Introduction
The Epstein-Barr virus is a fascinating human herpesvirus whose study has provided unique insight into host:pathogen interactions and complex cellular molecular processes. The virus is the first discovered human tumor virus and was initially identified in primary cell cultures of Burkitt lymphoma, an unusual African pediatric lymphoma [1]. The quickly developed serologic studies revealed that most people had antibodies to the virus but that patients with BL had elevated titers to specific antigenic components [2]. The serologic screenings also showed a link between elevated EBV titers and an unusual epithelial nasopharyngeal carcinoma (NPC) that developed with extraordinarily high incidence in Southern China [3]. The seroconversion of the young technician performing these early serologies following a bout with infectious mononucleosis led to definitive serologic studies that proved infectious mononucleosis was the disease manifestation associated with primary infection [4].
The continuing studies of EBV have identified additional cancers and diseases linked to EBV [5]. An overarching goal has been to define in what way a virus that is carried by almost everyone contributes to intriguing and unique cancers. The study of EBV has illuminated the molecular biology of latent herpesvirus infection and new potent mechanisms through which cell growth can be modulated by viruses. This review will summarize key findings that form the basis for our understanding of EBV pathogenesis with special emphasis on newly identified potential mechanisms through which EBV alters cellular growth.
EBV Biology and Link to Human Cancers
The biologic properties of the virus were immediately intriguing as it was shown that the cell lines could be established from BL samples and could produce virus that could infect primary B cells with EBV and transform them into immortalized cell lines. This powerful molecular phenotype has led to the identification of the viral proteins that are essential for latent infection and required for cell transformation [6]. Virus production can be induced in the cell lines where most of infected cells do not produce virus and are considered to be latently infected. In latent infection the viral DNA is not usually integrated into the host chromosome but rather is stably maintained as an extrachromosomal episome [7]. This was the first molecular characterization of the herpesviral genome in cells that represented a “latent” or nonpermissive infection and provided a new understanding of how these viruses can readily reactivate from latent to permissive infection. The episomal DNA had nucleosomal structure and was maintained using a specific origin for plasmid DNA replication, orip, that was activated by binding of one of the EBV nuclear antigens (EBNAs) [8].
Early studies revealed that in transformed cell lines and within the EBV associated tumors, EBV DNA was present in every cell which also contained viral RNAs and proteins. The first RNAs to be identified were entitled EBERs (EBV encoded RNA) [9]. These enigmatic noncoding RNAs can be present at a million copies per cell but are not required for B-cell transformation [10]. Their high stability and abundance have made them a superb marker for EBV latent infection and their detection is one criteria for determining the association of different tumors with EBV infection. Another criteria is the detection of clonal viral EBV genomes. Viral clonality is based on restriction enzyme analysis of the terminal restriction enzyme fragments [11]. These fragments are quite heterogeneous due to varying numbers of terminal repeats (TR) at each end of the linear genome. In linear, virion DNA the terminal restriction enzyme fragments migrate as a ladder array with each band representing a fragment with a certain number of TRs and can be identified on Southern blots using a probe representing unique DNA from the left or right end of the genome. In the circular episomal form the right and left ends of the genome are fused such that the left and right DNA probes hybridize to the same fragment. In tumor samples with clonal episomal DNA, a single fragment is detected by both probes. If viral replication has reactivated in some cells, faint ladder arrays of terminal fragments are detected. The detection of clonal episomes revealed that every EBV genome within a cell was identical and suggested by extension that the malignant cells were also clonal [11]. This prediction and correlation has been confirmed in lymphoid cell lines where immunoglobulin gene rearrangement can be used as a marker of cellular clonality.
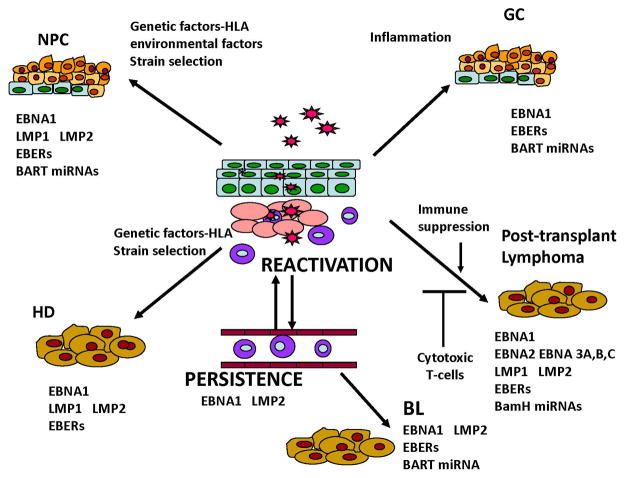
EBV is now considered an etiologic factor in multiple types of cancer that primarily develop in lymphocytes and epithelial cells (Figure 1). These include cancers that develop in the immunocompromised such as AIDS-associated lymphomas and post-transplant lymphoproliferative disease and also unusual cancers that develop in the immunocompotent [5]. Some of the cancers have very distinct patterns of incidence such as BL, NPC, T-cell lymphoma, and salivary gland carcinoma. These malignancies develop in specific geographic and ethnic populations and environmental, genetic, and immune factors potentially contribute. Additionally, EBV is also found in a consistent subset of some cancers worldwide including Hodgkin lymphoma and gastric carcinoma.
Figure 1.
EBV Transformation and Viral Oncoproteins
Interestingly, differences in viral expression comparing EBV expression in transformed lymphocytes to that in tumor samples were identified in early studies. Comparison of sequences encoding polyadenylated RNA from Burkitt lymphoma biopsy material identified abundant transcription from specific restriction enzyme fragments that was not detected in transformed cell lines [12]. Similar studies of NPC biopsy samples indicated that the sequences later shown to encode EBNA2 and EBNA3 were not transcribed in NPC but there was abundant transcription of sequences that encode latent membrane proteins 1 and 2 (LMP1, and LMP2), and the BamHI A restriction fragment (BamHI A rightward transcripts - BARTs) [13]. These findings led to the identification of three distinct types of latent infection with differing patterns of viral expression. The malignancies and some cell lines are characterized by different patterns of viral expression and are classified into types I, II and III [14]. Type I latency, typical of Burkitt lymphoma, has the most restricted expression profile such that only the EBV nuclear antigen 1 (EBNA1), the untranslated nonpolyadenylated EBERs, and variable levels of BART miRNAs are expressed (Figure 1). Type II latency is associated with nasopharyngeal carcinoma and Hodgkin lymphoma, with expression of (LMP1, LMP2A and LMP2B) in addition to the transcripts expressed in type I latency. The BART transcripts and the BART miRNAs are particularly abundant in NPC and gastric carcinoma. Expression is the most complex in Type III latency which is characteristic of transformed B-cells in culture and is only found in cancers linked to immunosuppression such as post-transplant lymphoma and AIDS-associated lymphoma [5].
Molecular analysis of the transformed cell lines maintained in vitro has revealed that transformation is dependent upon the carefully regulated expression of the viral genes [6]. The EBNA1 protein binds to orip and is required for maintenance of the viral episome and its replication by the host cell DNA polymerase machinery [8]. EBNA2, EBNA3A, 3B, 3C, and leader protein (LP) regulate transcription of cellular genes and LMP1 and LMP2 through interactions with the major transcription factor for the NOTCH pathway, RBPJκ [15,16]. In addition to being the major transcriptional regulators during latent infection, EBNA2 and particularly the EBNA3 proteins are the primary targets of cytotoxic lymphocytes. Thus in immunocompromised individuals, the latently infected cells can switch into the transforming latency III pattern of expression leading to the development of polyclonal and subsequent monoclonal lymphoma. These tumors can respond to reduced immunosuppression and can be prevented or eliminated by EBV-specific cytotoxic T-cell preparations.
In the immunocompetent, one event that likely contributes to the development of cancer is to have more restricted expression of viral proteins with the expression of the viral transforming proteins, LMP1 and LMP2 in the absence of the EBNA proteins that are recognized by the immune system. In the more restricted infections in Type I or II latency, the noncoding miRNAs or EBER RNAs may have increased etiologic contributions.
Latent Membrane Protein 1
Latent membrane protein 1 (LMP1) is considered the major oncogene of EBV as it has transforming properties in cultured cell lines and is essential for B-lymphocyte transformation [5]. LMP1 transcription is also consistently detected in several of the EBV-associated cancers including post-transplant lymphoma, Hodgkin Disease (HD), and nasopharyngeal carcinoma (NPC). LMP1 functions as a constitutively active member of the tumor necrosis factor receptor family and activates multiple signaling pathways including mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK), phosphatidylinositol 3-kinase (PI3K)/Akt, and NF-κB. LMP1 induces the expression of specific genes that are involved with apoptosis, cell cycle progression, cell proliferation, and migration. Inhibition of NF-κB induces apoptosis of EBV transformed cells and fibroblast and epithelial cell transformation require activation of PI3kinase/Akt. An additional important target of LMP1 is EGFR, a member of the ErbB receptor tyrosine kinase family [17]. Thus LMP1 activates a wealth of signaling pathways that could be specifically targeted in EBV infected tumors.
Two regions, CTAR1 and CTAR2, have been identified within the cytoplasmic carboxy terminal domain that activates NF-κB. C-terminal activating region 2 (CTAR2) activates the canonical NF-κB pathway which includes p50/p65 complexes while C-terminal activating region 1 (CTAR1) can activate both the canonical and noncanonical pathways to produce multiple distinct NF-κB dimers, including p52/p50, p52/p65, and p50/p50 [17]. CTAR1 also uniquely upregulates the epidermal growth factor receptor (EGFR) in epithelial cells. Increased p50-Bcl-3 complexes have been detected by chromatin precipitation on the NF-κB consensus motifs within the egfr promoter in CTAR1-expressing epithelial cells and NPC cells [18]. The study of LMP1 activation of NF-κB has been particularly informative for determining potential specificities of this important family of transcription factors. Many studies have relied on reporter assays to identify NF-κB activation, however, analyses of LMP1 mediated activation have led to the identification of canonical and noncanonical activation and distinct genes that are regulated by the distinct forms
To explore the possibility that specific EBV variants may be linked to cancer development and potentially explain the geographic differences in incidence of some cancers, many studies have analyzed EBV variants. To determine if variants of LMP1 existed, perhaps analogous to the oncogenes of high risk papillomavirus, analysis of sequence variation within LMP1 identified consistent signature amino acids that distinguished at least seven variants of LMP1 [19]. The CTAR1 and CTAR2 motifs are highly conserved and unchanged in all variants and comparison of the biologic and signaling properties of the variants indicated that all of the variants were capable of transforming Rat-1 fibroblasts as measured by blockage of contact-inhibited and anchorage-independent growth, and inducing homotypic adhesion in EBV-negative B-cells [20]. Interestingly, most of the conserved sequence changes in the variants were within known and predicted potential Human Leukocyte Antigen (HLA)-restricted epitopes, suggesting a potential immune-modulated selection mechanism [19]. All of the variants have been detected in various malignancies, however, the CAO/China 1 LMP1 variant is significantly more prevalent in NPC tumors from the southern endemic region of China. Functional immune recognition studies have shown that the China 1 sequence changes within a strong A2/A24 epitope eliminate presentation and these HLA types are among the most prevalent in patients with NPC. Although LMP1 induces a relatively weak HLA restricted CTL response, the prevalence of strains lacking the strongest LMP1 HLA restricted epitope in NPC tumor samples suggests that immune escape of viral oncogenes may be one contributing factor in the development of EBV associated cancers (Figure 1).
Latent Membrane Protein 2
The Epstein-Barr virus (EBV) latent membrane protein 2A (LMP2A) is important for maintenance of latency in infected B lymphocytes and also modulates epithelial cell growth [21]. Through its immunoreceptor tyrosine-based activation motif (ITAM) and PY motifs, LMP2A is able to block B cell receptor (BCR) signaling, bind BCR-associated kinases, and manipulate the turnover of itself and these kinases via a PY-mediated interaction with the Nedd4 family of ubiquitin ligases. In B cells, LMP2A provides cell survival signals by mimicking B cell receptor signaling and activating Syk through the ITAM domain. The Akt pathway is also activated by LMP2A in B-cells and contributes to their survival [22]. In epithelial cells, LMP2A has been shown to activate the phosphatidylinositol 3′-OH kinase (PI3K)/Akt and β-catenin signaling pathways [23]. These effects block differentiation of epithelial cells and in some epithelial cell lines, LMP2A can induce transformation and anchorage independence [24] [23].
LMP2 is expressed in NPC and at particularly high levels in HD [5]. Several HLA restricted epitopes have been identified in LMP2 with a particularly prevalent epitope presented by HLA-0201 expressing individuals [25]. This finding may also underlie the higher risk of developing NPC in HLA-A2 as the HLA-A-0201 subtype is rare among Chinese compared to other HLA-A2 subtypes. Perhaps, similarly to LMP1, impaired immune presentation and recognition of LMP2 is a factor that enables its expression and its potent effects on cell growth.
Novel Mechanisms to Modulate Cell Growth
Viral miRNAs
One of the most exciting recent findings in molecular biology has been the identification of microRNAs (miRNAs) that mediate another type of regulation of cellular gene expression [26]. miRNAs are approximately 22-nucleotide long single stranded RNAs that are closely related to small interfering RNAs (siRNAs) [26]. It is thought that most miRNAs inhibit the translation of mRNAs by targeting an inhibitory complex to specific mRNAs based on base pair complementarity. The first viral miRNAs to be identified were cloned from the prototype EBV infected B cell line, B95 [27]. Five miRNAs were identified as being derived from the EBV genome, including two from the introns of the BARTs. Three of the miRNAs were located near the BHRF1 ORF that encodes a bcl2 homologue. These miRNAs have subsequently been shown to only be present in cells in Type 3 latency that express the primary EBNA transcript that initiates from the Wp or Cp promoters [28]. Recent studies indicate that they make a significant contribution to EBV induced growth transformation and strains deleted for their expression are impaired [29].
The majority of the BART sequences are deleted in B95 so to identify likely additional miRNAs, subsequent studies cloned and sequenced from Kaposi sarcoma associated herpesvirus (KSHV) and EBV coinfected pleural effusion lymphoma (PEL) cell lines and NPC tumors [28]. These studies identified at least 44 miRNAs produced from the BART transcripts. Some of the miRNAs are directly opposite to several genes involved in replication and the EBV DNA polymerase is a target of BART2. However, most of the BART miRNAs do not appear regulate viral genes and most likely function in downregulating transcripts from the host cell [30]. Presently, some of the identified targets function as tumor suppressors indicating one potential mechanism through which the BART miRNAs may contribute to EBV-induced oncogenesis.
Several of the identified miR-BART targets identified contribute to apoptosis and the miRNAs in general have anti-apoptotic affects. The BH3-only protein, PUMA, which contributes to cytochrome c release from the mitochondria is targeted by miR-BART5 [31]. The effects on PUMA by miR-BART5 were also shown using 3′UTR reporter assays and by immunoblotting. Microarray analysis looking for transcripts that decreased in abundance in cells expressing the BART miRNAs identified another BH3-only protein, Bim, that was targeted by multiple BART miRNAs [32]. Bim is considered a tumor suppressor and has been shown to be targeted by several oncogenic cellular miRNAs in some cancers. EBV miRNA targets have also been identified by immunoprecipitating the RNA-induced silencing complex (RISC). This approach identified TOMM22 which is part of the mitochondrial pore receptor complex for the proapoptotic protein Bax as a potential target for miR-BART16. Additional tumor suppressor genes have also been shown to be targets for the BART miRNAs including WIF1 (miR-BART19-3p) and APC (miR-BART7, 19-3p, and 17-5p. It is thought that the individual miRNAs likely target multiple genes and pathways and many additional cellular targets for the EBV miRNAs will likely be identified. The effects of the miRNAs will clearly vary considerably between cell lines, cell types, and individual cells as a result of differences in their individual abundance, the abundance of potential target transcripts, the co-targeting by cellular miRNAs, potential allelic differences in target sites, and also structural differences in the mRNA with 3′ UTR shortening a characteristic of some cancer cells [30].
A recent study has shown that the EBV negative AGS cell line when infected with a recombinant EBV becomes anchorage independent, a hallmark of transformation [33]. The cells have very little viral protein expression but express high levels of the BART miRNAs, similar to EBV positive gastric cancer (Figure 1). Importantly, microarray analysis revealed dramatic changes in expression patterns after infection with EBV with highly significant enrichment for previously identified and bioinformatically predicted BART miRNA targets in the large numbers of genes that were downregulated. These findings suggest that the BART miRNAs, in the absence of expression of the EBV transforming proteins, are major contributors to the cellular expression changes and can alter cell growth. Thus the viral miRNAs may be an etiologic factor in cancer development in immunocompetent individuals that would bypass a requirement for viral protein expression and the consequential recognition by the immune system.
Exosomes
Exosomes are small vesicles of unique size and shape that are released from cells and have been shown to contain and transfer RNA and proteins including integrin and growth factor receptors and immune signaling molecules including MHCI and MHCII, ICAM1 and LFA3 [34]. Initial studies showed that exosomes containing LMP1 could inhibit monocyte proliferation and that NPC cells secreted exosomes that contain LMP1 and galectin 9, a protein that specifically interacted with membrane receptor Tim1 and induced apoptosis in T cells [35]. LMP1 induces secretion of exosomes that contain fibroblast growth factor, and EGFR into exosomes [36]. The purified exosomes containing LMP1 and EGFR are taken up by epithelial, endothelial, and fibroblast cells leading to the activation of ERK and PI3K/Akt pathways [37]. It has always been noted that LMP1 is not expressed in all cells within tumors and in some cases its expression is barely detectable. However, through exosomes, limited expression of viral oncoproteins could alter growth regulation in neighboring and distant cells. Additionally, EBV miRNAs are secreted in exosomes and shown to affect known targets in recipient cells [38]. Through these effects on secretion of key signaling molecules, and viral-encoded proteins and miRNAs, EBV could modulate both the tumor microenvironment and also potentially impact the infected host.
Conclusions
The molecular studies of EBV transforming proteins have shown them to be a molecular treasure treasure trove that has revealed key regulatory mechanism in critical cell pathways. The EBNA proteins have helped clarify Notch regulated signaling and the properties of the major Notch DNA binding protein, RBPJκ. LMP1 and its interactions with ubiquitin ligases have enabled dissection of NF-κB regulation and led to the identification of canonical, noncanonical, and bcl3 mediated targets. Future studies of the effects of EBV on cellular processes will likely uncover mechanisms that regulate miRNA synthesis and exosome secretion.
Importantly, studies of EBV associated cancers have revealed the multiple ways that EBV can alter cell growth. In the absence of immune recognition and control, EBV can mediate highly regulated growth transformation with expression of all of its growth inducing proteins. In other scenarios, environmental factors may enable expression of the less antigenic but potent oncogenes, LMP1 and LMP2. The unfortunate combination of specific EBV variants and different HLA types may enable these proteins to go unrecognized and the EBV induced proliferating cells to escape immune control. Alternatively, their expression may become significantly downregulated, however, the potential expression in a rare cell and exosomal delivery of the proteins or activated cellular proteins to many cells may induce growth and alter the tumor environment. Finally, in some situations with the right levels of expression of cell targets and viral and cellular miRNAs, EBV may alter growth without any viral protein expression.
The complex growth inducing potential of EBV may initially seem daunting. However, as our understanding increases for these many processes, each mechanism represents a potential point for therapeutic targeting. These would include EBV protein-specific cytotoxic T-cell immunotherapy, inhibiting critical pathways activated by EBV such as NFkB, PI3kinase, EGFR, PKC block the critical activation sites of the EBV oncogenes. EBV and other oncogenic viruses have provided target-rich environments to be exploited. These findings suggest that the eventual control of virally associated cancers is likely to be more accessible than cancers that lack these unique viral targets.
Highlights.
Factors that contribute to EBV oncogenesis
Variation and reduced viral expression
HLA and Immune recognition of viral proteins
Growth regulation by miRNAs
Exosome transfer of oncogenes and signaling molecules
Acknowledgments
The summarized studies have been supported by grants from the National Cancer Institute, CA19014, CA138811, and CA32979 to NR-T
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein MA, Achong BG, Barr YM. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 2.Henle G, Henle W, Clifford P, Diehl V, Kafuko GW, Kirya BG, Klein G, Morrow RH, Munube GM, Pike P, et al. Antibodies to Epstein-Barr virus in Burkitt’s lymphoma and control groups. J Natl Cancer Inst. 1969;43:1147–1157. [PubMed] [Google Scholar]
- 3.Henle W, Henle G, Ho HC, Burtin P, Cachin Y, Clifford P, de Schryver A, de-The G, Diehl V, Klein G. Antibodies to Epstein-Barr virus in nasopharyngeal carcinoma, other head and neck neoplasms, and control groups. J Natl Cancer Inst. 1970;44:225–231. [PubMed] [Google Scholar]
- 4.Henle G, Henle W, Diehl V. Relation of Burkitt’s tumor-associated herpes-type virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968;59:94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raab-Traub N. EBV-induced oncogenesis. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. 2007. [PubMed] [Google Scholar]
- 6.Kieff E, Rickinson AB. Lippincott Williams & Wilkins Publishers, editor. Epstein-Barr Virus and Its Replication. In: Fields BNPMH, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, Knipe DM, editors. Field’s Virology. 4. II pp. 2511–2573. [Google Scholar]
- 7.Nonoyama M, Pagano JS. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972;238:169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- 8.Yates J, Warren N, Reisman D, Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984;81:3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrand JR, Rymo L. Characterization of the major Epstein-Barr virus-specific RNA in Burkitt lymphoma-derived cells. J Virol. 1982;41:376–389. doi: 10.1128/jvi.41.2.376-389.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci U S A. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47:883–889. doi: 10.1016/0092-8674(86)90803-2. [DOI] [PubMed] [Google Scholar]
- 12.Dambaugh T, Nkrumah FK, Biggar RJ, Kieff E. Epstein-Barr virus RNA in Burkitt tumor tissue. Cell. 1979;16:313–322. doi: 10.1016/0092-8674(79)90008-4. [DOI] [PubMed] [Google Scholar]
- 13.Raab-Traub N, Hood R, Yang CS, Henry B, 2nd, Pagano JS. Epstein-Barr virus transcription in nasopharyngeal carcinoma. J Virol. 1983;48:580–590. doi: 10.1128/jvi.48.3.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickinson A, Kieff E. Epstein-Barr Virus and Its Replication. In: Knipe ID, editor. Field’s Virology. 4. Vol. 2 Lippincott Williams & Wilkins Publishers; 2001. pp. 2511–2573. [Google Scholar]
- 15.Robertson ES, Lin J, Kieff E. The amino-terminal domains of Epstein-Barr virus nuclear proteins 3A, 3B, and 3C interact with RBPJ(kappa) J Virol. 1996;70:3068–3074. doi: 10.1128/jvi.70.5.3068-3074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henkel T, Ling PD, Hayward SD, Peterson MG. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein J kappa. Science. 1994;265:92–95. doi: 10.1126/science.8016657. [DOI] [PubMed] [Google Scholar]
- 17.Miller WE, Cheshire JL, Raab-Traub N. Interaction of tumor necrosis factor receptor-associated factor signaling proteins with the latent membrane protein 1 PXQXT motif is essential for induction of epidermal growth factor receptor expression. Mol Cell Biol. 1998;18:2835–2844. doi: 10.1128/mcb.18.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornburg NJ, Raab-Traub N. Induction of epidermal growth factor receptor expression by Epstein-Barr virus latent membrane protein 1 C-terminal-activating region 1 is mediated by NF-kappaB p50 homodimer/Bcl-3 complexes. J Virol. 2007;81:12954–12961. doi: 10.1128/JVI.01601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Edwards RH, Sitki-Green D, Moore DT, Raab-Traub N. Potential selection of LMP1 variants in nasopharyngeal carcinoma. J Virol. 2004;78:868–881. doi: 10.1128/JVI.78.2.868-881.2004. This is the first identification of specific LMP1 variants which reveals that the sequence changes lie within known and predicted HLA epitopes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mainou BA, Raab-Traub N. LMP1 strain variants: biological and molecular properties. J Virol. 2006;80:6458–6468. doi: 10.1128/JVI.00135-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longnecker R, Miller CL. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol. 1996;4:38–42. doi: 10.1016/0966-842x(96)81504-6. [DOI] [PubMed] [Google Scholar]
- 22.Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-Kinase/Akt pathway. J Virol. 2000;74:10838–10845. doi: 10.1128/jvi.74.22.10838-10845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74:10681–10689. doi: 10.1128/jvi.74.22.10681-10689.2000. This is the first identification of transforming properties for LMP2A and its activation of Akt kinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda M, Longnecker R. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J Virol. 2007;81:9299–9306. doi: 10.1128/JVI.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lautscham G, Haigh T, Mayrhofer S, Taylor G, Croom-Carter D, Leese A, Gadola S, Cerundolo V, Rickinson A, Blake N. Identification of a TAP-independent, immunoproteasome-dependent CD8+ T-cell epitope in Epstein-Barr virus latent membrane protein 2. J Virol. 2003;77:2757–2761. doi: 10.1128/JVI.77.4.2757-2761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26**.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. Clear review of miRNA production and function. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, et al. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 28**.Cai X, Schafer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. This study identifies the abundant BART miRNAs and shows that the seed sequences are highly conserved in the rhesus EBV homologue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Feederle R, Linnstaedt SD, Bannert H, Lips H, Bencun M, Cullen BR, Delecluse HJ. A viral microRNA cluster strongly potentiates the transforming properties of a human herpesvirus. PLoS Pathog. 2011;7:e1001294. doi: 10.1371/journal.ppat.1001294. First genetic evidence of a contribution of the EBNA transcript miRNAs to B-cell transformation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Marquitz AR, Raab-Traub N. The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol. 2012;22:166–172. doi: 10.1016/j.semcancer.2011.12.001. Comprehensive review of our current understanding of EBV miRNA functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, Kwong DL, Tsao SW, Jin DY. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marquitz AR, Mathur A, Nam CS, Raab-Traub N. The Epstein-Barr Virus BART microRNAs target the pro-apoptotic protein Bim. Virology. 2011;412:392–400. doi: 10.1016/j.virol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquitz AR, Shair Kathy HY, Raab-Traub Nancy. Infection of Epstein-Barr Virus in a gastric carcinoma cell line induces anchorage independence and global changes in gene expression. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1202910109. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Meckes DG, Jr, Raab-Traub N. Microvesicles and viral infection. J Virol. 2011;85:12844–12854. doi: 10.1128/JVI.05853-11. Thorough review of microvesicles and exosomes during viral infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 36.Ceccarelli S, Visco V, Raffa S, Wakisaka N, Pagano JS, Torrisi MR. Epstein-Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer. 2007;121:1494–1506. doi: 10.1002/ijc.22844. [DOI] [PubMed] [Google Scholar]
- 37.Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. First identification of miRNA functional transfer through exosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]