Abstract
Purpose of Review
Oxygen (O2) delivery, the maintenance of which is fundamental to supporting those with critical illness, is a function of blood O2 content and flow. Here, we review red blood cell (RBC) physiology relevant to disordered O2 delivery in the critically ill.
Recent Findings
Flow (rather then content) is the focus of O2 delivery regulation: O2 content is relatively fixed, whereas flow fluctuates by several orders of magnitude. Thus, blood flow volume and distribution vary to maintain coupling between O2 delivery and demand. The trapping, processing and delivery of nitric oxide (NO) by RBCs has emerged as a conserved mechanism through which regional blood flow is linked to biochemical cues of perfusion sufficiency. We will review conventional RBC physiology influencing O2 delivery (O2 affinity & rheology) and introduce a new paradigm for O2 delivery homeostasis based on coordinated gas transport and vascular signaling by RBCs.
Summary
By coordinating vascular signaling in a fashion that links O2 and NO flux, RBCs couple vessel caliber (and thus blood flow) to O2 need in tissue. Malfunction of this signaling system is implicated in a wide array of pathophysiologies and may be explanatory for the dysoxia frequently encountered in the critical care setting.
Keywords: erythrocyte, red blood cell, O2 delivery, nitric oxide
Introduction: the Erythron
Recently, the red blood cell (RBC) series, from progenitor cells to mature erythrocytes, have been termed the Erythron. This convention serves to reinforce the concept of integrated tissue function as an independent organ responsible for transport of oxygen from lungs to tissue[1]. In adults, the total number of circulating RBCs is in steady state unless perturbed by pathologic or environmental insult. This is not so during the early stages of embryonic development. Mature RBCs have a life span of approximately 120 days, the majority of which is spent traversing capillary channels of the microcirculation. It is estimated that RBCs travel approximately 300 miles prior to senescence and clearance from the circulation, completing 170,000 circuits through the heart[2] and losing 15-20% of it's hemoglobin content[3]. RBC survival during is attributed to the unique composition of it's membrane and it's ability to rotate around the intracellular contents[4]. RBCs achieve gas transport to and from tissues, but that is not their sole function. RBCs also play an important role in regulation of regional vascular tone, vascular antioxidant systems, immune regulation and self-recognition and the physiologic response to hypoxia on both a regional and whole body level.
RBC Clearance
The estimated normal life span of a mature RBC is 110-120 days[5]. To date, clearance of normal senescent RBCs has not been clearly understood. Two mechanisms have been proposed, clustering of the band 3 (B3) membrane protein[6-9] and externalization of membrane phosphatidyl serine (PS)[10-13], both of these processes may be accelerated in the setting of critical illness, impairing oxygen transport capacity. Oxidatively modified hemoglobin (Hb) forms hemichrome aggregates, which associate with the cytoplasmic domain of the abundant membrane protein B3. Subsequent, clustering of B3 exofascial domains increases affinity of naturally occurring anti-B3 autoantibodies, which activates the complement system – leading to RBC uptake and destruction by macrophages[14]. Normally, PS is asymmetrically distributed in the plasma membrane (a process regulated by flippases). Disruption of this pattern is a well-documented mark of RBC senescence[10-13], signaling RBC removal by the reticulo-endothelial system[13]. Alternatively, RBCs may proceed through a form of ‘stimulated suicide’ similar to apoptosis (termed eryptosis), which is characterized by cell shrinkage and cell membrane scrambling, that is stimulated by Ca2+ entry through Ca2+-permeable, PGE2-activated cation channels, by ceramide, caspases, calpain, complement, hyperosmotic shock, energy depletion, oxidative stress, and deranged activity of several kinases (e.g. AMPK, GK, PAK2, CK1α, JAK3, PKC, p38-MAPK). Eryptosis has been described in the setting of ETOH intoxication, malignancy, hepatic failure, diabetes, chronic renal insufficiency, hemolytic uremic syndrome, dehydration, phosphate depletion, fever, sepsis, mycoplasma infection, malaria, iron deficiency, sickle cell anemia, thalassemia, glucose 6-phosphate dehydrogenase deficiency, and Wilson's disease[13, 15, 16].
Capture and Release of Oxygen by RBCs
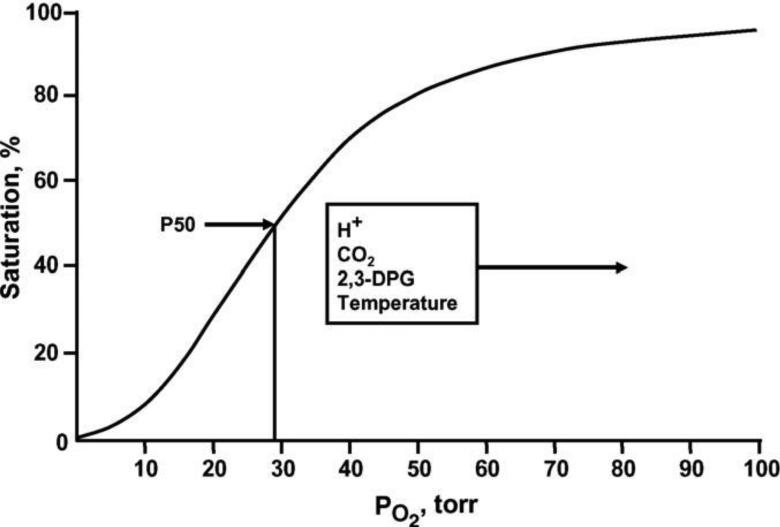
Hemoglobin (Hb) is formed of 2 α and 2 β polypeptide chains each carrying a heme prosthetic group, comprised of a porphyrin ring bearing a ferrous atom that can reversibly bind an oxygen (O2) molecule. In the deoxygenated state, the Hb tetramer is electrostatically held in a tense (T) conformation. Binding of the first O2 molecule leads to mechanical disruption of these bonds, an increase in free energy and transition to the relaxed (R) conformation. Each successive O2 captured by T-state Hb shifts the Hb tetramer closer to the R state, which has an estimated 500-fold increase in O2 affinity[17]. This concept of thermodynamically coupled “cooperativity” in O2 binding was first described by Bohr[18] and explains the sigmoidal appearance of the O2-Hb binding curve, also known as the oxy-Hemoglobin dissociation curve (ODC) (Figure 1)[19]. Moreover, understanding of allosteric influence of protein function by ‘heterotropic effectors’ (e.g. For Hb, O2, which binds to the ‘active’ site (heme) is the homotropic ligand and all other molecules influencing the Hb~O2 binding relationship are termed heterotropic effectors.) was first achieved following description of the variation in Hb~O2 affinity[20]. In addition to the homotropic effects of ligand binding on quaternary conformational changes (e.g. cooperativity), primary ligand binding affinity (O2) is also affected by multiple heterotropic effectors of significant physiologic relevance. The major heterotropic effectors that influence Hb O2 affinity are hydrogen ion (H+), chloride ion (Cl−), carbon dioxide (CO2) and 2,3-diphosphoglycerate (DPG)[17].
Figure 1.
The normal whole blood oxygen equilibrium curve (OEC)[19]. P50 is the pO2 at which hemoglobin is half-saturated with O2. The principle effectors that alter the position and shape of the curve under physiological conditions are indicated.
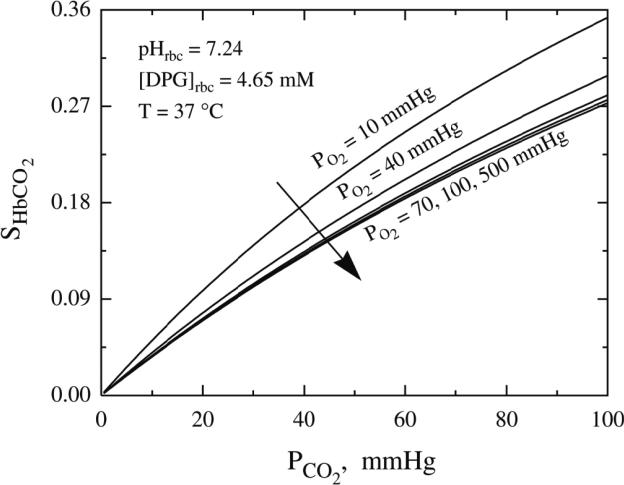
P50, the oxygen tension at which 50% of Hb binding sites are saturated, is used as a standard means to quantify change in Hb~O2 affinity and is inversely related to the binding affinity of Hb for O2[19]. Elevated levels of H+, Cl− and CO2 reduce O2 binding affinity (e.g. raise P50). This allosteric shift in O2 affinity, called the Bohr effect[21], arises from the interactions among the above heterotropic effectors bound to different sites on hemoglobin – all of which serve to stabilize the low energy, low affinity, T-state Hb conformation[22]. This effect is achieved by complex interactions amongst carbonic anhydrase (CA) and the B3 membrane protein (also known as anion exchange protein 1, AE1). Specifically, CA generates H+ and HCO3− from CO2 encountered in the microcirculation; HCO3− then exchanges for Cl− across the RBC membrane through AE1. As a consequence, extra erythrocytic CO2 is converted into intra-erythrocytic HCl by the CA-AE1 complex, thus acidifying RBC cytoplasm and raising p50 (lowering affinity, also termed ‘right’ shifting the ODC). Additionally, through the Haldane effect, CO2 more directly lowers O2 affinity (by binding to the N-terminus of the globin chains to form a carbamino, further stabilizing T-state Hb); carbamino formation also releases another hydrogen ion (further reinforcing the ‘right shift’ in ODC)[17] (Figure 2)[23]. This set of reactions is reversed in the alkaline (and low CO2) milieu in the pulmonary circulation, leading to increased Hb~O2 binding affinity (lower P50). In sum, this physiology vastly improves O2 transport efficiency by enhancing gas capture in the lung and release to tissue – and does so in proportion to perfusion sufficiency (in the setting of perfusion lack, acidosis and hypercpanea improve O2 release). Of note, this tightly regulated modulation of O2 affinity may become impaired in the setting of critical illness[24-27] and may, in part explain the dysoxia commonly observed in this setting.
Figure 2.
The quantitative behavior of the Carbaminohemoglobin (HbCO2) dissociation curves at various oxygen tension levels[23].
Less acute modulation of P50 is achieved by DPG, a glycolytic intermediate that binds in an electrically charged pocket between the β chains of hemoglobin, which stabilizes the T conformation, decreasing O2 affinity and elevating P50. DPG binding also releases protons, lowering intracellular pH and further reinforcing the Bohr effect. DPG in RBCs increases whenever O2 availability is diminished (as in hypoxia or anemia) or when glycolytic flux is stimulated[28]. Lastly, temperature significantly influences Hb~O2 affinity. As body temperature increases, affinity lessens (P50 increases, ODC shifts right); the reverse happens in hypothermia. This feature is of physiological importance during heavy exercise, fever or induced hypothermia. It should be noted that clinical co-oximetry results and blood gas values are reported at 37°C and not at true in vivo temperature and can lead to either under or over estimation of true HbSO2% values and blood O2 tension[29].
RBC Biophysical factors Influencing tissue perfusion
Hemorheology describes flow and deformation properties of blood and its formed elements (RBCs, WBCs and platelets). Plasma is a newtonian fluid (viscosity is independent of shear rate); its viscosity is closely related to protein content[30] and in critical illness, physiologically significant changes in viscosity may vary with concentration of acute phase reactants[31-33]. Whole blood, however, is considered a non-newtonian suspension (fluidity cannot be described by a single viscosity value)[34]; whole blood fluidity is determined by combined rheological properties of plasma and the cellular components. There is increasing evidence that pathophysiologic variation in hemorheology is a major determinant of tissue perfusion and as such, of O2 delivery by RBCs[35].
The cellular components of blood, particularly RBCs, influence blood viscosity as a function of both number and deformability. RBC concentration in plasma (hematocrit) has an exponential relationship with viscosity and meaningfully diminishing tissue perfusion when Hct exceeds ~ 60-65[36]. RBC deformability, or behavior under shear stress, also strongly influences blood fluidity. Normal RBCs behave like fluid drops under most conditions, are highly deformable under shear and orient with flow streamlines[37]. However, during inflammatory stress, RBC tend to aggregate into linear arrays like a stack of coins (rouleaux); fibrinogen and other acute phase reactants in plasma stabilize such aggregates, significantly increasing blood viscosity – such a change in viscosity is most impactful upon O2 delivery during low flow (e.g. low shear) states (such as in critical illness) in the microcirculation[38]. RBC biomechanics and aggregation impact blood viscosity, strongly influencing the volume and distribution of O2 delivery (again more so, in the low-shear microcirculation, or when vessel tone is abnormal)[34]. This hemorheologic physiology is perturbed by oxidative stress (common in critical illness)[39, 40] and in sepsis[41-46]. This has been attributed to increased intracellular 2,3-DPG concentration[47], intracellular free Ca2+ [48] and decreased intra-erythrocytic ATP with subsequent decreased sialic acid content in RBC membranes[49]. Both increased direct contact between RBCs and WBCs and reactive oxygen species released during sepsis have also been shown to alter RBC membrane properties [50, 51].
Blood viscosity and subsequent tissue blood flow is altered in several patho-physiological states. A well-known example is catecholamine discharge, which under acute stressful conditions reduces circulating blood volume and elevates blood pressure. The resultant fluid shift leads to a higher hematocrit and increased plasma protein and overall increase in blood viscosity. Catecholamine discharge also increases the absolute circulating RBC mass secondary to reintroduction of the “reserve” RBC volume from the splanchnic circulation[52].
Under normal conditions, RBC adherence to endothelial cells (EC) is insignificant and RBC deformability permits efficient passage through the microcirculation. Again, under normal conditions, enhanced EC adherence plays a role removal of senescent RBCs in the spleen. However, during critical illness RBC~endothelial interactions are altered by RBC injuries associated with sepsis[43, 44, 53, 54] and/or oxidative stress[40] (more so, with ‘activated’ endothelium, as occurs in critical illness)[54-56] and such RBC~endothelial aggregates create a physiologically significant increase in apparent blood viscosity[34]. Moreover, RBC adhesion directly damages endothelium[57-60] and augments leukocyte adhesion[61-64] further impairing apparent viscosity and microcirculatory flow. This phenomenon is commonly appreciated in the pathophysiology of vaso-occlusive crises in sickle cell disease patients [65], malaria[66], diabetic vasculopathy[67], polycythemia vera[68] and central retinal vein thrombosis[69], but may be more widespread than originally appreciated.
Influence of RBC signaling upon tissue perfusion
Normal physiologic regulation of microcirculatory blood flow instantaneously matches O2 delivery to metabolic demand and is exquisitely responsive to change in O2 consumption across tissue region and within regions, across time[70]. This programmed physiological response to relative tissue hypoperfusion[71] (e.g. hypoxic vasodilation; HVD) is effected in a fashion which suggests the presence of an O2 sensor, detecting point-to-point variations in arteriolar O2 content, and subsequently initiating a signaling mechanism capable of immediate modulation of vascular tone. Stein and Ellsworth[72] originally identified Hb as a potential circulating O2 sensor, a fact later established in vivo following the discovery that HbSO2, rather than plasma or tissue pO2, directly correlated with blood flow[73]. RBCs were thus identified as vascular control elements that actively coordinate modulation of blood flow to resolve perfusion insufficiency (rather than simple transporters without a role in regulatory signaling): to date, three HbSO2-dependent RBC-derived signaling mechanisms have been proposed: (1) formation and export of S-nitrosthiols, ‘catalyzed’ by hemoglobin (SNO-Hb hypothesis)[74, 75], (2) reduction of nitrite to NO by deoxygenated Hb (nitrite hypothesis)[76] and (3) hypoxia-responsive release of ATP (ATP hypothesis)[77]. Each (probably non-exclusive) mechanism has been described to play a role in blood flow misdistribution during various pathologic states and this newly appreciated feature of RBC physiology is centrally relevant to understanding tissue dysoxia in the critically ill.
Mechanistic appreciation of the above physiology has been achieved only after a fundamental shift in our understanding of nitric oxide (NO) biology and chemistry and this issue requires some attention here. Since the original identification of NO of as endothelium-derived relaxing factor (EDRF)[78, 79], our understanding of NO-based vascular signaling has advanced immeasurably[80, 81]. The apparent brief lifetime and fate of EDRF was originally explained by facile diffusion of NO “gas” in solution and its rapid terminal reactions (1) in vascular smooth muscle cells with the ferrous heme iron (Fe2+) of soluble guanylate cyclase (sGC), and (2) in the vessel lumen with oxygenated Hb (forming MetHb and nitrate), deoxygenated Hb (forming nitrosyl hemoglobin) and superoxide (forming peroxynitrite)[82]. We have since come to appreciate much broader biological chemistry of endothelial NO[83], a large portion of which we now understand to occur through the covalent binding of NO+ to cysteine thiols, forming S-nitrosothiols (SNO). SNO signaling follows oxidation of NOS-generated NO radicals to a NO+ (nitrosonium) equivalent, which can then cascade across thiols in peptides and proteins to regulate protein function in a tightly regulated fashion by enzymatic trans-S-nitrosylation reactions (akin to phosphorylation[84-86]); thereby preserving NO bioactivity[80, 81] (in fact, Hb was the first described protein to catalyze S-transnitrosylation reactions and it is this function that is essential to regulation of blood flow by RBCs)[87-91]. This far broader signaling repertoire enabled awareness that heme in sGC is not the sole (or even the principal) target of NO generated by endothelium, with a wide array of alternative sGC (cyclic guanosine monophosphate)-independent reactions following endothelial NOS (eNOS) activation[83, 84].
To recognize the central importance of vasoregulation by RBCs, it is essential to appreciate that it is now broadly accepted that (contrary to the original paradigm) endothelium-derived NO plays no direct role in the HVD response that underlies blood flow regulation[73, 92]. In fact, because of substrate (O2) limitation, NO production by eNOS is most likely attenuated by hypoxia[93, 94]. Moreover, NOS inhibitors do not block the acute change in blood flow coupled to Hb desaturation[95]. However, NO groups captured, transported, processed and subsequently deployed by RBCs do originally arise from eNOS[74] and perhaps other NOS isoforms[96] and/or nitrite[97]. As such eNOS derived NO groups are transported by RBCs to effect HVD at a time and place remote from the original site of NO synthesis, initiating immediate modulation of vascular tone in concert with cues perfusion insufficiency, including hypoxia, hypercarbia, and acidosis[74, 75].
Processing and export of S-nitrosothiols by RBCs
The discovery that Hb could sustain S-nitrosylation (HbSNO)[74], later characterized by both mass spectrometry[98] and X-ray crystallography[99], provided an explanation as to how NO could circumvent terminal reactions with Hb. Rather than acting solely as scavengers of NO (as originally described), this chemistry enables RBCs to conserve NO bioactivity, allowing its transport throughout the circulation (Figure 3)[74, 75, 91]. The formation and export of NO groups by Hb is governed by the transition in Hb conformation that occurs in the course of O2 loading/unloading during arteriovenous (A-V) transit[74, 75, 100, 101]. In a tightly regulated fashion, Hb captures and binds NO at its ß-hemes and subsequently converts ß-heme NO into Cys-ß93-SNO[102]. The passing of NO between heme and thiol requires heme-redox coupled activation of the NO group, which is controlled by its allosteric transition across the lung[103]. NO group export from Hb occurs when steep O2 gradients are encountered in the periphery (HVD). This promotes NO transfer to receptor thiols, including those associated with the erythrocytic membrane protein AE-1 (band 3)[104] and extra erythrocytic thiols[90, 105] to form plasma or other cellular SNOs, which are vasoactive at low nM concentrations[74, 75]. Importantly all NO transfers in this process involve NO+[74, 106], which protects bioactivity from Fe2+ heme recapture and/or inactivation (S-nitrosothiols are the only known endogenous NO compounds that retain bioactivity in the presence of Hb[74, 105, 107]). Extensive evidence supports SNO-Hb biology, whereby RBCs exert graded vasodilator and vasoconstrictor responses across the physiological microcirculatory O2 gradient. RBCs dilate pre-constricted aortic rings at low pO2 (1% O2), while constricting at high pO2 (95% O2)[75, 100, 107, 108]. The vasodilatory response at low O2 is enhanced following the addition of NO (or SNO) to RBCs, commensurate with SNO-Hb formation[74, 104, 107, 109]. Additionally, the vasodilatory response is enhanced in the presence of extra cellular free thiol[107], occurs in the absence of endothelium[106, 107] (which is consistent with in vivo observation that HVD is endothelium independent [110]) and transpires in the time frame of normal circulatory transit (is effected over seconds), as confirmed by measurements of A-V gradients in SNO-Hb[74, 90, 100, 108]. Finally, numerous groups have demonstrated that bioactivity of inhaled NO is commensurate with SNO-Hb formation[111-116].
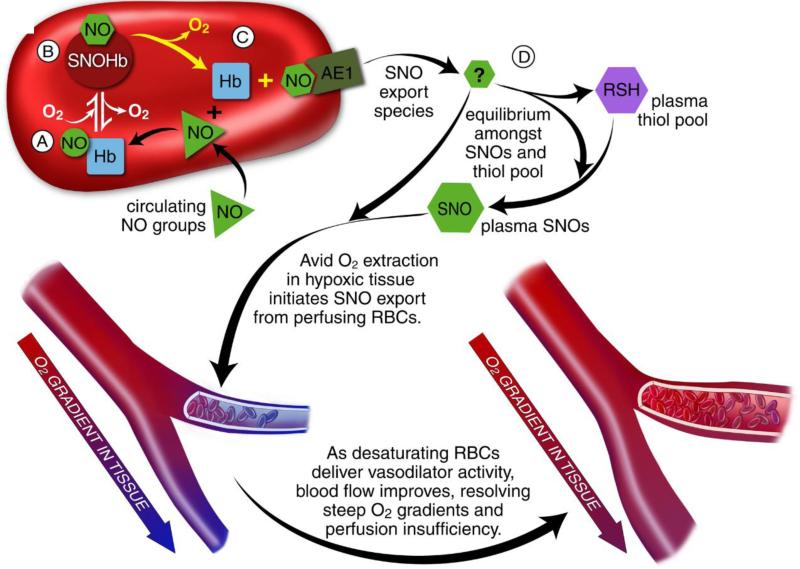
Figure 3.
RBCs transduce regional O2 gradients in tissue to control NO bioactivity in plasma by trapping or delivering NO groups as a function of HbO2 saturation[91]. In this fashion, circulating NO groups are processed by Hb into the highly vasoactive (thiol-based) NO congener, S-nitrosothiol (SNO). By exporting SNOs as a function of Hb deoxygenation, RBCs precisely dispense vasodilator activity in direct proportion to regional blood flow lack. Because oxy- and deoxy-Hb process NO differently, allosteric transitions in Hb conformation afford context-responsive (O2-coupled) control of NO bioavailability, linking the sensor and effector arms of this system. Specifically, Hb conformation governs the equilibria among deoxyHbFeNO (A; NO sink), oxySNOHb (B; NO store), and acceptor thiols including the membrane protein SNO-AE1 (C; bioactive NO source). Direct SNO export from RBCs or S-transnitrosylation from RBCs to plasma thiols (D) yields vasoactive SNOs, which influence resistance vessel caliber and close this signaling loop. Thus, RBCs either trap (A) or export (D) NO groups in response to physiologic cues, linking vessel tone to tissue pO2 in a fashion that calibrates blood flow to tissue respiration.
Metabolism of nitrite by RBCs
Nitrite, long viewed as an inactive end product of NO metabolism[117], has recently been identified as another potential store of bioactive NO in the circulation[118]. Rationale for this proposal relies on the reduction of nitrite to (and subsequent export of) NO radical by RBCs. Several RBC nitrite “reductases” have been identified, including Hb[118-120], xanthine oxidoreductase[121], and carbonic anhydrase[122]. Some have suggested that the reduction of nitrite to NO radical by deoxyHb may serve as the RBC derived signaling mechanism regulating HVD[120, 123-125]. However, this hypothesis has two major shortcomings in terms of known NO chemistry/biochemistry and HVD physiology. Firstly, to influence vascular tone, the NO radical produced from nitrite must escape RBCs at low O2 tension in order to elicit a vasodilatory response. Established experimental evidence, however, unambiguously refutes the possibility of NO escaping RBCs as an authentic radical, especially given the proximity, high concentration, and rapid reaction kinetics (107M−1s−1) of authentic NO with deoxyHb. The only plausible reconciliation of this chemistry enabling bioactivity from deoxyHb-catalyzed nitrite reduction, would be that bioactivity instead - derives from heme captured NO (HbFe2+NO) being further converted into SNO-Hb[97, 102]. The second shortcoming relates to the fact that the nitrite reductase activity of deoxyHb is in fact symmetrical across the physiological O2 gradient[125, 126], with maximal activity occurring at Hb p50 (the pO2 at which Hb is 50% O2 saturated, which for intra-erythrocytic Hb in vivo is ~ 27 mmHg)[124, 125]. This pO2 does not align with peak HVD response, which also of course, increases in a steadily graded fashion as pO2 falls in the physiological range from 100mmHg down to approximately 5mmHg (HbSO2 ~ 1-2%) [71, 73]. Instead, ff RBC based vasoactivity were maximal at Hb p50, then blood flow would be diverted away from regions with pO2 below 27 mmHg (where it would be needed most). Additionally, based upon the symmetry of Hb nitrite reductase activity at the p50, RBCs traversing vascular beds with pO2 at p25 or p75 (or p10 and p90, etc.) would generate equal NO-based activity [123], where, in fact, gradually progressive (rather than equal) HVD responses are observed.
Adenosine Triphosphate (ATP)
ATP (but not its degradation products ADP, AMP, or adenosine [127]) has long been known to act as an endothelium dependent vasodilator in humans[77], binding to P2Y purinergic receptors to induce local vascular tone[128, 129] and to influence conducted vasodilation[130, 131] via stimulation of vasoactive signals including endothelial NO, prostaglandins, and endothelial-derived hyperpolarization factors (EDHFs).
More recently, RBCs have been identified as potential sources of vascular ATP[77, 132, 133], with release being stimulated by conditions associated with diminished oxygen supply relative to demand; i.e., hypoxia, hypercapnia, and low pH[77, 132, 134]. Despite its seeming potential as a mediator of HVD, RBC derived ATP falls short on a couple of fronts. Firstly, HVD is unaltered by both endothelial denudation and eNOS deletion[106]; however, ATP vasoactivity is endothelial dependent. Secondly, blood levels of ATP rise and fall over minutes, which is not commensurate with the physiologic time scale for the RBC-based HVD response that occurs in the course of A-V transit over seconds. Despite its shortcomings in terms of acting as the primary mediator of HVD, it is more likely that Hb and ATP serve complementary vasoactive roles, in acute local and prolonged systemic hypoxia respectively[106, 135-137].
Conclusion: Blood flow disruption during critical illness by maladaptive RBC-based signaling
Evidence is mounting in support of a causal relationship between acquired RBC dysfunction and a host of perfusion-related morbidities that complicate critical illness[41, 108, 138-152]. Recently, it has been observed that levels of SNO-Hb are altered in several disease states characterized by disordered tissue oxygenation[108, 109, 153-160]. In addition, where examined, RBCs from such patients exhibit impaired vasodilatory capacity[90, 108, 109, 157, 159-161]. These data suggest that altered RBC-derived NO bioactivity may contribute to human pathophysiology. Specifically, alterations in thiol-based RBC NO metabolism have been reported in congestive heart failure[108], diabetes[109, 156], pulmonary hypertension[100, 155] and sickle cell disease[157, 162], all of which are conditions characterized by inflammation, oxidative stress and dysfunctional vascular control. Moreover, known cross-talk between SNO signaling and cellular communication via carbon monoxide[163-165], serotonin[86, 166, 167], prostanoids[168, 169], catecholamines[170-172] and endothelin[173-175] may permit broad dispersal of signals generated by dysfunctional RBCs. Precise understanding of the roles of dysregulated RBC-based NO transport in the spread of vasomotor dysfunction from stressed vascular beds may open novel therapeutic approaches to a range of pathologies.
Key Points.
RBC dysfunction may contribute to the dysoxia commonly observed in the critically ill, by impairing delivery of RBCs to tissue (altered rheology and adhesion) or by impairing O2 delivery from perfusing RBCs (altered p50, Bohr and Haldane shifts).
RBCs are newly appreciated to capture, process, transport and release NO in a tightly regulated fashion that links regional blood flow to metabolic demand in support of O2 delivery homeostasis.
Likewise, malfunction of RBC-based control of vasoactive effectors may contribute to disordered perfusion commonly observed in the critically ill.
Acknowledgments
Financial support and sponsorship:
This work was supported by NIH 1R01GM113838-01 & UL1 RR024992WUSM, American Heart Association GIA 0950133G and Children's Discovery Institute CH-LI-2013-420.
Footnotes
Conflicts of Interest:
AD has received research funding and/or consulting fees from: Viasys Inc., Entegrion Inc., Terumo BCT, Galleon Pharmaceuticals, Nitrox LLD, Nitric BioTherapeutics, Galera Inc., and Novartis. AS and SR have no conflicts to declare.
References
- 1.Kaushansky K. Williams Hematology. McGraw-Hill; 2010. [Google Scholar]
- 2.Allison AC. Turnovers of erythrocytes and plasma proteins in mammals. Nature. 1960;188:37–40. doi: 10.1038/188037a0. [DOI] [PubMed] [Google Scholar]
- 3.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–751. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 4.Schmid-Schoenbein H, Wells R. Fluid drop-like transition of erythrocytes under shear. Science. 1969;165:288–291. doi: 10.1126/science.165.3890.288. [DOI] [PubMed] [Google Scholar]
- 5.Landaw SA. Factors that accelerate or retard red blood cell senescence. Blood cells. 1988;14:47–67. [PubMed] [Google Scholar]
- 6.Kay MM. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984;81:5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kay MM, Bosman GJ, Johnson GJ, Beth AH. Band-3 polymers and aggregates, and hemoglobin precipitates in red cell aging. Blood cells. 1988;14:275–295. [PubMed] [Google Scholar]
- 8.Low PS, Waugh SM, Zinke K, Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 9.Lutz HU, Bussolino F, Flepp R, et al. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc Natl Acad Sci U S A. 1987;84:7368–7372. doi: 10.1073/pnas.84.21.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci U S A. 1998;95:3077–3081. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bratosin D, Mazurier J, Tissier JP, et al. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–195. doi: 10.1016/s0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 12.Kiefer CR, Snyder LM. Oxidation and erythrocyte senescence. Curr Opin Hematol. 2000;7:113–116. doi: 10.1097/00062752-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kuypers FA, de Jong K. The role of phosphatidylserine in recognition and removal of erythrocytes. Cellular and molecular biology. 2004;50:147–158. [PubMed] [Google Scholar]
- 14.Lutz HU. Naturally occurring anti-band 3 antibodies in clearance of senescent and oxidatively stressed human red blood cells. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2012;39:321–327. doi: 10.1159/000342171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Lang F, Abed M, Lang E, Foller M. Oxidative stress and suicidal erythrocyte death. Antioxid Redox Signal. 2014;21:138–153. doi: 10.1089/ars.2013.5747. [This paper describes the mechanism and significance of eryptosis, a newly appreciated process of RBC clearance in the critically ill.] [DOI] [PubMed] [Google Scholar]
- 16.Lang KS, Lang PA, Bauer C, et al. Mechanisms of suicidal erythrocyte death. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2005;15:195–202. doi: 10.1159/000086406. [DOI] [PubMed] [Google Scholar]
- 17.Hsia CC. Respiratory function of hemoglobin. N Engl J Med. 1998;338:239–247. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 18.Edsall JT. Understanding blood and hemoglobin: an example of international relations in science. Perspectives in biology and medicine. 1986;29:S107–123. doi: 10.1353/pbm.1986.0052. [DOI] [PubMed] [Google Scholar]
- 19.Winslow RM. The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol. 2007;158:121–127. doi: 10.1016/j.resp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Edsall JT. Hemoglobin and the origins of the concept of allosterism. Federation proceedings. 1980;39:226–235. [PubMed] [Google Scholar]
- 21.Bohr C HK, Krogh A. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Skand Arch Physiol. 1904;16:402–412. [Google Scholar]
- 22.Margaria R GA. The first dissociation constant, pK 1, of carbonic acid in hemoglobin solutions and its relation to the existence of a combination of hemoglobin with carbon dioxide. J Biol Chem. 1933;102:611–634. [Google Scholar]
- 23.Dash RK, Bassingthwaighte JB. Erratum to: Blood HbO2 and HbCO2 dissociation curves at varied O2, CO2, pH, 2,3-DPG and temperature levels. Annals of biomedical engineering. 2010;38:1683–1701. doi: 10.1007/s10439-010-9948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Leon K, Pichavant-Rafini K, Quemener E, et al. Oxygen blood transport during experimental sepsis: effect of hypothermia. Crit Care Med. 2012;40:912–918. doi: 10.1097/CCM.0b013e3182373134. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim Eel D, McLellan SA, Walsh TS. Red blood cell 2,3-diphosphoglycerate concentration and in vivo P50 during early critical illness. Crit Care Med. 2005;33:2247–2252. doi: 10.1097/01.ccm.0000181675.39370.3d. [DOI] [PubMed] [Google Scholar]
- 26.Tuynman HA, Thijs LG, Straub JP, et al. Effects of glucose-insulin-potassium (GIK) on the position of the oxyhemoglobin dissociation curve, 2.3-diphosphoglycerate, and oxygen consumption in canine endotoxin shock. The Journal of surgical research. 1983;34:246–253. doi: 10.1016/0022-4804(83)90067-7. [DOI] [PubMed] [Google Scholar]
- 27.Johnson G, Jr., McDevitt NB, Proctor HJ. Erythrocyte 2,3-diphosphoglycerate in endotoxic shock in the subhuman primate: response to fluid and-or methylprednisolone succinate. Ann Surg. 1974;180:783–786. doi: 10.1097/00000658-197411000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunn HF, Jandl JH. Control of hemoglobin function within the red cell. N Engl J Med. 1970;282:1414–1421. doi: 10.1056/NEJM197006182822507. [DOI] [PubMed] [Google Scholar]
- 29.Severinghaus JW. Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J Appl Physiol. 1958;12:485–486. doi: 10.1152/jappl.1958.12.3.485. [DOI] [PubMed] [Google Scholar]
- 30.Somer T, Meiselman HJ. Disorders of blood viscosity. Annals of medicine. 1993;25:31–39. doi: 10.3109/07853899309147854. [DOI] [PubMed] [Google Scholar]
- 31.Tek I, Toprak SK, Hasdemir E, et al. Influence of febrile neutropenia period on plasma viscosity at malignancy. TheScientificWorldJournal. 2013;2013:507270. doi: 10.1155/2013/507270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwaan HC. Role of plasma proteins in whole blood viscosity: a brief clinical review. Clin Hemorheol Microcirc. 2010;44:167–176. doi: 10.3233/CH-2010-1271. [DOI] [PubMed] [Google Scholar]
- 33.Kesmarky G, Kenyeres P, Rabai M, Toth K. Plasma viscosity: a forgotten variable. Clin Hemorheol Microcirc. 2008;39:243–246. [PubMed] [Google Scholar]
- 34.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Seminars in thrombosis and hemostasis. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 35.Copley AL. Fluid mechanics and biorheology. Thrombosis research. 1990;57:315–331. doi: 10.1016/0049-3848(90)90248-b. [DOI] [PubMed] [Google Scholar]
- 36.S C. Biophysical behavior of red cells in suspension. In: DM S, editor. Red Blood Cell. Academic Press; New York: 1975. pp. 1031–1133. [Google Scholar]
- 37.Schmid-Schonbein H, Wells RE, Goldstone J. Fluid drop-like behaviour of erythrocytes--disturbance in pathology and its quantification. Biorheology. 1971;7:227–234. doi: 10.3233/bir-1971-7406. [DOI] [PubMed] [Google Scholar]
- 38.McHedlishvili G, Varazashvili M, Gobejishvili L. Local RBC aggregation disturbing blood fluidity and causing stasis in microvessels. Clin Hemorheol Microcirc. 2002;26:99–106. [PubMed] [Google Scholar]
- 39.Wang X, Wu Z, Song G, et al. Effects of oxidative damage of membrane protein thiol groups on erythrocyte membrane viscoelasticities. Clin Hemorheol Microcirc. 1999;21:137–146. [PubMed] [Google Scholar]
- 40.Baskurt OK, Temiz A, Meiselman HJ. Effect of superoxide anions on red blood cell rheologic properties. Free Radic Biol Med. 1998;24:102–110. doi: 10.1016/s0891-5849(97)00169-x. [DOI] [PubMed] [Google Scholar]
- 41.Reggiori G, Occhipinti G, De Gasperi A, et al. Early alterations of red blood cell rheology in critically ill patients. Crit Care Med. 2009;37:3041–3046. doi: 10.1097/CCM.0b013e3181b02b3f. [DOI] [PubMed] [Google Scholar]
- 42.Poschl JM, Leray C, Ruef P, et al. Endotoxin binding to erythrocyte membrane and erythrocyte deformability in human sepsis and in vitro. Crit Care Med. 2003;31:924–928. doi: 10.1097/01.CCM.0000055366.24147.80. [DOI] [PubMed] [Google Scholar]
- 43.Aird WC. The hematologic system as a marker of organ dysfunction in sepsis. Mayo Clinic proceedings. Mayo Clinic. 2003;78:869–881. doi: 10.4065/78.7.869. [DOI] [PubMed] [Google Scholar]
- 44.Goyette RE, Key NS, Ely EW. Hematologic changes in sepsis and their therapeutic implications. Seminars in respiratory and critical care medicine. 2004;25:645–659. doi: 10.1055/s-2004-860979. [DOI] [PubMed] [Google Scholar]
- 45.Condon MR, Kim JE, Deitch EA, et al. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol. 2003;284:H2177–2184. doi: 10.1152/ajpheart.01069.2002. [DOI] [PubMed] [Google Scholar]
- 46.Piagnerelli M, Boudjeltia KZ, Vanhaeverbeek M, Vincent JL. Red blood cell rheology in sepsis. Intensive Care Med. 2003;29:1052–1061. doi: 10.1007/s00134-003-1783-2. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki Y, Nakajima T, Shiga T, Maeda N. Influence of 2,3-diphosphoglycerate on the deformability of human erythrocytes. Biochim Biophys Acta. 1990;1029:85–90. doi: 10.1016/0005-2736(90)90439-u. [DOI] [PubMed] [Google Scholar]
- 48.Todd JC, 3rd, Mollitt DL. Effect of sepsis on erythrocyte intracellular calcium homeostasis. Crit Care Med. 1995;23:459–465. doi: 10.1097/00003246-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Piagnerelli M, Boudjeltia KZ, Brohee D, et al. Alterations of red blood cell shape and sialic acid membrane content in septic patients. Critical care medicine. 2003;31:2156–2162. doi: 10.1097/01.CCM.0000079608.00875.14. [DOI] [PubMed] [Google Scholar]
- 50.Betticher DC, Keller H, Maly FE, Reinhart WH. The effect of endotoxin and tumour necrosis factor on erythrocyte and leucocyte deformability in vitro. Br J Haematol. 1993;83:130–137. doi: 10.1111/j.1365-2141.1993.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 51.Machiedo GW, Powell RJ, Rush BF, Jr., et al. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 52.Isbister JP. The contracted plasma volume syndromes (relative polycythaemias) and their haemorheological significance. Bailliere's clinical haematology. 1987;1:665–693. doi: 10.1016/s0950-3536(87)80020-3. [DOI] [PubMed] [Google Scholar]
- 53.Eichelbronner O, Sibbald WJ, Chin-Yee IH. Intermittent flow increases endotoxin-induced adhesion of human erythrocytes to vascular endothelial cells. Intensive Care Med. 2003;29:709–714. doi: 10.1007/s00134-003-1698-y. [DOI] [PubMed] [Google Scholar]
- 54.Tissot Van Patot MC, MacKenzie S, Tucker A, Voelkel NF. Endotoxin-induced adhesion of red blood cells to pulmonary artery endothelial cells. Am J Physiol. 1996;270:L28–36. doi: 10.1152/ajplung.1996.270.1.L28. [DOI] [PubMed] [Google Scholar]
- 55.Anniss AM, Sparrow RL. Variable adhesion of different red blood cell products to activated vascular endothelium under flow conditions. American journal of hematology. 2007;82:439–445. doi: 10.1002/ajh.20837. [DOI] [PubMed] [Google Scholar]
- 56.Chappey O, Wautier MP, Boval B, Wautier JL. Endothelial cells in culture: an experimental model for the study of vascular dysfunctions. Cell biology and toxicology. 1996;12:199–205. doi: 10.1007/BF00438146. [DOI] [PubMed] [Google Scholar]
- 57.Zoukourian C, Wautier MP, Chappey O, et al. Endothelial cell dysfunction secondary to the adhesion of diabetic erythrocytes. Modulation by iloprost. International angiology : a journal of the International Union of Angiology. 1996;15:195–200. [PubMed] [Google Scholar]
- 58.Rattan V, Shen Y, Sultana C, et al. Diabetic RBC-induced oxidant stress leads to transendothelial migration of monocyte-like HL-60 cells. Am J Physiol. 1997;273:E369–375. doi: 10.1152/ajpendo.1997.273.2.E369. [DOI] [PubMed] [Google Scholar]
- 59.Sultana C, Shen Y, Rattan V, et al. Interaction of sickle erythrocytes with endothelial cells in the presence of endothelial cell conditioned medium induces oxidant stress leading to transendothelial migration of monocytes. Blood. 1998;92:3924–3935. [PubMed] [Google Scholar]
- 60.Sirois E, Charara J, Ruel J, et al. Endothelial cells exposed to erythrocytes under shear stress: an in vitro study. Biomaterials. 1998;19:1925–1934. doi: 10.1016/s0142-9612(98)00094-5. [DOI] [PubMed] [Google Scholar]
- 61.Munn LL, Melder RJ, Jain RK. Role of erythrocytes in leukocyte-endothelial interactions: mathematical model and experimental validation. Biophys J. 1996;71:466–478. doi: 10.1016/S0006-3495(96)79248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun C, Migliorini C, Munn LL. Red blood cells initiate leukocyte rolling in postcapillary expansions: a lattice Boltzmann analysis. Biophys J. 2003;85:208–222. doi: 10.1016/S0006-3495(03)74467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Migliorini C, Qian Y, Chen H, et al. Red blood cells augment leukocyte rolling in a virtual blood vessel. Biophys J. 2002;83:1834–1841. doi: 10.1016/S0006-3495(02)73948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zennadi R, Chien A, Xu K, et al. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112:3474–3483. doi: 10.1182/blood-2008-01-134346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wautier JL, Galacteros F, Wautier MP, et al. Clinical manifestations and erythrocyte adhesion to endothelium in sickle cell syndrome. American journal of hematology. 1985;19:121–130. doi: 10.1002/ajh.2830190203. [DOI] [PubMed] [Google Scholar]
- 66.Treutiger CJ, Heddini A, Fernandez V, et al. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 67.Wautier JL, Paton RC, Wautier MP, et al. Increased adhesion of erythrocytes to endothelial cells in diabetes mellitus and its relation to vascular complications. N Engl J Med. 1981;305:237–242. doi: 10.1056/NEJM198107303050501. [DOI] [PubMed] [Google Scholar]
- 68.Wautier MP, El Nemer W, Gane P, et al. Increased adhesion to endothelial cells of erythrocytes from patients with polycythemia vera is mediated by laminin alpha5 chain and Lu/BCAM. Blood. 2007;110:894–901. doi: 10.1182/blood-2006-10-048298. [DOI] [PubMed] [Google Scholar]
- 69.Wautier MP, Heron E, Picot J, et al. Red blood cell phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. Journal of thrombosis and haemostasis : JTH. 2011;9:1049–1055. doi: 10.1111/j.1538-7836.2011.04251.x. [DOI] [PubMed] [Google Scholar]
- 70.Roy CS, Brown JG. The Blood-Pressure and its Variations in the Arterioles, Capillaries and Smaller Veins. J Physiol. 1880;2:323–446. 321. doi: 10.1113/jphysiol.1880.sp000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. Am J Physiol. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 72.Stein JC, Ellsworth ML. Capillary oxygen transport during severe hypoxia: role of hemoglobin oxygen affinity. J Appl Physiol (1985) 1993;75:1601–1607. doi: 10.1152/jappl.1993.75.4.1601. [DOI] [PubMed] [Google Scholar]
- 73.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 75.Stamler JS, Jia L, Eu JP, et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science (New York, N.Y. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 76.Gladwin MT, Shelhamer JH, Schechter AN, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol. 1995;269:H2155–2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 78.Ignarro LJ, Buga GM, Wood KS, et al. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 80.Gow AJ, Farkouh CR, Munson DA, et al. Biological significance of nitric oxide-mediated protein modifications. American journal of physiology. Lung cellular and molecular physiology. 2004;287:L262–268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 81.Hess DT, Matsumoto A, Kim SO, et al. Protein S-nitrosylation: purview and parameters. Nature reviews. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 82.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 83.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science (New York, N.Y. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 84.Arnelle DR, Stamler JS. NO+, NO, and NO- donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 85.Gaston B. Nitric oxide and thiol groups. Biochim Biophys Acta. 1999;1411:323–333. doi: 10.1016/s0005-2728(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 86.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 87.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 88.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 89.Singel DJ, Stamler JS. Chemical Physiology of Blood Flow Regulation by Red Blood Cells: Role of Nitric Oxide and S-Nitrosohemoglobin. Annual Review of Physiology. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 90.Doctor A, Platt R, Sheram ML, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci U S A. 2005;102:5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91*.Doctor A, Stamler JS. Nitric oxide transport in blood: a third gas in the respiratory cycle. Compr Physiol. 2011;1:541–568. doi: 10.1002/cphy.c090009. [This is a detailed review of the mechanisms and physiology of vascular signaling by red blood cells in health and disease.] [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Alonso J, Mortensen SP, Dawson EA, et al. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kantrow SP, Huang YC, Whorton AR, et al. Hypoxia inhibits nitric oxide synthesis in isolated rabbit lung. Am J Physiol. 1997;272:L1167–1173. doi: 10.1152/ajplung.1997.272.6.L1167. [DOI] [PubMed] [Google Scholar]
- 94.Rengasamy A, Johns RA. Determination of Km for oxygen of nitric oxide synthase isoforms. The Journal of pharmacology and experimental therapeutics. 1996;276:30–33. [PubMed] [Google Scholar]
- 95.Frandsenn U, Bangsbo J, Sander M, et al. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N(G)-nitro-L-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mamone G, Sannolo N, Malorni A, Ferranti P. In vitro formation of S-nitrosohemoglobin in red cells by inducible nitric oxide synthase. FEBS Lett. 1999;462:241–245. doi: 10.1016/s0014-5793(99)01527-6. [DOI] [PubMed] [Google Scholar]
- 97.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferranti P, Malorni A, Mamone G, et al. Characterisation of S-nitrosohaemoglobin by mass spectrometry. FEBS Lett. 1997;400:19–24. doi: 10.1016/s0014-5793(96)01258-6. [DOI] [PubMed] [Google Scholar]
- 99.Chan NL, Rogers PH, Arnone A. Crystal structure of the S-nitroso form of liganded human hemoglobin. Biochemistry. 1998;37:16459–16464. doi: 10.1021/bi9816711. [DOI] [PubMed] [Google Scholar]
- 100.McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 101.Sonveaux P, Lobysheva II, Feron O, McMahon TJ. Transport and peripheral bioactivities of nitrogen oxides carried by red blood cell hemoglobin: role in oxygen delivery. Physiology (Bethesda) 2007;22:97–112. doi: 10.1152/physiol.00042.2006. [DOI] [PubMed] [Google Scholar]
- 102.Luchsinger BP, Rich EN, Gow AJ, et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci U S A. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 104.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 105.Palmer LA, Doctor A, Chhabra P, et al. S-nitrosothiols signal hypoxia-mimetic vascular pathology. The Journal of clinical investigation. 2007;117:2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 107.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: evidence for an snitrosothiol-based signal. Circ Res. 2008;103:545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Datta B, Tufnell-Barrett T, Bleasdale RA, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–1342. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 109.James PE, Lang D, Tufnell-Barret T, et al. Vasorelaxation by red blood cells and impairment in diabetes: reduced nitric oxide and oxygen delivery by glycated hemoglobin. Circ Res. 2004;94:976–983. doi: 10.1161/01.RES.0000122044.21787.01. [DOI] [PubMed] [Google Scholar]
- 110.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- 111.Fox-Robichaud A, Payne D, Kubes P. Inhaled NO reaches distal vasculatures to inhibit endothelium- but not leukocyte-dependent cell adhesion. Am J Physiol. 1999;277:L1224–1231. doi: 10.1152/ajplung.1999.277.6.L1224. [DOI] [PubMed] [Google Scholar]
- 112.Fox-Robichaud A, Payne D, Hasan SU, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kubes P, Payne D, Grisham MB, et al. Inhaled NO impacts vascular but not extravascular compartments in postischemic peripheral organs. Am J Physiol. 1999;277:H676–682. doi: 10.1152/ajpheart.1999.277.2.H676. [DOI] [PubMed] [Google Scholar]
- 114.Cannon RO, 3rd, Schechter AN, Panza JA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McMahon TJ, Ahearn GS, Moya MP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci U S A. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McMahon TJ, Doctor A. Extrapulmonary Effects of Inhaled Nitric Oxide: Role of Reversible SNitrosylation of Erythrocytic Hemoglobin. Proceedings of the American Thoracic Society. 2006;3:153–160. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lauer T, Preik M, Rassaf T, et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci U S A. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reutov VP, Sorokina EG. NO-synthase and nitrite-reductase components of nitric oxide cycle. Biochemistry (Mosc) 1998;63:874–884. [PubMed] [Google Scholar]
- 119.Brooks J. The Action of Nitrite on Haemoglobin in the Absence of Oxygen. Proceedings of the Royal Society of London. Series B, Biological Sciences. 1937;123:368–382. [Google Scholar]
- 120.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 121.Ghosh SM, Kapil V, Fuentes-Calvo I, et al. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61:1091–1102. doi: 10.1161/HYPERTENSIONAHA.111.00933. [DOI] [PubMed] [Google Scholar]
- 122.Aamand R, Dalsgaard T, Jensen FB, et al. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H2068–2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 123.Gladwin MT. Evidence mounts that nitrite contributes to hypoxic vasodilation in the human circulation. Circulation. 2008;117:594–597. doi: 10.1161/CIRCULATIONAHA.107.753897. [DOI] [PubMed] [Google Scholar]
- 124.Crawford JH, Isbell TS, Huang Z, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang Z, Shiva S, Kim-Shapiro DB, et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rifkind JM, Ramasamy S, Manoharan PT, et al. Redox reactions of hemoglobin. Antioxidants & redox signaling. 2004;6:657–666. doi: 10.1089/152308604773934422. [DOI] [PubMed] [Google Scholar]
- 127*.Gonzalez-Alonso J. ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J Physiol. 2012;590:5001–5013. doi: 10.1113/jphysiol.2012.235002. [This paper evaluates RBC-mediated control of regional blood flow in skeletal muscle.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Headrick JP, Ashton KJ, Rose'meyer RB, Peart JN. Cardiovascular adenosine receptors: expression, actions and interactions. Pharmacology & therapeutics. 2013;140:92–111. doi: 10.1016/j.pharmthera.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 129.Hellsten Y, Nyberg M, Mortensen SP. Contribution of intravascular versus interstitial purines and nitric oxide in the regulation of exercise hyperaemia in humans. J Physiol. 2012;590:5015–5023. doi: 10.1113/jphysiol.2012.234963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dora KA, Xia J, Duling BR. Endothelial cell signaling during conducted vasomotor responses. Am J Physiol Heart Circ Physiol. 2003;285:H119–126. doi: 10.1152/ajpheart.00643.2002. [DOI] [PubMed] [Google Scholar]
- 131.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 132.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 133.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 134.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 135.Goldman D, Fraser GM, Ellis CG, et al. Toward a multiscale description of microvascular flow regulation: o(2)-dependent release of ATP from human erythrocytes and the distribution of ATP in capillary networks. Frontiers in physiology. 2012;3:246. doi: 10.3389/fphys.2012.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ellis CG, Milkovich S, Goldman D. What is the efficiency of ATP signaling from erythrocytes to regulate distribution of O(2) supply within the microvasculature? Microcirculation. 2012;19:440–450. doi: 10.1111/j.1549-8719.2012.00196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sprague RS, Bowles EA, Achilleus D, Ellsworth ML. Erythrocytes as controllers of perfusion distribution in the microvasculature of skeletal muscle. Acta physiologica (Oxford, England) 2011;202:285–292. doi: 10.1111/j.1748-1716.2010.02182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 139.Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 140.Hart RG, Kanter MC. Hematologic disorders and ischemic stroke. A selective review. Stroke. 1990;21:1111–1121. doi: 10.1161/01.str.21.8.1111. [DOI] [PubMed] [Google Scholar]
- 141.Cirillo M, Laurenzi M, Trevisan M, Stamler J. Hematocrit, blood pressure, and hypertension. The Gubbio Population Study. Hypertension. 1992;20:319–326. doi: 10.1161/01.hyp.20.3.319. [DOI] [PubMed] [Google Scholar]
- 142.Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal hemoglobin concentration during pregnancy and risk of stillbirth. JAMA. 2000;284:2611–2617. doi: 10.1001/jama.284.20.2611. [DOI] [PubMed] [Google Scholar]
- 143.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 144.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 145.Crawford JH, Chacko BK, Kevil CG, Patel RP. The Red Blood Cell and Vascular Function in Health and Disease. Antioxidants & Redox Signaling. 2004;6:992–999. doi: 10.1089/ars.2004.6.992. [DOI] [PubMed] [Google Scholar]
- 146.Rao SV, Jollis JG, Harrington RA, et al. Relationship of Blood Transfusion and Clinical Outcomes in Patients With Acute Coronary Syndromes. JAMA. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 147*.Qing DY, Conegliano D, Shashaty MG, et al. Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am J Respir Crit Care Med. 2014;190:1243–1254. doi: 10.1164/rccm.201406-1095OC. [This paper describes in detail a mechanism for RBC-mediated lung injury.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huertas A, Das SR, Emin M, et al. Erythrocytes induce proinflammatory endothelial activation in hypoxia. American journal of respiratory cell and molecular biology. 2013;48:78–86. doi: 10.1165/rcmb.2011-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149*.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Frontiers in physiology. 2014;5:84. doi: 10.3389/fphys.2014.00084. [This paper reviews acquired energy failure and early clearance for RBCs in critical illness.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kiefmann R, Rifkind JM, Nagababu E, Bhattacharya J. Red blood cells induce hypoxic lung inflammation. Blood. 2008;111:5205–5214. doi: 10.1182/blood-2007-09-113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151*.Purtle SW, Moromizato T, McKane CK, et al. The association of red cell distribution width at hospital discharge and out-of-hospital mortality following critical illness. Crit Care Med. 2014;42:918–929. doi: 10.1097/CCM.0000000000000118. [This paper describes the association between abnormal RBC sub-populations and poor outcome in critical illness.] [DOI] [PubMed] [Google Scholar]
- 152.Bazick HS, Chang D, Mahadevappa K, et al. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913–1921. doi: 10.1097/CCM.0b013e31821b85c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Crawford JH, Chacko BK, Pruitt HM, et al. Transduction of NO-bioactivity by the red blood cell in sepsis: novel mechanisms of vasodilation during acute inflammatory disease. Blood. 2004;104:1375–1382. doi: 10.1182/blood-2004-03-0880. [DOI] [PubMed] [Google Scholar]
- 154.Liu L, Yan Y, Zeng M, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 155.McMahon TJ, Ahearn GS, Moya MP, et al. A nitric oxide processing defect of red blood cells created by hypoxia: Deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proceedings of the National Academy of Sciences. 2005;102:14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Milsom AB, Jones CJ, Goodfellow J, et al. Abnormal metabolic fate of nitric oxide in Type I diabetes mellitus. Diabetologia. 2002;45:1515–1522. doi: 10.1007/s00125-002-0956-9. [DOI] [PubMed] [Google Scholar]
- 157.Pawloski JR, Hess DT, Stamler JS. Impaired vasodilation by red blood cells in sickle cell disease. Proceedings of the National Academy of Sciences. 2005;102:2531–2536. doi: 10.1073/pnas.0409876102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sonveaux P, Kaz AM, Snyder SA, et al. Oxygen Regulation of Tumor Perfusion by SNitrosohemoglobin Reveals a Pressor Activity of Nitric Oxide. Circulation Research. 2005;96:1119–1126. doi: 10.1161/01.RES.0000168740.04986.a7. [DOI] [PubMed] [Google Scholar]
- 159.Reynolds JD, Ahearn GS, Angelo M, et al. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proceedings of the National Academy of Sciences. 2007;104:17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proceedings of the National Academy of Sciences. 2007;104:17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.James PE, Tufnell-Barret T, Milsom AB, et al. Red Blood Cell-Mediated Hypoxic Vasodilatation: A Balanced Physiological Viewpoint. Circulation Research. 2004;95:e8–e9. [PubMed] [Google Scholar]
- 162.Hammerman SI, Klings ES, Hendra KP, et al. Endothelial cell nitric oxide production in acute chest syndrome. AJP - Heart and Circulatory Physiology. 1999;277:H1579–H1592. doi: 10.1152/ajpheart.1999.277.4.H1579. [DOI] [PubMed] [Google Scholar]
- 163.Foresti R, Clark JE, Green CJ, Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. Journal of Biological Chemistry. 1997;272:18411–18417. doi: 10.1074/jbc.272.29.18411. [DOI] [PubMed] [Google Scholar]
- 164.Ryter SW, Morse D, Choi AMK. Carbon Monoxide: To Boldly Go Where NO Has Gone Before. Science's STKE. 2004;2004:re6. doi: 10.1126/stke.2302004re6. [DOI] [PubMed] [Google Scholar]
- 165.Motterlini R, Foresti R, Bassi R, et al. Endothelial Heme Oxygenase-1 Induction by Hypoxia. modulation by inducible nitric oxide synthase and S-nitrosothiols. Journal of Biological Chemistry. 2000;275:13613–13620. doi: 10.1074/jbc.275.18.13613. [DOI] [PubMed] [Google Scholar]
- 166.Nozic-Grayck E, McMahon T, Huang YCT, et al. Pulmonary vasoconstriction by serotonin is inhibited by S-nitrosoglutathione. American Journal of Physiology. 2001;282:L1057–L1065. doi: 10.1152/ajplung.00081.2001. [DOI] [PubMed] [Google Scholar]
- 167.Owen JA, Bates JN, Lewis SJ. Endogenous nitrosyl factors may inhibit the desensitization of 5-HT3 receptors on vagal cardiopulmonary afferents. Brain Research. 2005;1059:167–172. doi: 10.1016/j.brainres.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 168.Vidwans AS, Uliasz TF, Hewett JA, Hewett SJ. Differential modulation of prostaglandin H synthase-2 by nitric oxide-related species in intact cells. Biochemistry. 2001;2001:11533–11542. doi: 10.1021/bi0108960. [DOI] [PubMed] [Google Scholar]
- 169.Kim SF, Huri DA, Snyder SH. Inducible Nitric Oxide Synthase Binds, S-Nitrosylates, and Activates Cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 170.Rigobello MP, Scutari G, Boscolo R, Bindoli A. Oxidation of adrenaline and its derivatives by S-nitrosoglutathione. Nitric Oxide. 2001;5:39–46. doi: 10.1006/niox.2000.0323. [DOI] [PubMed] [Google Scholar]
- 171.Satoh S, Kimura T, Toda M, et al. NO donors stimulate noradrenaline release from rat hippocampus in a calmodulin-dependent manner in the presence of L-cysteine. Journal of Cellular Physiology. 2003;169:87–96. doi: 10.1002/(SICI)1097-4652(199610)169:1<87::AID-JCP9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 172.Nozik-Grayck E, Whalen EJ, Stamler JS, et al. S-nitrosoglutathione inhibits {alpha}1-adrenergic receptor-mediated vasoconstriction and ligand binding in pulmonary artery. AJP - Lung Cellular and Molecular Physiology. 2006;290:L136–L143. doi: 10.1152/ajplung.00230.2005. [DOI] [PubMed] [Google Scholar]
- 173.Sand AE, Andersson AE, Fried G. Effects of nitric oxide donors and inhibitors of nitric oxide signalling on endothelin- and serotonin-induced contractions in human placental arteries. Acta Physiologica Scandinavica. 2003;174:217–223. doi: 10.1046/j.1365-201x.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- 174.Brunner F, Leonhard B, Kukovetz WR, Mayer B. Role of endothelin, nitric oxide and L-arginine release in ischaemia/reperfusion injury of rat heart. Cardiovascular Research. 1997;36:60–66. doi: 10.1016/s0008-6363(97)00138-7. [DOI] [PubMed] [Google Scholar]
- 175.Okishio M, Ohikawa S, Ichimori Y, Kondo K. Interaction between endothelium-derived relaxing factors, S-nitrosothiols, and endothelin-1 on Ca2+ mobilization in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1992;183:849–855. doi: 10.1016/0006-291x(92)90561-x. [DOI] [PubMed] [Google Scholar]