Abstract
Prior to the first description of hay fever in 1870 there was very little awareness of allergic disease, which is actually similar to the situation in pre-hygiene villages in Africa today. The best explanation for the appearance and subsequent increase in hay fever at that time is the combination of hygiene and increased pollen secondary to changes in agriculture. However, it is important to remember that the major changes in hygiene in Northern Europe and the USA were complete by 1920. Asthma in children did not start to increase until 1960, but by 1990 it had clearly increased to epidemic numbers in all countries where children had adopted an indoor lifestyle. There are many features of the move indoors that could have played a role; these include: increased sensitization to indoor allergens, diet, and decreased physical activity as well as the effects of prolonged periods of shallow breathing. Since 1990 there has been a remarkable increase in food allergy which has now reached epidemic numbers. Peanut has played a major role in the food epidemic and there is increasing evidence that sensitization to peanut can occur through the skin. This suggests the possibility that changes in lifestyle in the last 20 years could have influenced the permeability of the skin. Overall, the important conclusion is that sequential changes in lifestyle have led to increases in different forms of allergic diseases. Equally it is clear that the consequences of hygiene, indoor entertainment, changes in diet or in physical activity have never been predicted.
Keywords: Hay fever, Asthma, Peanut, Lifestyle, Hygiene, Indoor environment
INTRODUCTION
The human race has come to dominate its environment so completely that any analysis of the increase or appearance of a disease has to take changes in our lifestyle into account. In the case of allergic disease changes in our environment, diet, water quality and personal behavior over the last 150 years have played a dominant role in the specificity of these diseases, as well as in the prevalence and severity. The first thing to address is when “the epidemic” started and how much the increase in different allergic diseases has occurred separately. It should be noted that some or most previous reviews have implied that the increase in allergic disease has been unimodal, but actually that has never been a tenable analysis. Not only have increases occurred or are currently occurring at different times in different countries but hay-fever, asthma and peanut allergy have had strikingly different time courses both in Europe and North America.
Occasional descriptions of allergic disease occurred in antiquity such as the suggestion that one of the Pharaoh’s died of anaphylaxis after a bee sting1. The first convincing description of hay fever was by John Bostock who described his own symptoms in 1828. The first investigations of hay fever were published in the 1870’s by Blackley who studied grass pollen in the UK and Wyman who studied ragweed pollen in the USA2, 3. At that stage, the only recognized allergic disease was hay fever and reports of an increase came from Germany, as well as the UK and USA. It is important to recognize that there were no clear reports of an increase in pediatric asthma until 1970. Further the current “epidemic” of food allergy does not appear to have started until after 1990. This review will attempt to evaluate both the evidence for those increases and the changes that have occurred in lifestyle that could have contributed to sequential rises of different allergic diseases.
The Hay-Fever Epidemic
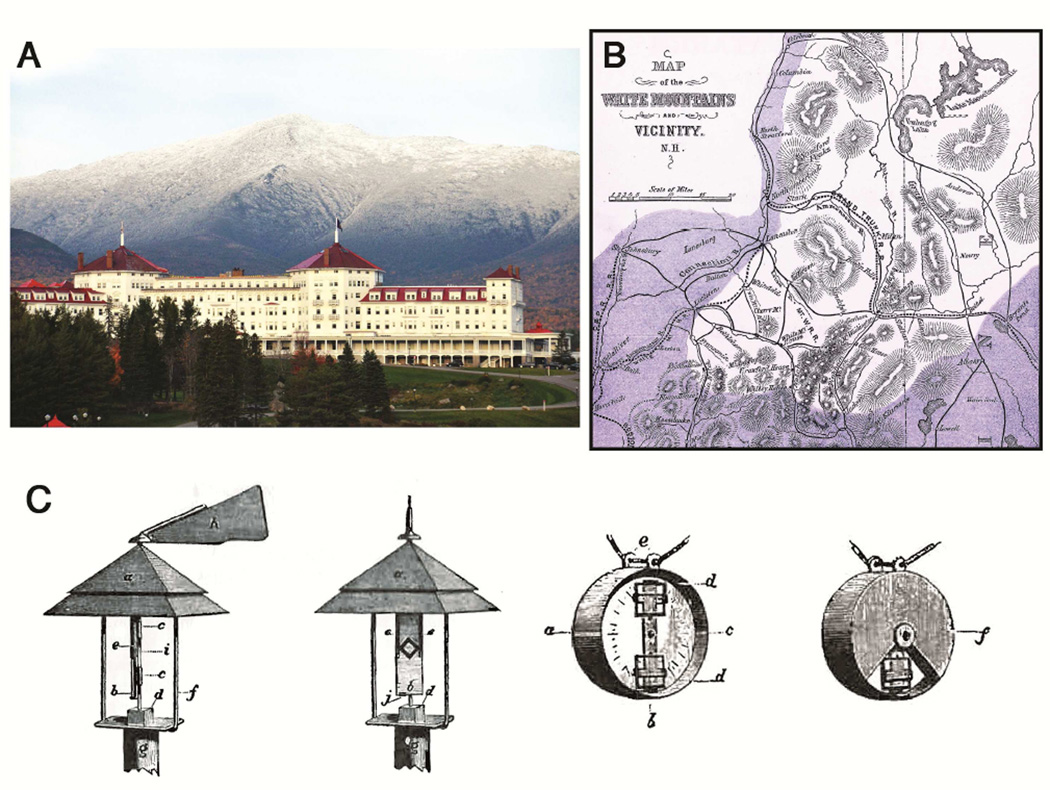
In 1982 Lady Simon, with a startling level of confidence about her facts, asked the author of this review “Why did hay fever start in 1870?”4. She then explained that her father had developed symptoms of allergic rhinitis and conjunctivitis in Germany in June of 1875 but after several years of symptoms he could not find a physician who was aware of the condition. By 1890, he knew a group of sufferers but none of them had had symptoms before 1870. Blackley had started studying the disease in Manchester, UK in the 1860’s but his studies including skin tests and challenge tests with grass pollen out of season, were primarily performed on himself2 [Fig 1]. By 1900, the disease was well recognized and sufficiently severe for two developments.
Identification of sites where hay fever sufferers could go during the season to avoid exposure to pollen: Thus the island of Heligoland in the North Sea was kept free of grass pollen and Bretton Woods resort in New Hampshire was recognized as a retreat from the ragweed season by the United States hay fever association5 [Fig 1]
The earliest investigations of the effects of injections of pollen extract were carried out with the objective of establishing immunity against pollen toxin. Those experiments were published by Dunbar in Germany and most significantly by Noon in the UK6, 7
Fig 1.
The Resort at Bretton Woods, which was recognized before 1900 as a retreat for hay-fever suffers during the ragweed pollen season [A]. A map of the pollen free areas in the White Mountains published in the 1870s [B]. The apparatuses used by Charles Blackley in his pollen counting experiments in 1872 [C]
Attribution: Panel A: Mount Washington Hotel Resort Bretton Woods New Hampshire
By user Buddymydog1972. Licensed under CC BY 2.0 via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Mount_Washington_Hotel_Resort_Bretton_Woods_New_Hampshire.JPG
Panel B: Autumnal catarrh (hay fever), map of White Mountains 1872 By user Fæ Licensed under CC BY 2.0 via Wikimedia Commons - http://commons.wikimedia.org/wiki/File:Autumnal_catarrh_(hay_fever),_map_of_White_Mountains_1872_Wellcome_L 0040001.jpg
Panel C: From book “Experimental Researches on the Causes and Nature of Catarrhus Aestivus”, C. H. Blackley, 1873. ISBN 1-871395-00-3.
The question to address is what happened in the 2nd half of the 19th century that could have contributed to the appearance and progressive rise in seasonal allergic rhinitis. It seems likely that changes in both airborne pollen and public hygiene contributed. In England, major changes in agriculture followed the reform of the corn laws in 18478. That reform allowed the import of cheap wheat from Odessa in the Ukraine, as a result of which much of English farm land lay fallow9. Between 1850 and 1880 dairy herds increased and Italian Rye grass (Lolium perene) was introduced which pollinated more heavily than any of the traditional grasses10, 11. In the USA, the progressive increase in arable farming is thought to have increased the growth of ragweed. Certainly ragweed became the most import cause of seasonal rhinitis in the USA3, 12.
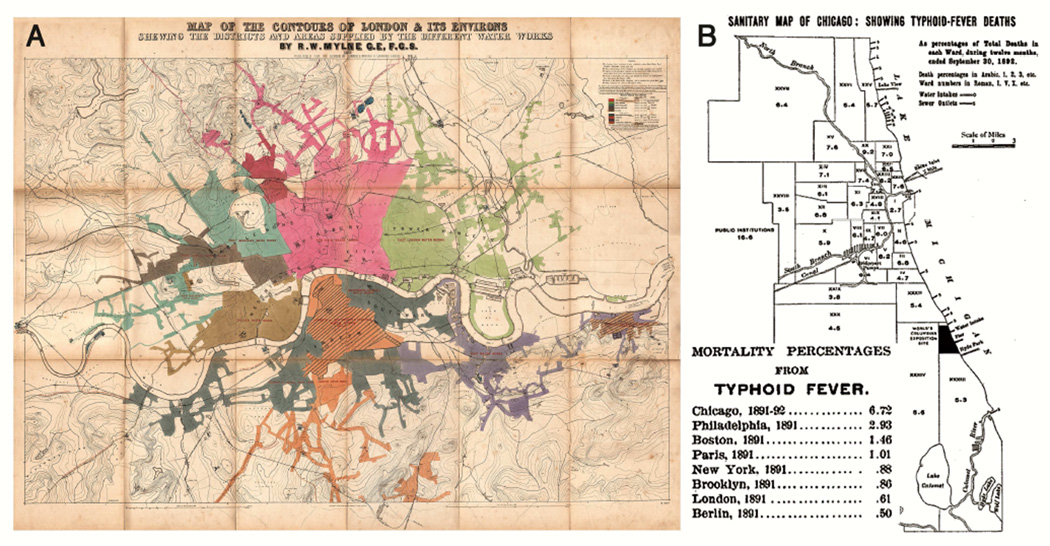
Major changes in public hygiene started during the 19th century. Given the fact that the Greeks and Romans understood the need for clean water supplies, it is difficult to believe that London in 1854 and Chicago as late as 1890 were collecting “drinking water” from the same site that was used to discharge untreated sewage13, 14 [Table 1]. The critical studies that lead to the acceptance of the relationship between sewage and enteric disease were carried out by John Snow in London. Starting with the evidence about the Soho pump and cholera, and following with epidemiological comparison in 1854 of typhoid cases among populations who obtained their water from the London River compared to those whose water came from further up the river13, 15 [Fig 2]. However as late as 1880, there was still only limited acceptance of the germ theory of disease even among physicians. Indeed in 1881, President James Garfield was “murdered” by his physicians who repeatedly probed a non-fatal gunshot wound, using non-sterile instruments and fingers16. Starting in 1892, the city of Chicago reversed the course of the Chicago River so that sewage flowed into the Mississippi rather than into Lake Michigan which was the source of drinking water14. By 1920, chlorination of water was widespread and all the major cities in the USA had clean water, as a result of which typhoid and cholera became rare. If you look at New York City, you could argue that the critical changes in hygiene were complete by 1920 [Table 2]. In keeping with that, allergy became more common and by 1946 ragweed hay-fever was such a severe problem in New York that the city council initiated a ragweed eradication campaign17, 18 [Table 2] Equally in London, Dr. Frankland’s allergy clinic had hundreds of patients in the 1950’s and he and Dr. Augustin carried out the first controlled trial of immunotherapy for grass pollen hay fever19. In fact, the rise in allergic disease was already obvious when Dr. Swineford was appointed as professor of allergy and rheumatology at the University of Virginia in 1935. He had been called back from doing pathology research in Vienna to “help deal with the allergy epidemic” and he opened the first sub-specialty clinic in the Medical School in 193620.
Table 1.
The Essence of Hygiene: What elements are likely to be relevant to the onset of allergic disease?
| Primary Measures |
Clean Water:
|
Uncontaminated Food:
|
Helminth Eradication:
|
| Secondary Elements |
| Decreased Exposure to Farm Animals – decreased diversity of bacterial exposure |
| Decreased exposure to older siblings due to small family sizes with resulting decreased transmissible infections (exposure in daycare may have the opposite effect) |
| Decreased Exposure to Soil Bacteria |
Fig 2.
London water supplies in 1854 used by John Snow as evidence that typhoid and Cholera were spread through the water [A] Typhoid fever deaths in Chicago 1892, which were controlled by extending the water intake into the lake and pumping 407 million gallons per day from the Chicago River into the Mississippi. [B]
Figure Attribution: Panel A: Mylne, Robert W. - Map of the Contours of London and Its Environs, showing the Districts and Areas supplied by the Nine Metropolitan Water Companies, Published for the Author by Edward Stanford, Charing Cross, London. Published by Waterlow and Sons, 1856. Accessed via: http://www.ph.ucla.edu/epi/snow/watermap1856/watermap_1856.html
Panel B: From book “Annual Report of the Department of Health of the City of Chicago for the Year Ended December 31, 1894”. Published by the Department of Health, City of Chicago, 1895 (public domain)
Table 2.
Allergic Diseases in New York City
| 1900 | Shoes universal Sources of clean water identified |
| 1920 | Helminths and malaria eradicated on Staten Island Water chlorination complete in New York City |
| 1924 | Last abattoir closed Horses becoming less common |
| 1932–1950 | Allergic disease increases up to 10–13% |
| 1946 | Ragweed eradication campaign started in Manhattan because of the severity of hay fever in the city |
| 1982 | Asthma was rated #1 medical problem of the city, but this was reversed because of the explosion of HIV during the year |
| 1996 | “Emerging epidemic of asthma” in New York schools; 200 or 1,100 students in East Harlem on treatment (See New York Times; Sept. 29th, 2996) |
| 1997 | Mayor Giuliani declares war on rats in New York City |
The epidemic rise of asthma among children: 1960–2000 [Table 3]
Table 3.
Changes That Have Been Suggested As Explanations For The Progressive Rise In Pediatric Asthma, 1955–2000
| I. | Increased number of immunizations in early childhood and possible changes in vaccines |
| II. | Progressive increase in the use of broad spectrum antibiotics |
| III. | Use of paracetamol to treat fever in childhood, which replaced aspirin, following identification of Reyes Syndrome in 1979 |
| IV. | Changes that occurred either due to or in parallel with the introduction and increase in indoor entertainment: primarily television programs for children, 1955 onwards
|
| V. | Increased exposure to indoor allergens, secondary to less time outdoors and higher quantities indoors |
Prior to 1960, most text books of pediatrics did not regard asthma as common let alone epidemic. During the 1960’s, there were occasional reports that asthma appeared to be becoming more common, but the first convincing publication came in 1969. Smith, et al carried out a population based study on school children in Birmingham, UK which demonstrated a sharp increase in asthma between 1958 and 196821. In addition, they reported that many of the children with asthma had positive skin tests to dust mites. Over the next few years, reports on the increasing prevalence of asthma came from several countries but predominantly from countries where dust mites were the dominant allergen. Thus increases were reported from Australia, New Zealand, and Japan as well as the UK21–23. Indeed by the 1980’s, it was possible to argue that increasing growth of dust mites in houses was an important cause of the increase in asthma24, 25. That argument was helped by the fact that homes in the UK, Australia and New Zealand had become warmer, tighter and had more carpets. This in turn was thought to have provided improved conditions for the growth of dust mites and for the accumulation of debris from dust mite growth26. However, it is important to recognize that a large part of the reason for wanting homes warmer and less drafty was because of the rise in indoor entertainment.
Although it is well known today that asthma has increased in all western countries, it may be forgotten that this did not become clear until 1990. In that year, the data on asthma among recruits to the Finnish and the Swedish armies came out, showing a progressive rise over 20 years27, 28. However, in large parts of Sweden the dominant allergens associated with asthma are those associated with cats or dogs29, 30. In addition, evidence was accumulating that cockroach was a major allergen related to asthma among African Americans living in poverty in the United States31–33. By 1995, it was accepted that both prevalence and hospitalization for asthma had increased among children living in climates or living conditions where several different allergens dominated both exposure and sensitization34–36. At this point, it became very difficult to argue that the increase had occurred simply because of an increase in allergens in homes since there was no reason to think that dust mite, cockroach, cat and Alternaria had all increased in parallel. It is important to recognize that the best evidence about the role of allergens in asthma came between 1970 and 1980 with the convincing demonstrations that chronic allergen exposure could make a major contribution to nonspecific bronchial hyper-reactivity (BHR)24, 37–39.
Any attempt to explain the increase in pediatric asthma has to deal with the progressive nature of the increase. While major changes were present by 1980, the increase continued for at least two more decades. Although there is evidence for many different aspects of the rise in asthma prevalence and severity most of these arguments cannot explain either the time course or the scale of the increase [Fig 3]. A typical example is the change from aspirin to paracetamol in 1979 following the identification of Reyes syndrome. This change may well have contributed to the severity of asthma but did not occur until half way through the increase40. In most studies, the children with asthma were found to be allergic to one or more of the common perennial allergens. In Australia Peat and Woolcock reported detailed studies on the “modifiable risk factors for asthma,” including diet and immunization, and they concluded that dust mite allergy was by far the most important of these factors41, 42.
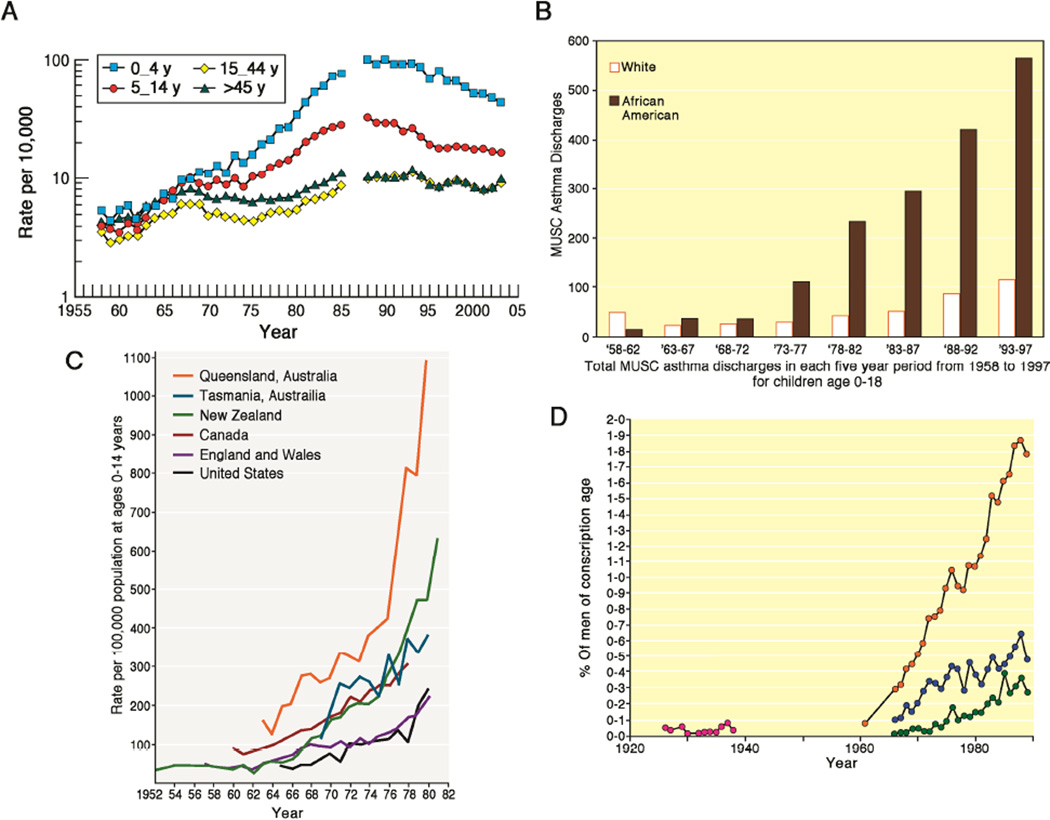
Fig 3.
Published data on the rise in hospitalizations of children and young adults due to asthma in four countries during the 20th century: asthmatics in the United Kingdom [A, See Ref 34]; children with asthma at the Medical College of South Carolina [B, See Ref 35]; children in Australia, New Zealand, Canada and the USA [C, See Ref 36]; and Finnish army recruits [D, See Ref 27]. All figures are used with permission but have been colorized differently than the original source material.
Taking everything into account the changes that fit the time course best are the ultimately disastrous changes that occurred following the introduction of television programs designed for children in the 1950’s [Table 3]. There are three questions that need addressing in relation to this period:
Were there changes in hygiene during this period that could explain the timing, the scale, or the severity of the increase in asthma?
Could decreased physical activity or simply changes in breathing patterns secondary to sitting and watching a screen have had a major effect?
Could any of the other changes explain more than a minor part of the epidemic; these include obesity, increasing schedules of immunization of children and the change from aspirin to acetaminophen.
What is Hygiene? Could changes in hygiene explain the rise in pediatric asthma 1960–2000
In 1980 David Strachan, proposed that repeated exposure to respiratory or other infections could decrease allergic disease43. At that time, his observations were primarily related to infections transmitted by older siblings. However, those observations were made on the basis of data within a country (the UK) where water had been clean for many years, parasitic helminths were not a major problem, and only a small proportion of the population were exposed to farm animals. By contrast, in Africa, India, and South America there are today many “pre-hygiene” communities where i) water supplies are contaminated by untreated sewage, ii) helminth infection is common, iii) children go barefoot and iv) the houses are not a place where children would choose to stay during the day44–47.
In addition, to studying differences between countries with a fully modern pattern of allergic disease and pre-hygiene communities there are three models where it is possible to study the effect of differences in “hygiene” within a community:
Countries such as Kenya, Ghana or Ecuador where changes are occurring currently44–47.
Villages and small towns in Europe where farming families live close to non-farming families48, 49.
There are two halves to these analyses: firstly what are the features of modern allergic disease that characterize a post hygiene state and second what elements of hygiene are essential for this change [Table 1]. In pre-hygiene villages, children and adults generally have elevated total serum IgE, low titer IgE antibodies to mite and other allergens, and if tested will often have IgE antibodies to parasite related antigens such as Ascaris or the tick related galactose alpha-1, 3-galactose44, 46, 47. In post hygiene society mean total IgE levels are lower, and specific IgE antibodies to common inhalant allergens are common and often present in high titer. In addition, the presence of high titer IgE antibodies correlates with both hay fever and asthma30, 54. In some studies, the mean total serum IgE among non-atopic individuals is as low as 20 IU/ml. In the post hygiene communities asthma is often severe and many children require regular treatment. By contrast, in pre-hygiene communities although wheezing is not uncommon, it is generally not severe and treatment is unusual44, 45. Given that the major changes in hygiene had occurred in London, New York and Munich by 1920, it is difficult to ascribe the massive changes in prevalence of asthma between 1960 and 2000 to the minor changes in hygiene that occurred, over that period.
The original observations about family size have not been confirmed consistently30, 55. Indeed a major study in Denmark found that the presence of increased bacteria in the upper airways was associated positively with the development of asthma. Furthermore, they reported that the presence of older siblings increased the risk, i.e. the opposite of the original observations56, 57. Overall, the best definition of the hygiene effect relates to changes that started in 1870 and were largely complete in the major cities of Europe and the USA by 1920. Similar changes are occurring today in Africa, India and South America. However, the shift to a western model of asthma appears to occur rapidly i.e. within 5 years following a major change in hygiene. Thus there is no reason to think that the changes in public hygiene that occurred in 1920 can be used to explain the timing of the increase in asthma from 1960 – 2000 [Fig 3].
The relevance of decreased physical activity and changed breathing patterns to the rise in pediatric asthma
One of the most obvious effects of “indoor entertainment” was a progressive increase in the number of hours children spent sitting each day. There are a large number of secondary consequences of this change that include increased obesity, changes in diet, more exposure to indoor allergens, etc. However our main concern here is with the lungs. Any form of physical activity will lead to full expansion of the lungs, but in addition normal breathing includes periodic deep breaths or sighs58. Studies on the effects of deep breathing have been of two major types:
Firstly: Fredberg and his colleagues carried out detailed studies on the physiology of bronchial smooth muscle. Those studies concluded that bronchial smooth muscle does not obey Starling’s law, and will start to contract at a shorter length if not stretched regularly59. He went so far as to state that, “stretching smooth muscle is a more potent bronchodilator than isoprenaline” and to describe sighs as the primary protection against bronchospasm60.
Secondly: The alternative form of the same experiment has been to study the effects of prolonged shallow breathing in human volunteers. Those studies by several different groups have shown that prolonged shallow breathing will result in increased lung resistance and increased BHR61–63. The question then is whether watching a screen can influence the breathing pattern. At present, the correct study on children watching a TV program compared to playing a computer game or texting has not been reported. However, students watching a screen have a significantly lower sigh rate compared to the same students reading a book64. Thus it is reasonable to propose that children watching a screen, without interaction with the program not only lack physical activity but may also experience prolonged periods of shallow breathing of exactly that form that has been shown to increase BHR.
Remarkably, regular exercise is not part of the standard treatment for asthma65. This despite the fact that aerobic activity is recommended for cystic fibrosis, COPD and a wide range of cardiological conditions. Many studies have shown a positive effect of exercise on asthma or on BHR, but these have not been converted into a consistent recommendation about exercise as part of the treatment66. In addition, it is not clear whether the primary effect of exercise would be to decrease the inflammation in the lungs or a physiological effect secondary to regular stretching of smooth muscle.
Other factors or Changes that have been suggested as playing a role in the increase in asthma
The list of explanations for the increase in asthma is not short [Table 3]. Most of these could be relevant to the increase but only a small number could have played a major role. Typical examples include: broad spectrum antibiotics; air pollution; global warming; obesity, and acetaminophen.
Broad spectrum antibiotics were widely available by 1965 and although their use has increased steadily, the major changes occurred very early in the epidemic. Clearly, it is possible that use of antibiotics early in life has played a role in changing the fecal biome; however, such changes tend to be transient, and most epidemiological studies on antibiotic use have only shown a modest effect on the prevalence of allergic disease67, 68.
Increases in air pollution could well have played a role in asthma in places like Los Angeles. However, asthma has increased in many other areas where air pollution is an insignificant problem e.g. coastal towns in New Zealand, or in places where air pollution has progressively decreased e.g. London. There is good evidence about the possible effects of diesel particulates, both in relation to sensitization and as a cause of direct irritation of the lungs69, 70. On the other hand, industrial pollution related to coal smoke is not a convincing cause of asthma. Indeed, in a town such as Katowice in Poland where the industrial pollution was very severe the children developed bronchitis but asthma was less common71.
Obesity has been one of the major consequences of the indoor lifestyle which includes a role for both dietary changes and decreased physical activity. Furthermore, there are strong correlations between time spent watching screens, asthma and obesity72, 73. So the question is whether obesity itself has made a contribution to asthma prevalence? There are two issues here; first, what is the evidence about an association with wheezing? And secondly what is the mechanism if there is an association? The main problem with the epidemiological data is that asthma diagnosis is generally based on questions such as “do you, (or your child), become short of breath on exercise”? The problems with such a question in an obese population are obvious74, 75. Our group recently reported that we could not find a difference in lung function between obese teenagers with a diagnosis of asthma and those without. The conclusion of our study was that many obese children receive a diagnosis of asthma because of symptoms that are primarily due to them being unfit76.
Global warming is having major effects in many fields, but the changes in temperate climates, so far, have been modest compared with the changes in exposure associated with the adoption of an indoor lifestyle. There is interesting data showing that increased concentrations of CO2 in the air can increase growth and pollen production by some of the important plants related to allergy including ragweed77. However, those data do not relate to the indoor allergens which are most strongly associated with asthma.
Increased use of acetaminophen instead of aspirin to treat fever in childhood. This change occurred rapidly following the discovery in 1979 that aspirin could induce Reyes Syndrome. Since then multiple studies have provided evidence that acetaminophen use can increase both the severity and the prevalence of asthma78–80. However, if we look at the time course of the increase in asthma major increases had occurred before 1980. Thus, if acetaminophen played a role it was in the continued increase, not related to the onset of the epidemic.
Progressive increase in the recommended immunization of children 1950–2010. The number of injections that children receive in early childhood is a concern for many parents and pediatricians. In addition, several authors have suggested a possible role of these injections in the increase in allergic disease or in food allergy in particular. There are several elements that have been identified. First, there was the possible protective role of BCG immunization; however, after the first report several studies did not confirm the effect81, 82. In addition, there was evidence that the increase in asthma prevalence did not look significantly different in countries where BCG vaccination was routine, (e.g., Brazil or Ireland) compared to those such as the United States where BCG was never adopted. Second, many of the vaccines contain alum and some investigators have implied that the total quantity of alum used could play a significant role in enhancing Th2 responses. Thirdly, there was an important change in the Pertussis vaccine from a cellular form to an acellular form. This last change took place around 1992 i.e. too late to play a significant role in the asthma epidemic but in time to be relevant to food allergy. Interestingly, the effects of pertussis vaccination have been investigated both prior to the change to the acellular form and also after the change83–85. In the investigation by Dr. Aalberse and his colleagues they found that the cellular vaccine downregulated IgE and IgG4 antibodies to tetanus toxoid and diphtheria toxoid83. By contrast, two separate groups have reported a strong pro-Th2 effect of the acellular vaccine84, 85. Needless to say, a proper controlled trial comparing the effects of cellular versus acellular pertussis vaccine on allergic disease has not yet been conducted.
The Dramatic Rise in Food Allergy 1990 to the present
Allergic or anaphylactic reactions to peanuts and other foods have been recognized for many years. However starting about twenty years ago most clinics in the USA and the UK observed an increase in the number of cases. Further, it was clear that at least for peanut the titers of IgE antibodies to the relevant proteins were often very high. The observations made in the clinics have been confirmed in population based birth cohorts86, 87. This “epidemic” cannot possibly be ascribed to the changes in water quality, etc. that occurred seventy years earlier or to changes in physical activity that started at least 30 years earlier. The cause of the increase in the USA and in London is not clear but several elements of the argument have been clarified. Firstly, it is clear that early exposure is not the cause of increased peanut allergy. Indeed it is now certain that oral exposure during the first five years can be protective88, 89 whether exposure in utero can also be protective remains a question90, 91. Secondly, there is now good evidence both for peanut and for wheat that sensitization can occur from exposure through the skin92, 93. In some studies, this is restricted to children who have eczema and/or a defect in Filaggrin94. The important implication is that for children who avoid oral exposure the presence of peanut products in the house that will inevitably get on the child’s skin can increase the risk of sensitization95. However, it is not clear that there have been big enough changes in the presence of peanut products or the preparation of peanut products over the period of the increase. If the skin is an important route, for sensitization is it possible that there have been changes in the skin secondary to diet or to skin care? The skin care issue is interesting because the washing of babies has undoubtedly increased. With progressively smaller family size there is a tendency to wash babies daily which was certainly not normal 50 or even 25 years ago. Thus it is conceivable that skin permeability to foreign proteins has changed96, 97. Interestingly the proposed explanations for the increase in food allergy relate to changes that could be reversed.
Regional Outbreaks of Asian Lady Beetle Allergy and Delayed Anaphylaxis to red meat
In the United States, there have been two regional outbreaks of allergic disease in the last ten years. One was caused by infestation of homes by the Asian lady beetle (Harmonia axyridis), which had been introduced to control aphids98, 99. Interestingly, lady beetles had not previously been identified as a source of allergens. The diagnosis had to be made with locally or individually made extracts and thus it is difficult to know anything about the overall prevalence99. The more recent “outbreak” has been of delayed anaphylaxis to red meat100. In this case, the cause appears to be a major increase in tick bites from the lone star tick. This increase is best explained by the truly dramatic increase of deer in both rural and suburban areas of the east coast101. At present, it is not clear how far this epidemic will go but it is occurring in Australia as well as Germany, France and Sweden102–105. This novel form of delayed allergic reaction is already the commonest cause of anaphylaxis among adults presenting to clinics in Virginia106. It is not clear how this epidemic could be controlled, because both larval and adult lone star ticks are remarkably enthusiastic about biting humans107.
Conclusions
The rise in allergic disease did not start until the most important changes in hygiene had been achieved. In keeping with that, the forms of allergic disease that are most common in developed countries are not present today in Kenyan, Ethiopian, and Ecuadorian villages or in poor areas of a major city in Ghana44–47. However, the real epidemics require more than hygiene alone. Hay fever appeared in the latter part of the 19th century and the first half of the 20th century when grass pollen in the UK and ragweed pollen in the USA, were already present at high levels [Fig 4]. The rise in pediatric asthma started in 1960 and has become obvious in all post-hygiene societies. However, the timing does not match any major changes in hygiene. Indeed, the changes that fit the timing of the increase in asthma relate to the move of children indoors that started with the introduction of television programs for children. The move indoors has had many consequences, including the rise in obesity and decline in physical fitness. However, the consequences that seem most relevant are the steady increase in sensitization to perennial indoor allergens, the decline in outdoor exercise and the remarkable amount of time children spend watching a screen.
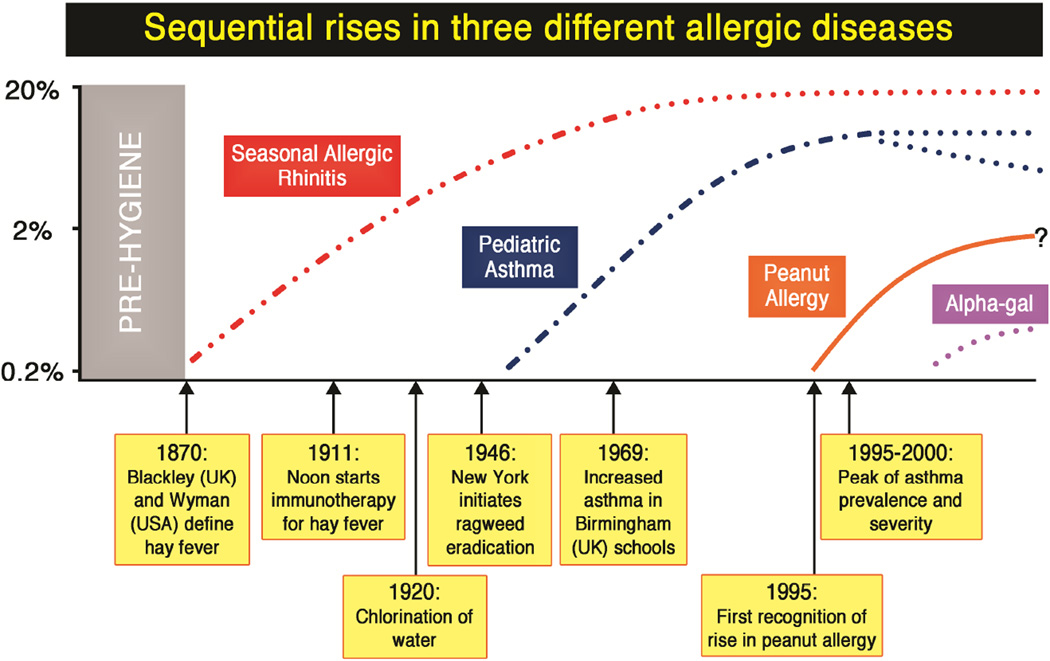
Fig 4.
Sequential rises in allergic rhinitis, pediatric asthma and peanut allergy with respect to key events in the adoption of modern hygiene of Western society.
We are all familiar with the time course and scale of the increase in symptomatic wheezing as documented so well by the ISAAC studies49. However, the rise in asthma has also been seen in treatment and hospital admissions (see Fig 3). Furthermore, the shift to poor populations in the United States was also clear in mortality statistics between 1970 and 1990108. The evidence that allergic rhinitis increased over that same period was based on soft questions about nasal symptoms. By contrast several surveys on hay fever confirmed a prevalence of around 10% before 1970, and two surveys in 1969 and 1997 suggest that the increase was not significant over that period109, 110. Over the period 1960–2000 the rise in symptomatic asthma was matched by rises in exacerbations requiring acute treatment (See Fig 3 A, B, C)34–36. Many or most of these admissions in children and young adults are triggered by viral infections. In infants and toddlers many different viruses are involved, but in children over age 3 and young adults more than 90% of the viral induced episodes are triggered by rhinoviruses54, 111–114. The central role of allergy in these acute episodes has been reinforced by the evidence that omalizumab can control these exacerbations115. Given that these viral infections have always been very common the increase in acute episodes is best explained by changes in the number of subjects with increased BHR and inflammation of their lungs. Taken together, the best explanation for the increase in asthma is that it resulted from an increase in sensitization to indoor allergens and the loss of a lung specific protective effect of regular deep inspiration.
Although some studies suggest that both hay fever and asthma prevalence have continued to increase there is other evidence that the severity of both hay fever and asthma have decreased. In the case of hay fever, several developments may have contributed to a decrease in the severity of symptoms. These include, improved anti-histamines, and nasal sprays, but equally less time spent outdoors. The move of adults indoors has been facilitated by the availability of home air-conditioning (starting in ~1965), which makes it possible to exclude pollen from houses. The decrease in prevalence and severity of asthma in the United States could have had several different causes. The most obvious is the introduction of combination inhalers, which include both steroids and long acting beta-2-agonists. However, it is also possible, that the breathing patterns of children are different when they are working on a computer or texting. As was mentioned earlier, the correct study comparing children’s breathing patterns while on a computer compared to a television program has not been reported. However, in addition, there is considerable public awareness of the significance of allergen exposure in the home, and it is not difficult to obtain full advice about avoidance measures for dust mite from an allergist or online.
The real conclusion of this review is that allergic disease has developed in large part as a result of changes in lifestyle. The development of public hygiene was driven by a logical desire to avoid enteric, insect borne and helminth infections. However, that has had the major consequence of allowing up to 50% of the population to become sensitive to otherwise irrelevant foreign proteins. Initially, this was predominantly the inhaled pollens associated with hay fever, and then extended to the perennial indoor allergens associated so strongly with asthma. Most recently a range of foods has become the focus. Although avoidance may have a role in relation to pollens and indoor allergens, it is now clear that avoiding oral exposure is the wrong strategy for foods. What is also clear is that none of the consequences of changes in the way we live have been predicted. John Snow was not worrying about hay fever. The inventors of the Mickey-Mouse Club did not imagine that they would help to put thousands of children in hospital with asthma. Equally, those of us (in fact most of us), who earnestly advised mothers to avoid peanuts for their child’s first two years did not imagine that the strategy would make the situation worse. Equally, when leash laws were introduced into thousands of suburban subdivisions, no one warned that an increased deer population would increase tick bites and sensitization to the oligosaccharide alpha-gal. Unfortunately, it seems most unlikely that we will correctly predict the allergic consequences of future so-called improvements in the way we live. However, we should do our best to continue “the unequal attempt to keep up with the consequences of real changes in life style”.108,116–118.
Table 4.
Pre-existing factors and changes that could be relevant to the rise of peanut allergy in the USA: 1990 to the present
| I. | Differences in the preparation of peanut products:
|
| II. | Delayed oral consumption of peanut proteins:
|
| III. | Changes in skin as a result of daily bathing with soap or detergents:
|
| IV. | Changes in vaccination policy:
|
What do we know?
The major changes relevant to hygiene i.e. clean water and helminth eradication, started in 1850 and were established in the major cities of the USA and Europe by 1920.
The relevance of pollen to hay-fever was first defined in 1870, and by 1900 the disease was common among the “leisured classes”. By 1940, hay fever was epidemic.
Pediatric asthma rose steadily from 1960 to 2000 and the clearest correlation is with the move of children indoors.
What is still unknown?
Which consequences of the move indoors were most important to the rise in asthma: i) increased sensitization to indoor allergens ii) long periods of time spent sitting with inadequate expansion of the lungs: iii) changes in diet.
The reasons why peanut allergy has become more common may include: i) changes in vaccines particularly the change from cellular to acellular pertussis iii) excessive washing of the skin that could have increased penetration of the skin by peanut proteins iv) attempts to avoid oral peanut.
After the primary changes in hygiene, has the move indoors added a further element that can best be reversed by having a dog in the house?
Acknowledgements
I thank the many colleagues who have contributed over the years to understanding the history as well as for many helpful comments on the text. In particular, I would like to thank Matt Perzanowski and Philip Copper for discussions about the nature of the hygiene effect, and I thank Jane El-Dahr, Scott Commins, and Pat Holt for helpful discussions about the impact of vaccination.
Abbreviations
- BHR
Bronchial Hyper-Reactivity
- COPD
Chronic Obstructive Pulmonary Disease
- BCG
Bacillus Calmette – Guerin
- ISAAC
International Study of Asthma and Allergy in Childhood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergmann K, Ring J. History of Allergy in Antiquity in History of Allergy by Karger in Unionville, CT. 2014 [Google Scholar]
- 2.Blackley CH. Experiments and researches on the causes of nature of catarrhus aestivas. Balliere, London: 1873. [Google Scholar]
- 3.Wyman M. Autumnal catarrh (Hay fever) Cambridge, Mass: Huro & Houghton; 1872. [Google Scholar]
- 4.Platts-Mills T. 1983. London University PhD Thesis: Local Production of IgG, IgA & IgE antibodies to pollen allergen in patients with hay fever; p. 163. [Google Scholar]
- 5.Mitman G. Hay fever holiday: Health, leisure, and place in gilded-age america. Bull. Hist. Med. 2003;77:600–635. doi: 10.1353/bhm.2003.0127. [DOI] [PubMed] [Google Scholar]
- 6.Dunbar W. The present state of knowledge of hay fever. Journal of Hygiene. 1913;13:105. doi: 10.1017/s0022172400005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noon L. Prophylactic innoculation for hay fever. Lancet. 1911;i:1572. [Google Scholar]
- 8.Briggs A. The making of modern England 1783–1867: The age of improvement. 1959:314. [Google Scholar]
- 9.deWaal E. The Hare with Amber Eyes. Picador, Farrr, Straus and Giroux, NY. 2010 [Google Scholar]
- 10.Johnson P, Marsh DG. 'Isoallergens' from rye grass pollen. Nature. 1965;206:935–937. doi: 10.1038/206935b0. [DOI] [PubMed] [Google Scholar]
- 11.Davies R. Grass pollen counts: London 1961–1980. Clinical Allergy. 1982;12:511–512. [Google Scholar]
- 12.King T, Norman P. Isolation studies of allergens from ragweed pollen. Biochemistry. 1962;1:709. doi: 10.1021/bi00910a027. [DOI] [PubMed] [Google Scholar]
- 13.Johnson SB. The Ghost Map. Riverhead, London: 2006. [Google Scholar]
- 14.Reynolds A, Hazen A. The Water-Supply of Chicago: Its Source and Sanitary Aspects. Public Health Pap Rep. 1893;19:146–151. [PMC free article] [PubMed] [Google Scholar]
- 15.Hempel S. John Snow. The Lancet. 2013;381:1269. doi: 10.1016/s0140-6736(13)60830-2. [DOI] [PubMed] [Google Scholar]
- 16.Millard C. A tale of madness, medicine, and the murder of a president. New York, NY: Doubleday; 2011. Destiny of the Republic. [Google Scholar]
- 17."4000 Acres that breed sneezes, target in City's hay fever war". New York Times. 1949 Jun 3; [Google Scholar]
- 18.Walzer M, Siegel BB. The effectiveness of the ragweed eradication campaigns in New York City; a 9-year study; 1946–1954. J Allergy. 1956;27:113–126. doi: 10.1016/0021-8707(56)90002-8. [DOI] [PubMed] [Google Scholar]
- 19.Frankland A, Augustin R. Prophylaxis of summer hay fever and asthma: Controlled trial comparing crude grass pollen extracts with isolated main component. Lancet. 1954;i:1055. doi: 10.1016/s0140-6736(54)91620-7. [DOI] [PubMed] [Google Scholar]
- 20.Swineford O., Jr . Asthma and hay fever. Springfield, IL: Charles C Thomas; 1971. [Google Scholar]
- 21.Smith JM, Disney ME, Williams JD, Goels ZA. Clinical significance of skin reactions to mite extracts in children with asthma. Br Med J. 1969;2:723–726. doi: 10.1136/bmj.2.5659.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke CW, Aldons PM. The nature of asthma in Brisbane. Clin Allergy. 1979;9:147–152. doi: 10.1111/j.1365-2222.1979.tb01535.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto T, Johansson SG, Ito K, Horiuchi Y. Atopic allergy in Japanese subjects: studies primarily with radioallergosorbent test. J Allergy Clin Immunol. 1974;53:9–19. doi: 10.1016/0091-6749(74)90094-3. [DOI] [PubMed] [Google Scholar]
- 24.Platts-Mills TAE, Mitchell EB, Tovey ER, Chapman MD, Wilkins SR. Airborne allergen exposure, allergen avoidance and bronchial hyperreactivity. In: Kay AB, Austen KF, Lichtenstein LM, editors. Asthma: physiology, immunopharmacology and treatment, Third International Symposium. London: Academic Press; 1984. pp. 297–314. [Google Scholar]
- 25.Platts-Mills TA, Tovey ER, Mitchell EB, Moszoro H, Nock P, Wilkins SR. Reduction of bronchial hyperreactivity during prolonged allergen avoidance. Lancet. 1982;2:675–678. doi: 10.1016/s0140-6736(82)90709-7. [DOI] [PubMed] [Google Scholar]
- 26.Tovey ER, Chapman MD, Wells CW, Platts-Mills TA. The distribution of dust mite allergen in the houses of patients with asthma. Am Rev Respir Dis. 1981;124:630–635. doi: 10.1164/arrd.1981.124.5.630. [DOI] [PubMed] [Google Scholar]
- 27.Haahtela T, Lindholm H, Bjorksten F, Koskenvuo K, Laitinen LA. Prevalence of asthma in Finnish young men. BMJ. 1990;301:266–268. doi: 10.1136/bmj.301.6746.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braback L, Hjern A, Rasmussen F. Trends in asthma, allergic rhinitis and eczema among Swedish conscripts from farming and non-farming environments. A nationwide study over three decades. Clin Exp Allergy. 2004;34:38–43. doi: 10.1111/j.1365-2222.2004.01841.x. [DOI] [PubMed] [Google Scholar]
- 29.Erwin EA, Ronmark E, Wickens K, Perzanowski MS, Barry D, Lundback B, et al. Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. J Allergy Clin Immunol. 2007;119:359–365. doi: 10.1016/j.jaci.2006.12.648. [DOI] [PubMed] [Google Scholar]
- 30.Ronmark E, Bjerg A, Perzanowski M, Platts-Mills T, Lundback B. Major increase in allergic sensitization in schoolchildren from 1996 to 2006 in northern Sweden. J Allergy Clin Immunol. 2009;124:357–363. 63 e1–63 e15. doi: 10.1016/j.jaci.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulett A, Dockhorn R. House dust, mite (D. farinae) and cockroach allergy in a midwestern population. Ann Allergy Asthma Immunol. 1979;43:160–165. [PubMed] [Google Scholar]
- 32.Gelber L, Seltzer L, Bouzoukis J, Pollart S, Chapman M, Platts-Mills T. Sensitization and exposure to indoor allergens as risk factors for asthma among patients presenting to hospital. Am Rev Respir Dis. 1993;147:573–578. doi: 10.1164/ajrccm/147.3.573. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 34.Anderson H, Gupta R, Strachan D, Limb E. 50 years of asthma: UK trends from 1955 to 2004. Thorax. 2007;62:85–90. doi: 10.1136/thx.2006.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crater DD, Heise S, Perzanowski M, Herbert R, Morse CG, Hulsey TC, et al. Asthma hospitalization trends in Charleston, South Carolina, 1956 to 1997: twenty-fold increase among black children during a 30-year period. Pediatrics. 2001;108:E97. doi: 10.1542/peds.108.6.e97. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell E. International trends in hospital admission rates for asthma. Arch Dis Child. 1985;60:376–378. doi: 10.1136/adc.60.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altounyan REC. Changes in histamine and atropine responsiveness as a guide to diagnosis and evaluation of therapy in obstructive airways disease. In: Pepys J, Frankland AW, editors. Disodium Chromoglycate in Allergic Airways Disease. London: Butterworth; 1970. [Google Scholar]
- 38.Kerrebijn K. Endogenous factors in childhood CNSLD: methodological aspects in population studies. The Netherlands: Royal Vangorcum Assesn; 1970. pp. 38–48. [Google Scholar]
- 39.Cockcroft D, Ruffin R, Dolovich J, Hargreave F. Allergen-induced increase in non-allergic bronchial reactivity. Alergy. 1977;7:503–513. doi: 10.1111/j.1365-2222.1977.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 40.Shaheen S, Newson R, Ring S, Rose-Zerilli M, Holloway J, Henderson A. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allegy Clin Immunol. 2010:1141. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peat JK, Tovey E, Mellis CM, Leeder SR, Woolcock AJ. Importance of house dust mite and Alternaria allergens in childhood asthma: an epidemiological study in two climatic regions of Australia. Clin Exp Allergy. 1993;23:812–820. doi: 10.1111/j.1365-2222.1993.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 42.Peat JK, Li J. Reversing the trend: reducing the prevalence of asthma. J Allergy Clin Immunol. 1999;103:1–10. doi: 10.1016/s0091-6749(99)70517-8. [DOI] [PubMed] [Google Scholar]
- 43.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perzanowski MS, Ng'ang'a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr. 2002;140:582–588. doi: 10.1067/mpd.2002.122937. [DOI] [PubMed] [Google Scholar]
- 45.Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 46.Stevens W, Addo-Yobo E, Roper J, Woodcock A, James H, Platts-Mills T, et al. Differences in both prevalence and titre of specific immunoglobulin E among children with asthma in affluent and poor communities within a large town in Ghana. Clin Exp Allergy. 2011;41:1587–1594. doi: 10.1111/j.1365-2222.2011.03832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endara P, Vaca M, Platts-Mills TA, Workman L, Chico ME, Barreto ML, et al. Effect of urban versus rural residence on the association between atopy and wheeze in Latin America: findings from a case-control analysis. Clin Exp Allergy. 2014 doi: 10.1111/cea.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 49.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 50.Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29:611–617. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 51.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 52.Wegienka G, Johnson CC, Havstad S, Ownby DR, Nicholas C, Zoratti EM. Lifetime dog and cat exposure and dog-and cat-specific sensitization at age 18 years. Clin Exp Allergy. 2011;41:979–986. doi: 10.1111/j.1365-2222.2011.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man's best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126:410–412. 2 e1–2 e3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol. 2012;129:1499–1505. e5. doi: 10.1016/j.jaci.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strachan D. Family size, infection and atopy: the first decade of the "hygiene hypothesis". Thorax. 2000;55(Suppl 1):S2–S10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 57.Stallings AP, Commins SP, Platts-Mills TA. Asthma and neonatal airway colonization. N Engl J Med. 2008;358:423–424. author reply 4–5. [PubMed] [Google Scholar]
- 58.Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchiprotection is stronger than bronchodilation. Am J Respir Crit Care Med. 2000;162(3 Pt 1):910–916. doi: 10.1164/ajrccm.162.3.9907048. [DOI] [PubMed] [Google Scholar]
- 59.Fredberg J, Inouye D, Mijailovich S, Butler J. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med. 1999;159:959–967. doi: 10.1164/ajrccm.159.3.9804060. [DOI] [PubMed] [Google Scholar]
- 60.Fredberg JJ. Airway smooth muscle in asthma: flirting with disaster. Eur Respir J. 1998;12:1252–1256. doi: 10.1183/09031936.98.12061252. [DOI] [PubMed] [Google Scholar]
- 61.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadel J, Tierney D. Effect of a previous deep inspiration on airway resistance in man. J Appl Physiol. 1961;16:717–719. doi: 10.1152/jappl.1961.16.4.717. [DOI] [PubMed] [Google Scholar]
- 63.Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol. 1981;50:1079–1086. doi: 10.1152/jappl.1981.50.5.1079. [DOI] [PubMed] [Google Scholar]
- 64.Hark WT, Thompson WM, McLaughlin TE, Wheatley LM, Platts-Mills TA. Spontaneous sigh rates during sedentary activity: watching television vs reading. Ann Allergy Asthma Immunol. 2005;94:247–250. doi: 10.1016/S1081-1206(10)61303-8. [DOI] [PubMed] [Google Scholar]
- 65.National institues of Health. Expert panel report 3: guidelines for the diagnosis and management of asthma. Bethesda: National Institues of Health; 2007. National Heart, Lung, and Blood Institute: National Asthma Education Prevention Program. NIH publication no. 07-4051. [Google Scholar]
- 66.Shaaban R, Leynaert B, Soussan D, Anto J, Chinn S, de Marco R, et al. Physical activity and bronchial hyperresponsiveness: European Community Respiratory Health Survey II. Thorax. 2006;62:403–410. doi: 10.1136/thx.2006.068205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoskin-Parr L, Teyhan A, Blocker A, Henderson A. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose=dependent relationship. Pediatr Allergy Immunol. 2013;24:762–771. doi: 10.1111/pai.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cullinan P, Harris J, Mills P, Moffat S, White C, Figg J, et al. Early prescriptions of antibiotics and the risk of allergic disease in adults: a cohort study. Thorax. 2004;59:11–15. [PMC free article] [PubMed] [Google Scholar]
- 69.Diaz-Sanchez D, Garcia M, Wang M, Jyrala M, Saxon A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J Allergy Clin Immunol Pract. 1999;104:1183–1188. doi: 10.1016/s0091-6749(99)70011-4. [DOI] [PubMed] [Google Scholar]
- 70.Ghio A, Smith C, Madden M. Diesel exhaust particles and airway inflammation. Curr Opin Pulm Med. 2012;18:144–150. doi: 10.1097/MCP.0b013e32834f0e2a. [DOI] [PubMed] [Google Scholar]
- 71.von Mutius E, Martinez F, Fritzsch C, Nicolai T, Roell G, Thiemann H. Prevalence of asthma and atopy in two areas of West and East Germany. Am J Respir Crit Care Med. 1994;149(2 Pt 1):358–364. doi: 10.1164/ajrccm.149.2.8306030. [DOI] [PubMed] [Google Scholar]
- 72.Sherriff A, Maitra A, Ness AR, Mattocks C, Riddoch C, Reilly JJ, et al. Association of duration of television viewing in early childhood with the subsequent development of asthma. Thorax. 2009;64:321–325. doi: 10.1136/thx.2008.104406. [DOI] [PubMed] [Google Scholar]
- 73.Epstein L, Roemmich J, Robinson J, Pauluch R, Winiewica D, Fuerch J, et al. A randomized trial of the effects of reducing television viewing and computer use on body mass index in young children. Arch Pediatr Adolesc Med. 2008;162:239–245. doi: 10.1001/archpediatrics.2007.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cottrell L, Neal WA, Ice C, Perez MK, Piedimonte G. Metabolic abnormalities in children with asthma. Am J Respir Crit Care Med. 2010;183:441–448. doi: 10.1164/rccm.201004-0603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Granell R, Henderson A, Evans D, Smith G, Ness A, Lewis S, et al. Effects of BMI, Fat Mass, and Lean Mass on Asthma in Childhood: A mendelian Randomization Study. PLoS Med. 2014;11:e1001669. doi: 10.1371/journal.pmed.1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shim YM, Burnette A, Lucas S, Herring RC, Weltman J, Patrie JT, et al. Physical deconditioning as a cause of breathlessness among obese adolescents with a diagnosis of asthma. PLoS One. 2013;8:e61022. doi: 10.1371/journal.pone.0061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wayne P, Foster S, Connolly J, Bazzaz F, Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atomospheres. Ann Allergy Asthma Immunol. 2002;88:279–282. doi: 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- 78.Shaheen S, Newson R, Ring S, Rose-Zerilli M, Holloway J, Henderson A. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol Pract. 2010;126:1141. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perzanowski MS, Miller RL, Tang D, Ali D, Garfinkel RS, Chew GL, et al. Prenatal acetaminophen exposure and risk of wheeze at age 5 years in an urban low-income cohort. Thorax. 2010;65:118–123. doi: 10.1136/thx.2009.121459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varner A, busse W, Lemanske RJ. Hypothesis: decreased use of pediatric aspirin ha contributed to the increaing prevalence of childhood asthma. Ann Allergy Asthma Immunol. 1998;81:347–351. doi: 10.1016/S1081-1206(10)63127-4. [DOI] [PubMed] [Google Scholar]
- 81.Shirakawa T, Enomoto T, Shimazu S, Hopkin JM. The inverse association between tuberculin responses and atopic disorder. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 82.Linehan M, Nurmatov U, Frank T, Niven R, Baxter D, Sheikh A. Does BCG vaccination protect against childhood asthma? Final results from the Manchester Community Asthma Study retrospective cohort study and updated systematic review and meta-analysis. JACI. 2014;133:688–695. doi: 10.1016/j.jaci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 83.Gruber C, Lau S, Dannemann A, Sommerfeld C, Wahn U, Aalberse R. Down-regulation of IgE and IgG4 antibodies to tetanus toxoid and diphtheria toxoid by covaccination with cellular Bordetella pertussis vaccine. J Immunol. 2001;167:2411–2417. doi: 10.4049/jimmunol.167.4.2411. [DOI] [PubMed] [Google Scholar]
- 84.Ausiello C, Urbani F, la Sala A, Lande R, Cassone A. Vaccine- and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997;65:2168–2174. doi: 10.1128/iai.65.6.2168-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowe J, Yerkovich SRP, Suriyaarachchi D, Fisher E, Feddema L, Loh R, et al. Th2-Associated Local Reactions to the Acellular Diphtheria-Tetanus-Pertussis Vaccine in 4- to 6-Year-Old Children. Infect Immun. 2005;73:8130–8135. doi: 10.1128/IAI.73.12.8130-8135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Gillman MW, et al. Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. J Allergy Clin Immunol. 2014;134:753–755. doi: 10.1016/j.jaci.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sicherer S, Munoz-Furlong A, Godbold J, Sampson H. US prevalence of self-reported peanut, tree nut, sesame allergy: 11-year follow-up. J Allergy Clin Immunol Pract. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 88.Du Toit G, Roberts G, Sayre P, Plaut M, Bahnson H, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol Pract. 2013;131:135–143. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 89.Du Toit G, Roberts G, Sayre P, Bahnson H, Radulovic S, Santos A, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–813. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bunyavanich S, Rifas-Shiman S, Platts-Mills TA, Workman L, Sordillo JE, Camargo CJ, et al. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. JACI. 2014;133:1373–1382. doi: 10.1016/j.jaci.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sicherer S, Wood R, Stablein D, Lindblad R, Burk A, Liu A, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol Pract. 2010;126:1191–1197. doi: 10.1016/j.jaci.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–985. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 93.Yokooji T, Kurihara S, Murakami T, Chinuki Y, Takahashi H, Morita E. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol Int. 2013;64:435–445. doi: 10.2332/allergolint.13-OA-0561. [DOI] [PubMed] [Google Scholar]
- 94.Brough H, Simpson A, Makinson K, Hankinson J, Brown S, Douiri A, et al. Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol Pract. 2014;134:867–875. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki S, Fisher H, et al. Early consumption of peanuts in infancy is association with a low prevalence of peanut allergy. J Allergy Clin Immunol Pract. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 96.de Benedetto A, Kubo A, Beck L. Skin barrier disruption: a requirement for allergen sensitzation? J Invest Dermatol. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Savage J, Matsui E, Wood R, Keet C. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J Allergy Clin Immunol Pract. 2012;130:453–460. doi: 10.1016/j.jaci.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yarbrough J, Armstrong J, Blumberg M, Phillips A, McGahee E, Dolen W. Allergic rhinoconjunctivitis caused by Harmonia axyridis (Asian lady beetle, Japanese lady beetle, or lady bug) J Allergy Clin Immunol. 1999;104(3 Pt 1):704–705. doi: 10.1016/s0091-6749(99)70347-7. [DOI] [PubMed] [Google Scholar]
- 99.Nakazawa T, Satinover SM, Naccara L, Goddard L, Dragulev BP, Peters E, et al. Asian ladybugs (Harmonia axyridis): a new seasonal indoor allergen. J Allergy Clin Immunol. 2007;119:421–427. doi: 10.1016/j.jaci.2006.11.633. [DOI] [PubMed] [Google Scholar]
- 100.Steinke J, Platts-Mills T, Commins S. The alpha-gal story: Lessons learned from connecting the dots. JACI. 2015 doi: 10.1016/j.jaci.2014.12.1947. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Commins S, James H, Kelly E, Pochan S, Workman L, Perzanowski M, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactose. JACI. 2011;127:1286–1293. doi: 10.1016/j.jaci.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Nunen SA, O'Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190:510–511. doi: 10.5694/j.1326-5377.2009.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 103.Commins SP, James H, Stevens W, Pochan S, Land M, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1, 3-galactose. JACI. 2014;14:180–188. doi: 10.1016/j.jaci.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamsten C, Tran T, Starkhammar M, Brauner A, Commins SP, Platts-Mills TA, et al. Red meat allergy in Sweden: association with tick sensitization and B-negative blood groups. JACI. 2013;132:1431–1434. doi: 10.1016/j.jaci.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J Allergy Clin Immunol. 2014;133:755–759. e1. doi: 10.1016/j.jaci.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 106.Tripathi A, Commins SP, Heymann P, Platts-Mills TA. Delayed anaphylaxis to red meat masquerading as idiopathic anaphylaxis. JACI in Practice. 2014 May-Jun;:259–265. doi: 10.1016/j.jaip.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr Top Microbiol Immunol. 2007;315:289–324. doi: 10.1007/978-3-540-70962-6_12. [DOI] [PubMed] [Google Scholar]
- 108.Weiss KB, Gergen PJ, Wagener DK. Breathing better or wheezing worse? The changing epidemiology of asthma morbidity and mortality. Annu Rev Public Health. 1993;14:491–513. doi: 10.1146/annurev.pu.14.050193.002423. [DOI] [PubMed] [Google Scholar]
- 109.Hagy G, Settipane G. Bronchial athma, allergic rhinitis, and allergy skin tests among college students. J Allergy. 1969;44:323–332. doi: 10.1016/0021-8707(69)90024-0. [DOI] [PubMed] [Google Scholar]
- 110.Nathan R, Meltzer E, Selner J, Storms W. Prevalence of allergic rhinitis in the United States, Asthma and Allergy Associates, Colorado Springs. J Allergy Clin Immunol. 1997:S808S14. [Google Scholar]
- 111.Johnston S, Pattemore P, Sanderson G, Smith S, Campbell M, Josephs L, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–660. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 112.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 113.Heymann PW, Carper HT, Murphy DD, Platts-Mills TA, Patrie J, McLaughlin AP, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. J Allergy Clin Immunol. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gern J. How rhinovirus infections cause exacerbations of asthma. Clin Exp Allergy. 2015;45:32–42. doi: 10.1111/cea.12428. [DOI] [PubMed] [Google Scholar]
- 115.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith R, Lourie B. Slow Death by Rubber Duck: How the toxic chemistry of everyday life affects our health. Counterpoint Press. 2010 [Google Scholar]
- 117.Platts-Mills TA, Woodfolk JA, Chapman MD, Heymann PW. Changing concepts of allergic disease: the attempt to keep up with real changes in lifestyles. J Allergy Clin Immunol. 1996;98:S297–S306. [PubMed] [Google Scholar]
- 118.Lucas SR, Platts-Mills TA. Physical activity and exercise in asthma: relevance to etiology and treatment. J Allergy Clin Immunol. 2005;115:928–934. doi: 10.1016/j.jaci.2005.01.033. [DOI] [PubMed] [Google Scholar]