Abstract
A key feature of substance use disorders is continued drug consumption despite aversive consequences. This has been modeled in the animal laboratory by pairing drug self-administration with electric shock, thereby punishing drug intake (Deroche-Gamonet et al. 2004). In the present experiments, we examined the effects of punishment on i.v. cocaine self-administration by adding histamine to the cocaine solution in three different animal models of high and low vulnerability to drug abuse: rats selectively bred for high (HiS) and low (LoS) saccharin consumption, rats selected for high (HiI) and low (LoI) impulsivity, and sex differences. Animals were allowed to self-administer cocaine (0.4 mg/kg/infusion) to establish a baseline of operant responding. Histamine (4.0 mg/kg/infusion) was then added directly into the cocaine solution and its consequent effects on self-administration were compared to baseline. The histamine + cocaine solution was then replaced with a cocaine-only solution, and the rats' operant responding was again measured and compared to baseline. Concurrent histamine exposure was effective in reducing cocaine consumption in all groups of rats; however, LoS and female rats took longer to return to baseline levels of cocaine consumption after histamine was removed compared to HiS and male rats. These data suggest the reduction of drug self-administration by aversive consequences may differ in groups that vary in drug use vulnerability, and these results may inform pharmacological strategies that enhance the negative aspects of drug consumption.
Keywords: Cocaine, Histamine, Impulsivity, Punishment, Saccharin, Sex differences
1. Introduction
The regular, continued use of a drug despite aversive consequences (e.g., health complications, hangovers, social repercussions, etc.) is a criterion for the diagnosis of a substance dependence disorder according to the DSM-IV [1]. One way this aspect of compulsive drug use is modeled in the animal laboratory is by applying electric shock to an organism before or after it makes an operant response for a drug delivery [2-4]. For instance, Deroche-Gamonet et al. [5] showed that a small subset of rats in a large heterogeneous population continued to press a lever for i.v. cocaine infusions despite the delivery of shock after the lever-press response. The results of this study have translational relevance, as in the case with rats, only a similarly small subset of humans who try drugs end up meeting the criteria for dependence (i.e., drug use despite aversive consequences) [6].
This difference in resistance to punishment may be attributable to elevated incentive-motivational characteristics of the more vulnerable groups, coupled with a decreased sensitivity to aversive stimuli. In general, preclinical literature has supported this notion by demonstrating a converse relationship between individual differences in vulnerability to substance use, proclivity to natural rewards, and responsiveness to aversive events [for reviews, see 7, 8]. Clinical studies have also illustrated this relationship; for instance, Vogel-Sprott et al. [9] found that alcoholics were less sensitive to electric shock compared to nonalcoholics, and Duffy et al. [10] found that alcoholics and their offspring are less sensitive to the aversive qualities of the bitter tastant 6-n-propylthiouracil (PROP) than nonalcoholics. Together, these data suggest a genetic link between phenotypic variation in substance dependence disorders and multiple behavioral indices following noxious events.
Based on these findings, the goal of the present study was to compare the effects of punishment on cocaine self-administration in rats with biologically-mediated differences (high and low) in vulnerability to the initiation [11-13] and escalation of drug self-administration [14-16], and subsequent drug-seeking in a reinstatement model [14, 17, 18] using animal populations commonly studied in our laboratory. One of these models was comprised of rats that have been selectively bred for high (HiS) or low (LoS) intake of a saccharin solution [for review, see 19]. Similar to human populations, high and low sweet preference between these lines also predicts corresponding high and low measures of substance abuse. Other groups that were compared on punished cocaine self-administration were rats screened for high (HiI) or low (LoI) impulsivity as assessed with a delay-discounting task. Impulsive behavioral baselines are typically derived from this measure or the 5-choice serial reaction-time task, and organisms exhibiting greater impulsivity also show greater drug abuse vulnerability [for review, see 20]. Last, we examined punished cocaine seeking in male and female rats, as sex also predicts relative addiction vulnerability (females > males) in human and non-human animals [for review, see 21].
Histamine (i.v.) was used as the aversive event in the present study with the goal of improving the face validity of the animal model of punished drug-seeking. Histamine administration simulates the negative consequences of drug use typically encountered by the human addict that involves long-term emotional and physical distress (i.e., hangovers, anxiety, anhedonia, etc.), such as that evoked by neurological pruritus. This condition is an aversive consequence of protracted cocaine (and opioid) use in humans that is characterized by aversive, delocalized itching sensations throughout the body that is mediated by mu and kappa opioid receptors [22]. In the laboratory, i.v. histamine is effective at reducing drug self-administration and other reward-driven behaviors in nonhuman primates [23, 24], presumably by mimicking or enhancing this aversive sensation. In accordance with previous research, such as a recent study showing that drug abuse-prone rats selected for high novelty preference (HNP) self-administered more cocaine despite electric shock following the lever-press response compared to drug-resilient rats with a low preference for novelty (LNP) [25], we hypothesized that the less drug-prone groups would exhibit greater decreases in histamine-punished cocaine self-administration.
From a therapeutic perspective, histamine punishment may model a particular approach to treating cocaine self-administration in which the treatment drug enhances the aversive aspects of drug consumption, thereby punishing drug intake. For example, disulfiram is a pharmacological agent that has been investigated as a treatment for alcoholism and cocaine addiction that acts, in part, by enhancing the aversive, interoceptive aspects of these drugs [26, 27]. Therefore, the present studies were designed to determine if phenotypes differing in vulnerability to drug consumption also differ in their response to pharmacological treatments such as histamine punishment of drug taking. With histamine, however, we may be able to better isolate similar interoceptive punishing effects of such a strategy, as it does not readily cross the blood brain barrier when administered i.v. [28]. In contrast, systemic (i.p.) disulfiram administration has complex interactions with drugs of abuse within the brain [29].
2. Materials and Methods
2.1 Subjects
Sixty-three adult rats were used in the present study. Of the total, 20 were female Sprague Dawley rats selectively bred for high (HiS; n = 10) or low (LoS; n = 10) saccharin intake at the University of Minnesota [30] from lines originating at Occidental College (Los Angeles, California, USA). The HiS and LoS lines were maintained through selectively breeding pairs based on extreme saccharin phenotype scores as described previously [19]. Fourteen days following experimental procedures, saccharin phenotype was verified using a saccharin preference test developed by Badia-Elder et al. [31] [saccharin score = ((saccharin mL – water baseline mL) × 100) / body weight]. HiS and LoS saccharin scores were 28.6 (± 2.7 SEM) and 22.6 (± 5.4 SEM), respectively. Twenty-six female Wistar rats were screened for high (HiI; n = 13) or low (LoI; n = 13) impulsivity with a delay discounting procedure. female rats were used to assess phenotypic differences in saccharin intake and impulsivity (i.e., HiS vs. LoS, HiI vs. LoI), respectively, because these characteristics, and their associated drug-seeking profiles, are typically more divergent between females than between males. Thus, it was reasoned that phenotypic differences in histamine-punished cocaine seeking would be more likely to appear in females than males, and this seemed like a prudent way to test our novel hypothesis. A delay discounting task, modified from a procedure described by Perry et al. [11], was used to screen rats for either high or low impulsivity. Rats with stable mean adjusted delays (MADs) of < 9 sec were classified as HiI rats, and those with stable MADs > 13 seconds were classified as LoI rats. Five-day average MADs for HiI and LoI rats were 4.5 (± 0.7 SEM) and 33.7 (± 2.4 SEM), respectively. Seven male and 10 female Wistar rats were used to assess sex differences. These were separate groups of rats that were not screened for impulsivity before the experiment. Prior to experimental sessions, rats were pair-housed in plastic cages and allowed to acclimate for a minimum of 3 days while they had free-access to food (Purina Laboratory Chow, Purina Mills, Minneapolis, MN) and water. All rodent holding rooms were maintained at 24°C and at 40-50% humidity under a constant 12/12-hour light/dark cycle with room lights on at 6:00 am. During the experimental conditions, female and male rats were food-restricted to 16 g and 20 g, respectively. The experimental protocol # 1008A87754 was approved by the University of Minnesota Institutional Care and Use Committee. Experiments were conducted in accordance with the Principles of Laboratory Animal Care [32], and all facilities were AAALAC accredited.
2.2 Drugs
Prior to catheterization, rats were anesthetized with a combination of ketamine (60 mg/kg) and xylazine (10 mg/kg). Atropine (0.15 mL), and doxapram (5 mg/kg) were administered to facilitate respiration under anesthesia. Following catheterization surgery, catheter patency was assessed every 5-7 days by the administration of a 0.1 mL solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline, and a second catheter was implanted in the left jugular vein if a loss of the righting reflex was not manifest during the catheter patency check. Cocaine HCl was provided by the National Institute on Drug Abuse (Research Triangle Institute, Research Triangle Park, NC), dissolved in a sterile 0.9% saline at a concentration of 1.6 mg cocaine HCl/ 1 mL saline, and refrigerated. The anticoagulant heparin (1 mL heparin/200 mL of saline; 190 USP units of heparin/kg) was added to the cocaine solution. The cocaine solution was infused at a volume of 0.025 mL/100 g of body weight following a lever press during cocaine self-administration sessions, and the duration of each infusion was 1 sec/100 g of body weight (the average infusion time was 2.5 sec). Histamine (16 mg/mL; Sigma Aldrich) was added to the cocaine solution during the histamine self-administration phase of the study. This dose was chosen based on unpublished dose-response pilot data from our laboratory.
2.3 Apparatus
Custom-made operant conditioning chambers with alternating stainless steel and Plexiglas walls were used to conduct both the delay-discounting and self-administration studies. As described in Perry et al. [11], each chamber contained slots that allowed for the insertion of stainless steel panels, lights, and operant fixtures. For the delay-discounting procedure, a 45-mg pellet feeder was attached to a pellet-delivery trough (Coulbourn Instruments, Allentown, NJ, USA) on one of the stainless steel panels. There were also two response levers mounted on stainless steel panels 4 cm above the cage floor on either side of the pellet receptacle. Tri-colored (red, green, and yellow) stimulus lights (4.6 W) were located above each lever, and a white house light (4.6 W) was positioned at the top of chamber. Operant conditioning chambers were housed within wooden sound-attenuating boxes and MED-PC software (Med Associates, St. Albans, VT, USA) was used to program experiments and collect data.
Operant conditioning chambers used for the self-administration component have been described previously [33] and were identical to those described above with the exception that the two response levers were located on opposite ends of the chamber and a pellet feeder was not used. Additionally, a syringe pump (PHM-100; Med Associates Inc., St. Albans, Vermont, USA) containing a 35-mL syringe was located outside of the operant conditioning chamber, and it was used to deliver response-contingent infusions of cocaine.
2.4 Procedure
2.4.1 Cocaine Self-Administration
Rats were allowed to recover for 3 days in their designated operant conditioning chambers following catheterization surgery. Subsequently, they were trained to lever press for cocaine infusions (0.4 mg/kg/infusion, i.v.) under an FR 1 schedule of reinforcement during daily 2-hr sessions. All self-administration sessions began at 9:00 am with the illumination of the house light and ended at 11:00 am. During sessions, responses on the left lever (active lever) produced one cocaine infusion and the simultaneous illumination of the stimulus lights located above the lever for the duration of the infusion. Responses on the other lever (inactive lever) illuminated the stimulus lights above it but had no other programmed consequences. During self-administration training, the active lever was baited with peanut butter (0.5 – 1.0g) and three non-contingent infusions were given at the beginning of each session until stable cocaine self-administration was reached. Once rats reached stability (> 25 or more infusions for three sessions and active responses exceeded the amount of inactive responses 2:1) peanut butter and non-contingent infusions were discontinued and rats were allowed to self-administer 0.4 mg/kg cocaine for 10 sessions. This constituted the Pre-Histamine phase. Histamine (4.0 mg/kg/infusion) was then added to the cocaine solution and infusions were monitored for 10 additional sessions, constituting the Histamine phase. Subsequently, the histamine/cocaine solution was removed and rats were allowed to self-administer 0.4 mg/kg cocaine for an additional 20 sessions, constituting the Post-Histamine phase.
2.5 Data Analysis
For each subject, infusions were averaged into 5-day blocks (2 pre-histamine blocks, 2 histamine blocks, and 4 post-histamine blocks; see Figures 1, 3 and 5). These blocks of average infusions served as the dependent measure in the present experiments. HiS vs. LoS, HiI vs. LoI, and male vs. female data were collected and analyzed independently as separate experiments. Data were analyzed with a mixed linear model (MIXED procedure) using SAS software (SAS Institute Inc., Cary, NC). Fixed effects in this analysis consisted of phenotype or sex (e.g., HiS vs. LoS; HiI vs. LoI; female vs. male), block (1-8), as well as a phenotype × block or sex × block interactions. Blocks of infusions were further analyzed using preplanned within- and between-subjects contrasts. The Bonferroni correction was applied to these contrasts, and they were therefore considered significant if p < .0025. Changes in self-administration were also analyzed during the Histamine and Post-Histamine phases relative to Pre-Histamine cocaine self-administration by computing percent change of average infusions self-administered during blocks 3-8 compared to block 2 ([block 2 infusions - block x infusions] / [block 2 infusions] × 100). Percent changes for each Histamine and Post-Histamine block were compared between groups using Student's t-tests. Results were considered significant if p < .05.
Figure 1.
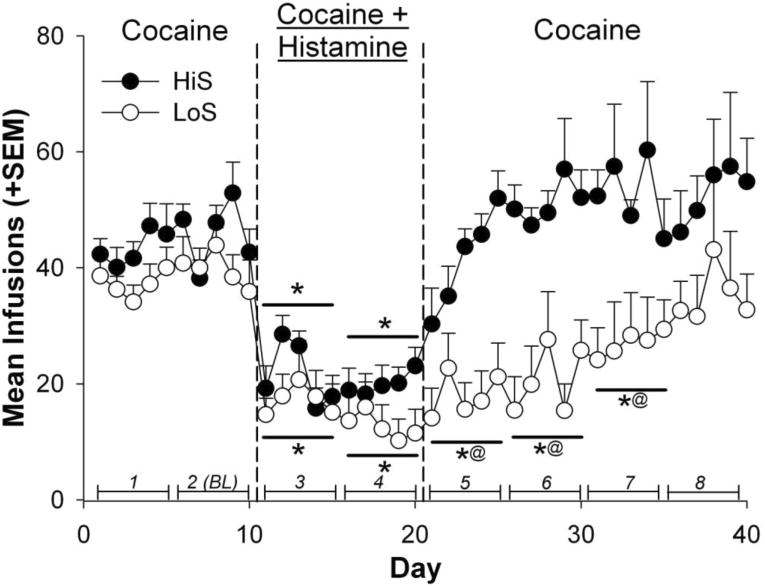
Mean infusions self-administered by HiS and LoS rats. Line segments with italicized numbers directly above the horizontal axis illustrate blocks of 5-day infusion averages. Blocks 1 and 2 constituted the Pre-Histamine phase, blocks 3 and 4 the Histamine phase, and blocks 5-8 the Post-Histamine phase. Block 2 served as the baseline reference measure (BL) of cocaine self-administration prior to histamine exposure. The * indicates a significant difference from baseline (BL, block 2) within each phenotype. The @ indicates phenotype differences in infusions self-administered between the phenotypes (p < .05).
Figure 3.
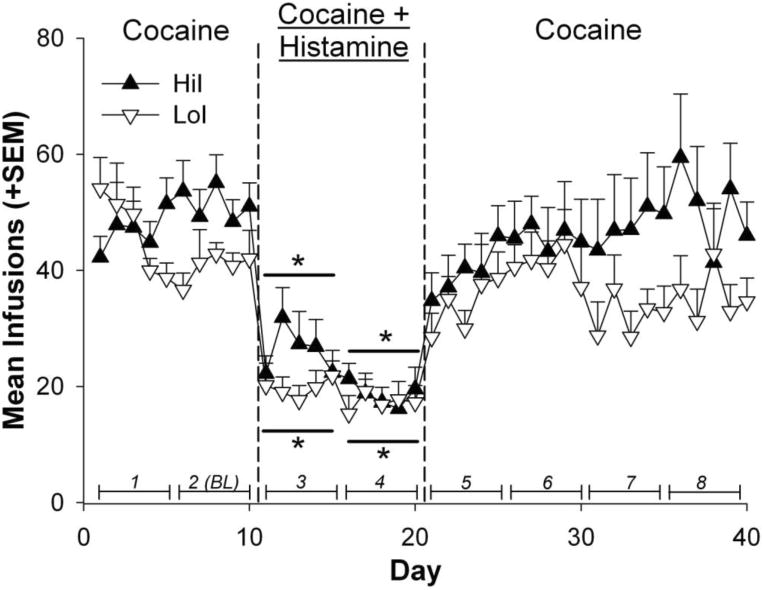
Mean infusions self-administered by HiI and LoI rats. Line segments with italicized numbers directly above the horizontal axis illustrate blocks of 5-day infusion averages. Blocks 1 and 2 constituted the Pre-Histamine phase, blocks 3 and 4 the Histamine phase, and blocks 5-8 the Post-Histamine phase. Block 2 served as the baseline reference measure (BL) of cocaine self-administration prior to histamine exposure. The * indicates a significant difference from baseline (BL, block 2) within each phenotype sex (p < .001).
Figure 5.
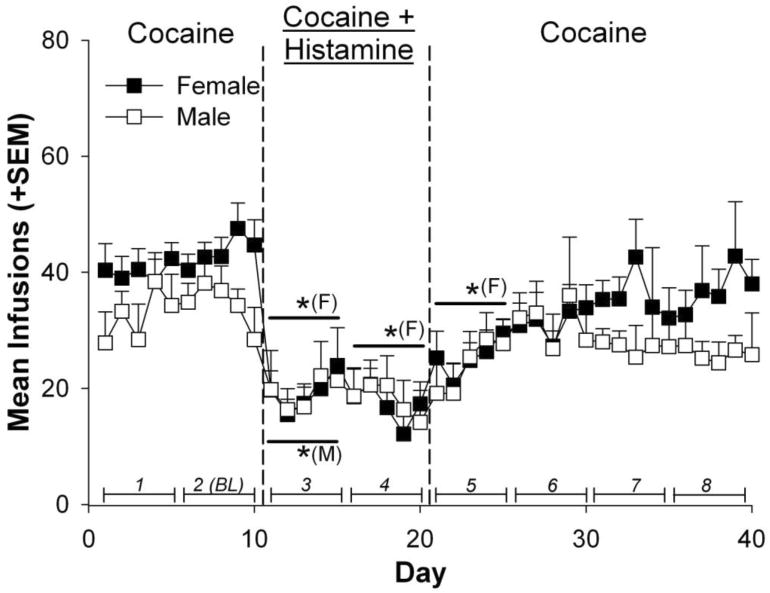
Mean infusions self-administered by female and male rats. Line segments with italicized numbers directly above the horizontal axis illustrate blocks of 5-day infusion averages. Blocks 1 and 2 constituted the Pre-Histamine phase, blocks 3 and 4 the Histamine phase, and blocks 5-8 the Post-Histamine phase. Block 2 served as the baseline reference measure (BL) of cocaine self-administration prior to histamine exposure. The * indicates a significant difference from baseline (BL, block 2) within each phenotype sex (* above data points = Females (F), * below = Males (M); p < .01).
3. Results
3.1 HiS vs. LoS
Figure 1 shows mean cocaine infusions that were self-administered throughout the experimental procedure. Results from these comparisons indicated main effects for phenotype [F(1,17) = 20.26, p < .001] and block [F(7,110) = 26.43, p < .0001], as well as an interaction effect between phenotype and block [F(7,110) = 3.03, p < .01]. Both HiS and LoS rats self-administered fewer infusions during blocks 3 and 4 (Histamine phase) compared to their respective baseline infusion means (p < .0001). LoS animals also had fewer infusions compared to baseline (block 2BL) during blocks 5 and 6 of the Post-Histamine phase (p < .0001). HiS animals self-administered more infusions than LoS animals during blocks 5 (p < .001), 6 (p < .0001), and 7 (p < .0001). There were no differences between the two groups during the Pre-Histamine phases. When percent reduction in infusions from baseline was compared across groups, there were no significant differences between HiS and LoS rats during the Histamine phase (Figure 2). However, LoS rats showed a greater percent reduction in infusions than HiS rats during blocks 5 [t(17) = 2.73, p < .05] and 6 [t(15) = 4.28, p < .001] of the Post-Histamine phase.
Figure 2.
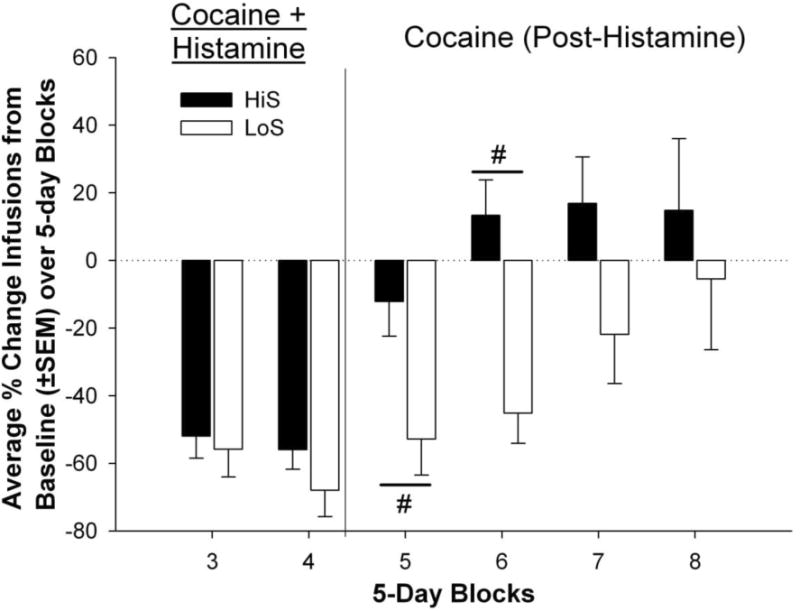
Percent change of infusions self-administered by HiS and LoS rats averaged into 5-day blocks compared to a 5-day baseline average. The # indicates phenotype differences in percent change of infusions self-administered between the phenotypes compared to baseline (p < .05).
3.2 HiI vs. LoI
Figure 3 shows cocaine infusions self-administered throughout the experimental phases by HiI and LoI rats. Results from these comparisons indicated a main effect of block [F(7,152) = 43.75, p < .0001]. Compared to baseline, both HiI and LoI rats self-administered significantly fewer infusions during blocks 3 and 4 of the Histamine phase (p < .0001). The HiI and LoI groups did not differ during the Pre-Histamine phase (Blocks 1 and 2 BL), or in the percent change of infusions between HiI and LoI rats (Figure 4).
Figure 4.
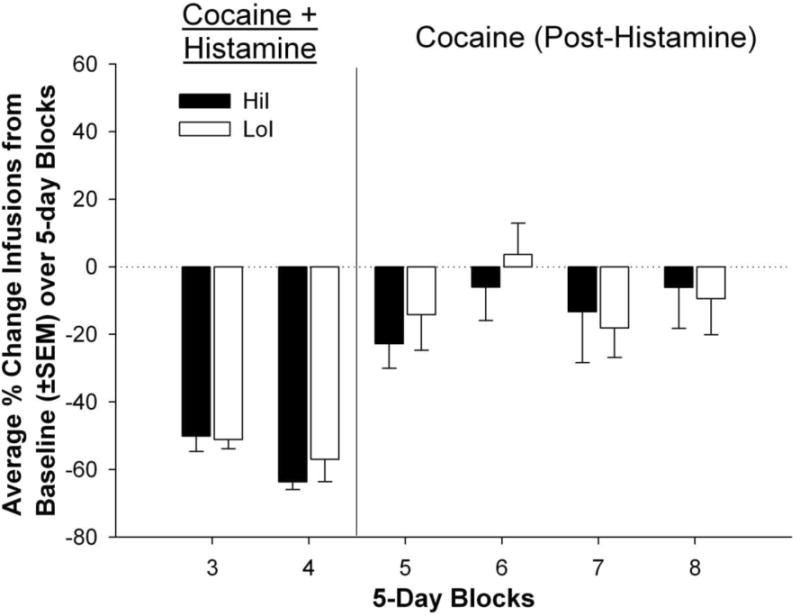
Percent change of infusions self-administered by HiI and LoI rats averaged into 5-day blocks compared to a 5-day baseline average.
3.3 Females vs. Males
Figure 5 shows cocaine infusions self-administered throughout the experimental procedure by female and male rats. There were no differences between the two groups during the Pre-Histamine phase (Blocks 1 and 2 BL), nor were there sex differences in percent change of infusions from the pre-histamine to histamine and post-histamine phases (Figure 6). When comparing self-administered infusions, results indicated a main effect of block [F(7,91) = 12.92, p < .0001]. Compared to baseline (2 BL), female rats self-administered significantly fewer infusions during blocks 3 and 4 of the Histamine phase, as well as block 5 of the Post-Histamine phase (p < .0001). Compared to the 2BL, male rats self-administered significantly fewer infusions during block 3 of the Histamine phase (p < .001).
Figure 6.
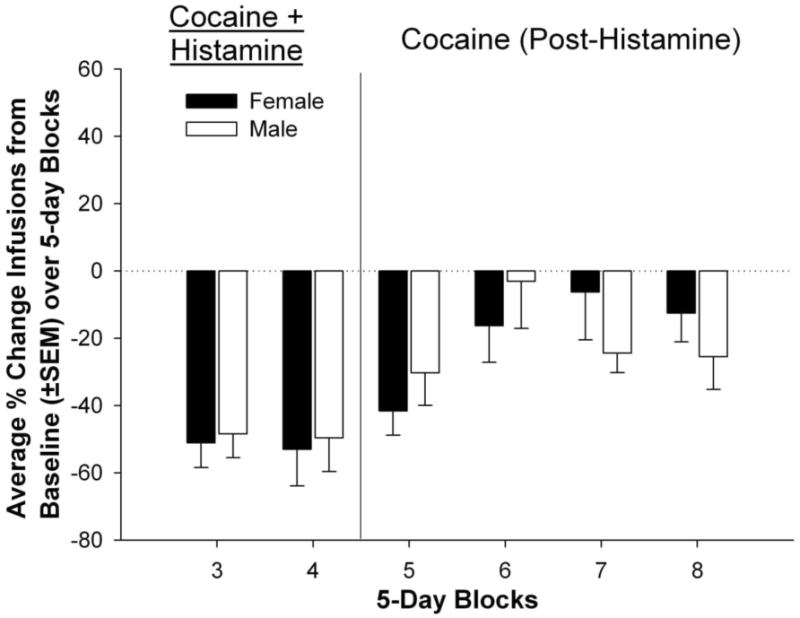
Percent change of infusions self-administered by female and male rats averaged into 5-day blocks compared to a 5-day baseline average.
4. Discussion
Results from the present experiments indicated that histamine was an effective punisher of cocaine self-administration in all groups tested: HiS and LoS, HiI and LoI, and female and male rats. However, the only group differences that emerged were in HiS vs. LoS and male vs. female. The LoS rats exhibited a delay in reestablishing baseline rates of cocaine self-administration during the Post-Histamine phase. In contrast, post-histamine cocaine self-administration rates were not different in the HiS animals. Additionally, female rats suppressed cocaine self administration below the pre-histamine baseline (block 2) compared to the post-histamine period, while males' pre- and post-histamine cocaine infusions did not differ. Since there were no differences in percent change from baseline cocaine consumption during the Histamine and Post-Histamine phases between males and females, this is considered a modest effect.
The differential action of histamine in HiS vs. LoS was consistent with other assays of aversive responsiveness in these selectively bred lines. For instance, LoS rats show greater measures of ethanol [34] and glucose [35] withdrawal compared to HiS rats. LoS (vs. HiS) rats also display greater acoustic startle response [36], social subordination [37], latency of emergence and increased defection in the novel open field, as well as heightened stress-induced anorexia [38] and analgesia [36]. The present results may offer a direct link between such variance in emotional reactivity and resilience of drug consumption despite aversive consequences. Recently, we showed that baclofen, an agent that reduces cocaine-induced dopamine increase in the nucleus accumbens [39], reduces cocaine self-administration in LoS animals while it potentiates the escalation of cocaine intake in HiS animals [40]. Interestingly, two clinical studies have shown that sweet preference was predictive of treatment efficacy in alcoholics [41, 42]. Together these data suggest that covarying behavioral traits, such as sweet preference and emotional reactivity, may help us predict response to pharmacological treatments that either decrease the rewarding effects, or increase the negative effects of abused drugs.
One interpretation of the delayed return to baseline in the LoS rats is that they were more sensitive to the long-term punishing effects of histamine on cocaine self-administration through an associative link between the stimulus properties of cocaine and the aversive effects of histamine. Using the conditioned taste aversion paradigm, differences in such associative sensitivity have been established in other drug-prone and –resilient phenotypes, namely the inbred Lewis and Fischer rats [43]. These studies have established that the drug-resilient Fischer rats show more aversion to a tastant previously paired with the administration of a variety of abused drugs than the drug-prone Lewis rats. In future work, it would be interesting to examine the effects of histamine punishment on cocaine-motivated behavior by assessing post-histamine progressive ratio (PR) ratio responding for cocaine. Furthermore, while histamine is rapidly metabolized and short-acting [44, 45], we also need to rule out the possibility that the decrease in Post-Histamine intake in the LoS animals is the result of a differential, nonspecific, non-associative somatic effect (e.g., lingering malaise, peripheral inflammation, etc.) of histamine exposure on general motor activity or motivation. Future studies may examine the effects of non-contingent histamine administration on operant responding in the HiS vs. LoS rats to address this issue.
While histamine had an enduring suppressant effect on cocaine self-administration in LoS and female but not HiS and male rats after its removal, it cannot be ruled out that the HiS and LoS or male and female animals metabolize histamine differently, or that histamine may have qualitatively different aversive effects between the lines or sexes. The absence of differences in the direct punishing effects of histamine (during the Histamine phase) in the HiS vs. LoS animals suggests that it served equally well as a punishing agent (had qualitatively similar aversive effects). Future studies could further support this notion by assessing the pharmacokinetic and discriminative stimulus properties of i.v. histamine between the phenotypes and sexes.
In contrast to results from the HiS and LoS animals, we did not find robust differences in the effects of histamine punishment on cocaine self-administration between male and female rats, nor any differences between HiI and LoI rats. One reason for this discrepancy may be attributable to the derivation of the HiS and LoS lines from Sprague-Dawley (SD) stock, while the rats used to examine female vs. male and HiI vs. LoI used rats were from Wistar stock. This was done to maintain consistency with the rat strains used in our laboratory to investigate individual differences in past experiments (HiS vs. LoS, SD; HiI vs. LoI, Wistar; Female vs. Male, Wistar), and potential differences in sensitivity to the present dose of histamine between the strains may have obscured sex- or impulsivity-mediated effects of histamine punishment (e.g., floor effects). Another possibility is that the phenotypic differences between the HiS and LoS rats may be more pronounced by virtue of the selective breeding process from which they were produced. That is, differential histamine punishment may be a subtle phenomenon that does not manifest in rats selected (not selectively bred) for behavioral (impulsivity) or physiological (sex) features from relatively heterogeneous stocks. However, by exaggerating drug-prone and -resilient phenotypes with selective breeding, we may be better able to reveal differential effects of histamine punishment. Alternatively, the HiS and LoS animals may represent unique phenotypes in which drug-prone and -resilient characteristics are mediated by different neurobiological mechanisms than those involved in female vs. male and HiI vs. LoI rats. This is supported by data showing that while HiS animals are more impulsive than LoS animals using the same delay discounting procedure [46], HiI and LoI animals do not differ in saccharin preference scores (Perry and Carroll, unpublished data). Additionally, sex differences have not been found in preference tests for saccharin using multiple concentrations in a number of rat strains, including SD and Wistar [47, but see 48]. It will be important to pursue additional ways in which characteristics of these and other models of individual differences in drug abuse vulnerability do and do not overlap, especially with regard to punished drug self-administration. Furthermore, dose-response functions for both cocaine and histamine under several different schedules of reinforcement within these vulnerable models will also prove to be important in broadening the interpretation of our present results.
Females showed significantly lower cocaine self-administration compared to baseline during the entire Histamine phase and part of the Post-Histamine phase, while male rats only showed a significant decrease during the first block of the Histamine phase. However, the effect sizes (i.e., percent change) of these decrements in consumption were not different between the sexes. Nonetheless, clinical and preclinical studies have shown that females are more sensitive to some of the aversive effects of abused drugs [for review, see 49], suggesting that sex differences in the effects of treatments that enhance the aversive interoceptive effects of drugs may be an intriguing issue to investigate. One clinical trial, for example, showed that disulfiram treatment was more effective at reducing cocaine consumption in men compared to women [50]. The authors noted that this difference might be confounded, as male participants in the study had greater instances of comorbid alcohol dependence. Animal models of punished drug-seeking may help resolve such issues.
The present difference in males and females is consistent with the outcomes in a few previous studies in which other pharmacological or behavioral treatments were compared in male and female rats or non-human primates; for example, spiradoline reduced cocaine-induced locomotor activity in female mice compared to males [51], and bremazocine, decreased phencyclidine self-administration in female rhesus monkeys compared to males [52]. Additionally, baclofen attenuated acquisition rates of i.v. cocaine self-administration in female rats more than male rats [53], and ketoconazole suppressed heroin consumption more in female than in male rats [54]. Furthermore, access to a saccharin solution reduced phencyclidine intake more in female compared to male rhesus monkeys [55], and access to a running wheel decreased cocaine self administration more in female rats compared to males [56]. These studies implicate sex as an important individual difference that, like variants in phenotype, may be used to guide more effective treatment strategies for substance use disorders.
4.1 Summary
In summary, we have shown that concurrent i.v. histamine exposure had a differential, enduring suppressant effect on subsequent cocaine self-administration in the absence of histamine exposure in rats that also vary in drug use vulnerability (e.g., LoS but not HiS). Future experiments could apply histamine punishment to other high-and low-vulnerable animal models (High-Responders/Low-Responders, Lewis/Fischer, etc.), as well as other phases of the animal model of addiction (reinstatement, escalation, acquisition, etc.). Similar to the present results, such studies may show that differential vulnerability to cocaine self-administration may result in different treatment effects. Thus, it may be necessary to customize treatment strategies by vulnerability characteristics.
Highlights.
-Females are generally more drug-abuse prone than males
-Impulsivity and sweet-preference are also positively associated with drug abuse vulnerability
-Enduring punishing effect of histamine on cocaine intake is less in saccharin-preferring rats
-Enduring punishing effect of histamine on cocaine intake greater in female rats
-Physiological and behavioral variation may predict punished drug self-administration
Acknowledgments
This research was supported by NIDA/NIH grants R01 DA003240, P20 DA024196 (Project 1), and K05 DA15267 (MEC). We thank Natalie Zlebnik, Amy Saykao, and Seth Johnson for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV. 4th. Washington, DC: American Psychiatric Association; 1994. Task Force on DSM-IV. [Google Scholar]
- 2.Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology (Berl) 2007;194:117–25. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnea-Ygael N, Yadid G, Yaka R, Ben-Shahar O, Zangen A. Cue-induced reinstatement of cocaine seeking in the rat “conflict model”: effect of prolonged home-cage confinement. Psychopharmacology (Berl) 2012;219:875–83. doi: 10.1007/s00213-011-2416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin SI, Bimle C, Miyauchi T. Differential effects of pentobarbital and cocaine on punished and nonpunished responding. J Exp Anal Behav. 1989;51:173–84. doi: 10.1901/jeab.1989.51-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 6.Health in the United States. Rockville, Md: U.S. Dept. of Health, Education, and Welfare, Public Health Service, Health Resources Administration; 2011. National Center for Health Statistics (U.S.), National Center for Health Statistics (U.S.) [Google Scholar]
- 7.Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–8. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 9.Vogel-Sprott MD, Banks RK. The effect of delayed punishment on an immediately rewarded response in alcoholics and nonalcoholics. Behav Res Ther. 1965;3:69–73. doi: 10.1016/0005-7967(65)90009-4. [DOI] [PubMed] [Google Scholar]
- 10.Duffy VB, Peterson JM, Bartoshuk LM. Associations between taste genetics, oral sensation and alcohol intake. Physiol Behav. 2004;82:435–45. doi: 10.1016/j.physbeh.2004.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 12.Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav. 2007;91:126–33. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 2008;197:237–46. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- 14.Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME. Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacology (Berl) 2006;186:235–45. doi: 10.1007/s00213-006-0371-x. [DOI] [PubMed] [Google Scholar]
- 15.Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93:343–8. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- 18.Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–77. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- 19.Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–60. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- 20.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- 21.Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Weisshaar E, Grull V, Konig A, Schweinfurth D, Diepgen TL, Eckart WU. The symptom of itch in medical history: highlights through the centuries. Int J Dermatol. 2009;48:1385–94. doi: 10.1111/j.1365-4632.2009.04117.x. [DOI] [PubMed] [Google Scholar]
- 23.Negus SS. Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 2005;181:244–52. doi: 10.1007/s00213-005-2266-7. [DOI] [PubMed] [Google Scholar]
- 24.Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self-administration in monkeys: effects of delayed punishment. Psychopharmacology (Berl) 2012;220:509–17. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–79. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen CH, Pedersen B, Tonnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35:1749–58. doi: 10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- 27.McCance-Katz EF, Kosten TR, Jatlow P. Chronic disulfiram treatment effects on intranasal cocaine administration: initial results. Biol Psychiatry. 1998;43:540–3. doi: 10.1016/S0006-3223(97)00506-4. [DOI] [PubMed] [Google Scholar]
- 28.Halpern BN, Neveu T, Wilson CW. The distribution and fate of radioactive histamine in the rat. J Physiol. 1959;147:437–49. doi: 10.1113/jphysiol.1959.sp006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devoto P, Flore G, Saba P, Cadeddu R, Gessa GL. Disulfiram stimulates dopamine release from noradrenergic terminals and potentiates cocaine-induced dopamine release in the prefrontal cortex. Psychopharmacology (Berl) 2012;219:1153–64. doi: 10.1007/s00213-011-2447-5. [DOI] [PubMed] [Google Scholar]
- 30.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–13. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 31.Badia-Elder N, Kiefer SW, Dess NK. Taste reactivity in rats selectively bred for high vs. low saccharin consumption. Physiol Behav. 1996;59:749–55. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- 32.National Research Council (U.S.) Guide for the care and use of laboratory animals. 8th. Washington, D.C.: National Academies Press; 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) [Google Scholar]
- 33.Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–7. [PubMed] [Google Scholar]
- 34.Dess NK, O'Neill P, Chapman CD. Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol. 2005;37:9–22. doi: 10.1016/j.alcohol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Yakovenko V, Speidel ER, Chapman CD, Dess NK. Food dependence in rats selectively bred for low versus high saccharin intake: implications for “food addiction”. Appetite. 2011;57:397–400. doi: 10.1016/j.appet.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Dess NK, Arnal J, Chapman CD, Siebal S, VanderWeele DA, Green KF. Exploring adaptations to famine: Rats selectively bred for differential intake of saccharin differ on deprivation-induced hyperactivity and emotionality. International Journal of Comparative Psychology. 2000;13:34–52. [Google Scholar]
- 37.Eaton J, Dess N, Chapman C. Sweet Success, Bitter Defeat: A Taste Phenotype Predicts Social Status in Selectively Bred Rats. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0046606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dess NK, Minor TR. Taste and emotionality in rats selectively bred for high versus low saccharin intake. Learning and Behavior. 1996;24:105–15. [Google Scholar]
- 39.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003;50:1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- 40.Holtz NA, Carroll ME. Baclofen has opposite effects on escalation of cocaine self-administration: Increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake in those selected for low (LoS) saccharin intake. Pharmacol Biochem Behav. 2011;100:275–83. doi: 10.1016/j.pbb.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laaksonen E, Lahti J, Sinclair JD, Heinala P, Alho H. Predictors for the efficacy of naltrexone treatment in alcohol dependence: sweet preference. Alcohol Alcohol. 2011;46:308–11. doi: 10.1093/alcalc/agq101. [DOI] [PubMed] [Google Scholar]
- 42.Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M, et al. Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol Alcohol. 2009;44:293–300. doi: 10.1093/alcalc/agn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Middleton M, Sarno M, Agarwala SS, Glaspy J, Laurent A, McMasters K, et al. Pharmacokinetics of histamine dihydrochloride in healthy volunteers and cancer patients: implications for combined immunotherapy with interleukin-2. J Clin Pharmacol. 2002;42:774–81. doi: 10.1177/009127002401102713. [DOI] [PubMed] [Google Scholar]
- 45.Rose B, Browne JSL. The distribution and rate of disappearance of intravenously injected histamine in the rat. Am J Physiol. 1938;124:412–20. [Google Scholar]
- 46.Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–29. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- 47.Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–32. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- 49.Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- 50.Nich C, McCance-Katz EF, Petrakis IL, Cubells JF, Rounsaville BJ, Carroll KM. Sex differences in cocaine-dependent individuals' response to disulfiram treatment. Addict Behav. 2004;29:1123–8. doi: 10.1016/j.addbeh.2004.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sershen H, Hashim A, Lajtha A. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res. 1998;801:67–71. doi: 10.1016/s0006-8993(98)00546-0. [DOI] [PubMed] [Google Scholar]
- 52.Cosgrove KP, Carroll ME. Differential effects of bremazocine on oral phencyclidine (PCP) self-administration in male and female rhesus monkeys. Exp Clin Psychopharmacol. 2004;12:111–7. doi: 10.1037/1064-1297.12.2.111. [DOI] [PubMed] [Google Scholar]
- 53.Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend. 2002;66:61–9. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 54.Carroll ME, Campbell UC, Heideman P. Ketoconazole suppresses food restriction-induced increases in heroin self-administration in rats: sex differences. Exp Clin Psychopharmacol. 2001;9:307–16. doi: 10.1037//1064-1297.9.3.307. [DOI] [PubMed] [Google Scholar]
- 55.Cosgrove KP, Carroll ME. Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology (Berl) 2003;170:9–16. doi: 10.1007/s00213-003-1487-x. [DOI] [PubMed] [Google Scholar]
- 56.Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73:663–71. doi: 10.1016/s0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]


