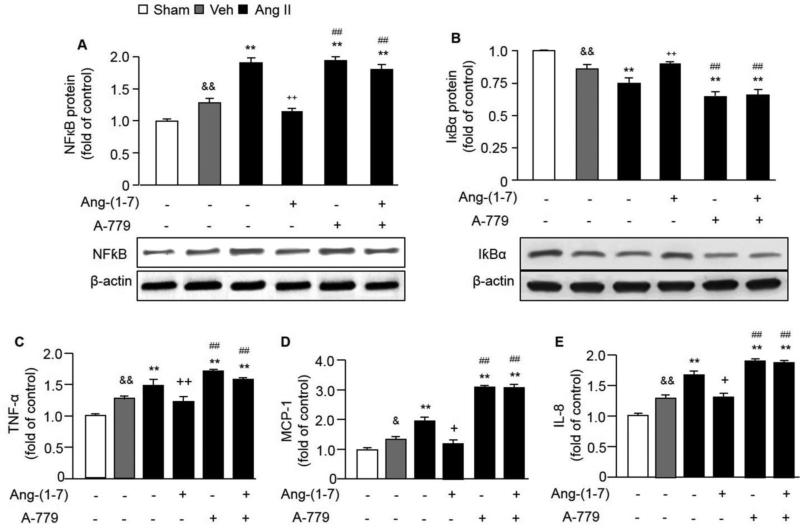
Ang II up-regulated the protein expression of NFκB by about 200% and down-regulated the expression of IκBα by 70% in HBVSMCs. Ang-(1-7) co-incubation with Ang II decreased NFκB expression by 50% and increased IκBα expression by about 300% in HBVSMCs when compared with Ang II incubation group. A-779 blocked those effects of Ang-(1-7) on HBVSMCs (P<0.01; Figure 3 A and B). There were no significant differences between Ang II and Ang II + A-779 groups in NFκB and IκBα expressions in HBVSMCs.
Fig. 3. The effects of Ang-(1-7) on regulating gene expressions and cytokines release of HBVSMCs.
Ang II induced the up-regulation of NFκB (A) and down-regulation of IκBα (B) in HBVSMCs. In addition, Ang II induced the release of TNF-α (C), MCP-1 (D) and IL-8 (E) from HBVSMCs. Ang-(1-7) counteracted these effects of Ang II, which could be aborted by A-779. **P< 0.01 vs. veh; ++P< 0.01 vs. Ang II, ##P< 0.01 vs. Ang II + Ang-(1-7), n=6/group. TNF-α: tumor necrosis factor; MCP-1: monocyte chemoattractant protein 1; IL-8: interleukin-8.
3.4. Ang-(1-7) Decreases Ang II-induced Secretion of Inflammatory Cytokines from HBVSMCs
Ang II increased the release of proinflammatory cytokines (TNF-α, MCP-1 and IL-8) from HBVSMCs. Ang-(1-7) co-incubation with Ang II decreased the levels of TNF-α, MCP-1 and IL-8 in the HBVSMC culture condition medium (P<0.01; Figure 3 D-E) compared with Ang II incubation group. This effect can be abolished by Ang-(1-7) antagonist (A-779) (P<0.01). There were no significant differences between Ang II and Ang II + A-779 groups in inflammatory cytokine levels in HBVSMCs.
3.5. Infusion of Ang-(1-7) Decreases Ang II-induced MCA Remodeling without Affecting MAP
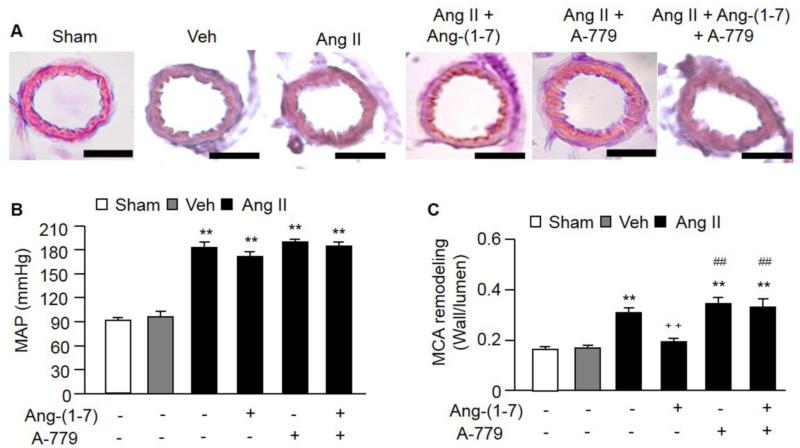
After minipump infusion, the Ang II and Ang-(1-7) levels were increased in the circulation (Table 1). Ang II infusion increased the MAP (P<0.01; Figure 4A), while infusion of Ang-(1-7) did not affect the level of MAP. Moreover, infusion of Ang II increased the MCA remodeling by 50% compared to the vehicle group (P<0.01; Figure 4B and C). Infusion of Ang-(1-7) attenuated the MCA remodeling induced by Ang II (P<0.01; Figure 4C). Infusion of A-779 totally blocked the effect of Ang-(1-7) (P<0.01), which further demonstrated the protective effect of Ang-(1-7) on MCA remodeling. These data suggest that Ang-(1-7) has a protective effect on vascular remodeling by counteracting the effect of Ang II via a BP-independent manner. There was no significant difference between Ang II and Ang II + A-779 groups in MAP.
Table 1.
Plasma levels of Ang II and Ang-(1-7) in different infusion groups
| Groups | Ang II (pg/ml) | Ang-(1-7) (pg/ml) |
|---|---|---|
| Sham | 54 ± 23 | 64 ± 18 |
| vehicle | 66 ± 11 | 84 ± 9 |
| Ang II | 185 ± 33** | 118 ± 11** |
| Ang II + Ang-(1-7) | 165 ± 39** | 189 ± 33**++ |
| Ang II + Ang-(1-7) + A-779 | 172 ± 26** | 185 ± 24**++ |
Data are means ± SE.
P< 0.01, vs. vehicle
P< 0.01 vs. Ang II, n=20/group.
Ang II: Angiotensin II, Ang-(1-7): Angiotensin-(1-7).
Fig. 4. The effects of Ang-(1-7) on MAP and MCA remodeling.
A, Representative pictures of HE staining for MCA remodeling. Scale bars: 50 μm. B, Ang II infusion increased MAP. Ang-(1-7) infusion had no effect on MAP. C, Ang II infusion induced MCA remodeling in mice, while Ang-(1-7) could alleviate Ang II-induced MCA remodeling. Mas receptor (A-779) could block the effects of Ang-(1-7). **P< 0.01 vs. veh; ++P< 0.01 vs. Ang II, ##P< 0.01 vs. Ang II + Ang-(1-7), n=10/group. MAP: mean arterial pressure; MCA: middle cerebral artery.
3.6. Infusion of Ang-(1-7) Decreases Ang II-enlarged Hemorrhage Volume and Improved Neurological Function after ICH
Figure 5A shows the representative images of hemorrhage volume in each group. Infusion of Ang II increased the hemorrhage volume when compared to vehicle group (P<0.01; Figure 5B). Moreover, Ang II infusion exacerbated the neurological function after ICH. Chronic infusion of Ang-(1-7) could decreased the enlarged hemorrhage volume and increased neurological deficit scores induced by Ang II (P<0.01). Infusion of A-779 abolished the effect of Ang-(1-7) (P<0.01), which further demonstrated the protective effect of Ang-(1-7) on brain injury and functional improvement in an ICH mouse model. These data suggest that Ang-(1-7) plays a role in protecting brain from hemorrhagic injury and improving neurological function by counteracting the effect of Ang II. There were no significant differences between Ang II and Ang II + A-779 groups in hemorrhage volume and neurological deficit scores, suggesting that the endogenous Ang-(1-7) did not have significant effects on enlarged hemorrhage volume and increased neurological deficit scores induced by Ang II.
Fig. 5. The effects of Ang-(1-7) on hemorrhage volume in ICH.
A, Representative pictures of hemorrhage volume in different treatment groups. Scale bars: 2 mm. B, Ang II infusion enlarged the hemorrhage volume, while Ang-(1-7) infusion decreased the hemorrhage volume. C, Neurological deficits were scored in ICH mice. Ang II infusion exaggerated the neurological deficits, while Ang-(1-7) infusion alleviated the neurological deficits. A-779 could abolish the protective effects of Ang-(1-7). &&P< 0.01 vs. sham; **P< 0.01 vs. veh; ++P< 0.01 vs. Ang II, ##P< 0.01 vs. Ang II + Ang-(1-7), n=10/group.
3.7. Infusion of Ang-(1-7) Inhibits Ang II-induced Activation of NFκB Pathway in Cerebral Microvessels
Ang II infusion increased the expression of NFκB and decreased the expression of IκBα in cerebral microvessels (P<0.01; Figure 6A and B). Infusion of Ang-(1-7) and Ang II decreased NFκB expression and increased IκBα expression when compared with Ang II infusion group (P<0.01). Infusion of A-779 abolished the effects of Ang-(1-7) on regulating NFκB and IκBα expressions (P<0.01), suggesting that Ang-(1-7) exerts counteractive effects to Ang II probably via regulating NFκB pathway. There were no significant differences between Ang II and Ang II + A-779 groups in NFκB and IκBα expressions in cerebral mirovessels.
Fig. 6. The effects of Ang-(1-7) on regulating gene expressions and cytokine levels in cerebral microvessels.
The protein expression of NFκB (A) in cerebral microvessels was up-regulated by Ang II infusion, while the expression of IκBα (B) was down-regulated. The levels of TNF-α (C), MCP-1 (D) and IL-8 (E) in the cerebral microvessels were increased by Ang II infusion. Ang-(1-7) blocked all these effects of Ang II. A-779 treatment reversed the effects of Ang-(1-7). &P< 0.05, &&P< 0.01 vs. sham; **P< 0.01 vs. veh; +P< 0.05, ++P< 0.01 vs. Ang II, ##P< 0.01 vs. Ang II + Ang-(1-7), n=10/group.
3.8. Infusion of Ang-(1-7) Decreases Ang II-induced Up-regulation of Inflammatory Cytokines in Cerebral Microvessels
The levels of inflammatory cytokines (TNF-α, MCP-1 and IL-8) in the cerebral microvessels were increased by Ang II infusion (P<0.01; Figure 6D-E). Infusion of Ang-(1-7) decreased the levels of TNF-α, MCP-1 and IL-8 which were increased by Ang II (P<0.01). Infusion of A-779 abolished the effects of Ang-(1-7) on regulating the inflammatory cytokine levels (P<0.01). There were no significant differences between Ang II and Ang II + A-779 groups in inflammatory cytokine levels in cerebral microvessels. These data suggest that Ang-(1-7) exerts counteractive effects to Ang II probably via regulating NFκB related inflammatory pathway.
4. Discussion
Primary ICH is caused by small arteriole ruptures which stem from vascular pathology changes [27]. Though hypertension is a usual inducer of vascular ruptures, vascular dysfunction is the key initiator for vascular pathology changes [28]. VSMCs are the main components of vascular parenchyma and maintain significant vascular plasticity [6, 7]. Emerging evidence demonstrates that VSMC function and phenotypic modulation play significant roles in cerebral aneurysm formation and rupture [8]. Therefore, protection of VSMCs should facilitate the prevention and treatment of ICH.
Ang II has been reported to induce aortic VSMC proliferation/migration and apoptosis [14, 19]. In the current study, we further demonstrated that Ang II induces human brain VSMC proliferation/migration and apoptosis. Previous studies also showed the inhibition of Ang II/AT1 pathway has protective effects on VSMCs [13, 14]. As the counteractive pathway of Ang II/AT1, Ang-(1-7)/Mas pathway has been reported to inhibit Ang II-induced mouse aortic VSMC proliferation and migration [29]. In the present study, we demonstrated the protective effects of Ang-(1-7) on human brain VSMCs by counteracting the effects of Ang II. Besides the proliferation and migration abilities, we also determined the protective effects of Ang-(1-7) on Ang II-induced HBVSMC apoptosis (Figure 2). We performed dose-response experiments to determine the effective dose of Ang-(1-7) in protecting HBVSMCs from Ang II-induced apoptosis, which are 10−7 M and 10−6 M. So we chose the lower concentration of Ang-(1-7) for the in vitro experiments. Moreover, we used Ang-(1-7)/Mas pathway inhibitor (A-779) to further confirm the protective effects of Ang-(1-7) pathway on HBVSMCs.
To further explore the protective effects of Ang-(1-7) on ICH, we conducted experiments to determine the counteractive effects of Ang-(1-7) to Ang II in a collagenase-induced ICH mouse model. Ang II and Ang-(1-7) infusions led to a significant increase in plasma Ang II and Ang-(1-7) levels, which indicate the success of minipump infusions (Table 1). Ang II infusion led to a significant increase in both plasma Ang II and Ang-(1-7) levels. We propose that increased Ang II level activates ACE2, which catalyzes Ang II into Ang-(1-7) and subsequetially increases the plasma Ang-(1-7) level. Vaibhav et al. showed that Ang II infusion in wild-type mice results in increased plasma ACE2 activity [30]. Moreover, the intracerebral hemorrhage itself might have an independent effects on the levels of RAS components. The Ang-(1-7)-Mas axis has been shown to undergo dynamic changes in ischemic stroke [31]. Levels of Ang II are increased after ischemic stroke in the ventral cortex [32]. In the present study, we have a sham group and we measured the Ang II/Ang-(1-7) levels in the sham group. Results (Table 1) showed that ICH itself did increase the Ang II and Ang-(1-7) levels, however there are no significant differences. The reason could be that the animal model we used is the collagenase-induced ICH model which is different from the ischemic stroke model used in previous studies [31, 32]. Also, the angiotensin levels we measured are the plasma levels, while the levels tested in previous reports are the local brain levels.
As expected, Ang II infusion significantly increased the MAP in C57BL/6 mice. However, the infusion of Ang-(1-7) had no effect on MAP (Figure 4). The lack of effect of Ang-(1-7) on MAP in the present study may be due to the dose and the duration of Ang-(1-7) used, and the animal model. The dose of Ang-(1-7) we used was 24 μg/kg/h which is based on a previous study showed that only a high dose (24 μg/kg/h) of Ang-(1-7) is able to attenuate the aortic lesions in ApoE−/− mice with chronic angiotensin II infusion and fed with high-fat diet [19]. They also reported that the chronic infusion (4 weeks) of Ang-(1-7) had no effects on BP in an atherosclerosis mouse model, which is the same infusion duration as our study. Therefore, the effects of Ang-(1-7) on BP appear to be more dependent on the model of hypertension than on the dose of Ang-(1-7) used. Recent studies found that Ang-(1-7) lowered BP only when it was used in normotensive Sprague-Dawley rats treated with losartan [33], in spontaneous hypertensive rats (SHR) treated with the nitric oxide synthase inhibitor N-nitro-L-arginine methyl ester [34], and in Wistar-Kyoto rats and SHR treated with the AT1 receptor antagonist candesartan [35].
Ang II has been reported to induce vascular injury [10] and cardiac remodeling [36]. In the present study, we demonstrated the first time that chronic infusion of Ang II could induce MCA remodeling, enlarge hemorrhage volume and exaggerate neurological deficits in ICH. Interestingly, we also found that infusion of Ang-(1-7) decreases Ang II-induced MCA remodeling (Figure 4). This provides new information to previous studies showing that Ang-(1-7) attenuates neointimal formation in abdominal aorta after angioplasty [37] or stent implantation [38] in rats. A previous study showed that Ang-(1-7) treatment is able to counteract Ang II-induced atherosclerosis by targeting VSMCs [19]. Our study indicates that Ang-(1-7) decreases Ang II-induced MCA remodeling probably by targeting brain VSMCs, because Ang-(1-7) could decrease Ang II-induced brain VSMC proliferation and migration which are important processes in the formation of atherosclerotic plaques and in the development of vascular remodeling [39, 40]. More recently, a paper reported that Ang-(1-7) reduces mortality and rupture of intracranial aneurysms in mice [41]. In the present study, we further demonstrated that Ang-(1-7) decreases hemorrhage volume and NDS in an ICH mouse model by counteracting the effects of Ang II (Figure 5). Taken together, we demonstrated that infusion of Ang-(1-7) can counteract the detrimental effects of Ang II by attenuating the MCA remodeling and hemorrhage volume. It is the first time we show the protective effects of Ang-(1-7) on small cerebral vessels and ICH. Since the infusion of Ang-(1-7) did not change the BP, we propose that there is a BP-independent mechanism underlying the protective effects of Ang-(1-7) on vascular remodeling and ICH.
Vascular inflammation, as well as some cytokine stimulation could affect the function of VSMCs [42] and has been suggested as a major mechanism involved in cerebral aneurysm formation and rupture and subsequential ICH [11]. Extensive researches have been reported the role of Ang II in promoting inflammation by up-regulating various proinflammatory signals, such as TNF-α, NFκB, and MCP-1 in VSMCs [12, 43, 44]. A previous study showed that NFκB activation is a mechanism for Ang II-induced proliferation and migration of VSMCs [44]. Our findings provide further information that Ang II activates the NFκB inflammatory pathway by up-regulating NFκB expression and down-regulating IκBα expression in both HBVSMCs and cerebral microvessels. IκBα is the inhibitor of degradation of the cytosolic NFκB. Activation of the NFκB is initiated by the signal-induced degradation of IκB proteins. Interestingly, Ang-(1-7) decreased the up-regulation of NFκB and increased the down-regulation of IκBα in both HBVSMCs and cerebral microvessels (Figure 3 and 6). Ang II-induced proinflammatory cytokine secretion is also could be the mechanism for vascular inflammation and injury, which includes TNF-α, MCP-1, IL-8 and IL-1β [45, 46]. Our research provides more evidence by showing that Ang II increases the release of TNF-α, MCP-1, IL-8 from HBVSMCs and increases their levels in cerebral microvessels. More importantly, co-incubation or infusion of Ang-(1-7) decreased the inflammatory cytokine levels. This is consistent with a previous study showed that Ang-(1-7) induced an attenuation of the NFκB pathway in renal tissue in SHRSP together with a down-regulation of the expression of several proinflammatory cytokine genes, including IL-6 and TNF-a [47]. The mechanisms for the counteractive beneficial actions of Ang-(1-7) probably include inhibiting Ang II-stimulated NFκB signaling pathways through kinase inhibition and a potential inhibitory effect on the signaling cascades leading to the synthesis/production of inflammatory cytokines. In addition, role of Ang-(1-7) in activating phosphatases was reported in rat primary astrocytes subjected to radiation-induced inflammation [48].
Moreover, the effect of endogenous Ang-(1-7) was investigated by including Ang II + A-779 group in in vitro and in vivo. The results suggest that endogenous Ang-(1-7) might have a biologically relevant effect, however, there are no significant differences found among Ang II, Ang II + A-779 and Ang II + Ang-(1-7) + A-779 groups in our study. The reason might be the model we used. Since the main objective of the present study is to determine the protective role of Ang-(1-7) in ICH by counteracting the effects of Ang II, we used the Ang II incubation to induce HBVSMC dysfunction and apoptosis, and Ang II infusion to induce hypertension and high level of plasma Ang II in animal model. The effects of infused high dose of Ang II and Ang-(1-7) might cover up the effects of endogenous Ang-(1-7).
In summary, Ang-(1-7) protects VSMCs from Ang II-induced activation and apoptosis by inhibiting the NFκB inflammatory pathway. In a mouse model of ICH, Ang-(1-7) alleviates MCA remodeling and brain injury by counteracting the effects of Ang II via inhibiting the NFκB inflammatory pathway in cerebral microvessels, independent of BP. Our data suggest that Ang-(1-7) may be protective in vascular remodeling and ICH.
Acknowledgements
The author would like to thank Dr. Gengxin Li (Assistant Professor, Mathematics & Statistics Department, Wright State University) for her help in animal sample size analysis and statistical analysis.
Grant supports
This work was supported by the National Heart, Lung, and Blood Institute (HL-098637, Y.C.), American Heart Association (13POST14780018, J.B.) and the National Natural Science Foundation of China (NSFC, #81300079).
Abbreviations
- Ang-(1-7)
Angiotensin-(1-7)
- Ang II
Angiotensin II
- RAS
renin angiotensin system
- ACE2
angiotensin converting enzyme 2
- BP
blood pressure
- VSMCs
vascular smooth muscle cells
- HBVSMCs
human brain vascular smooth muscle cells
- ICH
intracerebral hemorrhagic stroke
- SHRSP
stroke-prone spontaneously hypertensive
- NDS
Neurological deficit score
- MTT
methyl thiazolyltetrazolium
- MCA
middle cerebral artery
- MAP
mean arterial pressure
- TNF-α
tumor necrosis factor α
- MCP-1
monocyte chemoattractant protein 1
- IL-8
interleukin-8
- NFκB
nuclear factor-KappaB
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
We declare that none of the authors has any kind of conflict of interest related to the present work.
References
- 1.Guo YJ, Li WH, Wu R, Xie Q, Cui LQ. ACE2 overexpression inhibits angiotensin II-induced monocyte chemoattractant protein-1 expression in macrophages. Arch Med Res. 2008 Feb;39(2):149–54. doi: 10.1016/j.arcmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Regenhardt RW, Bennion DM, Sumners C. Cerebroprotective action of angiotensin peptides in stroke. Clin Sci (Lond) 2014 Feb;126(3):195–205. doi: 10.1042/CS20130324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mecca AP, Regenhardt RW, O'Connor TE, Joseph JP, Raizada MK, Katovich MJ, et al. Cerebroprotection by angiotensin-(1-7) in endothelin-1-induced ischaemic stroke. Exp Physiol. 2011 Oct;96(10):1084–96. doi: 10.1113/expphysiol.2011.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regenhardt RW, Mecca AP, Desland F, Ritucci-Chinni PF, Ludin JA, Greenstein D, et al. Centrally administered angiotensin-(1-7) increases the survival of stroke-prone spontaneously hypertensive rats. Exp Physiol. 2014 Feb;99(2):442–53. doi: 10.1113/expphysiol.2013.075242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, et al. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology. 2014 Apr;79:550–8. doi: 10.1016/j.neuropharm.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S, Song S, Lee J, Yoon J, Park J, Choi S, et al. Phenotypic modulation of primary vascular smooth muscle cells by short-term culture on micropatterned substrate. PLoS One. 2014;9(2):e88089. doi: 10.1371/journal.pone.0088089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudijanto A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med Indones. 2007 Apr;39(2):86–93. [PubMed] [Google Scholar]
- 8.Starke RM, Chalouhi N, Ding D, Raper DM, Mckisic MS, Owens GK, et al. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res. 2014 Jun;5(3):338–46. doi: 10.1007/s12975-013-0290-1. [DOI] [PubMed] [Google Scholar]
- 9.Lim S, Park S. Role of vascular smooth muscle cell in the inflammation of atherosclerosis. BMB Rep. 2014 Jan;47(1):1–7. doi: 10.5483/BMBRep.2014.47.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugherty A, Cassis L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc Med. 2004 Apr;14(3):117–20. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, et al. Tumor necrosis factor-alpha modulates cerebral aneurysm formation and rupture. Transl Stroke Res. 2014 Apr;5(2):269–77. doi: 10.1007/s12975-013-0287-9. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt SR, Lokhandwala MF, Banday AA. Vascular oxidative stress upregulates angiotensin II type I receptors via mechanisms involving nuclear factor kappa B. Clin Exp Hypertens. 2014;36(6):367–73. doi: 10.3109/10641963.2014.943402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colie S, Pecher C, Girolami JP, Blaes N. Modulation by bradykinin and nitric oxide of angiotensin II-induced apoptosis in a vascular smooth muscle cell phenotype. Int Immunopharmacol. 2008 Feb;8(2):231–6. doi: 10.1016/j.intimp.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz E, Redondo S, Padilla E, Gordillo-Moscoso A, Salaices M, Balfagon G, et al. Importance of intracellular angiotensin II in vascular smooth muscle cell apoptosis: inhibition by the angiotensin AT1 receptor antagonist irbesartan. Eur J Pharmacol. 2007 Jul 19;567(3):231–9. doi: 10.1016/j.ejphar.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Gu S, Zhang W, Chen J, Ma R, Xiao X, Ma X, et al. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS One. 2014;9(1):e85396. doi: 10.1371/journal.pone.0085396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarajapu YP, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, et al. Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes. 2013 Apr;62(4):1258–69. doi: 10.2337/db12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaghini FA, Song CY, Lavrentyev EN, Ghafoor HU, Fang XR, Estes AM, et al. Angiotensin II-induced vascular smooth muscle cell migration and growth are mediated by cytochrome P450 1B1-dependent superoxide generation. Hypertension. 2010 Jun;55(6):1461–7. doi: 10.1161/HYPERTENSIONAHA.110.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Costa Goncalves AC, Fontes MA, Klussmann E, Qadri F, Janke J, Gollasch M, et al. Spinophilin regulates central angiotensin II-mediated effect on blood pressure. J Mol Med (Berl) 2011 Dec;89(12):1219–29. doi: 10.1007/s00109-011-0793-8. [DOI] [PubMed] [Google Scholar]
- 19.Yang JM, Dong M, Meng X, Zhao YX, Yang XY, Liu XL, et al. Angiotensin-(1-7) dose-dependently inhibits atherosclerotic lesion formation and enhances plaque stability by targeting vascular cells. Arterioscler Thromb Vasc Biol. 2013 Aug;33(8):1978–85. doi: 10.1161/ATVBAHA.113.301320. [DOI] [PubMed] [Google Scholar]
- 20.Chang CF, Chen SF, Lee TS, Lee HF, Chen SF, Shyue SK. Caveolin-1 deletion reduces early brain injury after experimental intracerebral hemorrhage. Am J Pathol. 2011 Apr;178(4):1749–61. doi: 10.1016/j.ajpath.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Tsirka SE. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain. 2005 Jul;128(Pt 7):1622–33. doi: 10.1093/brain/awh489. [DOI] [PubMed] [Google Scholar]
- 22.Clark W, Gunion-Rinker L, Lessov N, Hazel K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 1998 Oct;29(10):2136–40. doi: 10.1161/01.str.29.10.2136. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS, Wang J. Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One. 2014;9(5):e97423. doi: 10.1371/journal.pone.0097423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Chen S, Chen Y, Zhang C, Wang J, Zhang W, et al. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E62–E71. doi: 10.1152/ajpendo.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquez-Martin A, Jimenez-Altayo F, Dantas AP, Caracuel L, Planas AM, Vila E. Middle cerebral artery alterations in a rat chronic hypoperfusion model. J Appl Physiol. 2012 Feb;112(3):511–8. doi: 10.1152/japplphysiol.00998.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foerch C, Arai K, Jin G, Park KP, Pallast S, van LK, et al. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008 Dec;39(12):3397–404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elijovich L, Patel PV, Hemphill JC., III. Intracerebral hemorrhage. Semin Neurol. 2008 Nov;28(5):657–67. doi: 10.1055/s-0028-1105974. [DOI] [PubMed] [Google Scholar]
- 28.Miller AA, Budzyn K, Sobey CG. Vascular dysfunction in cerebrovascular disease: mechanisms and therapeutic intervention. Clin Sci (Lond) 2010 Jul;119(1):1–17. doi: 10.1042/CS20090649. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Hu Y, Xu Q, Ye S. Different effects of angiotensin II and angiotensin-(1-7) on vascular smooth muscle cell proliferation and migration. PLoS One. 2010;5(8):e12323. doi: 10.1371/journal.pone.0012323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel VB, Clarke N, Wang Z, Fan D, Parajuli N, Basu R, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014 Jan;66:167–76. doi: 10.1016/j.yjmcc.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Lu J, Jiang T, Wu L, Gao L, Wang Y, Zhou F, et al. The expression of angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas receptor axis are upregulated after acute cerebral ischemic stroke in rats. Neuropeptides. 2013 Oct;47(5):289–95. doi: 10.1016/j.npep.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Kagiyama T, Kagiyama S, Phillips MI. Expression of angiotensin type 1 and 2 receptors in brain after transient middle cerebral artery occlusion in rats. Regul Pept. 2003 Feb 28;110(3):241–7. doi: 10.1016/s0167-0115(02)00223-9. [DOI] [PubMed] [Google Scholar]
- 33.Collister JP, Hendel MD. The role of Ang (1-7) in mediating the chronic hypotensive effects of losartan in normal rats. J Renin Angiotensin Aldosterone Syst. 2003 Sep;4(3):176–9. doi: 10.3317/jraas.2003.028. [DOI] [PubMed] [Google Scholar]
- 34.Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006 Feb;290(2):H684–H691. doi: 10.1152/ajpheart.00632.2005. [DOI] [PubMed] [Google Scholar]
- 35.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1-7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005 May;45(5):960–6. doi: 10.1161/01.HYP.0000160325.59323.b8. [DOI] [PubMed] [Google Scholar]
- 36.Grobe JL, Mecca AP, Lingis M, Shenoy V, Bolton TA, Machado JM, et al. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1-7). Am J Physiol Heart Circ Physiol. 2007 Feb;292(2):H736–H742. doi: 10.1152/ajpheart.00937.2006. [DOI] [PubMed] [Google Scholar]
- 37.Zeng W, Chen W, Leng X, He JG, Ma H. Chronic angiotensin-(1-7) administration improves vascular remodeling after angioplasty through the regulation of the TGF-beta/Smad signaling pathway in rabbits. Biochem Biophys Res Commun. 2009 Nov 6;389(1):138–44. doi: 10.1016/j.bbrc.2009.08.112. [DOI] [PubMed] [Google Scholar]
- 38.Langeveld B, van Gilst WH, Tio RA, Zijlstra F, Roks AJ. Angiotensin-(1-7) attenuates neointimal formation after stent implantation in the rat. Hypertension. 2005 Jan;45(1):138–41. doi: 10.1161/01.HYP.0000149382.83973.c2. [DOI] [PubMed] [Google Scholar]
- 39.Lusis AJ. Atherosclerosis. Nature. 2000 Sep 14;407(6801):233–41. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenig JB, Jaffe IZ. Direct role for smooth muscle cell mineralocorticoid receptors in vascular remodeling: novel mechanisms and clinical implications. Curr Hypertens Rep. 2014 May;16(5):427. doi: 10.1007/s11906-014-0427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pena Silva RA, Kung DK, Mitchell IJ, Alenina N, Bader M, Santos RA, et al. Angiotensin 1-7 reduces mortality and rupture of intracranial aneurysms in mice. Hypertension. 2014 Aug;64(2):362–8. doi: 10.1161/HYPERTENSIONAHA.114.03415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen S, Ding Y, Tao W, Zhang W, Liang T, Liu C. Naringenin inhibits TNF-alpha induced VSMC proliferation and migration via induction of HO-1. Food Chem Toxicol. 2012 Sep;50(9):3025–31. doi: 10.1016/j.fct.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Lee D, Lee KH, Park H, Kim SH, Jin T, Cho S, et al. The effect of soluble RAGE on inhibition of angiotensin II-mediated atherosclerosis in apolipoprotein E deficient mice. PLoS One. 2013;8(8):e69669. doi: 10.1371/journal.pone.0069669. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Zahradka P, Werner JP, Buhay S, Litchie B, Helwer G, Thomas S. NF-kappaB activation is essential for angiotensin II-dependent proliferation and migration of vascular smooth muscle cells. J Mol Cell Cardiol. 2002 Dec;34(12):1609–21. doi: 10.1006/jmcc.2002.2111. [DOI] [PubMed] [Google Scholar]
- 45.Ebrahimian T, Li MW, Lemarie CA, Simeone SM, Pagano PJ, Gaestel M, et al. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension: regulation of oxidative stress. Hypertension. 2011 Feb;57(2):245–54. doi: 10.1161/HYPERTENSIONAHA.110.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011 Dec 1;92(3):401–8. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giani JF, Munoz MC, Pons RA, Cao G, Toblli JE, Turyn D, et al. Angiotensin-(1-7) reduces proteinuria and diminishes structural damage in renal tissue of stroke-prone spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2011 Jan;300(1):F272–F282. doi: 10.1152/ajprenal.00278.2010. [DOI] [PubMed] [Google Scholar]
- 48.Moore ED, Kooshki M, Metheny-Barlow LJ, Gallagher PE, Robbins ME. Angiotensin-(1-7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP kinase signaling. Free Radic Biol Med. 2013 Dec;65:1060–8. doi: 10.1016/j.freeradbiomed.2013.08.183. [DOI] [PMC free article] [PubMed] [Google Scholar]