Abstract
Mammalian oocytes and embryos are exquisitely sensitive to a wide range of insults related to physical stress, chemical exposure, and exposures to adverse maternal nutrition or health status. Although cells manifest specific responses to various stressors, many of these stressors intersect at the endoplasmic reticulum, where disruptions in protein folding and production of reactive oxygen species initiate downstream signaling events. These signals modulate mRNA translation and gene transcription, leading to recovery, activation of autophagy, or with severe and prolonged stress, apoptosis. ER stress signaling has recently come to the fore as a major contributor to embryo demise. Accordingly, agents that modulate or inhibit ER stress signaling have yielded beneficial effects on embryo survival and long-term developmental potential. We review here the mechanisms of ER stress signaling, their connections to mammalian oocytes and embryos, and the promising indications that interventions in this pathway may provide new opportunities for improving mammalian reproduction and health.
1. INTRODUCTION
The maturing oocyte and early mammalian embryos are notable for their unique cellular physiologies and unique mechanisms of developmental regulation. Oocytes and early embryos lack many of the mechanisms that exist in somatic cells to perform basic metabolic and homeostatic functions, such as free radical scavengers, ion transporters, and osmoregulatory mechanisms. Oocytes and embryos also undergo unique cellular events not seen in somatic cells. For example, fertilization results in massive calcium release and extensive changes to the cell membrane. Meiotic cell cycle progression leads to asymmetric cell division, with attendant mechanisms that must position and orient the meiotic spindle appropriately. The cell cycle of the early cleavage stage embryo is unique in that DNA replication and cytokinesis occur in the absence of substantial cell growth. Oocyte maturation encompasses global transcriptional repression, so that maturing oocytes and early embryos rely predominantly on post-transcriptional mechanisms to sustain and modify protein content of the cell and to execute key developmental transitions.
These unique characteristics of maturing oocytes and early embryos create unique challenges. Indeed, these unique challenges may underlie the relative sensitivity of these cells to exogenous insults. Although the early mammalian embryo is often noted for its apparent plasticity, enabling it to compensate for dramatic perturbations such as cell extirpation, the maturing oocyte and early embryo are quite sensitive to exogenous stresses. It is becoming increasingly apparent that insults to oocytes and early embryos underlie long-term phenotypic alterations observed during both fetal and post-natal life (Latham et al., 2012). The simplest interpretation of these observations is that oocytes and early embryos can undergo physiological adaptations to environmental perturbations, and that these adaptations likely involve epigenetic changes that permanently modify cellular properties by establishing abnormal genome programming.
Such adaptations highlight the fascinating interplay between the environment and developmental biology, particularly the sensitivity of early embryonic genomes undergoing early developmental programming processes. However, such adaptations to environmental stress are only possible when the oocyte or embryo survives the insult.
This chapter focuses on the role of unfolded protein response (UPR) and endoplasmic reticulum stress signaling (ERSS) in the responses of oocytes and embryos to environmental stress, the unique consequences that ERSS may have in oocytes and early embryos, and the potential for novel approaches to manage ERSS in enhancing oocyte and embryo quality and survival. The latter possibility stands at the frontier of modern mammalian embryology, and offers many exciting new possibilities for enhancing clinical and applied outcomes in humans and other mammalian species.
2. OVERVIEW OF UPR AND ERSS
Sensing and responding to exogenous stress is a vital part of cellular physiology. It has become increasingly apparent that one of the key mechanisms of initiating cellular response to a variety of exogenous stressors resides in the endoplasmic reticulum (ER). Secreted proteins and membrane-associated proteins are synthesized in the ER, and must then undergo proper folding, glycosylation, and disulfide bond formation in order to generate functional proteins. A quality control mechanism that detects and eliminates incorrectly processed or unprocessed proteins is thus vital to overall cellular functioning, including cell division, homeostasis, functional responses and cell-cell interactions, and differentiation.
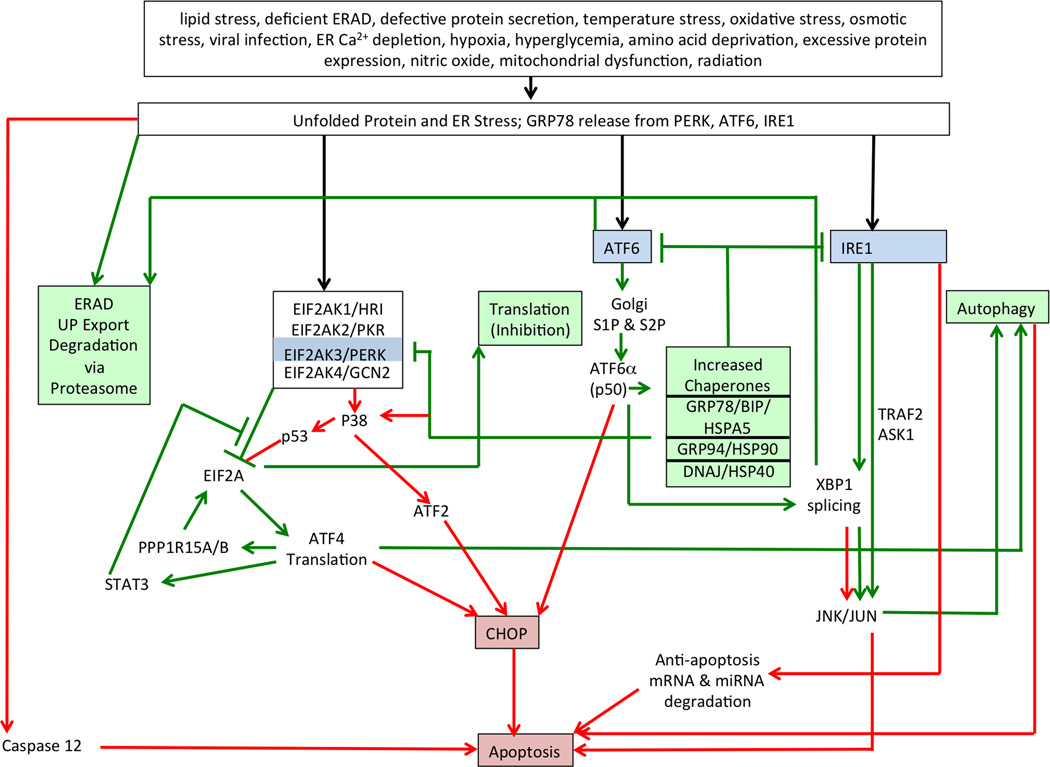
The unfolded protein response fills this need (Bernales et al., 2006). But UPR also fills a much greater role in the cell by providing an indirect means of detecting and responding to stress, because many exogenous stressors negatively impact the ER environment and protein processing (Fig. 1), for example by altering amino acid availability affecting rates of protein synthesis, carbon substrate availability for glycosylation, Ca2+ concentration required for proper folding, cellular redox state related to disulfide bond formation and macromolecular oxidation states, ATP availability for biosynthesis, protein denaturation, lipid availability for protein lipidation, and rates of protein trafficking and secretion.
Figure 1.
Summary of unfolded protein response and endoplasmic reticulum stress signaling pathways. Stress mediated by diverse agents (top box) causes accumulation of unfolded protein, which then bind to GRP78/BIP/HSPA5, releasing the three primary transducers (blue boxes). Green boxes and green lines/arrows designate pathway components that promote survival and recovery. Red boxes and red lines/arrows designate pathway components that promote apoptosis when stress is too severe or prolonged to allow survival.
Disruptions in any of these protein-processing steps by any of a wide variety of stressors leads to accumulation of unfolded or incorrectly folded proteins in the ER. Whereas under normal conditions unfolded and incorrectly folded proteins are removed from the ER by the ER associated protein degradation machinery (ERAD) and targeted for degradation in the cytosol by the ubiquitin-proteasome pathway, any exogenous stress that increases the abundance of such defective proteins in the ER initiates a series of responses that lead either to cellular recovery or cell death (Fig. 1). This sequence is initiated by the transfer of glucose regulated protein 78 (GRP78 a.k.a. HSPA5, BIP) from three primary responder ER membrane proteins eukaryotic translation initiation factor 2A (EIF2A) kinase 3 (PERK), activating transcription factor 6 (ATF6), and endoplasmic reticulum-to-nucleus signaling protein (ERN, a.k.a. IRE1)(Fig. 1, blue boxes) to the accumulating unfolded proteins. Disruption of GRP78 leads to preimplantation arrest, blastocyst failure to hatch, cell division defects and apoptosis in the inner cell mass (Luo et al., 2006), attesting to the importance of ERSS in the early embryo. Once PERK, ATF6 and/or IRE1 are activated, they initiate an early adaptive response to unfolded proteins that, if the stress is short-lived, can facilitate clearance of the unfolded proteins and cell survival—specifically, inhibition of protein translation via EIF2A phosphorylation to reduce the rate of unfolded protein production, transcriptional induction and increased expression of chaperones to facilitate protein folding and negatively feed back to the primary responders, and increased expression of ERAD components to enhance clearance from the ER (Fig. 1, green arrows and boxes). Certain stressors can lead to translational inhibition via alternative EIF2A kinases, thereby augmenting the basic ERSS response. Early ATF4 expression can activate autophagy and enhance cell survival.
With prolonged stress, however, additional responses are initiated, including continued accumulation of ATF4 and ATF6α (p50), leading to transcriptional induction of CHOP, transcriptional induction of JNK/JUN (v-JUN oncogene homolog and kinase) signaling, and activation of p38MAPK14 (mitogen-activated kinase 14) and p53, all of which promote cell apoptosis (Fig. 1 red arrows and boxes). Apoptosis is driven by both mitochondrial dependent and independent pathways (Logue et al., 2013; Minamino and Kitakaze, 2010). IRE1 mediated activation of XBP1 (X-box binding protein) mRNA splicing to produce functional (short form) XBP1 can either promote autophagy or activate transcriptional responses leading to apoptosis via JNK/JUN. Prolonged or increased induction of autophagy, however, can lead to apoptosis rather than survival. Consequently the balance between ERSS and autophagy is a key determinant of survival, and ERSS can diminish to cell survival in conjunction with autophagy induction. One other possible consequence of ERSS is induction of caspase-12, which distinguishes apoptosis deriving from ERSS from other causes of apoptosis (Nakagawa et al., 2000).
3. INTERACTIONS WITH OTHER SIGNALING PATHWAYS
The above summary of ERSS (Fig. 1) addresses a limited set of pathways and interactions that lie at the core of ERSS, mediating a common set of responses to diverse stressors. But the ERSS response is integrated into overall cellular physiology so that it interacts with many other pathways, and can in turn be modulated by these other pathways. A detailed summary of all such interactions is not possible here, but particular interactions are worth mentioning due to potential relevance to oocytes and embryos.
3.1 Interaction with TOR pathway
One key pathway that interacts with ERSS is the TOR (Target of Rapamycin) signaling pathway. This pathway is relevant particularly in the context of effects of diabetes, hyperglycemia, insulin resistance, and insulin signaling. TOR signaling inhibits autophagy under normal conditions but TOR promotes apoptosis during ER stress, and inhibition of TOR promotes autophagy and cell survival under ER stress (Kapuy et al., 2014). ERSS can negatively affect TOR signaling (Qin et al., 2010). The nature of the agent causing ER stress and the duration of the stress affect outcome (autophagy vs. apoptosis) and the activation state of TOR contributes to this choice (Kapuy et al., 2014). Amino acid deprivation, for example, may activate ERSS whilst biasing a cell toward apoptosis by activating TOR. Activation of TOR signaling can promote phosphorylation of the translation inhibitors EIF4EBP1 and 2 (eukaryotic translation initiation factor 4E binding proteins 1 and 2) (Jansson et al., 2012) and other translation factors (e.g., ribosomal protein S6) to promote translation of certain mRNAs that increase translation of particular mRNA classes. Multiple signals linked to nutrient signaling operate through controlling EIF2A phosphorylation, including PI3/AKT (v-AKT oncogene homolog), TOR, GCN2 (general control non-derepressible 2, a.k.a. EIF2AK4), AMPK (AMP protein kinase), and GSK3β (glycogen synthase kinase 3 beta), and these same signals can directly or indirectly modulate TOR activity, so that diverse signals may modulate the translation response to TOR signaling. AMPK can diminish ERSS downstream events (Salvado et al., 2013; Terai et al., 2005; Tirupathi Pichiah et al., 2011) but can also activate ERSS (Lin et al., 2014b; Yang et al., 2013). PPARβ/δ (peroxisome proliferator activated receptors) inhibit events downstream of ERSS by AMPK activation (Salvado et al., 2014). Transcriptional responses to TOR signaling affecting genes related to amino acid transport, lipid metabolism, nucleotide metabolism, and protein synthesis can also be affected by other regulators such as hypoxia inducible factor HIF1A (Jansson et al., 2012). The overall effect is that the status of TOR activation and signaling in a cell is modulated by many factors. Whether cells arrest growth and either survive (autophagy) or undergo apoptosis under ER stress, and the magnitude and duration of ER stress tolerated before initiating apoptosis vary in this context of TOR signaling and interacting pathways such as AMPK signaling.
3.2 Interaction with SRC pathway
Another interacting pathway is the cell growth, proliferation, migration and differentiation regulator SRC (v-SRC oncogene homolog). ER stress induced by thapsigargin or tunicamycin activates SRC kinase (Moon et al., 2014; Yu and Kim, 2010), and this has been implicated in significant cellular responses such as epithelial-to-mesenchymal transition (Ulianich et al., 2008) and other changes (Yu and Kim, 2010). ERSS inhibitors (e.g., salubrinal, guanabenz) reduce SRC activation (Wan et al., 2014). Conversely, overexpression of the SRC homology domain adapter protein NCK1 reduces ERSS mediated EIF2A phosphorylation by inhibiting PERK and recruiting phosphatases (Kebache et al., 2004; Latreille and Larose, 2006).
3.3 Interaction with NRF2 pathway
Another key stress response pathway is linked to ERSS, as PERK activates NRF2 (NFE2 related factor 2) (Cullinan and Diehl, 2004; Cullinan et al., 2003). NRF2 activation leads to increased glutathione levels and buffering of reactive oxygen species (Cullinan and Diehl, 2004) providing a valuable connection between unfolded protein and oxidative stress responses. As oxidized lipids also induce ERSS (Chen et al., 2013; Garbin et al., 2014), activation of NRF2 may also help protect from this source of oxidative stress. Interestingly, reactive oxygen species can be generated during ER stress via oxidative protein folding in the ER (Wang et al., 2014b), so that activation of NRF2-mediated buffering of reactive oxygen species by PERK may minimize damage arising from protein oxidation. Additionally, fatty acid oxidation in the ER inhibits oxidative protein folding, and inhibition of fatty acid oxidation can reduce ERSS (Tyra et al., 2012).
3.4 Interaction with NFκB pathway
There is also extensive interaction between ERSS and the NFκB (nuclear factor kappa B) pathway. NFκB is involved in many processes such as cell adhesion, proliferation and differentiation, cell morphology changes, apoptosis, and inflammation (Pahl and Baeuerle, 1995; Prell et al., 2014). Activation of the NFκB pathway by ERSS can arise with some selectivity (e.g., accumulated protein, oxidative stress and calcium chelation, but not unfolded protein in ERSS) (Pahl and Baeuerle, 1995, 1996). NFκB pathway activation is mediated by IRE1 and TRAF2 (Kaneko et al., 2003; Mauro et al., 2006) or by inhibition of production of the IκBα (NFκB inhibitor) (Deng et al., 2004). Other signaling pathways and kinases [e.g., GSK3β and CK2 (casein kinase type II); (Piazza et al., 2013)] connect to the NFκB pathway and modulate cell survival. NFκB can protect cells from stress by modulating reactive oxygen species generation (Mauro et al., 2006). Additionally, NFκB and TOR may cooperate to initiate unfolded protein aggregation in the cytoplasm to protect cells from ER stress (Liu et al., 2012).
3.5 Interaction with p53 pathway
P53 acting downstream of p38/MAPK14 activation, can contribute to the EIF2A and EIF4E phosphorylation (Jiang et al., 2014). P53 can also contribute to cell cycle arrest and apoptosis. ERSS can inhibit p53-mediated apoptosis via GSK3b signaling and de-stabilize p53 via synoviolin (an E3 ubiquitin ligase used in ERAD, ER associated degradation) during mild ER stress, but severe stress leads to p53 activation and stabilization (Yamasaki et al., 2007).
One lesson that emerges from the foregoing considerations is that ERSS is complex and is an integral part of cellular responses to many different stressors, and to many different signaling pathways or combinations of pathways. Thus, ERSS can operate in parallel with multiple signaling mechanisms in different situations, and the overall outcome in terms of autophagy, cell survival, or cell apoptosis depends on the additive effects on downstream effectors.
4. CHEMICAL ACTIVATORS AND INHIBITORS OF ER STRESS
As is clear above, ERSS is complex, and moreover intersects with many key regulatory pathways in the cell. As a result, many compounds have been identified that directly or indirectly regulate ERSS. Some of these are described in Table 1. Activators of ER stress include agents that inhibit protein glycosylation or export from the ER, induce oxidative stress, affect mitochondrial function, diminish ER calcium stores, activate PERK, increase NO production, or disrupt GRP78 binding. Some agents have multiple effects in cells. Importantly, some agents impact interacting pathways, further attesting to the close integration of ERSS with other cellular processes. One interesting class of agents that inhibit ER stress comprises inhibitors of EIF2A dephosphorylation. EIF2A phosphorylation is one aspect of the early ER stress response. Continued protein translation contributes to later events such as ATF4 mRNA translation and proteins synthesis following transcriptional induction. Continued protein synthesis may slow the recovery from ER stress. The inhibitors of EIF2A dephosphorylation appear to inhibit ER stress by reducing the rate of protein synthesis to minimize the genesis of unfolded proteins, as well as by inhibiting protein synthesis-dependent aspects of ERSS that lead to apoptosis. Activators/inhibitors of autophagy can have opposing effects, due to the relationship between short-and long-term autophagy induction and ERSS. One other feature of ERSS-modulating agents is that many are derived from natural sources. A variety of natural compounds function as antioxidants or can induce ROS generation. Others can inhibit the proteasome, inhibit TOR signaling, or modulate ER calcium stores.
Table 1.
Chemical Activators and Inhibitors of ER Stress Response and Cell Death
| Agent | Probable Modes of Action | Coments | Reference |
|---|---|---|---|
| Activators | |||
| 2-deoxy-D glucose | activation of AMPK as stress sensor, inhibit autophagy, ATP lowering, oxidative stress |
Xi et al. 2011; Xi et al. 2013; Saez et al. 2014; Wang et al. 2014 |
|
| 2,4-Dichlorophenol | EIF2A de phosphorylation | Zhang et al. 2014 | |
| Ampelopsin | ROS generation | falvanol from Ampelopsis grossedentata |
Wang et al. 2014; Zhou et al. 2014 |
| Asymmetric dimethylarginine | inhibits nitric oxide production | Hong et al. 2014 | |
| bichalcone analog TSWU-CD4 | Ca ER depletion, suppression PI3/Akt signaling |
Lin et al. 2014 | |
| Brefeldin A | Inhibits protein transport from ER to Golgi | Rao et al. 2001 | |
| Calcium ionophores | Ca depletion | Rao et al. 2001 | |
| CCT020312 | Activates PERK | Stockwell et al. 2012 | |
| Celastrol, triterpene | Ca release | Matos et al. 2014; Yoon et al. 2014 | |
| Clofoctol | unknown | Gram+ bactierial antibiotic | Wang et al. 2014 |
| CuSO4 | Disrupt GRP78 protein binding, induces GRP78 recomparmentalization |
Qian et al. 2005; Matos et al. 2014 | |
| Flouride | intracisternal granule accumulation | Ito, Nakagawa et al. 2009, | |
| Gadolinium | effects on Ca stores | Feng et al. 2011 | |
| H2O2 | oxidative stress | Suyama et al. 2014 | |
| Homocysteine | reductive stress | Outinen et al. 1998; Yang et al. 2014 | |
| Indium, Indium tin oxide | oxidative stress | Brun et al. 2014 | |
| Indomethacin | oxphos uncoupler, cyclooxygenase inhibitor,Ca channel blockade |
Tsutsumi et al. 2004; Narabayashi, Ito et al. 2014 | |
| Lead | Disrupt GRP78 protein binding, induces GRP78 recomparmentalization |
Qian and Tiffany-Castiglioni 2003; Qian et al. 2005; Shinkai et al. 2010 | |
| L-NAME | nitric oxide diminishment | Shen et al. 2014 | |
| Neferine | oxidative stress | alkaloid from lotus seed embryo |
Yoon et al. 2013 |
| Nitric oxide | PPAR-delta | Cheang et al., 2014 | |
| Palm itate | ER Ca depletiobn, accumulation unfolded proteins |
Laybutt et al. 2007; Cnop et al. 2010; Park et al. 2014 | |
| Paraquat | Hausberg et al., 2005 | ||
| Quinones | Inhibit proteasome | derived from xenobiotics and endogenous molecules |
Xiong et al. 2014 |
| Sarsasapogenin | oxidative stress | from the Chinese medical herb Anemarrhena asphodeloides Bunge; a steroidal sapogenin; anti- diabetic |
Shen et al. 2013; Shen et al. 2013 |
| SNX-2112 | HSP90 inhibition | Wang et al. 2014 | |
| Thapsigargin | SERCA inhibition, Ca depletion in ER | Humeres et al. 2014; Zhang et al. 2014 | |
| Tunicamycin | GlcNAc phosphotransferase Inhibitor; blockds N-linked glycosylation |
Perez-Martin et al. 2014; Saez et al. 2014; Zhang et al. 2014 | |
| Inhibitors | |||
| 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) |
serine protease inhibitor | Okada et al. 2003 | |
| 4-phenylbutyric acid PBA | chemical chaperone | Cadavez et al. 2014; Humeres et al. 2014; Yao et al. 2014 | |
| Baicalin | nitric oxide synthase induction | Shen et al. 2014 | |
| Betaine | inhibits hyperhomocysteinemia | Ji and Kaplowitz 2003 | |
| Cajaninstilbene acid | antioxidant | a natural stilbene isolated from Cajanus cajan leaves |
Liu et al. 2014 |
| Emodin | Ca regulation | Wu et al. 2014 | |
| Exendin-4 | GLP1R agonist | Younce et al. 2013 | |
| Glucagon like peptide 1 | activation of SERCA2 | effective against hyperglycemia and lipid induced ERSS |
Cnop et al. 2010; Younce et al. 2013 |
| Guanabenz | inhibits EIF2A dephosphorylation | Wan et al. 2014 | |
| Honiokol | GRP78 inducer | Kudo et al. 2008 | |
| Imipramine | works via Sigma1 receptor which is a ER chaperone that modulates Ca release |
Ono et al. 2012 | |
| Metallothionein | antioxidant | Guo et al. 2009 | |
| Metformin | increases nitric oxide bioavailability, activates AMPK |
Cheang et al. 2014 | |
| Metyrapone | inhibits TOR to promote autophagy and inhibit apoptosis |
Kapuy et al. 2014 | |
| N-acetylcysteine | antioxidant | Yang et al. 2014 | |
| Naltrexone | opioid receptor antagonist; works via Sigma1 receptor which is a ER chaperone that modulates Ca release |
Moslehi et al. 2014 | |
| Nitric oxide | PPAR-delta | Cheang et al. 2014; Narabayashi et al. 2014 | |
| Rapamycin | inhibits TOR to promote autophagy and inhibit apoptosis |
bacterial macrolide | Li et al. 2014 |
| Salubrinal | inhibits EIF2A dephosphorylation | Boyce et al. 2005; Kapuy et al. 2014 | |
| sodium tanshinone IIA sulfonate | reduces reactive oxygen species in irradiated cells |
Gu et al. 2014 | |
| tauroursodeoxycholic acid (TUDCA) |
ER chaperone properties; prevents BAX transport to mitochondria |
Rivard et al. 2007; Cadavez et al. 2014; Yan et al. 2014 | |
| Trolox | antioxidant | analog of vitamin E | Brito et al. 2014 |
5. RELATIONSHIP OF ERSS PATHWAYS TO OOCYTE MATURATION AND EARLY EMBRYOGENESIS
The foregoing discussion of ERSS highlights the complexity of the process. Indeed, ERSS occupies a nodal regulatory role in the cell, serving to integrate a myriad of environmental cues and other cellular signaling pathways into a network of events that coordinate numerous events in diverse cellular organelles, including the nucleus, mitochondria, the ER, and the Golgi, and coordinating diverse stress responses with a variety of cell functions including transit through the cell cycle, growth and metabolism.
In the multicellular organism, the ERSS achieves an overall objective of either repairing or eliminating damaged cells. In the oocyte and fertilized zygote, however, cell death eliminates the entire organism; in the cleaving embryo cell death may severely compromise embryo viability. Reduced rates of cell division may likewise lead to more long-term effects.
Additionally, the development of high-quality oocytes and the elaboration of high-quality embryos are linked to correct dialog between the developing oocyte, the supporting cumulus cells and other cells within the follicle (Hao et al., 2014; Matzuk et al., 2002). Thus, stressors that impact follicle cells can also indirectly affect oocyte and embryo viability. Successful mammalian development also relies on successful implantation and placentation and efficient maternal-fetal exchange of materials. Stressors that impinge on formation and function of the placenta can thus exert severe detrimental effects on developmental outcomes. The following sections review what has been discovered with respect to ERSS in mammalian oocytes, embryos, and follicle cells, and what measures may be taken to enhance reproductive outcomes. It is noted that there is an extensive body of literature on ERSS in trophoblast and placenta, describing the relationships between inflammation, ERSS, maternal health state, and pregnancy outcomes. Though too extensive to cover in this chapter, those studies provide additional insight into the link between ERSS and mammalian reproduction.
6. ACTIVATION AND INHIBITION OF ER STRESS IN OOCYTES AND EMBRYOS
Many in vivo and in vitro stressors impact oocyte and preimplantation embryo health and developmental potential, and long-term developmental outcomes. These include heat, DNA damage or DNA damaging agents, osmotic stress and availability of organic osmolytes, oxygen and oxidative stress, hyperglycemia and carbon substrate availability, hyperlipidemia and oxidized lipids, calcium ionophores, cytokines, amino acid deprivation, insulin signaling, and serum components. The overall effects of these stresses range from delayed cleavage to developmental arrest at the 2-cell stage, to significant fetal and post-natal effects, such as intrauterine growth restriction, post-natal compensatory growth, abnormal developmental programming of metabolism, hypertension, diabetes, obesity, and potential epigenetic changes that may even be transmitted transgenerationally. Such responses underlie some of what are now recognized as developmental origins of adult disease (DOADs) (Latham et al., 2012). Immediate embryo responses to these stressors can be multi-pronged, invoking multiple parallel signaling pathways as discussed above; these can include p38/MAPK14 activation, AMPK activation, DNA damage response, TOR, and GSK3β. ERSS occupies a central role in mediating overall cellular responses and cell survival/death, and thus may be linked to the long-term effects on developmental programing and developmental origins of disease. As such, it is worthwhile to summarize activation of ERSS in oocytes and embryos subsequent to a range of different insults.
6.1 Temperature
Maternal heat stress impairs cleavage in mouse embryos in vivo, with most embryos arresting at the 2-cell stage (Bellve, 1973), and severe heat stress can inhibit oocyte maturation (Kim et al., 2002). Similar effects are seen in other mammals (Edwards and Hansen, 1996; Isom et al., 2007; Makarevich et al., 2007; Payton et al., 2004). Heat stress is associated with increased reactive oxygen species and apoptosis (Paula-Lopes and Hansen, 2002; Sakatani et al., 2004; Tseng et al., 2006). A variety of exogenous factors such as antioxidants, melatonin, retinol, IGF1 and sphingosine-1-phosphate can protect embryos from heat stress (Cebrian-Serrano et al., 2013; Lawrence et al., 2004; Namekawa et al., 2010; Roth and Hansen, 2004; Sakatani et al., 2007). Heat stress induces changes in gene expression in surviving embryos (Hickman et al., 2013). Cold stress associated with cryopreservation, particularly slow freezing can also affect embryo development and gene regulation (Larman et al., 2011; Saenz-de-Juano et al., 2012). Prolonged exposure to room temperature can inhibit cleavage and alter the Golgi complex in early embryos (Hegele-Hartung et al., 1991), and can degrade meiotic spindle structure in ovulated oocytes (Van der Elst et al., 1988). In addition to generating ROS, negative effects on intracellular structures could impact ER function and lead to ERSS.
6.2 Osmotic stress
Osmotic stress has profound effects on early embryos, including induction of 2-cell arrest in mouse embryos (Wang et al., 2011), a response that is genotype-dependent and that underlies the classical 2-cell arrest of outbred mouse lines in sub-optimum culture conditions. Early embryos lack some of the osmoregulatory mechanisms that operate in somatic cells, and rely instead on amino acids as internal organic osmolytes (Baltz and Tartia, 2010; Petronini et al., 1992; Steeves and Baltz, 2005). Availability of amino acids in the culture medium can ameliorate stress responses, such as translational repression, and facilitate survival (Petronini et al., 1992; Richards et al., 2010). Thus, amino acid starvation may not only affect protein synthesis and other metabolic processes, but may also compromise osmoregulatory ability in the early embryo. Hyperosmolarity induces activation of p38/MAPK14 and JNK signaling, and induction of HSP70.1 (Baltz and Tartia, 2010; Fiorenza et al., 2004; Xie et al., 2013). Interestingly, organic osmolytes can also reduce negative effects of other stressors (Zander-Fox et al., 2013). Osmotic stress affects ER to Golgi trafficking (Jiang and Storrie, 2005; Lee and Linstedt, 1999) and may affect calcium sequestration, which may activate ERSS via calreticulin involvement (Jiang and Storrie, 2005; Samak et al., 2011; Wang et al., 2012a). Thus, specific cellular responses to cell shrinking/swelling under changes in external osmolarity can be accompanied by ERSS and associated downstream events.
Sorbitol supplementation (25 µM) of mouse embryo culture medium to create hyperosmolarity decreases blastocyst formation and induces ERSS, including XBP1s expression and increased nuclear localization, as does tunicamycin treatment at 1–2 µg/ml (Zhang et al., 2012). Higher concentrations of tunicamycin (5 µg/ml) and sorbitol (50mM) arrest embryos at the 2-cell stage and increase cytoplasmic but not nuclear XBP-1 staining (Zhang et al., 2012). The higher concentrations applied to blastocysts increase apoptosis. All of these ERSS mediated responses are inhibited by treatment with tauroursodeoxycholic acid (TUDCA), which improves embryo development in vitro (Abraham et al., 2012; Kim et al., 2012; Zhang et al., 2012).
6.3 pH stress
Alkalinized medium during the zygote stage can alter post-natal growth in mice (Banrezes et al., 2011). Zygotes and embryos prior to compaction have lesser abilities to regulate intracellular pH, and treatment with ammonium may contribute to inter-blastomere heterogeneity and stress-mediated effects on gene transcription, cell lineage commitment and cell survival (Brison et al., 2014; Zander et al., 2006). Exposure to acid to reduce intracellular pH by less than 0.2 pH units for either brief period or a prolonged period impairs blastocyst formation in mouse embryos; exposure of zygotes does not reduce implantation of these blastocysts but alters fetal growth, indicating the exquisite sensitivity of zygotes to mild stress and the impact on developmental programming of later development (Zander-Fox et al., 2010). Importantly, early embryos lack active mechanisms for alleviating acid loads, and can only alleviate alkaline loads by sodium-independent bicarbonate/chloride exchange (Baltz et al., 1991a, b), making them susceptible to perturbations in pH. Because pH can affect protein folding, calcium signaling and sequestration, and other processes, ERSS may contribute to effects on viability and long-term developmental programming following pH insults.
6.4 Maternal nutrition and physiology, and nutrient availability in vitro
The preceding sections touched upon the connections between glucose availability, lipid exposure, oxidative stress and the ERSS in affecting oocyte quality and embryo development. Other aspects of maternal nutrition and physiology, and in vitro availability of nutrients also contribute to embryonic ERSS activation. Maternal hyperglycemia and insulin resistance are significant factors. Maternal insulin resistance is harmful to embryos, due to downregulation of IGF1R (insulin like growth factor 1 receptor) and reduced glucose uptake (Louden et al., 2008). Nutrient availability is sensed in part by activation of AMPK in response to elevated AMP/ATP ratio, and AMPK inhibits TOR activity (Rehman et al., 2014). Activation of AMPK in blastocysts rescues them from the effects of maternal insulin resistance (Louden et al., 2008), implicating TOR signaling in the detrimental effects of nutrient stress on early embryos. TOR may enhance amino acid transport in placentae (Jansson et al., 2012). Maternal dietary protein intake is also important. Low protein diets during oocyte maturation and preimplantation development can perturb embryo metabolism, alter epigenetic information, and contribute to post-natal abnormalities (Fleming et al., 2011; Kwong et al., 2006; Kwong et al., 2007; Mitchell et al., 2009; Watkins et al., 2010, 2008a, 2008b; Williams-Wyss et al., 2014). The stress of in vitro embryo growth can perturb normal cellular physiology. Availability of nutrients in the medium such as amino acids and vitamins can ameliorate negative effects of culture (Lane and Gardner, 1998). Such effects may be mediated in part by modulating ERSS.
6.5 Oxidative stress, oxygen availability, and glucose availability
Oocyte development is sensitive to oxidative stress in the ovary (Devine et al., 2012). In embryos, oxidative stress can be problematic as early embryos may not express all the same factors that help protect somatic cells from reactive oxygen species (ROS) (Johnson and Nasr-Esfahani, 1994; Nasr-Esfahani and Johnson, 1992). Genes related to oxidative stress response (and ERSS) are expressed in immature and mature oocytes and in embryos, but may be subject to translational control as well as induction in response to stress (El Mouatassim et al., 1999).
Oxidative stress can arise for many reasons. Oxidative phosphorylation in mitochondria generates ROS. There is an intimate connection between ER stress and oxidative stress. ERSS itself can generate ROS (Landau et al., 2013). Oxidative stress then impedes correct protein folding and transport, and calcium homeostasis, and can trigger ERSS (Malhotra and Kaufman, 2007). Embryo culture in elevated (atmospheric) oxygen levels, elevated glucose, and other factors that alter oxidative phosphorylation and mitochondrial function can induce oxidative stress. Environmental toxins induce oxidative stress in embryos (Chu et al., 2013). Transient exposure to elevated glucose concentrations (or older media formulations with higher glucose concentrations) in vitro can increase ROS generation, and alter gene expression and energy metabolism (Cagnone et al., 2012; Karja et al., 2006; Rinaudo et al., 2006), as can exposure to maternal hyperglycemia (Moley, 2001; Moley et al., 1998). Maternal hyperglycemia degrades oocyte and preimplantation embryo quality, impacts meiosis, and alters mitochondria in embryos (Wang and Moley, 2010; Wyman et al., 2008). These conditions can contribute to diabetes, growth abnormalities, and other long-term effects in progeny (Wyman et al., 2008). Responses to other maternal stressors that may compromise oocyte quality and early embryogenesis can be accompanied by oxidative stress and increase in ROS production (Koyama et al., 2012; Lian et al., 2013; Ozawa et al., 2002). Embryo glutathione levels change during development and are altered in vitro (Gardiner and Reed, 1994). Oxidative stress in embryos can lead to DNA damage (Takahashi et al., 2000). Paraquat, an inducer of oxidative stress and ER stress, inhibits preimplantation development (Hausburg et al., 2005), highlighting the importance of ERSS in early development.
The benefits of reduced oxygen availability during embryo culture have long been recognized (Rinaudo et al., 2006; Umaoka et al., 1991, 1992), as have the beneficial effects of adding antioxidants or glutathione to the culture medium, particularly in overcoming cleavage stage developmental arrest (Hu et al., 2012; Lee et al., 2000; Leese, 2012; Legge and Sellens, 1991; Nonogaki et al., 1991; Takahashi, 2012; Umaoka et al., 1991). Antioxidants can improve oocyte quality and enhance embryo development (Huang et al., 2013; Ideta et al., 2012; Orsi and Leese, 2001; Takeo et al., 2014; Wang et al., 2014a) and also protect against peroxide-induced damage (Yu et al., 2014) and other stress induced damage (Cebrian-Serrano et al., 2013; Koyama et al., 2012; Sakatani et al., 2007) in embryos and oocytes (Devine et al., 2012). Additionally, reduced glucose availability in vitro has long been recognized as beneficial to embryos (Chatot et al., 1989; Leese, 2012), although glucose starvation can actually increase ROS production and is not beneficial (Jansen et al., 2009). Other maternal stressors that may compromise oocyte quality and early embryogenesis can be redressed by antioxidant treatments (Lian et al., 2013). Growth factors may also improve development after oxidative stress (Kurzawa et al., 2002).
Embryos respond to oxidative stress in part via a p66(Shc)-dependent pathway (Betts et al., 2014; Favetta et al., 2007; Ren et al., 2014). ERSS and oxidative stress response often accompany each other, ERSS can mediate response to oxidative stressors (Xu et al., 2012), and treatments that alleviate one often alleviate both. Separate cellular responses to oxidative stress and ER stress combine to dictate developmental outcomes (Yoon et al., 2014b). Interestingly, the mouse zygote apoptotic response to oxidative stress resides in the cytoplasm (Liu and Keefe, 2000), consistent with the absence of early gene transcription. Overall, the observations to date indicate that many different stressors are accompanied by oxidative stress and ROS production, and that the response to these stressors arises form a combination of oxidative stress response involving p66 and activation of ERSS, and that inhibition of ERSS as well as suppression of ROS can enhance embryo viability.
6.6 Lipids
Fatty acid oxidation (β-oxidation) is essential for oocyte maturation, providing an important energy source and availability of acetyl-CoA (Dunning et al., 2014; Paczkowski et al., 2014). Inhibiting fatty acid oxidation in oocytes impairs maturation (Downs et al., 2009), and stimulating fatty acid oxidation reduces glucose metabolism, increases lipid stores, and improves embryo development after fertilization (Johnson and Nasr-Esfahani, 1994; Paczkowski et al., 2014). Changing the serum lipid profile can change lipid availability in the ovarian follicle and affect embryogenesis after fertilization (Johnson and Nasr-Esfahani, 1994). Dietary fatty acids can enhance oocyte maturation and quality (Moallem et al., 2013). But excess circulating lipids and excess amounts of non-esterified fatty acids can increase ROS and negatively affect oocyte quality and embryo development (Leroy et al., 2005; Shehab-El-Deen et al., 2009). Palmitic acid can induce ERSS (Jung et al., 2012). Elevated levels of some polyunsaturated fatty acids in vivo can adversely affect oocyte quality and embryo development (Wakefield et al., 2008). Exposure of embryos in vitro to elevated serum lipid negatively impacts pluripotency gene expression in bovine embryos, but does not reduce blastocyst formation (Cagnone and Sirard, 2014). A high fat diet can exert subtle changes in DNA methylation and gene expression in oocytes of the treated mother, and also in the oocytes and tissues of their offspring (Ge et al., 2014). Maternal obesity negatively affects clinical outcome in human in vitro fertilization, and also degrades oocyte and embryo developmental in mice, and this is associated with abnormalities in fetal growth and changes in oocyte mitochondrial properties (Grindler and Moley, 2013; Jungheim et al., 2010; Luzzo et al., 2012). Oxidized oil and lipids are detrimental and can induce ER stress (Guo et al., 2013; Larroque-Cardoso et al., 2013; Otsuki et al., 2007), and oxidized low density lipoprotein is associated with ER stress in adipocytes (Chen et al., 2013). Hypercholesterolemia can induce ER stress in some tissues (Cai et al., 2013). These latter observations indicate that excess lipid availability may activate ERSS and possibly oxidative stress response in oocytes, and thereafter negatively affect embryogenesis. Potential negative effects of excess lipid availability may combine with negative effects of hyperglycemia in maternal diabetes, obesity, and altered nutrition status.
6.7 Cytokines
Inflammatory cytokines can activate SRC signaling, which interacts with the ERSS pathway. This can be attenuated with inhibitors of ERSS (Wan et al., 2014). Interleukins can activate ERSS, which can be pro-survival, and can also influence cellular responses to other stimuli, such as activators of NFκB signaling (Lee et al., 2014). Additionally, ERSS can activate interleukin and cytokine expression in paracrine interactions (Meares et al., 2014). However, IL-10 deficiency combined with NADPH oxidase deficiency can induce ERSS (Treton et al., 2014). Cytokines such as CSF1 and GMCSF positively affect preimplantation development in mice and improve in vitro survival, and CSF1 deficiency can impair pre- and post-implantation development (Bhatnagar et al., 1995; Karagenc et al., 2005; Robertson et al., 2001; Sjoblom et al., 2005). The beneficial effects of CSF1 and GMCSF are mediated in part by suppressing ERSS (Chin et al., 2009).
6.8 Shear stress
Shear stress during embryo handling can induce a stress response. Mouse embryo pipetting generates shear stress that is ameliorated by the presence of the zona pellucida (Xie et al., 2006). The stress activated protein kinases MAPK8 (also known as SAPK or JNK1) is activated by prolonged shear stress, and more so in hatched embryos (Xie et al., 2007). Transient stress may not lead to negative effects on the embryo, but prolonged or repeated handling or handling without the zona pellucida may be more deleterious (Xie et al., 2007).
6.9 Autophagy
Induction of autophagy as opposed to apoptosis leads to cell survival. Fertilization is accompanied by increase in autophagic activity and mutation of autophagy genes impedes early development (Song et al., 2012). Induction of autophagy in bovine embryos by rapamycin enhances embryo development in vitro whereas inhibition of autophagy with 3-methyladenine reduces development, and treatment with TUDCA ameliorated the effects of autophagy inhibition (Song et al., 2012). Thus, increased ER stress in early embryos may diminish the beneficial effects of autophagy and tip the scale in favor of apoptosis.
6.10 Electrofusion
One study in cloning by somatic cell nuclear transfer revealed a potential effect of electrofusion in the induction of ERSS in bovine oocytes and early embryos, including activation of ERSS related genes (Song et al., 2011). Electrofusion yielded embryos with fewer cells and different nuclear remodeling changes as compared to embryos produced by Sendai virus fusion. The negative effects of electrofusion could be prevented with TUDCA treatment to inhibit ERSS. ERSS induction by electrofusion could be arise following calcium depletion in the ER. It is noted, however, that whereas electrofusion in the bovine activates the oocyte, this does not occur in the mouse; hence it is has not been determined to what degree electrofusion or electrical pulses will elicit the ERSS in different species or using different parameters across protocols.
7. UNIQUE CONSIDERATIONS FOR ERSS IN OOCYTES AND EMBRYOS-DEVELOPMENTAL OUTCOMES
Induction of ER stress in mammalian oocytes and embryos may conflict with normal developmental processes. The progression of oocytes and early cleavage stage embryos is controlled in large part by the timely recruitment, translation and degradation of maternal mRNAs in the ooplasm. Thus, although utilizing ERSS to cope with stress may help maintain cell and embryo survival, this may come at the cost of a disruption in the normal temporal coordination of events regulated by post-transcriptional mechanisms. Additionally, activation of apoptotic mechanisms in the early embryo may eliminate damaged cells that cannot recover from severe or prolonged stress, and although mammalian embryos have considerable regulative capacities, the loss of a substantial proportion of cells can greatly reduce developmental potential.
Another challenge in mammalian embryogenesis relates to genome integrity. Embryos may begin life with DNA damage in either or both parental genomes, and this DNA damage needs to be repaired. Activation of ERSS can interfere with DNA repair (Yamamori et al., 2013; Yasui et al., 2014). The “quiet embryo hypothesis” posits that a low metabolic rate, low glucose consumption, low glycolysis, low amino acid turnover, and low oxidative phosphorylation from the zygote to the morula stage limits ROS production and maximizes viability (Baumann et al., 2007; Leese, 2002). Stress activates AMPK, which in turn increases glucose transport and glycolysis, but which also leads to changes in gene regulation. High amino acid turnover correlates with increased DNA damage (Sturmey et al., 2009). Stress and excess exogenous nutrients can thus combine to increase metabolism and amino acid turnover (Leese, 2012), impede the repair of DNA damage, and alter gene regulation, leading either to embryo demise or long-term changes in developmental phenotype. One other link of ERSS to genome integrity is that XBP1 is observed associated abundantly in the oocyte germinal vesicle, then abundantly with the meiotic spindle in MI oocytes, but less so on spindles of MII stage oocytes, then in the cytoplasm of 1-cell embryos, and subsequently in the nuclei of 2-cell and 4-cell embryos (Zhang et al., 2012). This dynamic regulation of XBP1 localization may reflect a role in chromatin regulation during meiosis and cleavage as well as periods of greater or lesser capacity for ERSS-induced DNA repair (Tao et al., 2011).
Another unique aspect of early mammalian embryos relates to mitochondrial biology. Early mammalian embryos display reduced rates of oxygen consumption, which increases with cavitation, and much of the oxygen consumed by blastocysts can be via non-mitochondrial processes (Leese, 2012). Mature oocytes and early embryos display mitochondria with an inert appearance, which take on a more active appearance as development progresses, becoming elongated with more transverse cristae (Sathananthan and Trounson, 2000). Stressors such as toxins, hyperglycemia, and maternal high fat diet can induce ERSS, generate defective mitochondria in the oocyte and embryo, increase ROS, disrupt AMPK activity, and possibly inhibit the removal of defective mitochondria by mitophagy (Grindler and Moley, 2013; Yuzefovych et al., 2013). This suggests that stressors that activate ERSS may not only alter embryo metabolism but may also disrupt the normal pattern of mitochondrial biogenesis/elimination to ensure healthy embryonic cells.
Early embryogenesis is also a period of extensive chromatin remodeling and susceptibility to factors that may interfere with correct developmental programming of the genome. The ERSS encompasses multiple components of transcriptional response, including modifications of histone acetylation, histone methylation, and DNA methylation, which in turn regulate diverse genes including genes related to ER stress response, genes related to the ERAD process, and other stress regulatory genes such as NRF2 (Baumeister et al., 2009; Chen et al., 2014; Han et al., 2013; Martin et al., 2014; Nakajima and Kitamura, 2013; Tao et al., 2011). Additionally, histone deacetylases can modify transcription factor activity and other proteins (Fazi et al., 2009; Kimura et al., 2012; Palsamy et al., 2014; Sato et al., 2014). For example, HDAC4 sequesters ATF4 in the cytoplasm (Zhang et al., 2014a). Thus, ERSS may exert a special penalty on early embryonic cells by modifying the developmental programming, and this may be one aspect of how diverse stressors can exert long-term effects on progeny phenotype.
Another special consideration for mammalian embryos related to ERSS is the wide range of manipulations to which embryos may be subjected during in vitro experimentation, assisted reproduction, or genome manipulation. Nutrient makeup of culture media, shear stress and embryo pipetting or handling, thermal and osmotic stress, exposure to atmospheric oxygen level, and electrofusion are common aspects of in vitro oocyte and embryo handling. Additionally, maternal health status, maternal serum carbohydrate and lipid content, and maternal toxin exposure also impact oocyte and embryo quality and potential responses to these oocyte/embryo handling parameters. Thus, the combination of all of these parameters will work additively to determine the extent, severity, and duration of ERSS activation, and whether ERSS is activated in concert with other stress responses.
All of this ultimately impacts the ability of the embryo to eliminate unfolded protein, repair DNA damage, eliminate damaged mitochondria, undergo autophagic processes to repair cellular damage, execute normal transcriptional programs, execute the required maternal mRNA translational program, and survive with a sufficient number of viable cells to sustain normal long-term development. What emerges from all of these considerations is that, while mammalian embryos are remarkably resilient and can tolerate a range of exogenous stresses without dying, such regulative responses come at a potentially high cost of aberrant phenotypes lasting into adult life.
8. CONCLUDING REMARKS
The above considerations suggest that some portion of adult disease and health disorders might be preventable by minimizing insults to the oocyte and embryo in vivo, and also by taking precautions to minimize in vitro conditions that may activate ERSS. Indeed, exciting recent developments illustrate that embryo viability can be improved by managing embryonic ERSS. This includes managing the REDOX state of embryonic cells, using inhibitors of ERSS, using histone deacetylase inhibitors to modulate ERSS, promoting autophagy, and in some species avoiding procedures that deplete ER calcium stores (Song et al., 2011; Song et al., 2012; Song et al., 2014; Yoon et al., 2014b; Zhang et al., 2012). The specific interventions will need to be optimized according to species, genotype, and possibly maternal health status and specific in vitro culture conditions. One exciting prospect will be to discover whether in vitro manipulation of ERSS in oocytes and embryos might provide a way to repair damage or improve oocyte and embryo quality that may have been compromised by maternal age or adverse maternal health or nutrition states. Additionally, the need for managing ER stress in the oocyte and early embryo may extend to post-implantation stages. Recent studies reveal links between ER stress, placenta or trophoblast cell defects such as inflammation and preeclampsia, and adverse outcomes such as pregnancy loss, intrauterine growth restriction, and low birth weight (Jain et al., 2012; Kawakami et al., 2014; Lian et al., 2011; Liu et al., 2011; Redman and Sargent, 2009; Sankaralingam et al., 2006; Shi et al., 2012; Wang et al., 2012b; Yung et al., 2008; Yung et al., 2012). An overall strategy of minimizing the likelihood of activating ERSS in oocytes, early embryos, and the placenta should be beneficial for enhancing reproductive efficiencies clinically, but may also enhance overall reproductive health and reduce the incidence of adult disorders and diseases that have their origins in early embryonic stress. Further study of the benefits of avoiding exposures of oocytes, embryos, and placenta to exogenous stressors, and providing exogenous factors that inhibit ERSS (e.g., antioxidants or other compounds) before conception, during clinical and applied reproduction procedures, and during pregnancy, may yield many beneficial discoveries.
ACKNOWLEDGEMENTS
The work in the author’s laboratory is supported in part by grants from the National Institutes of Health, National Institute of Child Health and Development (RO1 HD-075093), and the Office of Research Infrastructure Programs, Division of Comparative Medicine Grants R24 OD-012221), and by MSU AgBioResearch and Michigan State University. The author thanks Ms. Ashley Severance for comments on the manuscript.
References
- Abraham T, Pin CL, Watson AJ. Embryo collection induces transient activation of XBP1 arm of the ER stress response while embryo vitrification does not. Mol. Hum. Reprod. 2012;18:229–242. doi: 10.1093/molehr/gar076. [DOI] [PubMed] [Google Scholar]
- Baltz JM, Biggers JD, Lechene C. Relief from alkaline load in two-cell stage mouse embryos by bicarbonate/chloride exchange. J. Biol. Chem. 1991a;266:17212–17217. [PubMed] [Google Scholar]
- Baltz JM, Biggers JD, Lechene C. Two-cell stage mouse embryos appear to lack mechanisms for alleviating intracellular acid loads. J. Biol. Chem. 1991b;266:6052–6057. [PubMed] [Google Scholar]
- Baltz JM, Tartia AP. Cell volume regulation in oocytes and early embryos: connecting physiology to successful culture media. Hum. Reprod. Update. 2010;16:166–176. doi: 10.1093/humupd/dmp045. [DOI] [PubMed] [Google Scholar]
- Banrezes B, Sainte-Beuve T, Canon E, Schultz RM, Cancela J, Ozil JP. Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS One. 2011;6:e29388. doi: 10.1371/journal.pone.0029388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CG, Morris DG, Sreenan JM, Leese HJ. The quiet embryo hypothesis: molecular characteristics favoring viability. Mol. Reprod. Dev. 2007;74:1345–1353. doi: 10.1002/mrd.20604. [DOI] [PubMed] [Google Scholar]
- Baumeister P, Dong D, Fu Y, Lee AS. Transcriptional induction of GRP78/BiP by histone deacetylase inhibitors and resistance to histone deacetylase inhibitor-induced apoptosis. Mol. Cancer Ther. 2009;8:1086–1094. doi: 10.1158/1535-7163.MCT-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR. Development of mouse embryos with abnormalities induced by parental heat stress. J. Reprod. Fertil. 1973;35:393–403. doi: 10.1530/jrf.0.0350393. [DOI] [PubMed] [Google Scholar]
- Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- Betts DH, Bain NT, Madan P. The p66(Shc) adaptor protein controls oxidative stress response in early bovine embryos. PLoS One. 2014;9:e86978. doi: 10.1371/journal.pone.0086978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar P, Papaioannou VE, Biggers JD. CSF-1 and mouse preimplantation development in vitro. Development. 1995;121:1333–1339. doi: 10.1242/dev.121.5.1333. [DOI] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Brison DR, Sturmey RG, Leese HJ. Metabolic heterogeneity during preimplantation development: the missing link? Hum. Reprod. Update. 2014;20:632–640. doi: 10.1093/humupd/dmu018. [DOI] [PubMed] [Google Scholar]
- Brito DC, Brito AB, Scalercio SR, Percario S, Miranda MS, Rocha RM, Diniz JA, Oskam IC, Van den Hurk R, Paris MC, Domingues SF, Santos RR. Vitamin E-analog Trolox prevents endoplasmic reticulum stress in frozen-thawed ovarian tissue of capuchin monkey (Sapajus apella) Cell Tissue Res. 2014;355:471–480. doi: 10.1007/s00441-013-1764-x. [DOI] [PubMed] [Google Scholar]
- Brun NR, Christen V, Furrer G, Fent K. Indium and Indium Tin Oxide Induce Endoplasmic Reticulum Stress and Oxidative Stress in Zebrafish (Danio rerio) Environ Sci Technol. 2014;48:11679–11687. doi: 10.1021/es5034876. [DOI] [PubMed] [Google Scholar]
- Cadavez L, Montane J, Alcarraz-Vizan G, Visa M, Vidal-Fabrega L, Servitja JM, Novials A. Chaperones ameliorate beta cell dysfunction associated with human islet amyloid polypeptide overexpression. PLoS One. 2014;9:e101797. doi: 10.1371/journal.pone.0101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnone G, Sirard MA. The impact of exposure to serum lipids during in vitro culture on the transcriptome of bovine blastocysts. Theriogenology. 2014;81:712–722. doi: 10.1016/j.theriogenology.2013.12.005. e711–713. [DOI] [PubMed] [Google Scholar]
- Cagnone GL, Dufort I, Vigneault C, Sirard MA. Differential gene expression profile in bovine blastocysts resulting from hyperglycemia exposure during early cleavage stages. Biol. Reprod. 2012;86:50. doi: 10.1095/biolreprod.111.094391. [DOI] [PubMed] [Google Scholar]
- Cai Z, Li F, Gong W, Liu W, Duan Q, Chen C, Ni L, Xia Y, Cianflone K, Dong N, Wang DW. Endoplasmic reticulum stress participates in aortic valve calcification in hypercholesterolemic animals. Arterioscler Thromb. Vasc. Biol. 2013;33:2345–2354. doi: 10.1161/ATVBAHA.112.300226. [DOI] [PubMed] [Google Scholar]
- Cebrian-Serrano A, Salvador I, Raga E, Dinnyes A, Silvestre MA. Beneficial effect of melatonin on blastocyst in vitro production from heat-stressed bovine oocytes. Reprod. Domest. Anim. 2013;48:738–746. doi: 10.1111/rda.12154. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, Yao X, Wang N, Huang Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5’ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor delta pathway. Arterioscler Thromb. Vasc. Biol. 2014;34:830–836. doi: 10.1161/ATVBAHA.113.301938. [DOI] [PubMed] [Google Scholar]
- Chen J, Guo Y, Zeng W, Huang L, Pang Q, Nie L, Mu J, Yuan F, Feng B. ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am. J. Physiol. Renal Physiol. 2014;306:F916–F925. doi: 10.1152/ajprenal.00697.2012. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen M, Wu Z, Zhao S. Ox-LDL induces ER stress and promotes the adipokines secretion in 3T3-L1 adipocytes. PLoS One. 2013;8:e81379. doi: 10.1371/journal.pone.0081379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin PY, Macpherson AM, Thompson JG, Lane M, Robertson SA. Stress response genes are suppressed in mouse preimplantation embryos by granulocyte-macrophage colony-stimulating factor (GM-CSF) Hum. Reprod. 2009;24:2997–3009. doi: 10.1093/humrep/dep307. [DOI] [PubMed] [Google Scholar]
- Chu DP, Tian S, Sun DG, Hao CJ, Xia HF, Ma X. Exposure to mono-n-butyl phthalate disrupts the development of preimplantation embryos. Reprod. Fertil. Dev. 2013;25:1174–1184. doi: 10.1071/RD12178. [DOI] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic beta-cell dysfunction. Diabetes Obes Metab. 2010;12(Suppl 2):76–82. doi: 10.1111/j.1463-1326.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity.. Biol. Reprod. 2012;86:27. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Mosey JL, Klinger J. Fatty acid oxidation and meiotic resumption in mouse oocytes. Mol. Reprod. Dev. 2009;76:844–853. doi: 10.1002/mrd.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning KR, Anastasi MR, Zhang VJ, Russell DL, Robker RL. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS One. 2014;9:e87327. doi: 10.1371/journal.pone.0087327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JL, Hansen PJ. Elevated temperature increases heat shock protein 70 synthesis in bovine two-cell embryos and compromises function of maturing oocytes. Biol. Reprod. 1996;55:341–346. doi: 10.1095/biolreprod55.2.341. [DOI] [PubMed] [Google Scholar]
- El Mouatassim S, Guerin P, Menezo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol. Hum. Reprod. 1999;5:720–725. doi: 10.1093/molehr/5.8.720. [DOI] [PubMed] [Google Scholar]
- Favetta LA, Madan P, Mastromonaco GF, St John EJ, King WA, Betts DH. The oxidative stress adaptor p66Shc is required for permanent embryo arrest in vitro. BMC Dev Biol. 2007;7:132. doi: 10.1186/1471-213X-7-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazi B, Melino S, De Rubeis S, Bagni C, Paci M, Piacentini M, Di Sano F. Acetylation of RTN-1C regulates the induction of ER stress by the inhibition of HDAC activity in neuroectodermal tumors. Oncogene. 2009;28:3814–3824. doi: 10.1038/onc.2009.233. [DOI] [PubMed] [Google Scholar]
- Feng XD, Xia Q, Yuan L, Huang HF, Yang XD, Wang K. Gadolinium triggers unfolded protein responses (UPRs) in primary cultured rat cortical astrocytes via promotion of an influx of extracellular Ca2+ Cell Biol. Toxicol. 2011;27:1–12. doi: 10.1007/s10565-010-9166-2. [DOI] [PubMed] [Google Scholar]
- Fiorenza MT, Bevilacqua A, Canterini S, Torcia S, Pontecorvi M, Mangia F. Early transcriptional activation of the hsp70.1 gene by osmotic stress in one-cell embryos of the mouse. Biol. Reprod. 2004;70:1606–1613. doi: 10.1095/biolreprod.103.024877. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Lucas ES, Watkins AJ, Eckert JJ. Adaptive responses of the embryo to maternal diet and consequences for post-implantation development. Reprod. Fertil. Dev. 2011;24:35–44. doi: 10.1071/RD11905. [DOI] [PubMed] [Google Scholar]
- Garbin U, Stranieri C, Pasini A, Baggio E, Lipari G, Solani E, Mozzini C, Vallerio P, Cominacini L, Fratta Pasini AM. Do oxidized polyunsaturated Fatty acids affect endoplasmic reticulum stress-induced apoptosis in human carotid plaques? Antioxid. Redox Signal. 2014;21:850–858. doi: 10.1089/ars.2014.5870. [DOI] [PubMed] [Google Scholar]
- Gardiner CS, Reed DJ. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol. Reprod. 1994;51:1307–1314. doi: 10.1095/biolreprod51.6.1307. [DOI] [PubMed] [Google Scholar]
- Ge ZJ, Luo SM, Lin F, Liang QX, Huang L, Wei YC, Hou Y, Han ZM, Schatten H, Sun QY. DNA methylation in oocytes and liver of female mice and their offspring: effects of high-fat-diet-induced obesity. Environ. Health Perspect. 2014;122:159–164. doi: 10.1289/ehp.1307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindler NM, Moley KH. Maternal obesity, infertility and mitochondrial dysfunction: potential mechanisms emerging from mouse model systems. Mol. Hum. Reprod. 2013;19:486–494. doi: 10.1093/molehr/gat026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Li HL, Wu HY, Gu M, Li YD, Wang XG, Ming HX, Dong XL, Liu K. Sodium tanshinone IIA sulfonate attenuates radiation-induced fibrosis damage in cardiac fibroblasts. J. Asian. Nat. Prod. Res. 2014;16:941–952. doi: 10.1080/10286020.2014.935769. [DOI] [PubMed] [Google Scholar]
- Guo H, Chen Y, Liao L, Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc. Drugs. Ther. 2013;27:189–198. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- Guo R, Ma H, Gao F, Zhong L, Ren J. Metallothionein alleviates oxidative stress-induced endoplasmic reticulum stress and myocardial dysfunction. J. Mol. Cell. Cardiol. 2009;47:228–237. doi: 10.1016/j.yjmcc.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Hu J, Lau MY, Feng M, Petrovic LM, Ji C. Altered methylation and expression of ER-associated degradation factors in long-term alcohol and constitutive ER stress-induced murine hepatic tumors. Front. Genet. 2013;4:224. doi: 10.3389/fgene.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Midic U, Garriga J, Latham KE. Contribution of CBX4 to cumulus oophorus cell phenotype in mice and attendant effects in cumulus cell cloned embryos. Physiol Genomics. 2014;46:66–80. doi: 10.1152/physiolgenomics.00071.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausburg MA, Dekrey GK, Salmen JJ, Palic MR, Gardiner CS. Effects of paraquat on development of preimplantation embryos in vivo and in vitro. Reprod. Toxicol. 2005;20:239–246. doi: 10.1016/j.reprotox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Hegele-Hartung C, Schumacher A, Fischer B. Effects of visible light and room temperature on the ultrastructure of preimplantation rabbit embryos: a time course study. Anat Embryol. (Berl.) 1991;183:559–571. doi: 10.1007/BF00187905. [DOI] [PubMed] [Google Scholar]
- Hickman CF, Clinton M, Ainslie A, Ashworth CJ, Rooke JA. Heat shock induces interferon-TAU gene expression by in vitro-produced bovine blastocysts. Am. J. Reprod. Immunol. 2013;70:177–181. doi: 10.1111/aji.12131. [DOI] [PubMed] [Google Scholar]
- Hong D, Gao HC, Wang X, Li LF, Li CC, Luo Y, Wang KK, Bai YP, Zhang GG. Asymmetric dimethylarginine triggers macrophage apoptosis via the endoplasmic reticulum stress pathway. Mol. Cell. Biochem. 2015;398:31–38. doi: 10.1007/s11010-014-2202-4. [DOI] [PubMed] [Google Scholar]
- Hu J, Cheng D, Gao X, Bao J, Ma X, Wang H. Vitamin C enhances the in vitro development of porcine pre-implantation embryos by reducing oxidative stress. Reprod. Domest. Anim. 2012;47:873–879. doi: 10.1111/j.1439-0531.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- Huang FJ, Chin TY, Chan WH. Resveratrol protects against methylglyoxal-induced apoptosis and disruption of embryonic development in mouse blastocysts. Environ. Toxicol. 2013;28:431–441. doi: 10.1002/tox.20734. [DOI] [PubMed] [Google Scholar]
- Humeres C, Montenegro J, Varela M, Ayala P, Vivar R, Letelier A, Olmedo I, Catalan M, Rivas C, Baeza P, Munoz C, Garcia L, Lavandero S, Diaz-Araya G. 4-Phenylbutyric acid prevent cytotoxicity induced by thapsigargin in rat cardiac fibroblast. Toxicol. In Vitro. 2014;28:1443–1448. doi: 10.1016/j.tiv.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Ideta A, Tsuchiya K, Aoyagi Y. Addition of erythrocytes to in vitro culture medium attenuates the detrimental effects of reactive oxygen species on bovine preimplantation embryo development. Anim. Sci. J. 2012;83:31–35. doi: 10.1111/j.1740-0929.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- Isom SC, Prather RS, Rucker EB., 3rd Heat stress-induced apoptosis in porcine in vitro fertilized and parthenogenetic preimplantation-stage embryos. Mol. Reprod. Dev. 2007;74:574–581. doi: 10.1002/mrd.20620. [DOI] [PubMed] [Google Scholar]
- Ito M, Nakagawa H, Okada T, Miyazaki S, Matsuo S. ER-stress caused by accumulated intracistanal granules activates autophagy through a different signal pathway from unfolded protein response in exocrine pancreas cells of rats exposed to fluoride. Arch. Toxicol. 2009;83:151–159. doi: 10.1007/s00204-008-0341-7. [DOI] [PubMed] [Google Scholar]
- Jain A, Olovsson M, Burton GJ, Yung HW. Endothelin-1 induces endoplasmic reticulum stress by activating the PLC-IP(3) pathway: implications for placental pathophysiology in preeclampsia. Am. J. Pathol. 2012;180:2309–2320. doi: 10.1016/j.ajpath.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Jansen S, Cashman K, Thompson JG, Pantaleon M, Kaye PL. Glucose deprivation, oxidative stress and peroxisome proliferator-activated receptor-alpha (PPARA) cause peroxisome proliferation in preimplantation mouse embryos. Reproduction. 2009;138:493–505. doi: 10.1530/REP-09-0038. [DOI] [PubMed] [Google Scholar]
- Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta. 2012;33(Suppl 2):e23–e29. doi: 10.1016/j.placenta.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li F, Shi K, Wu P, An J, Yang Y, Xu C. Involvement of p38 in signal switching from autophagy to apoptosis via the PERK/eIF2alpha/ATF4 axis in selenite-treated NB4 cells. Cell Death Dis. 2014;5:e1270. doi: 10.1038/cddis.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Storrie B. Cisternal rab proteins regulate Golgi apparatus redistribution in response to hypotonic stress. Mol. Biol. Cell. 2005;16:2586–2596. doi: 10.1091/mbc.E04-10-0861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16:31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- Jung TW, Lee KT, Lee MW, Ka KH. SIRT1 attenuates palmitate-induced endoplasmic reticulum stress and insulin resistance in HepG2 cells via induction of oxygen-regulated protein 150. Biochem. Biophys. Res. Commun. 2012;422:229–232. doi: 10.1016/j.bbrc.2012.04.129. [DOI] [PubMed] [Google Scholar]
- Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- Kapuy O, Vinod PK, Banhegyi G. mTOR inhibition increases cell viability via autophagy induction during endoplasmic reticulum stress - An experimental and modeling study. FEBS Open Bio. 2014;4:704–713. doi: 10.1016/j.fob.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagenc L, Lane M, Gardner DK. Granulocyte-macrophage colony-stimulating factor stimulates mouse blastocyst inner cell mass development only when media lack human serum albumin. Reprod. Biomed. Online. 2005;10:511–518. doi: 10.1016/s1472-6483(10)60829-2. [DOI] [PubMed] [Google Scholar]
- Karja NW, Kikuchi K, Fahrudin M, Ozawa M, Somfai T, Ohnuma K, Noguchi J, Kaneko H, Nagai T. Development to the blastocyst stage, the oxidative state, and the quality of early developmental stage of porcine embryos cultured in alteration of glucose concentrations in vitro under different oxygen tensions. Reprod. Biol. Endocrinol. 2006;4:54. doi: 10.1186/1477-7827-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Yoshimi M, Kadota Y, Inoue M, Sato M, Suzuki S. Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol. Appl. Pharmacol. 2014;275:134–144. doi: 10.1016/j.taap.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Kebache S, Cardin E, Nguyen DT, Chevet E, Larose L. Nck-1 antagonizes the endoplasmic reticulum stress-induced inhibition of translation. J. Biol. Chem. 2004;279:9662–9671. doi: 10.1074/jbc.M310535200. [DOI] [PubMed] [Google Scholar]
- Kim JS, Song BS, Lee KS, Kim DH, Kim SU, Choo YK, Chang KT, Koo DB. Tauroursodeoxycholic acid enhances the pre-implantation embryo development by reducing apoptosis in pigs. Reprod. Domest. Anim. 2012;47:791–798. doi: 10.1111/j.1439-0531.2011.01969.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Geum D, Khang I, Park YM, Kang BM, Lee KA, Kim K. Expression pattern of HSP25 in mouse preimplantation embryo: heat shock responses during oocyte maturation. Mol. Reprod. Dev. 2002;61:3–13. doi: 10.1002/mrd.1125. [DOI] [PubMed] [Google Scholar]
- Kimura K, Yamada T, Matsumoto M, Kido Y, Hosooka T, Asahara S, Matsuda T, Ota T, Watanabe H, Sai Y, Miyamoto K, Kaneko S, Kasuga M, Inoue H. Endoplasmic reticulum stress inhibits STAT3-dependent suppression of hepatic gluconeogenesis via dephosphorylation and deacetylation. Diabetes. 2012;61:61–73. doi: 10.2337/db10-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama H, Ikeda S, Sugimoto M, Kume S. Effects of folic acid on the development and oxidative stress of mouse embryos exposed to heat stress. Reprod. Domest. Anim. 2012;47:921–927. doi: 10.1111/j.1439-0531.2012.01992.x. [DOI] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H, Morimoto N, Morihara T, Kimura R, Tabira T, Imaizumi K, Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- Kurzawa R, Glabowski W, Baczkowski T, Brelik P. Evaluation of mouse preimplantation embryos exposed to oxidative stress cultured with insulin-like growth factor I and II, epidermal growth factor, insulin, transferrin and selenium. Reprod. Biol. 2002;2:143–162. [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, Anthony FW, Fleming TP. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction. 2006;132:265–277. doi: 10.1530/rep.1.01038. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Wilkins AP, Dear MS, Wright JN, Osmond C, Zhang J, Fleming TP. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol. Reprod. Dev. 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- Landau G, Kodali VK, Malhotra JD, Kaufman RJ. Detection of oxidative damage in response to protein misfolding in the endoplasmic reticulum. Methods Enzymol. 2013;526:231–250. doi: 10.1016/B978-0-12-405883-5.00014-4. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Amino acids and vitamins prevent culture-induced metabolic perturbations and associated loss of viability of mouse blastocysts. Hum. Reprod. 1998;13:991–997. doi: 10.1093/humrep/13.4.991. [DOI] [PubMed] [Google Scholar]
- Larman MG, Katz-Jaffe MG, McCallie B, Filipovits JA, Gardner DK. Analysis of global gene expression following mouse blastocyst cryopreservation. Hum. Reprod. 2011;26:2672–2680. doi: 10.1093/humrep/der238. [DOI] [PubMed] [Google Scholar]
- Larroque-Cardoso P, Swiader A, Ingueneau C, Negre-Salvayre A, Elbaz M, Reyland ME, Salvayre R, Vindis C. Role of protein kinase C delta in ER stress and apoptosis induced by oxidized LDL in human vascular smooth muscle cells. Cell Death Dis. 2013;4:e520. doi: 10.1038/cddis.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham KE, Sapienza C, Engel N. The epigenetic lorax: gene-environment interactions in human health. Epigenomics. 2012;4:383–402. doi: 10.2217/epi.12.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latreille M, Larose L. Nck in a complex containing the catalytic subunit of protein phosphatase 1 regulates eukaryotic initiation factor 2alpha signaling and cell survival to endoplasmic reticulum stress. J. Biol. Chem. 2006;281:26633–26644. doi: 10.1074/jbc.M513556200. [DOI] [PubMed] [Google Scholar]
- Lawrence JL, Payton RR, Godkin JD, Saxton AM, Schrick FN, Edwards JL. Retinol improves development of bovine oocytes compromised by heat stress during maturation. J. Dairy Sci. 2004;87:2449–2454. doi: 10.3168/jds.S0022-0302(04)73368-8. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- Lee CS, Koo DB, Fang N, Lee Y, Shin ST, Park CS, Lee KK. Potent and stage-specific action of glutathione on the development of goat early embryos in vitro. Mol. Reprod. Dev. 2000;57:48–54. doi: 10.1002/1098-2795(200009)57:1<48::AID-MRD7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Lee EG, Sung MS, Yoo HG, Chae HJ, Kim HR, Yoo WH. Increased RANKL-mediated osteoclastogenesis by interleukin-1beta and endoplasmic reticulum stress. Joint Bone Spine. 2014 doi: 10.1016/j.jbspin.2014.04.012. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Osmotically induced cell volume changes alter anterograde and retrograde transport, Golgi structure, and COPI dissociation. Mol. Biol. Cell. 1999;10:1445–1462. doi: 10.1091/mbc.10.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leese HJ. Quiet please, do not disturb: a hypothesis of embryo metabolism and viability. Bioessays. 2002;24:845–849. doi: 10.1002/bies.10137. [DOI] [PubMed] [Google Scholar]
- Leese HJ. Metabolism of the preimplantation embryo: 40 years on. Reproduction. 2012;143:417–427. doi: 10.1530/REP-11-0484. [DOI] [PubMed] [Google Scholar]
- Legge M, Sellens MH. Free radical scavengers ameliorate the 2-cell block in mouse embryo culture. Hum. Reprod. 1991;6:867–871. doi: 10.1093/oxfordjournals.humrep.a137442. [DOI] [PubMed] [Google Scholar]
- Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, Genicot G, Van Soom A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. 2005;130:485–495. doi: 10.1530/rep.1.00735. [DOI] [PubMed] [Google Scholar]
- Li GY, Fan B, Jiao YY. Rapamycin attenuates visible light-induced injury in retinal photoreceptor cells via inhibiting endoplasmic reticulum stress. Brain Res. 2014;1563:1–12. doi: 10.1016/j.brainres.2014.02.020. [DOI] [PubMed] [Google Scholar]
- Lian HY, Gao Y, Jiao GZ, Sun MJ, Wu XF, Wang TY, Li H, Tan JH. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction. 2013;146:559–568. doi: 10.1530/REP-13-0268. [DOI] [PubMed] [Google Scholar]
- Lian IA, Loset M, Mundal SB, Fenstad MH, Johnson MP, Eide IP, Bjorge L, Freed KA, Moses EK, Austgulen R. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta. 2011;32:823–829. doi: 10.1016/j.placenta.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ML, Chen SS, Huang RY, Lu YC, Liao YR, Reddy MV, Lee CC, Wu TS. Suppression of PI3K/Akt signaling by synthetic bichalcone analog TSWU-CD4 induces ER stress- and Bax/Bak-mediated apoptosis of cancer cells. Apoptosis. 2014a;19:1637–1653. doi: 10.1007/s10495-014-1031-y. [DOI] [PubMed] [Google Scholar]
- Lin YC, Wu MH, Wei TT, Lin YC, Huang WC, Huang LY, Lin YT, Chen CC. Metformin sensitizes anticancer effect of dasatinib in head and neck squamous cell carcinoma cells through AMPK-dependent ER stress. Oncotarget. 2014b;5:298–308. doi: 10.18632/oncotarget.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AX, He WH, Yin LJ, Lv PP, Zhang Y, Sheng JZ, Leung PC, Huang HF. Sustained endoplasmic reticulum stress as a cofactor of oxidative stress in decidual cells from patients with early pregnancy loss. J Clin Endocrinol Metab. 2011;96:E493–E497. doi: 10.1210/jc.2010-2192. [DOI] [PubMed] [Google Scholar]
- Liu L, Keefe DL. Cytoplasm mediates both development and oxidation-induced apoptotic cell death in mouse zygotes. Biol. Reprod. 2000;62:1828–1834. doi: 10.1095/biolreprod62.6.1828. [DOI] [PubMed] [Google Scholar]
- Liu XD, Ko S, Xu Y, Fattah EA, Xiang Q, Jagannath C, Ishii T, Komatsu M, Eissa NT. Transient aggregation of ubiquitinated proteins is a cytosolic unfolded protein response to inflammation and endoplasmic reticulum stress. J. Biol. Chem. 2012;287:19687–19698. doi: 10.1074/jbc.M112.350934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shen S, Li Z, Jiang Y, Si J, Chang Q, Liu X, Pan R. Cajaninstilbene acid protects corticosterone-induced injury in PC12 cells by inhibiting oxidative and endoplasmic reticulum stress-mediated apoptosis. Neurochem. Int. 2014;78C:43–52. doi: 10.1016/j.neuint.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Logue SE, Cleary P, Saveljeva S, Samali A. New directions in ER stress-induced cell death. Apoptosis. 2013;18:537–546. doi: 10.1007/s10495-013-0818-6. [DOI] [PubMed] [Google Scholar]
- Louden E, Chi MM, Moley KH. Crosstalk between the AMP-activated kinase and insulin signaling pathways rescues murine blastocyst cells from insulin resistance. Reproduction. 2008;136:335–344. doi: 10.1530/REP-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol. Cell. Biol. 2006;26:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarevich AV, Olexikova L, Chrenek P, Kubovicova E, Freharova K, Pivko J. The effect of hyperthermia in vitro on vitality of rabbit preimplantation embryos. Physiol. Res. 2007;56:789–796. doi: 10.33549/physiolres.931105. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Martin D, Li Y, Yang J, Wang G, Margariti A, Jiang Z, Yu H, Zampetaki A, Hu Y, Xu Q, Zeng L. Unspliced X-box binding protein 1 protects endothelial cell from oxidative stress through interaction with histone deacetylase 3. J. Biol. Chem. 2014;289:30625–30634. doi: 10.1074/jbc.M114.571984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos L, Gouveia AM, Almeida H. ER Stress Response in Human Cellular Models of Senescence. J. Gerontol. A. Biol. Sci. Med. Sci. 2014 doi: 10.1093/gerona/glu129. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296:2178–2180. doi: 10.1126/science.1071965. [DOI] [PubMed] [Google Scholar]
- Mauro C, Crescenzi E, De Mattia R, Pacifico F, Mellone S, Salzano S, de Luca C, D’Adamio L, Palumbo G, Formisano S, Vito P, Leonardi A. Central role of the scaffold protein tumor necrosis factor receptor-associated factor 2 in regulating endoplasmic reticulum stress-induced apoptosis. J. Biol. Chem. 2006;281:2631–2638. doi: 10.1074/jbc.M502181200. [DOI] [PubMed] [Google Scholar]
- Meares GP, Liu Y, Rajbhandari R, Qin H, Nozell SE, Mobley JA, Corbett JA, Benveniste EN. PERK-Dependent Activation of JAK1 and STAT3 Contributes to Endoplasmic Reticulum Stress-Induced Inflammation. Mol. Cell. Biol. 2014;34:3911–3925. doi: 10.1128/MCB.00980-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T, Kitakaze M. ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Mitchell M, Schulz SL, Armstrong DT, Lane M. Metabolic and mitochondrial dysfunction in early mouse embryos following maternal dietary protein intervention. Biol. Reprod. 2009;80:622–630. doi: 10.1095/biolreprod.108.072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moallem U, Shafran A, Zachut M, Dekel I, Portnick Y, Arieli A. Dietary alpha-linolenic acid from flaxseed oil improved folliculogenesis and IVF performance in dairy cows, similar to eicosapentaenoic and docosahexaenoic acids from fish oil. Reproduction. 2013;146:603–614. doi: 10.1530/REP-13-0244. [DOI] [PubMed] [Google Scholar]
- Moley KH. Hyperglycemia and apoptosis: mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol. Metab. 2001;12:78–82. doi: 10.1016/s1043-2760(00)00341-6. [DOI] [PubMed] [Google Scholar]
- Moley KH, Chi MM, Knudson CM, Korsmeyer SJ, Mueckler MM. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat. Med. 1998;4:1421–1424. doi: 10.1038/4013. [DOI] [PubMed] [Google Scholar]
- Moon SY, Kim HS, Nho KW, Jang YJ, Lee SK. Endoplasmic reticulum stress induces epithelial-mesenchymal transition through autophagy via activation of c-Src kinase. Nephron Exp. Nephrol. 2014;126:127–140. doi: 10.1159/000362457. [DOI] [PubMed] [Google Scholar]
- Moslehi A, Nabavizadeh F, Dehpou AR, Tavanga SM, Hassanzadeh G, Zekri A, Nahrevanian H, Sohanaki H. Naltrexone attenuates endoplasmic reticulum stress induced hepatic injury in mice. Acta Physiol. Hung. 2014;101:341–352. doi: 10.1556/APhysiol.101.2014.3.9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Kitamura M. Bidirectional regulation of NF-kappaB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 2013;65:162–174. doi: 10.1016/j.freeradbiomed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Namekawa T, Ikeda S, Sugimoto M, Kume S. Effects of astaxanthin-containing oil on development and stress-related gene expression of bovine embryos exposed to heat stress. Reprod. Domest. Anim. 2010;45:e387–e391. doi: 10.1111/j.1439-0531.2010.01584.x. [DOI] [PubMed] [Google Scholar]
- Narabayashi K, Ito Y, Eid N, Maemura K, Inoue T, Takeuchi T, Otsuki Y, Higuchi K. Indomethacin suppresses LAMP-2 expression and induces lipophagy and lipoapoptosis in rat enterocytes via the ER stress pathway. J Gastroenterol. 2014 doi: 10.1007/s00535-014-0995-2. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Nasr-Esfahani MH, Johnson MH. Quantitative analysis of cellular glutathione in early preimplantation mouse embryos developing in vivo and in vitro. Hum. Reprod. 1992;7:1281–1290. doi: 10.1093/oxfordjournals.humrep.a137843. [DOI] [PubMed] [Google Scholar]
- Nonogaki T, Noda Y, Narimoto K, Umaoka Y, Mori T. Protection from oxidative stress by thioredoxin and superoxide dismutase of mouse embryos fertilized in vitro. Hum. Reprod. 1991;6:1305–1310. doi: 10.1093/oxfordjournals.humrep.a137532. [DOI] [PubMed] [Google Scholar]
- Okada T, Haze K, Nadanaka S, Yoshida H, Seidah NG, Hirano Y, Sato R, Negishi M, Mori K. A serine protease inhibitor prevents endoplasmic reticulum stress-induced cleavage but not transport of the membrane-bound transcription factor ATF6. J. Biol. Chem. 2003;278:31024–31032. doi: 10.1074/jbc.M300923200. [DOI] [PubMed] [Google Scholar]
- Ono Y, Shimazawa M, Ishisaka M, Oyagi A, Tsuruma K, Hara H. Imipramine protects mouse hippocampus against tunicamycin-induced cell death. Eur. J. Pharmacol. 2012;696:83–88. doi: 10.1016/j.ejphar.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Orsi NM, Leese HJ. Protection against reactive oxygen species during mouse preimplantation embryo development: role of EDTA, oxygen tension, catalase, superoxide dismutase and pyruvate. Mol. Reprod. Dev. 2001;59:44–53. doi: 10.1002/mrd.1006. [DOI] [PubMed] [Google Scholar]
- Otsuki J, Nagai Y, Chiba K. Peroxidation of mineral oil used in droplet culture is detrimental to fertilization and embryo development. Fertil. Steril. 2007;88:741–743. doi: 10.1016/j.fertnstert.2006.11.144. [DOI] [PubMed] [Google Scholar]
- Outinen PA, Sood SK, Liaw PC, Sarge KD, Maeda N, Hirsh J, Ribau J, Podor TJ, Weitz JI, Austin RC. Characterization of the stress-inducing effects of homocysteine. Biochem. J. 1998;332(Pt 1):213–221. doi: 10.1042/bj3320213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Hirabayashi M, Kanai Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction. 2002;124:683–689. doi: 10.1530/rep.0.1240683. [DOI] [PubMed] [Google Scholar]
- Paczkowski M, Schoolcraft WB, Krisher RL. Fatty acid metabolism during maturation affects glucose uptake and is essential to oocyte competence. Reproduction. 2014;148:429–439. doi: 10.1530/REP-14-0015. [DOI] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. Activation of NF-kappa B by ER stress requires both Ca2+ and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- Palsamy P, Bidasee KR, Shinohara T. Valproic acid suppresses Nrf2/Keap1 dependent antioxidant protection through induction of endoplasmic reticulum stress and Keap1 promoter DNA demethylation in human lens epithelial cells. Exp Eye Res. 2014;121:26–34. doi: 10.1016/j.exer.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Sabetski A, Chan YK, Turdi S, Sweeney G. Palmitate induces ER stress and autophagy in H9c2 cells: implications for apoptosis and adiponectin resistance. J Cell Physiol. 2014;230:630–639. doi: 10.1002/jcp.24781. [DOI] [PubMed] [Google Scholar]
- Paula-Lopes FF, Hansen PJ. Apoptosis is an adaptive response in bovine preimplantation embryos that facilitates survival after heat shock. Biochem. Biophys. Res. Commun. 2002;295:37–42. doi: 10.1016/s0006-291x(02)00619-8. [DOI] [PubMed] [Google Scholar]
- Payton RR, Romar R, Coy P, Saxton AM, Lawrence JL, Edwards JL. Susceptibility of bovine germinal vesicle-stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol. Reprod. 2004;71:1303–1308. doi: 10.1095/biolreprod.104.029892. [DOI] [PubMed] [Google Scholar]
- Perez-Martin M, Perez-Perez ME, Lemaire SD, Crespo JL. Oxidative stress contributes to autophagy induction in response to endoplasmic reticulum stress in Chlamydomonas. Plant Physiol. 2014;N166:997–1008. doi: 10.1104/pp.114.243659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronini PG, De Angelis E, Borghetti P, Borghetti AF, Wheeler KP. Modulation by betaine of cellular responses to osmotic stress. Biochem. J. 1992;282:69–73. doi: 10.1042/bj2820069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza F, Manni S, Semenzato G. Novel players in multiple myeloma pathogenesis: role of protein kinases CK2 and GSK3. Leuk. Res. 2013;37:221–227. doi: 10.1016/j.leukres.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Prell T, Lautenschlager J, Weidemann L, Ruhmer J, Witte OW, Grosskreutz J. Endoplasmic reticulum stress is accompanied by activation of NF-kappaB in amyotrophic lateral sclerosis. J. Neuroimmunol. 2014;270:29–36. doi: 10.1016/j.jneuroim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Qian Y, Tiffany-Castiglioni E. Lead-induced endoplasmic reticulum (ER) stress responses in the nervous system. Neurochem. Res. 2003;28:153–162. doi: 10.1023/a:1021664632393. [DOI] [PubMed] [Google Scholar]
- Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. GRP78 compartmentalized redistribution in Pb-treated glia: role of GRP78 in lead-induced oxidative stress. Neurotoxicology. 2005;26:267–275. doi: 10.1016/j.neuro.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Qin L, Wang Z, Tao L, Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol. Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Rehman G, Shehzad A, Khan AL, Hamayun M. Role of AMP-activated protein kinase in cancer therapy. Arch. Pharm. (Weinheim) 2014;347:457–468. doi: 10.1002/ardp.201300402. [DOI] [PubMed] [Google Scholar]
- Ren K, Li X, Yan J, Huang G, Zhou S, Yang B, Ma X, Lu C. Knockdown of p66Shc by siRNA injection rescues arsenite-induced developmental retardation in mouse preimplantation embryos. Reprod. Toxicol. 2014;43:8–18. doi: 10.1016/j.reprotox.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Richards T, Wang F, Liu L, Baltz JM. Rescue of postcompaction-stage mouse embryo development from hypertonicity by amino acid transporter substrates that may function as organic osmolytes. Biol. Reprod. 2010;82:769–777. doi: 10.1095/biolreprod.109.081646. [DOI] [PubMed] [Google Scholar]
- Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil. Steril. 2006;86(Suppl 4):1252–1265. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Rivard AL, Steer CJ, Kren BT, Rodrigues CM, Castro RE, Bianco RW, Low WC. Administration of tauroursodeoxycholic acid (TUDCA) reduces apoptosis following myocardial infarction in rat. Am. J. Chin. Med. 2007;35:279–295. doi: 10.1142/S0192415X07004813. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Sjoblom C, Jasper MJ, Norman RJ, Seamark RF. Granulocyte-macrophage colony-stimulating factor promotes glucose transport and blastomere viability in murine preimplantation embryos. Biol. Reprod. 2001;64:1206–1215. doi: 10.1095/biolreprod64.4.1206. [DOI] [PubMed] [Google Scholar]
- Roth Z, Hansen PJ. Sphingosine 1-phosphate protects bovine oocytes from heat shock during maturation. Biol. Reprod. 2004;71:2072–2078. doi: 10.1095/biolreprod.104.031989. [DOI] [PubMed] [Google Scholar]
- Saenz-de-Juano MD, Marco-Jimenez F, Penaranda DS, Joly T, Vicente JS. Effects of slow freezing procedure on late blastocyst gene expression and survival rate in rabbit. Biol. Reprod. 2012;87:91. doi: 10.1095/biolreprod.112.100677. [DOI] [PubMed] [Google Scholar]
- Saez PJ, Villalobos-Labra R, Westermeier F, Sobrevia L, Farias-Jofre M. Modulation of endothelial cell migration by ER stress and insulin resistance: a role during maternal obesity? Front. Pharmacol. 2014;5:189. doi: 10.3389/fphar.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakatani M, Kobayashi S, Takahashi M. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol. Reprod. Dev. 2004;67:77–82. doi: 10.1002/mrd.20014. [DOI] [PubMed] [Google Scholar]
- Sakatani M, Suda I, Oki T, Kobayashi S, Kobayashi S, Takahashi M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J. Reprod. Dev. 2007;53:605–614. doi: 10.1262/jrd.18124. [DOI] [PubMed] [Google Scholar]
- Salvado L, Barroso E, Gomez-Foix AM, Palomer X, Michalik L, Wahli W, Vazquez-Carrera M. PPARbeta/delta prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 2014;57:2126–2135. doi: 10.1007/s00125-014-3331-8. [DOI] [PubMed] [Google Scholar]
- Salvado L, Coll T, Gomez-Foix AM, Salmeron E, Barroso E, Palomer X, Vazquez-Carrera M. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 2013;56:1372–1382. doi: 10.1007/s00125-013-2867-3. [DOI] [PubMed] [Google Scholar]
- Samak G, Narayanan D, Jaggar JH, Rao R. CaV1.3 channels and intracellular calcium mediate osmotic stress-induced N-terminal c-Jun kinase activation and disruption of tight junctions in Caco-2 CELL MONOLAYERS. J. Biol. Chem. 2011;286:30232–30243. doi: 10.1074/jbc.M111.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaralingam S, Arenas IA, Lalu MM, Davidge ST. Preeclampsia: current understanding of the molecular basis of vascular dysfunction. Expert Rev. Mol. Med. 2006;8:1–20. doi: 10.1017/S1462399406010465. [DOI] [PubMed] [Google Scholar]
- Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum. Reprod. 2000;15(Suppl 2):148–159. doi: 10.1093/humrep/15.suppl_2.148. [DOI] [PubMed] [Google Scholar]
- Sato A, Asano T, Isono M, Ito K, Asano T. Panobinostat synergizes with bortezomib to induce endoplasmic reticulum stress and ubiquitinated protein accumulation in renal cancer cells. BMC Urol. 2014;14:71. doi: 10.1186/1471-2490-14-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehab-El-Deen MA, Leroy JL, Maes D, Van Soom A. Cryotolerance of bovine blastocysts is affected by oocyte maturation in media containing palmitic or stearic acid. Reprod. Domest. Anim. 2009;44:140–142. doi: 10.1111/j.1439-0531.2008.01084.x. [DOI] [PubMed] [Google Scholar]
- Shen M, Wang L, Yang G, Gao L, Wang B, Guo X, Zeng C, Xu Y, Shen L, Cheng K, Xia Y, Li X, Wang H, Fan L, Wang X. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One. 2014;9:e88389. doi: 10.1371/journal.pone.0088389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Zhang Y, Zhang R, Gong X. Sarsasapogenin induces apoptosis via the reactive oxygen species-mediated mitochondrial pathway and ER stress pathway in HeLa cells. Biochem. Biophys. Res. Commun. 2013a;441:519–524. [PubMed] [Google Scholar]
- Shen S, Zhang Y, Zhang R, Gong X. Sarsasapogenin induces apoptosis via the reactive oxygen species-mediated mitochondrial pathway and ER stress pathway in HeLa cells. Biochem Biophys Res Commun. 2013b;441:519–524. [PubMed] [Google Scholar]
- Shi Z, Hou W, Hua X, Zhang X, Liu X, Wang X, Wang X. Overexpression of calreticulin in pre-eclamptic placentas: effect on apoptosis, cell invasion and severity of pre-eclampsia. Cell .Biochem. Biophys. 2012;63:183–189. doi: 10.1007/s12013-012-9350-5. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Yamamoto C, Kaji T. Lead induces the expression of endoplasmic reticulum chaperones GRP78 and GRP94 in vascular endothelial cells via the JNK-AP-1 pathway. Toxicol. Sci. 2010;114:378–386. doi: 10.1093/toxsci/kfq008. [DOI] [PubMed] [Google Scholar]
- Sjoblom C, Roberts CT, Wikland M, Robertson SA. Granulocyte-macrophage colony-stimulating factor alleviates adverse consequences of embryo culture on fetal growth trajectory and placental morphogenesis. Endocrinology. 2005;146:2142–2153. doi: 10.1210/en.2004-1260. [DOI] [PubMed] [Google Scholar]
- Song BS, Kim JS, Yoon SB, Lee KS, Koo DB, Lee DS, Choo YK, Huh JW, Lee SR, Kim SU, Kim SH, Kim HM, Chang KT. Inactivated Sendai-virus-mediated fusion improves early development of cloned bovine embryos by avoiding endoplasmic-reticulum-stress-associated apoptosis. Reprod. Fertil. Dev. 2011;23:826–836. doi: 10.1071/RD10194. [DOI] [PubMed] [Google Scholar]
- Song BS, Yoon SB, Kim JS, Sim BW, Kim YH, Cha JJ, Choi SA, Min HK, Lee Y, Huh JW, Lee SR, Kim SH, Koo DB, Choo YK, Kim HM, Kim SU, Chang KT. Induction of autophagy promotes preattachment development of bovine embryos by reducing endoplasmic reticulum stress. Biol. Reprod. 2012;87(8):1–11. doi: 10.1095/biolreprod.111.097949. [DOI] [PubMed] [Google Scholar]
- Song BS, Yoon SB, Sim BW, Kim YH, Cha JJ, Choi SA, Jeong KJ, Kim JS, Huh JW, Lee SR, Kim SH, Kim SU, Chang KT. Valproic acid enhances early development of bovine somatic cell nuclear transfer embryos by alleviating endoplasmic reticulum stress. Reprod. Fertil. Dev. 2014;26:432–440. doi: 10.1071/RD12336. [DOI] [PubMed] [Google Scholar]
- Steeves CL, Baltz JM. Regulation of intracellular glycine as an organic osmolyte in early preimplantation mouse embryos. J. Cell. Physiol. 2005;204:273–279. doi: 10.1002/jcp.20284. [DOI] [PubMed] [Google Scholar]
- Stockwell SR, Platt G, Barrie SE, Zoumpoulidou G, Te Poele RH, Aherne GW, Wilson SC, Sheldrake P, McDonald E, Venet M, Soudy C, Elustondo F, Rigoreau L, Blagg J, Workman P, Garrett MD, Mittnacht S. Mechanism-based screen for G1/S checkpoint activators identifies a selective activator of EIF2AK3/PERK signalling. PLoS One. 2012;7:e28568. doi: 10.1371/journal.pone.0028568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmey RG, Hawkhead JA, Barker EA, Leese HJ. DNA damage and metabolic activity in the preimplantation embryo. Hum. Reprod. 2009;24:81–91. doi: 10.1093/humrep/den346. [DOI] [PubMed] [Google Scholar]
- Suyama K, Watanabe M, Sakabe K, Otomo A, Okada Y, Terayama H, Imai T, Mochida J. GRP78 suppresses lipid peroxidation and promotes cellular antioxidant levels in glial cells following hydrogen peroxide exposure. PLoS One. 2014;9:e86951. doi: 10.1371/journal.pone.0086951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 2012;58:1–9. doi: 10.1262/jrd.11-138n. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology. 2000;54:137–145. doi: 10.1016/s0093-691x(00)00332-0. [DOI] [PubMed] [Google Scholar]
- Takeo S, Sato D, Kimura K, Monji Y, Kuwayama T, Kawahara-Miki R, Iwata H. Resveratrol improves the mitochondrial function and fertilization outcome of bovine oocytes. J. Reprod. Dev. 2014;60:92–99. doi: 10.1262/jrd.2013-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Chen H, Gao C, Xue P, Yang F, Han JD, Zhou B, Chen YG. Xbp1-mediated histone H4 deacetylation contributes to DNA double-strand break repair in yeast. Cell Res. 2011;21:1619–1633. doi: 10.1038/cr.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol. Cell. Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirupathi Pichiah PB, Suriyakalaa U, Kamalakkannan S, Kokilavani P, Kalaiselvi S, SankarGanesh D, Gowri J, Archunan G, Cha YS, Achiraman S. Spermidine may decrease ER stress in pancreatic beta cells and may reduce apoptosis via activating AMPK dependent autophagy pathway. Med. Hypotheses. 2011;77:677–679. doi: 10.1016/j.mehy.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Treton X, Pedruzzi E, Guichard C, Ladeiro Y, Sedghi S, Vallee M, Fernandez N, Bruyere E, Woerther PL, Ducroc R, Montcuquet N, Freund JN, Van Seuningen I, Barreau F, Marah A, Hugot JP, Cazals-Hatem D, Bouhnik Y, Daniel F, Ogier-Denis E. Combined NADPH oxidase 1 and interleukin 10 deficiency induces chronic endoplasmic reticulum stress and causes ulcerative colitis-like disease in mice. PLoS One. 2014;9:e101669. doi: 10.1371/journal.pone.0101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng JK, Tang PC, Ju JC. In vitro thermal stress induces apoptosis and reduces development of porcine parthenotes. Theriogenology. 2006;66:1073–1082. doi: 10.1016/j.theriogenology.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T. Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ. 2004;11:1009–1016. doi: 10.1038/sj.cdd.4401436. [DOI] [PubMed] [Google Scholar]
- Tyra HM, Spitz DR, Rutkowski DT. Inhibition of fatty acid oxidation enhances oxidative protein folding and protects hepatocytes from endoplasmic reticulum stress. Mol. Biol. Cell. 2012;23:811–819. doi: 10.1091/mbc.E11-12-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulianich L, Garbi C, Treglia AS, Punzi D, Miele C, Raciti GA, Beguinot F, Consiglio E, Di Jeso B. ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. J Cell Sci. 2008;121:477–486. doi: 10.1242/jcs.017202. [DOI] [PubMed] [Google Scholar]
- Umaoka Y, Noda Y, Narimoto K, Mori T. Developmental potentiality of embryos cultured under low oxygen tension with superoxide dismutase. J. In Vitro Fert. Embryo Transf. 1991;8:245–249. doi: 10.1007/BF01139778. [DOI] [PubMed] [Google Scholar]
- Umaoka Y, Noda Y, Narimoto K, Mori T. Effects of oxygen toxicity on early development of mouse embryos. Mol. Reprod. Dev. 1992;31:28–33. doi: 10.1002/mrd.1080310106. [DOI] [PubMed] [Google Scholar]
- Van der Elst J, Van den Abbeel E, Jacobs R, Wisse E, Van Steirteghem A. Effect of 1,2-propanediol and dimethylsulphoxide on the meiotic spindle of the mouse oocyte. Hum. Reprod. 1988;3:960–967. doi: 10.1093/oxfordjournals.humrep.a136826. [DOI] [PubMed] [Google Scholar]
- Wakefield SL, Lane M, Schulz SJ, Hebart ML, Thompson JG, Mitchell M. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am. J. Physiol. Endocrinol. Metab. 2008;294:E425–E434. doi: 10.1152/ajpendo.00409.2007. [DOI] [PubMed] [Google Scholar]
- Wan Q, Xu W, Yan JL, Yokota H, Na S. Distinctive subcellular inhibition of cytokine-induced SRC by salubrinal and fluid flow. PLoS One. 2014;9:e105699. doi: 10.1371/journal.pone.0105699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kooistra M, Lee M, Liu L, Baltz JM. Mouse embryos stressed by physiological levels of osmolarity become arrested in the late 2-cell stage before entry into M phase. Biol. Reprod. 2011;85:702–713. doi: 10.1095/biolreprod.111.090910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Tian X, Zhang L, He C, Ji P, Li Y, Tan D, Liu G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014a;101:577–586. doi: 10.1016/j.fertnstert.2013.10.041. [DOI] [PubMed] [Google Scholar]
- Wang J, Pareja KA, Kaiser CA, Sevier CS. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. Elife. 2014b;3:e03496. doi: 10.7554/eLife.03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shim JS, Li RJ, Dang Y, He Q, Das M, Liu JO. Identification of an old antibiotic clofoctol as a novel activator of unfolded protein response pathways and an inhibitor of prostate cancer. Br. J. Pharmacol. 2014c;171:4478–4489. doi: 10.1111/bph.12800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion. 2010;10:403–410. doi: 10.1016/j.mito.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int. J. Biochem. Cell Biol. 2012a;44:842–846. doi: 10.1016/j.biocel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang S, Liu Y, Ding W, Zheng K, Xiang Y, Liu K, Wang D, Zeng Y, Xia M, Yang D, Wang Y. The Hsp90 inhibitor SNX-2112 induces apoptosis of human hepatocellular carcinoma cells: the role of ER stress. Biochem. Biophys. Res. Commun. 2014d;446:160–166. doi: 10.1016/j.bbrc.2014.02.081. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li JR, Sun MX, Ni B, Huan C, Huang L, Li C, Fan HJ, Ren XF, Mao X. Triggering unfolded protein response by 2-Deoxy-D-glucose inhibits porcine epidemic diarrhea virus propagation. Antiviral Res. 2014e;106:33–41. doi: 10.1016/j.antiviral.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang H, Xu ZM, Ji YL, Chen YH, Zhang ZH, Zhang C, Meng XH, Zhao M, Xu DX. Cadmium-induced teratogenicity: association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol. Appl. Pharmacol. 2012b;259:236–247. doi: 10.1016/j.taap.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Lucas ES, Torrens C, Cleal JK, Green L, Osmond C, Eckert JJ, Gray WP, Hanson MA, Fleming TP. Maternal low-protein diet during mouse pre-implantation development induces vascular dysfunction and altered renin-angiotensin-system homeostasis in the offspring. Br. J. Nutr. 2010;103:1762–1770. doi: 10.1017/S0007114509993783. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY, Eckert JJ, Wild AE, Hanson MA, Osmond C, Fleming TP. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol. Reprod. 2008a;78:299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Wilkins A, Cunningham C, Perry VH, Seet MJ, Osmond C, Eckert JJ, Torrens C, Cagampang FR, Cleal J, Gray WP, Hanson MA, Fleming TP. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J. Physiol. 2008b;586:2231–2244. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Wyss O, Zhang S, MacLaughlin SM, Kleemann D, Walker SK, Suter CM, Cropley JE, Morrison JL, Roberts CT, McMillen IC. Embryo number and periconceptional undernutrition in the sheep have differential effects on adrenal epigenotype, growth, and development. Am. J. Physiol. Endocrinol. Metab. 2014;307:E141–E150. doi: 10.1152/ajpendo.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Cai B, Liu X, Cai H. Emodin attenuates calcium overload and endoplasmic reticulum stress in AR42J rat pancreatic acinar cells. Mol Med Rep. 2014;9:267–272. doi: 10.3892/mmr.2013.1773. [DOI] [PubMed] [Google Scholar]
- Wyman A, Pinto AB, Sheridan R, Moley KH. One-cell zygote transfer from diabetic to nondiabetic mouse results in congenital malformations and growth retardation in offspring. Endocrinology. 2008;149:466–469. doi: 10.1210/en.2007-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H, Barredo JC, Merchan JR, Lampidis TJ. Endoplasmic reticulum stress induced by 2-deoxyglucose but not glucose starvation activates AMPK through CaMKKbeta leading to autophagy. Biochem. Pharmacol. 2013;85:1463–1477. doi: 10.1016/j.bcp.2013.02.037. [DOI] [PubMed] [Google Scholar]
- Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, Savaraj N, Lampidis TJ. 2-Deoxy-D-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother. Pharmacol. 2011;67:899–910. doi: 10.1007/s00280-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Awonuga A, Liu J, Rings E, Puscheck EE, Rappolee DA. Stress induces AMP-dependent loss of potency factors Id2 and Cdx2 in early embryos and stem cells. Stem Cells Dev. 2013;22:1564–1575. doi: 10.1089/scd.2012.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol. Reprod. Dev. 2007;74:1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol. Reprod. 2006;75:45–55. doi: 10.1095/biolreprod.105.049791. [DOI] [PubMed] [Google Scholar]
- Xiong R, Siegel D, Ross D. Quinone-induced protein handling changes: Implications for major protein handling systems in quinone-mediated toxicity. Toxicol. Appl. Pharmacol. 2014;280:285–295. doi: 10.1016/j.taap.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu T, Zhang A, Huo X, Luo Q, Chen Z, Yu L, Li Q, Liu L, Lun ZR, Shen J. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect. Immun. 2012;80:2121–2132. doi: 10.1128/IAI.06295-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yamamori T, Meike S, Nagane M, Yasui H, Inanami O. ER stress suppresses DNA double-strand break repair and sensitizes tumor cells to ionizing radiation by stimulating proteasomal degradation of Rad51. FEBS Lett. 2013;587:3348–3353. doi: 10.1016/j.febslet.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Yagishita N, Nishioka K, Nakajima T. The roles of synoviolin in crosstalk between endoplasmic reticulum stress-induced apoptosis and p53 pathway. Cell Cycle. 2007;6:1319–1323. doi: 10.4161/cc.6.11.4277. [DOI] [PubMed] [Google Scholar]
- Yan F, Li J, Chen J, Hu Q, Gu C, Lin W, Chen G. Endoplasmic reticulum stress is associated with neuroprotection against apoptosis via autophagy activation in a rat model of subarachnoid hemorrhage. Neurosci. Lett. 2014;563:160–165. doi: 10.1016/j.neulet.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Yang L, Hu N, Jiang S, Zou Y, Yang J, Xiong L, Ren J. Heavy metal scavenger metallothionein attenuates ER stress-induced myocardial contractile anomalies: role of autophagy. Toxicol. Lett. 2014a;225:333–341. doi: 10.1016/j.toxlet.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Sha H, Davisson RL, Qi L. Phenformin activates the unfolded protein response in an AMP-activated protein kinase (AMPK)-dependent manner. J. Biol. Chem. 2013;288:13631–13638. doi: 10.1074/jbc.M113.462762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xu H, Hao Y, Zhao L, Cai X, Tian J, Zhang M, Han X, Ma S, Cao J, Jiang Y. Endoplasmic reticulum oxidoreductin 1alpha mediates hepatic endoplasmic reticulum stress in homocysteine-induced atherosclerosis. Acta Biochim. Biophys. Sin. (Shanghai) 2014b;46:902–910. doi: 10.1093/abbs/gmu081. [DOI] [PubMed] [Google Scholar]
- Yao S, Miao C, Tian H, Sang H, Yang N, Jiao P, Han J, Zong C, Qin S. Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression. J. Biol. Chem. 2014;289:4032–4042. doi: 10.1074/jbc.M113.524512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui H, Takeuchi R, Nagane M, Meike S, Nakamura Y, Yamamori T, Ikenaka Y, Kon Y, Murotani H, Oishi M, Nagasaki Y, Inanami O. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014;347:151–158. doi: 10.1016/j.canlet.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Yoon JS, Kim HM, Yadunandam AK, Kim NH, Jung HA, Choi JS, Kim CY, Kim GD. Neferine isolated from Nelumbo nucifera enhances anti-cancer activities in Hep3B cells: molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine. 2013;20:1013–1022. doi: 10.1016/j.phymed.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Lee AR, Jeong SA, Kim YS, Kim JY, Kwon YJ, Choi KS. Release of Ca2+ from the endoplasmic reticulum and its subsequent influx into mitochondria trigger celastrol-induced paraptosis in cancer cells. Oncotarget. 2014a;5:6816–6831. doi: 10.18632/oncotarget.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SB, Choi SA, Sim BW, Kim JS, Mun SE, Jeong PS, Yang HJ, Lee Y, Park YH, Song BS, Kim YH, Jeong KJ, Huh JW, Lee SR, Kim SU, Chang KT. Developmental competence of bovine early embryos depends on the coupled response between oxidative and endoplasmic reticulum stress. Biol. Reprod. 2014b;90:104. doi: 10.1095/biolreprod.113.113480. [DOI] [PubMed] [Google Scholar]
- Younce CW, Burmeister MA, Ayala JE. Exendin-4 attenuates high glucose-induced cardiomyocyte apoptosis via inhibition of endoplasmic reticulum stress and activation of SERCA2a. Am J Physiol Cell Physiol. 2013;304:C508–C518. doi: 10.1152/ajpcell.00248.2012. [DOI] [PubMed] [Google Scholar]
- Yu S, Long H, Lyu QF, Zhang QH, Yan ZG, Liang HX, Chai WR, Yan Z, Kuang YP, Qi C. Protective effect of quercetin on the development of preimplantation mouse embryos against hydrogen peroxide-induced oxidative injury. PLoS One. 2014;9:e89520. doi: 10.1371/journal.pone.0089520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM, Kim SJ. Endoplasmic reticulum stress (ER-stress) by 2-deoxy-D-glucose (2DG) reduces cyclooxygenase-2 (COX-2) expression and N-glycosylation and induces a loss of COX-2 activity via a Src kinase-dependent pathway in rabbit articular chondrocytes. Exp. Mol. Med. 2010;42:777–786. doi: 10.3858/emm.2010.42.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am. J. Pathol. 2008;173:451–462. doi: 10.2353/ajpath.2008.071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung HW, Cox M, Tissot van Patot M, Burton GJ. Evidence of endoplasmic reticulum stress and protein synthesis inhibition in the placenta of non-native women at high altitude. FASEB J. 2012;26:1970–1981. doi: 10.1096/fj.11-190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander DL, Thompson JG, Lane M. Perturbations in mouse embryo development and viability caused by ammonium are more severe after exposure at the cleavage stages. Biol. Reprod. 2006;74:288–294. doi: 10.1095/biolreprod.105.046235. [DOI] [PubMed] [Google Scholar]
- Zander-Fox D, Cashman KS, Lane M. The presence of 1 mM glycine in vitrification solutions protects oocyte mitochondrial homeostasis and improves blastocyst development. J. Assist. Reprod. Genet. 2013;30:107–116. doi: 10.1007/s10815-012-9898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander-Fox DL, Mitchell M, Thompson JG, Lane M. Alterations in mouse embryo intracellular pH by DMO during culture impair implantation and fetal growth. Reprod. Biomed. Online. 2010;21:219–229. doi: 10.1016/j.rbmo.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Diao YF, Kim HR, Jin DI. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS One. 2012;7:e40433. doi: 10.1371/journal.pone.0040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Sun Q, Zhao C, Ling S, Li Q, Chang YZ, Li Y. HDAC4 protects cells from ER stress induced apoptosis through interaction with ATF4. Cell Signal. 2014a;26:556–563. doi: 10.1016/j.cellsig.2013.11.026. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yuan Y, Jiang L, Zhang J, Gao J, Shen Z, Zheng Y, Deng T, Yan H, Li W, Hou WW, Lu J, Shen Y, Dai H, Hu WW, Zhang Z, Chen Z. Endoplasmic reticulum stress induced by tunicamycin and thapsigargin protects against transient ischemic brain injury: Involvement of PARK2-dependent mitophagy. Autophagy. 2014b;10:1801–1813. doi: 10.4161/auto.32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang X, Qi Y, Huang D, Zhang Y. 2,4-dichlorophenol induces ER stress-mediated apoptosis via eIF2alpha dephosphorylation in vitro. Environ Toxicol. 2014c doi: 10.1002/tox.22039. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu F, Liang X, Chang H, Shi L, Peng X, Zhu J, Mi M. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS One. 2014;9:e89021. doi: 10.1371/journal.pone.0089021. [DOI] [PMC free article] [PubMed] [Google Scholar]