Abstract
The stagnant state of drug development for Alzheimer’s disease demands new approaches for seeking promising candidates. In this report, we reasoned that non-covalent modification of amyloid beta (Aβ) peptide by crown ethers could inhibit its aggregation. To specifically target Aβs, a conjugate of Pittsburgh compound B (PiB) and 12-crown-4 ether (termed PiB-C) was synthesized. Our results indicated that the conjugate could significantly reduce the aggregation of Aβs in vitro. In addition, by two-photon microscopic imaging, we found that PiB-C could readily penetrate the BBB and efficiently label Aβ plaques and CAAs (cerebral amyloid angiophathy) in an APP-PS1 transgenic mouse.
No cure is currently available for Alzheimer’s disease (AD). Reducing amyloid beta (Aβ) loading is one of the main goals for AD drug development [1]. In past decades, several categories of Aβ-reducing agents have been developed, and some of them had advanced into clinical trials. These categories included aggregating inhibitors such as tramiprosate, humanized monoclonal antibodies such as bapineuzumab, β-secretase inhibitors LY2811376 and the γ-secretase inhibitor Flurizan. Unfortunately, these clinical trials, by and large, failed to demonstrate their efficacy and safety [1b, 2].
All these failures clearly imply that new strategies for developing drugs for AD are urgently needed. In this report, we propose a new strategy to attenuate the aggregation of Aβs through a non-covalent modification at its surface. We reasoned that crown ethers could be used to “neutralize” positive charges of the amino groups of Aβs through the formation of hydrogen bonds. To specifically target Aβs with the purpose of reducing aggregation, we proposed a conjugate (PiB-C) of a 12-crown-4 ether and PiB (Pittsburg Compound B, the widely used PET ligand for imaging Aβs [3]).
Crown ethers are well known for their capability to form complexes with alkali ions such as K+, Na+ and Li+. Apart from their high affinities for alkali ions, crown ethers also could form stable complexes with protonated amines through hydrogen bonds, a property that has been adapted for various applications of crown ethers. Beauchamp et al, Julian et al., and others developed the SNAPP (selective non-covalent adduct protein probing) technique using crown ether to probe protein sequences, which is based on the selectivity of crown ethers towards basic amino acids such as lysine, arginine, and histidine [4]. Using theoretical electronic structure calculations, Chen et al. recently demonstrated that crown ethers could form non-covalent complexes with lysine, arginine and histidine [5]. Clemmer et al. used crown ethers as shift reagents for ion mobility spectrometry [6]. Moreover, molecular recognition capacity of crown ethers has recently been utilized for the investigation of the protein-folding process [4a, 7]. We believe that non-covalent alteration of the folding property of the aggregation-prone protein/peptide could be a new approach for designing new anti-aggregation drugs.
Under physiological pH, basic amino acids of Aβ are partially positively charged. Yoshiike et al. reported that positively charged Aβ fibrils/profibrils are highly cytotoxic, and neutralization of the charges could reduce neuronal toxicity [8]. Moreover, covalent modification of the charged amino groups of Aβ peptides through glycation and acylation could significantly reduce cytotoxicity of Aβ species [8a]. In addition, Yang et al. showed that surface coating of Aβs with open-chain conjugates of polyether-thioflavin analogues could reduce the adhesion of Aβs towards Anti-Aβ IgG, and could ameliorate dendritic spine density and improve cognitive function in an AD mouse model [9].
Studies suggested that K16 and K28 are crucial amino acids for the mis-folding of Aβs, because K16 could form inter-sheet salt bridges and K28 can form intra-peptide salt bridges with Asparate23 (D23) leading to the stabilization of the misfolded peptides [10]. In this report, we hypothesized that crown ethers can form non-covalent complexes with Aβ peptides through the formation of hydrogen bonds with positively charged basic amino acids such as R5 (arginine-5), K16, K28 (lysine-16, 28), and H13, H14 (histidine-13, 14). We reasoned that crown ether has the capability to break down the salt bridge, and thus to attenuate the aggregation process of Aβs.
12-crown-4 ether was chosen over other crown-ethers in our studies, due to the following reasons: 1) it has insignificant disturbance of the homeostasis of the physiological ions such as K+, and Na+ [11]; 2) its low molecular weight can be beneficial for BBB penetration; 3) it can effectively form complexes with charged amino acids [6]. In our preliminary studies we used a non-conjugated 12-crown-4 ether to demonstrate its anti-aggregating properties in Aβ solution.
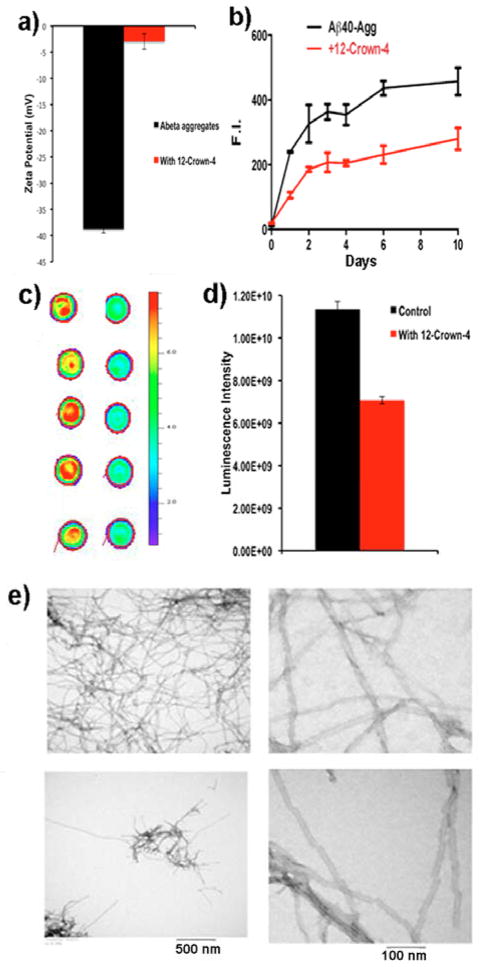
We first used zeta-potential measurement to investigate whether 12-crown-4 ether can non-covalently change the surface charges of Aβs. Zeta potential has been used to evaluate the potential change between the double layer and the sliding plane of particles, and has also been used for studying interactions between amyloid fibrils and its ligands [8b, 12]. Our zeta-potential test suggested that 12-crown-4 ether could change the surface charges of Aβs. Zeta potential of Aβ40 fibrils/aggregates was significantly changed once it was mixed with 12-crown-4 ether (from −48 mV to −4 mV), suggesting that the crown ether could modify the surface charges of Aβs (Fig. 1a).
Fig. 1.
Anti-aggregation testing of 12-crown-4 ether for Aβ40 in solutions. (a) Zeta potential measurements of Aβ40 aggregates with and without 12-crown-4 ether (n=3). (b) Change in fluorescence intensities of ThioT as a measurement of Aβ40 aggregation at different time points with and without 12-crown-4 ether (n=3). (c) Dot blotting of Aβ40 incubated without (left) and with (right) 12-crown-4 ether for 48-hours (n=5). (d) Quantitative analysis of the dots in (c). (e) Representative TEM images of Aβ40 without (upper) and with (bottom) 12-crown-4 ether on day10. Three images are shown with different scale bars.
Next, we investigated whether 12-crown-4 ether could inhibit the aggregation Aβ40 by incubating Aβ40 with 12-crown-4 ether in PBS (pH 7.4) for 10 days. Thioflavin T (ThioT) was used for monitoring the aggregation of Aβ40. We found that fluorescence intensities of the 12-crown-4-treated group were significantly (50%) lower than that of the control group (Fig. 1b). From TEM images, fewer Aβ deposits could be found in the samples treated with 12-crown-4 ether (Fig. 1e). Interestingly, we observed a different morphology of periodic twists in these images. Specifically, the twists of the Aβ fibrils were apparently rougher in the presence of 12-crown-4 ether than without it (Fig. 1e right panel). These data most likely suggest that 12-crown-4 ether could affect the aggregation process. It has been reported that different morphology of fibrillar Aβs could have different toxicity, and more twisted Aβs fibrils may have higher toxicity [13]. To further validate the effectiveness of 12-crown-4 ether, we used a dot blot for measuring the amount of profibrils/fibrils in solution with Aβ antibody 2H4, which is more specific for fibrillar/profibrillar Aβs than for monomers/oligomers [14]. Compared to the signal from the control group, a significantly lower signal (62%) was observed for the 12-crown-4 ether group after 48 hours of incubation (Fig. 1c, d), suggesting that it could attenuate the formation of profibrils/fibrils.
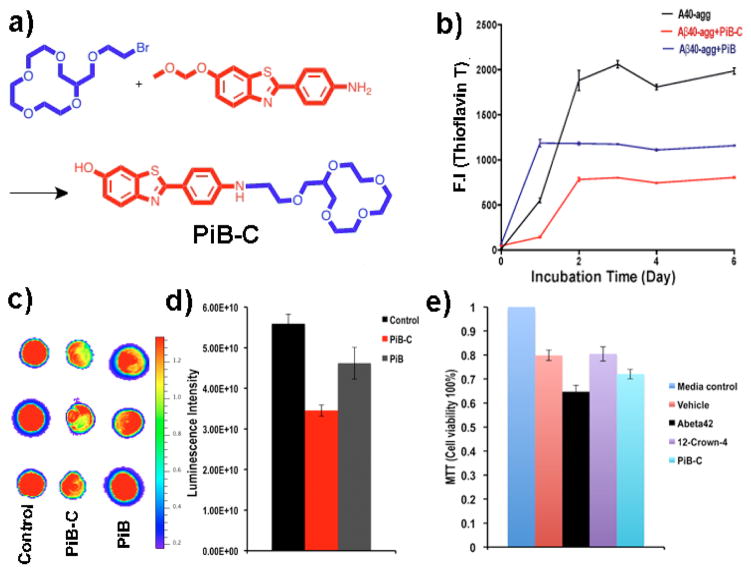
The above studies indicated that 12-crown-4 ether could efficiently inhibit the aggregation of Aβs; however this compound is not Aβ specific. For targeting, we used a well-studied Aβ ligand PiB, which is specific to Aβs, particularly to fibrillar Aβs [3]. A conjugate PiB-C was synthesized by attaching the 12-crown-4 ether moiety to the amino group of PiB (Fig. 2a). The synthesized PiB-C showed a similar fluorescence spectrum as PiB. However, once mixed with fibrillar Aβ40, it displayed a significant emission wavelength red-shift, which probably reflected polarization that was induced by the interaction of 12-crown-4 ether with the positively charged amino groups of Aβs (SI Fig. 1a).
Fig. 2.
Anti-aggregating studies with PiB-C. (a) The synthetic route for PiB-C. (b) Change in fluorescence intensities of ThioT as a measure of Aβ40 aggregation at different time points without (black line) and with PiB-C (red line), and with PiB (blue line) (n=3). (c) Dot blotting of Aβ40 incubated without (left) and with PiB-C (middle), and PiB (right) for 48-hours (n=3). (d) Quantitative analysis of the dots in (c). (e) MTT Testing of cell viability under different treatment conditions.
Like with 12-crown-4 ether, we found that PiB-C could change the zeta potential of Aβ fibrils (SI Fig. 1b). Before testing the anti-aggregating ability of PiB-C, we first examined the fluorescence contribution from PiB-C under the ThioT testing condition. We found that the contribution was minimal (data not shown), because the used 450nm excitation was suitable for ThioT, but not for PiB-C. To investigate the efficiency of PiB-C, we incubated PiB-C with Aβ40 in PBS for 6 days. Both the ThioT testing and dot blotting indicated that PiB-C could inhibit the aggregation (Fig. 2b–d). At day 6, F(ThioT) of the control samples was 2.47-fold higher than that of the PiB-C-treated samples (Fig. 2b). Dot blot with 2H4 antibody showed that the signal from the control group was 1.62-fold higher than that from the PiB-C group (Fig. 2c, d). Moreover, TEM images from the PiB-C-treated samples showed fewer fibrillar Aβ deposits than that from the control samples. We also observed that the detailed morphology of the fibrils was different, and the fibrils from PiB-C samples were less twisted (SI Fig. 2). We also used non-conjugated PiB as a control. Although PiB showed apparent aggregation inhibitory effects from ThioT testing and dot blotting, it was obviously weaker than PiB-C (Fig. 2b, c, d), indicating that the crown-ether moiety of PiB-C contributes to the inhibitory effects.
To examine whether 12-crown-4 ether and PiB-C treatment can reduce Aβ toxicity, we treated SH-SY5Y neuronal cells with Aβ42 (20μM) for 4 hours in the absence or presence of 12-crown-4 ether and PiB-C (40μM). The cells were treated under five different conditions, including 1) pure cell culture medium, 2) the vehicle that was consisted of 1% DMSO and 9% PBS and 90% cell culture medium, 3) Aβ42 and the vehicle, 4) 12-crown-4 ether, Aβ42 and the vehicle, and 5) PiB-C, Aβ42 and the vehicle. The cell viability from the pure culture medium treated group was considered to be 100%. Our results indicated that both 12-crown-4 ether (purple bar) and PiB-C (cyan bar) could lower the toxicity of Aβ42 (black bar) (Fig. 2e).
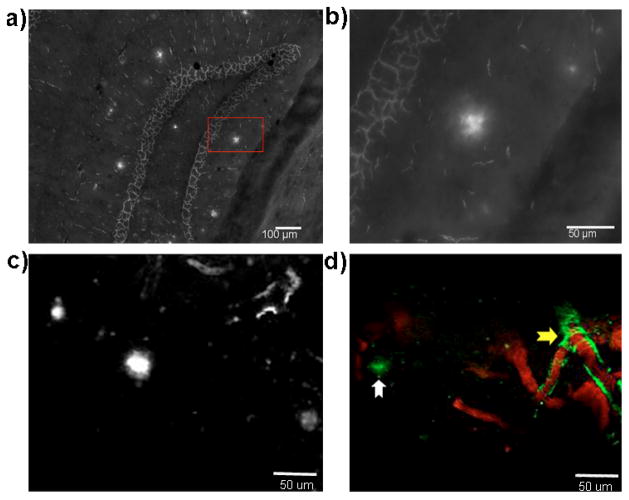
To investigate whether PiB-C could specifically interact with Aβs in a biologically relevant environment, we first incubated PiB-C with a mouse AD brain slice with characteristic Aβ plaques. As expected, PiB-C could specifically label the plaques (Fig. 3a, b). To further investigate whether PiB-C could potentially be used for in vivo studies, we first tested its BBB penetration in a wild type mouse using two-photon imaging. Our results indicated that PiB-C, like PiB, could efficiently cross BBB (SI Fig. 3). PiB-C reached its maximum in brain around 10 minutes after i.v. injection, and then slowly washed out. To further test whether PiB-C could specifically interact with Aβ plaques in vivo, we used two-photon imaging to validate. As expected, PiB-C can clearly highlight the plaques (Fig. 3c, d). Similar to PiB [15], PiB-C could also efficiently label CAAs (cerebral amyloid angiophathy). Taken together, all these data indicated that PiB-C could be used for future in vivo therapeutic studies.
Fig. 3.
Microscopic imaging of amyloid deposits with PiB-C in vitro and in vivo. (a–b) Microscopic images of PiB-C showing stained plaques in a hippocampus area of a brain slice from a 10-month APP-PS1 mouse. (b) Zoomed-in image of a plaque in the red box in (a). (c–d) Representative two-photon images of PiB-C in a 12-month old APP-PS1 mouse. (c) Aβ plaques were highlighted by PiB-C; (d) both CAAs (yellow arrow) and plaques (white arrow) were highlighted by PiB-C (green). The blood vessels were labeled with Texas red-dextran conjugates (MW 70,000) (red).
In summary, in this report we propose a novel approach to inhibit the aggregation of Aβs through neutralizing positive charges on amino acids of Aβs. Our approach is different from traditional approaches that include high throughput screening and other organic dye–based structure modification. Introduction of 12-crown-4 ether would not only neutralize positive charge, but could also change the hydrophobicity of the Aβ surface, which probably also contributed to the efficiency of 12-crown-4 ether. One of the limitations of PiB is its binding to white matter in the brain [16]. PiB-C may increase its specificity towards Aβs over white matter, because PiB-C has much higher hydropholicity due to the crown ether moiety. In addition, PiB-C could be considered as a theranostic agent, because it could be potentially used for both therapy and imaging. Further in vivo studies in mice are currently undergoing in our research group.
Supplementary Material
Acknowledgments
This work was supported by K25AG036760 award to C.R. The authors would also like to thank Pamela Pantazopoulos, B.S. for proofreading this manuscript.
Footnotes
Electronic Supplementary Information (ESI) available: [synthesis and detailed experimental procedures]. See DOI: 10.1039/c000000x/
References
- 1.a) Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]; b) Selkoe DJ. Nat Med. 2011;17:1060–1065. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 2.a) Haas C. J Alzh Dis. 2012;28:241–281. doi: 10.3233/JAD-2011-110986. [DOI] [PubMed] [Google Scholar]; b) Salloway S. CNS spectrums. 2009;14:4–7. doi: 10.1017/s1092852900024895. discussion 16–18. [DOI] [PubMed] [Google Scholar]
- 3.a) Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]; b) Klunk WE. Neurobio Aging. 2011;32(Suppl 1):S20–36. doi: 10.1016/j.neurobiolaging.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mathis CA, Mason NS, Lopresti BJ, Klunk WE. Semin Nucl Med. 2012;42:423–432. doi: 10.1053/j.semnuclmed.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Ly T, Julian RR. J Am Soc Mass Spectrom. 2008;19:1663–1672. doi: 10.1016/j.jasms.2008.07.006. [DOI] [PubMed] [Google Scholar]; b) Liu Z, Cheng S, Gallie DR, Julian RR. Anal Chem. 2008;80:3846–3852. doi: 10.1021/ac800176u. [DOI] [PubMed] [Google Scholar]; c) Julian RR, Beauchamp JL. J Am Soc Mass Spectrom. 2004;15:616–624. doi: 10.1016/j.jasms.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Rodgers MT. J Amer Chem Soc. 2012;134:2313–2324. doi: 10.1021/ja2102345. [DOI] [PubMed] [Google Scholar]
- 6.Hilderbrand AE, Myung S, Clemmer DE. Anal Chem. 2006;78:6792–6800. doi: 10.1021/ac060439v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Schneider HJ. Angewandte Chemie. 2009;48:3924–3977. doi: 10.1002/anie.200802947. [DOI] [PubMed] [Google Scholar]; b) Peczuh MW, Hamilton AD. Chem Rev. 2000;100:2479–2494. doi: 10.1021/cr9900026. [DOI] [PubMed] [Google Scholar]
- 8.a) Yoshiike Y, Akagi T, Takashima A. Biochemistry. 2007;46:9805–9812. doi: 10.1021/bi700455c. [DOI] [PubMed] [Google Scholar]; b) Bin Y, Li X, He Y, Chen S, Xiang J. Acta biochimica et biophysica Sinica. 2013;45:570–577. doi: 10.1093/abbs/gmt044. [DOI] [PubMed] [Google Scholar]
- 9.a) Inbar P, Li C, Takayama S, Bautista M, Yang J. Chem Bio Chem. 2006;7:1563–1566. doi: 10.1002/cbic.200600119. [DOI] [PubMed] [Google Scholar]; b) Song JM, DiBattista AM, Sung YM, Ahn JM, Turner RS, Yang J, Pak DT, Lee HK, Hoe HS. Exp Neurol. 2014;252:105–113. doi: 10.1016/j.expneurol.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO. Nat Struct Mol Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Otzen DE, Kristensen O, Oliveberg M. Proc Natl Acad Sci U S A. 2000;97:9907–9912. doi: 10.1073/pnas.160086297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen C. J Amer Chem Soc. 1967;89:2495–2496. [Google Scholar]
- 12.a) Fukuhara S, Nishigaki T, Miyata K, Tsuchiya N, Waku T, Tanaka N. Biochemistry. 2012;51:5394–5401. doi: 10.1021/bi3004236. [DOI] [PubMed] [Google Scholar]; b) Vetri V, Canale C, Relini A, Librizzi F, Militello V, Gliozzi A, Leone M. Biophys Chem. 2007;125:184–190. doi: 10.1016/j.bpc.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Petkova A, Leapman R, Guo Z, Yau W, Mattson M, Tycko R. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 14.a) Boye-Harnasch M, Cullin C. J Biotech. 2006;125:222–230. doi: 10.1016/j.jbiotec.2006.03.006. [DOI] [PubMed] [Google Scholar]; b) Fawver JN, Duong KT, Wise-Scira O, Petrofes Chapa R, Schall HE, Coskuner O, Zhu X, Colom LV, Murray J Alzh Dis. 2012;32:197–215. doi: 10.3233/JAD-2012-120880. [DOI] [PubMed] [Google Scholar]; c) Zhang X, Tian Y, Li Z, Tian X, Sun H, Liu H, Moore A, Ran C. J Amer Chem Soc. 2013;135:16397–16409. doi: 10.1021/ja405239v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacskai BJ, Hickey GA, Skoch J, Kajdasz ST, Wang Y, Huang GF, Mathis CA, Klunk WE, Hyman BT. Proc Natl Acad Sci U S A. 2003;100:12462–12467. doi: 10.1073/pnas.2034101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fodero-Tavoletti MT, Rowe CC, McLean CA, Leone L, Li QX, Masters CL, Cappai R, Villemagne VL. J Nucl Med. 2009;50:198–204. doi: 10.2967/jnumed.108.057984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.