Abstract
Neuroendocrine tumors (NETs) are associated with variable prognosis, with grade 1 and 2 NETs having a more favorable outcome than G3 ones (also called carcinoma). GEP-NET patients need highly individualized interdisciplinary evaluations and treatment. New treatment options have become available (i.e., sunitinib, mTOR inhibitors) with significant improvements in progression-free survival. Peptide receptor radionuclide therapy (PRRT) using 90Y or 177Lu-labeled somatostatin analogs has also shown promise in the treatment of advanced progressive NETs but randomized clinical trials comparing with other modalities are still lacking. SST-targeting represents the essence of theranostics. 68Ga-DOTA-SSTa can be used as companion imaging agents to assist in such a radionuclide therapy selection. 68Ga-DOTA-SSTa PET/CT might also provide critical information for prognosis, tumor response assessement to PRRT, and internal dosimetry. It is also expected that the development of novel receptor-targeting radiopharmaceuticals will contribute to the development of molecular-based personalized medicine approaches.
Keywords: positron emission tomography, gallium radioisotopes, somatostatin, gastroenteropancreatic, neuroendocrine
1. Current views on molecular origins of GEP-NETs
Neuroendocrine tumors are neural crest-derived neoplasms with predominant neuroendocrine differentiation and arise in most organs of the body. They account for 0.5% (incidence 2/100,000) of all malignancies. The most frequent primary sites are the pancreas (PanNET), gastrointestinal tract, and lungs. They share some common biological features such as overexpression of somatostatin receptors (SST) in 70–100% of cases.
The pathogenesis of most NETs starts with inherited or somatic driver mutations in the genes that specifically regulate neuroendocrine cell proliferation 1. Exonomic studies of sporadic pancreatic NETs (PanNETs) demonstrate somatic mutations in the MEN1 gene in 44% of these tumors, Daxx (death-domain-associated protein) and ATRX (α thalassemia/mental retardation syndrome X-linked) in 43%, and mTOR pathway genes (PTEN and TSC2) in 14% 2. Some of the mutations are related to a loss in the integrity of telomere chromatins 3. Recently, PHLDA3, a repressor of Akt activity, was proposed as a novel tumor suppressor of PanNETs 4.
The genetic pathogenesis of small intestine neuroendocrine tumors (midgut NETs) is less well understood. In contrast to PanNETs, exonomic studies demonstrate that somatic mutations are rare in midgut NETs 5.
2. Current management of metastatic GEP-NETs
The management of these tumors relies on several factors such as the presence of hormones/peptide hypersecretion-related symptoms, tumor stage, and grade. According to the ENETS recommendations, tumors are graded as follows: grade 1 (<2 mitoses/10 HPF i.e high power field on microscope and <3% Ki67 index), grade 2 (2–20 mitoses/10 HPF or 3–20% Ki67 index), and grade 3, also called NE carcinomas (>20 mitoses/10 HPF or >20% Ki67 index). In the case of disseminated disease, many treatment options are possible, with potential associations between systemic and locoregional approaches.
The systemic therapy of patients with progressive metastatic GEP-NETs has historically relied mainly on cytotoxic chemotherapy with some positive responses when using a combination of streptozotocin, 5FU, and doxorubicin (especially in PanNETs). In contrast, these treatments were found to have limited efficacy in midgut NETs. There has been breakthrough research in the last few years that has made rapid strides in the targeted therapies of NETs. Since most NETs are hypervascular, they can be targeted by antiangiogenic agents. Furthermore, they may also exhibit an activation of the mTOR signaling pathways and be treated with mTOR inhibitors.
In RADIANT-3, 410 patients with advanced PanNETs and progressive disease were randomly assigned to treatment with oral everolimus 10 mg/day or a placebo. Octreotide LAR was administered at the discretion of the investigator 6. Everolimus showed improved survival (11.0 months with everolimus compared to 4.6 months with the placebo) in the advanced, low-grade (Grade 1) or intermediate-grade (Grade 2) PanNETs with radiological progression 6. In the RADIANT-2 study, 429 patients with advanced progressive midgut NETs were randomized to receive everolimus 10 mg/day plus octreotide LAR 30 mg/month or octreotide LAR plus a placebo 7. In the study, the pre-defined threshold for statistical significance was not achieved. Therefore, the precise therapeutic activity of everolimus in advanced progressive midgut NETs has not been demonstrated 7. However, if the tumor was somatostatin receptor positive, the CLARINET trial group has shown that Lanreotide is associated with prolonged progression-free survival among patients with metastatic midgut and pancreatic NETs of Grade 1 or 2 8. Sunitinib has also been proven to improve progression free survival and overall survival among patients with advanced PanNETs 9. In summary, Grade 1 and 2 PanNETs and midgut NETs exihibit strikingly different drug response profiles.
3. SST PET/CT using 68Ga-labeled somatostatin analogues
Well-differenciated neuroendocine tumors (NETs) often overexpress somatostatin receptors (SSTR) on their cell surfaces that could be targeted for diagnostic and therapeutic purposes by radiolabeling somatostatin analogs. Octreoscan® (111In-DTPA-octreotide, Octreoscan®, Mallinckrodt), a synthetic octapeptide labeled with indium-111 was the first radiolabeled SST analog to be approved for scintigraphy of NETs and has been shown to be well suited for the scintigraphic localization of primary and metastatic NETs 10.
Beyond tumor localization, radionuclide imaging using radiolabeled peptides that target SST may be used to select who is likely to benefit from PRRT and to assess therepeutic responses to PRRT.
Scanning with Octreoscan® is usually performed at 4 and 24 hours after tracer injection. Repeat imaging may be required later. The sensitivity of Octreoscan® is widely dependent on SST density, tumor grade, and size. Also, in recent years, SPECT/CT has become more widely available and has the advantage of simultaneous acquisition of both anatomical and functional data, increasing diagnostic confidence in image interpretation and enhancing sensitivity in some cases. Octreoscan® scintigraphy is associated with practical constraints such as long imaging times, GI tract artifacts requiring bowel cleansing in some cases. The main disadvantage is the still low-resolution of the SPECT image, limiting the ability to detect tiny lesions. SPECT also does not provide a quantifiable estimate of the SST expression. Thus, PET imaging has been growing rapidly in the localization of paragangliomas (PGLs), paralleled by great efforts towards the development of new tracers.
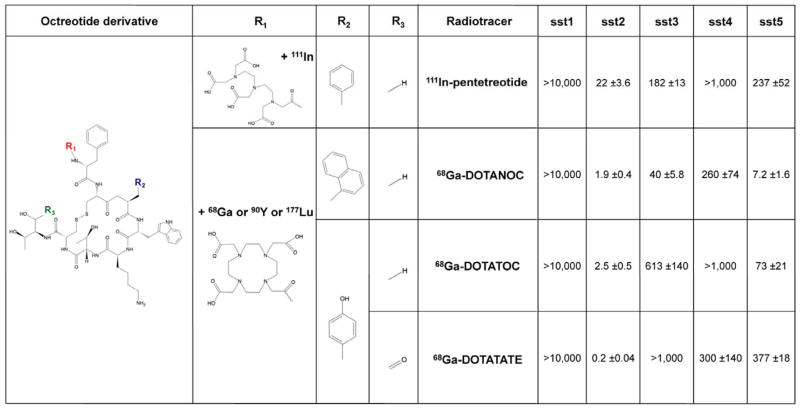
The design of the radiotracer (isotope, chelator, peptidic sequence) dramatically affects the SST affinity (Figure 1). The generator-produced, positron-emitting gallium-68 (68Ga) is a diagnostic trivalent radiometal with convenient labeling characteristics and is also easily available for the daily routine synthesis of 68Ga-labeled radiopharmaceuticals.
Figure 1.
1,4,7,10-Tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) was identified as a better chelator compared to pentetic acid (DTPA), increasing stability and SST targeting.
Numerous 68Ga DOTA-conjugated SST analogs have been designed in order to increase affinity of these compounds to SST receptors, but three are mostly described: 68Ga-DOTA0-[Tyr3]octreotide (68Ga-DOTATOC), 68Ga-DOTA0-1-NaI3-octreotide (68Ga-DOTANOC) and [68Ga-DOTA0-Tyr3]octreotate (68Ga-DOTATATE). All of these bind to SST2. 68Ga-DOTATATE has been shown to have the highest affinity for STT2 (IC50= 0.2 nM vs 2.5 nM for and DOTATOC and 1.9 nM for DOTANOC (Figure 1). DOTA-NOC also binds specifically to SST3, SST4 and SST5 receptors. DOTA-TOC binds to SST5 although with lower affinity than DOTA-NOC) 11–13. In direct comparisons between 68Ga-DOTA-SSTa PET/CT and 99mTc-HYNIC-Octreotide/111In-pentetreotide SPECT(/CT), 68Ga-DOTA-SSTa has performed better than other functional imaging technique, providing a compelling reason for switching from SPECT/CT to PET/CT imaging. PET/CT is also more suitable than SPECT/CT for quantifying the disease at a molecular level.
3. 68Ga-labeled somatostatin analogues as theranostics for GEP-NETs
Peptide receptor radionuclide therapy (PRRT) has shown promise in the treatment of metastatic Grades 1 and 2 NETs 14,15. 90Y-octreotide and 177Lu-octreotate (Lutathera®), have been shown to be efficient and effective therapeutic modalities 16 (Table 1). Response rates (mainly partial responses) have been 30–60% on average. Disease stabilization is frequent (20–50%) but more difficult to interpret 17–23. Independent predictors of survival in advanced grade 1/2 PanNETs treated by 177Lu-octreotate are the tumor proliferation index, the patient’s performance status, tumor burden, and baseline plasma NSE level 23. For advanced NET of the small intestine, tumor functional status and high plasma chromogranin A appeared to be independent predictors of unfavorable patient outcome 24.
Table 1.
| Type of intervention | Pooled number of patients |
Number of studies |
Complete remission CR |
Partial response PR |
Minor response MR |
Stable disease SD |
Favourable outcome CR+PR+MR+SD |
Progressive disease PD |
Median Progression Free Survival in months |
Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| 177Lu-DOTATATE | 988 | 12 | 14 (1.4%) | 310 (31.4%) | 112 (11.3%) | 390 (39.5%) | 826 (83.6%) | 162 (16.4%) | 32 |
|
| Re-treatment with 177Lu-DOTATATE | 88 | 3 | 3 (3.4%) | 10 (11.4%) | 7 (8%) | 37 (42%) | 57 (64.8%) | 31 (35.2%) | 18.5 |
|
| Dual tx 90Y-DOTATATE + 177Lu-DOTATATE | 88 | 2 | 2 (2%) | 26 (30%) | - | 45 (51%) | 73 (83%) | 15 (17%) | 18 |
|
| 177Lu-DOTATATE combined with chemotherapy | 86 | 2 | 6 (7%) | 28 (32%) | - | 48 (56%) | 82 (95%) | 4 (5%) | 39.5 |
|
Randomized clinical trials comparing with other modalities are still lacking 25.
It is worth noting the ongoing trials that compare the use peptide receptor radionuclide therapy with 177Lu-octreotate in GEPNETS against a range of other molecules. These were obtained from the database of clinicaltrials.gov.
The (CONTROL NETS) is an open label phase two study that involves two parallel trials, the first comparing PRRT therapy (177Lu-octreotate) plus Capecitabine/Temozolomide (CAP/TEM) against: (a) Lu177-DOTATATE alone in the treatment of low to intermediate grade mid gut neuroendocrine tumours; (b) CAP/TEM alone in the treatment of low to intermediate grade pancreatic neuroendocrine tumours. A further study is comparing treatment with 177Lu-octreotate against Octreotide LAR in patients with inoperable, progressive, somatostatin receptor positive midgut carcinoid tumours (NETTER-1).
The french OCLURANDOM study is a randomized, open-label, multicenter trial that assesses the safety and efficacy of 177Lu-octreotate versus Sunitinib in pre-treated progressive well differentiated pancreatic neuroendocrine tumours and is expected to be finalised in 2023. Another study is comparing 177Lu-octreotate against interferon α-2b in progressive non-pancreatic gastrointestinal neuroendocrine tumors that are non-resectable and resistant to therapy with somatostatin analogues, its estimated completion date is the end of 2016.
In 2014, the FDA defined a companion diagnostic device (CDD) as an “in vitro […] or an imaging tool that provides information that is essential for the safe and effective use of a corresponding therapeutic product” publishing an exhaustive list of consequent examples. Most of these were in vitro diagnostic devices like immunohistochemistry and FISH/CISH kits, with the aim of predicting the utility of therapeutic monoclonal antibodies upon one target (or more) expressed by the tumor. CDDs in imaging, and particularly in PRRT, are emerging.
PET/CT using gallium-68-labeled SST analogs is a prime example of a PET-based theranostic approach. A close correlation was found between SUVmax and immunohistochemical scores used for the quantitative assessment of the density of subtypes of SST 26. Until present, the Krenning scale remains the unique validated scoring system for selecting good candidates for PRRT 27. Validation of a new scoring system adapted to 68Ga-DOTA-SSTa PET/CT would be of particular interest.
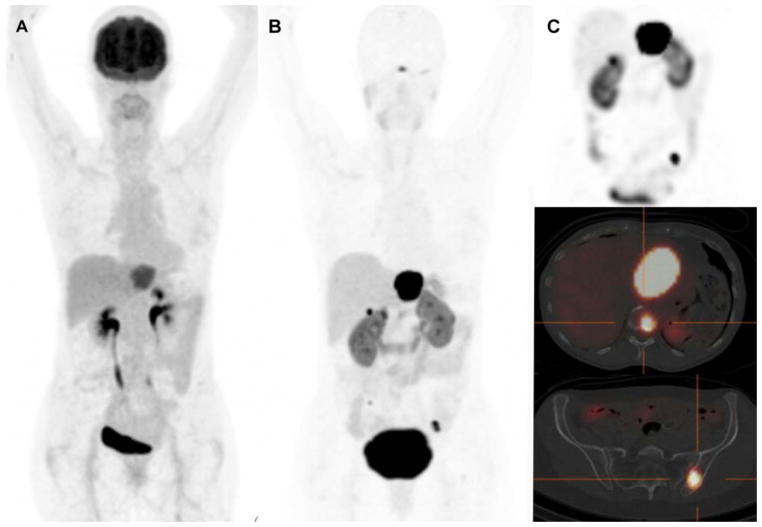
DOTANOC/TOC/TATE can be radiolabelled with lutetium-177 for PRRT with a lower energy deposit and a shorter tissue penetration than 90Y. It has to be underlined that exactly the same peptide should be used for CDD imaging to avoid potential discordances 28–30(Figure 2).
Figure 2.
4. Prognostic value and tumor response assessement to PRRT
PET/CT with 68Ga-DOTA-SSTa might also provide prognostic information. Increased tumor avidity for 68Ga-DOTA-SSTa was found to be associated with prolonged survival 31. Sequential evaluation of metastatic NET patients by 68Ga-DOTA-SSTa PET/CT and 18F-FDG PET/CT that is often also complementary and points towards aggressiveness of these tumors should be recommended at baseline for comprehensive NET grading of patients with these tumors. It was found that patients with high tumor 68Ga-DOTA-SSTa avidity and low 18F-FDG uptake have a better prognosis and are good candidates for PRRT (27). Decrease in tumor-to-spleen SUV ratio (and not SUVmax) after the first PRRT cycle is associated with a longer progression-free survival 32, although additional confirmatory studies are needed. However, PRRT in these tumors, in contrast to other tumor types, usually results in smaller tumor size responses compared to functional status responses (e.g., done by monitoring secretory status of these tumors), which are often very substantial. Thus, response evaluation criteria in solid tumors (RECIST) and World Health Organization criteria for classifying tumor response is less adapted to the evaluation of targeted therapies and PRRT since only a small percentage of patients show a significant decline in tumor size despite their clinical and biochemical improvements. Furthermore, it is widely recognized that molecular/functional responses precede morphological responses and therefore enable an earlier evaluation of overall therapy response.
5. Internal dosimetry
Internal dosimetry enables a personalized approach to patient treatment. The dream of a common dosimetry protocol applicable to all targeted radionuclide therapy (TRT) procedures is a widespread misconception, possibly derived from the wish to standardize therapeutic applications in nuclear medicine.
TRT dosimetry must be implemented in order to answer a clinical question. The fact is that dosimetry implementations, as seen in the literature, are diverse and depend on the clinical context (the aim of the therapy) and the radiopharmaceutical and its mode of administration. In addition, the isotope attached to the biological vector will impact the methodology that can be implemented. In other words, safety-related dosimetry will focus of organs at risk (OARs) whereas efficacy-related dosimetry will focus on tumors 33.
This is consistent with legal requirements, derived from EURATOM Directive 97/43 and the more recent 2013/59 that states: “For all medical exposure of patients for radiotherapeutic purposes, exposures of target volumes shall be individually planned and their delivery appropriately verified taking into account that doses to non-target volumes and tissues shall be as low as reasonably achievable and consistent with the intended radiotherapeutic purpose of the exposure.” In that respect, dosimetry that works should establish the relation between injected activity and observed biological or clinical effect: “The objective of dosimetry in targeted radionuclide therapy is to provide information that will help improve patient care. With this objective, estimated absorbed dose is useful to the extent that it relates to response” 34. In that sense, dosimetry is the missing link that allows for real treatment personalization.
The current administration scheme of 177Lu-labeled peptides is based on repeated administrations of fixed activities, most often 4 to 6 cycles of 7.4 GBq of radiophamarceutical.
The front-line OAR is the kidney, as toxicity has been observed in that kind of treatment, even though initially with 90Y-labelled peptides. This explains why the renal function is followed during the course of the treatment. Suspicion of kidney toxicity may lead to a decrease injected activities or even stop the treatment.
The second OAR is bone marrow.
Tumor dosimetry has been reported, even though to-date more as a way to document the therapy than as a means to define the posology. However, a significant correlation between absorbed dose and tumor reduction was reported 35.
Dosimetry that works in PRRT should aim at assessing kidneys, bone marrow, and tumor absorbed doses, with the aim of establishing the relationship between absorbed dose and observed effect.
Regarding clinical dosimetry, the well-known 90Y-DOTATOC trial yielded very important conclusions 36:
Activity quantification (and cumulated activity determination) is of paramount importance. 86Y-DOTATOC was used to assess pharmacokinetics 37, a far from trivial task as 86Y is a “dirty isotope” with low positron abundance and emits a high proportion of single gammas in the range of the coincidence window 38.
The model used for absorbed dose calculation also has a major impact: By moving from a “standard” kidney model for all patients to a better accounting of patient-specific kidney volume, the correlation between absorbed dose and kidney toxicity significantly improved.
Accounting for radiobiological parameters (computation of the Biologic Equivalent Dose – BED) allowed to further improve the correlation. It is remarkable that this was accomplished by deriving BED values from parameters issued from External Beam Radiotherapy, thereby illustrating the fact that a quite robust phenomenon is supporting that absorbed dose effect correlation 39.
In a retrospective study, Walrand et al. also demonstrate a good correlation between red marrow absorbed dose and platelet count reduction at the nadir.
For 177Lu-labelled PRRT, kidney toxicity is far less frequent 20, a fact that can be explained partly by the different range of radiation emitted by 177Lu as compared to 90Y 40. This makes the determination of the absorbed dose (or surrogate)–effect relationship more difficult to characterize.
Dosimetry with 177Lu-labelled peptides, when performed, is usually meant to insure that the absorbed dose (or surrogate) delivered to kidney will not exceed a certain threshold (safety). Nephrotoxicity is increased in patients with baseline impaired renal function and is more frequently observed in those who develop hematotoxicity during PRRT. Nephroptection by using positively charged molecules such as L-lysine and/or L–arginine (which competitively inhibit the proximal tubular reabsorption of the radiopeptide) is recommended.
There is usually no pre-therapeutic dosimetry: absorbed dose is assessed for every therapy cycle, in order to insure that the next cycle can be safely administered. This “conservative” approach relies on the hypothesis that intra-patient pharmacokinetics variability is inferior to inter-patient variability.
On principle, that scheme could be used to modulate injected activity—as is done, for example, in 131I-mIBG neuroblastoma Molecular Radiotherapy where the absorbed dose assessed for the first, fixed injection (444 MBq/kg) is used to derive the activity to administer for the second injection, under the constraint of limiting the whole body absorbed dose below 4 Gy 41.
The fact is that 177Lu-labelled PRRT, as currently delivered, is not very toxic: the maximum tolerated absorbed dose has probably not been reached, and therefore absorbed dose–toxicity correlations are difficult to put in evidence. In that context, it is difficult to conclude: a potential reason for that apparent absence of correlation could lie in methodological flaws in the dosimetric protocol implemented, but a more trivial reason could be that the lack of effect limits the possibility of evaluating the dose-response relationship.
The dosimetric protocols implemented suffer from a very high heterogeneity, and the comparison and appraisal of uncertainties is very difficult to get 42.
Most protocols implement 2D whole body dosimetry at different times after injection (3 to 7 time-points), even though this approach is known to be limited, essentially due to the overlap of source contribution in Ant-Post projections and the difficulty of correcting for background. Time sampling is also very variable, and this is known to markedly impact the determination of cumulated activities 43. Even for 3D approaches, protocols can hardly be compared.
A discussion of the current means to derive dosimetry for PRRT is given in an article from Cremonesi 42. In figure 2, they present the various possibilities offered for pre- and post-therapeutic dosimetry. The possibility of using 68Ga as a surrogate isotope for quantitative imaging PET studies is mentioned. However, due to the very short physical half-life of 68Ga (68 min) compared to 90Y and 177Lu, data collection can only be performed for up to a few hours after the injection (Table 1). This, on principle, should rule out 68Ga for dosimetric studies. However, some recent studies highlighted the potential of 68Ga for assessing the response to PRRT 32,44. This means that the “effect” of the absorbed dose–effect relationship can be identified. It is therefore tempting to see how 68Ga could be used in a dosimetric context. Velikyan proposed an elegant concept for combining the good activity quantification obtained from 68Ga PET imaging with late data acquisition from 177Lu blood sampling or quantitative SPECT imaging. This approach certainly deserves to be studied. Beyond internal dosimetry, there are also unidentified individual susceptibilities to radiation-associated disease 45.
Conclusion
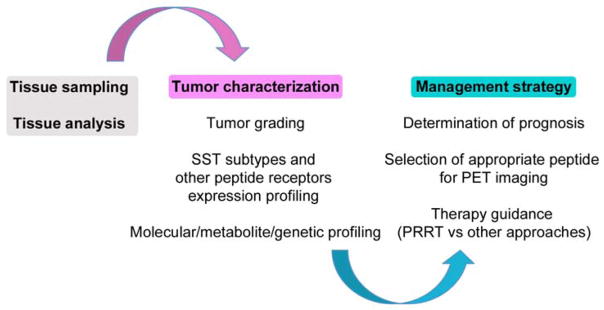
Theranostics of GEP-NETs based on 68Ga-labeled-SSTa PET imaging and targeted therapy applying PRRT with 90Y and/or 177Lu-labeled SSTa has paved the way to personalized medicine (Figure 3). Future directions include the clinical use of somatostatin antagonists as targeting peptides for imaging and therapy 46 and the development of novel receptor-targeting radiopharmaceuticals that will offer exciting perspectives for theranostics of NETs.
Figure 3.
Supplementary Material
Table 2.
| T1/2 (h) | Eβmax (Mev) | Max tissue penetration range (mm) | |
|---|---|---|---|
| 68Ga | 1.08 | 1.9 | 10 |
| 90Y | 64.1 | 2.3 | 12 |
| 177Lu | 160.4 | 0.5 | <2 |
Key points.
Peptide receptor radionuclide therapy (PRRT) using 177Lu-labeled somatostatin analogs has also shown promise in the treatment of advanced progressive grades 1 and 2 NETs with response rates (mainly partial responses) observed in approximately 30% of cases.
PET/CT using gallium-68-labeled SST analogs is a prime example of a PET-based theranostic approach by providing prognostic information, selecting good candidates for PRRT and enabling tumor response assessement to PRRT.
Footnotes
Disclosure summary: The authors have nothing to disclose.
References
- 1.Lewis MA, Yao JC. Molecular pathology and genetics of gastrointestinal neuroendocrine tumours. Curr Opin Endocrinol Diabetes Obes. 2014 Feb;21(1):22–27. doi: 10.1097/MED.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 2.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011 Mar 4;331(6021):1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology. 2014 Feb;146(2):453–460. e455. doi: 10.1053/j.gastro.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Ohki R, Saito K, Chen Y, et al. PHLDA3 is a novel tumor suppressor of pancreatic neuroendocrine tumors. Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):E2404–2413. doi: 10.1073/pnas.1319962111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banck MS, Kanwar R, Kulkarni AA, et al. The genomic landscape of small intestine neuroendocrine tumors. J Clin Invest. 2013 Jun 3;123(6):2502–2508. doi: 10.1172/JCI67963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. The New England journal of medicine. 2011 Feb 10;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011 Dec 10;378(9808):2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 8.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. The New England journal of medicine. 2014 Jul 17;371(3):224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 9.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. The New England journal of medicine. 2011 Feb 10;364(6):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 10.Baum RP, Kulkarni HR, Carreras C. Peptides and receptors in image-guided therapy: theranostics for neuroendocrine neoplasms. Semin Nucl Med. 2012 May;42(3):190–207. doi: 10.1053/j.semnuclmed.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Wild D, Macke HR, Waser B, et al. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging. 2005 Jun;32(6):724. doi: 10.1007/s00259-004-1697-4. [DOI] [PubMed] [Google Scholar]
- 12.Wild D, Schmitt JS, Ginj M, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003 Oct;30(10):1338–1347. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- 13.Reubi JC, Schar JC, Waser B, et al. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med. 2000 Mar;27(3):273–282. doi: 10.1007/s002590050034. [DOI] [PubMed] [Google Scholar]
- 14.Ezziddin S, Opitz M, Attassi M, et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2011 Mar;38(3):459–466. doi: 10.1007/s00259-010-1610-2. [DOI] [PubMed] [Google Scholar]
- 15.Ezziddin S, Attassi M, Yong-Hing CJ, et al. Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014 Feb;55(2):183–190. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 16.Bodei L, Cremonesi M, Kidd M, et al. Peptide receptor radionuclide therapy for advanced neuroendocrine tumors. Thoracic surgery clinics. 2014 Aug;24(3):333–349. doi: 10.1016/j.thorsurg.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Waldherr C, Pless M, Maecke HR, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med. 2002 May;43(5):610–616. [PubMed] [Google Scholar]
- 18.Kwekkeboom DJ, de Herder WW, Kam BL, et al. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008 May 1;26(13):2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 19.Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011 Jun 10;29(17):2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 20.Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011 Dec;38(12):2125–2135. doi: 10.1007/s00259-011-1902-1. [DOI] [PubMed] [Google Scholar]
- 21.Danthala M, Kallur KG, Prashant GR, Rajkumar K, Raghavendra Rao M. (177)Lu-DOTATATE therapy in patients with neuroendocrine tumours: 5 years’ experience from a tertiary cancer care centre in India. Eur J Nucl Med Mol Imaging. 2014 Jul;41(7):1319–1326. doi: 10.1007/s00259-014-2710-1. [DOI] [PubMed] [Google Scholar]
- 22.Paganelli G, Sansovini M, Ambrosetti A, et al. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: results from a phase II study. Eur J Nucl Med Mol Imaging. 2014 Oct;41(10):1845–1851. doi: 10.1007/s00259-014-2735-5. [DOI] [PubMed] [Google Scholar]
- 23.Ezziddin S, Khalaf F, Vanezi M, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014 May;41(5):925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 24.Sabet A, Dautzenberg K, Haslerud T, et al. Specific efficacy of peptide receptor radionuclide therapy with Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur J Nucl Med Mol Imaging. 2015 Mar 26; doi: 10.1007/s00259-015-3041-6. [DOI] [PubMed] [Google Scholar]
- 25.van der Zwan WA, Bodei L, Mueller-Brand J, de Herder W, Kvols L, Kwekkeboom D. GEP-NETS update: Radionuclide therapy in neuroendocrine tumors. European journal of endocrinology/European Federation of Endocrine Societies. 2014 Aug 12; doi: 10.1530/EJE-14-0488. [DOI] [PubMed] [Google Scholar]
- 26.Kaemmerer D, Peter L, Lupp A, et al. Molecular imaging with (6)(8)Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011 Sep;38(9):1659–1668. doi: 10.1007/s00259-011-1846-5. [DOI] [PubMed] [Google Scholar]
- 27.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med. 1993 Aug;20(8):716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 28.Wild D, Bomanji JB, Benkert P, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013 Mar;54(3):364–372. doi: 10.2967/jnumed.112.111724. [DOI] [PubMed] [Google Scholar]
- 29.Basu S, Abhyankar A, Kand P, et al. ‘Reverse discordance’ between 68Ga-DOTA-NOC PET/CT and 177Lu-DOTA-TATE posttherapy scan: the plausible explanations and its implications for high-dose therapy with radiolabeled somatostatin receptor analogs. Nucl Med Commun. 2011 Jul;32(7):654–658. doi: 10.1097/MNM.0b013e328346350f. [DOI] [PubMed] [Google Scholar]
- 30.Damle NA, Bal C, Gupta S, Singhal A. Discordance in 68Ga-DOTANOC and 177Lu-DOTATATE uptake in diagnostic and post-therapy scans in patients with medullary thyroid cancer-likely reasons. J Cancer Res Ther. 2013 Oct-Dec;9(4):754–755. doi: 10.4103/0973-1482.126489. [DOI] [PubMed] [Google Scholar]
- 31.Campana D, Ambrosini V, Pezzilli R, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010 Mar;51(3):353–359. doi: 10.2967/jnumed.109.066662. [DOI] [PubMed] [Google Scholar]
- 32.Haug AR, Auernhammer CJ, Wangler B, et al. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010 Sep;51(9):1349–1356. doi: 10.2967/jnumed.110.075002. [DOI] [PubMed] [Google Scholar]
- 33.Strigari L, Konijnenberg M, Chiesa C, et al. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur J Nucl Med Mol Imaging. 2014 Oct;41(10):1976–1988. doi: 10.1007/s00259-014-2824-5. [DOI] [PubMed] [Google Scholar]
- 34.Sgouros G. Toward patient-friendly cell-level dosimetry. J Nucl Med. 2007 Apr;48(4):496–497. doi: 10.2967/jnumed.106.036749. [DOI] [PubMed] [Google Scholar]
- 35.Ilan E, Sandstrom M, Wassberg C, et al. Dose response of pancreatic neuroendocrine tumors treated with peptide receptor radionuclide therapy using 177Lu-DOTATATE. J Nucl Med. 2015 Feb;56(2):177–182. doi: 10.2967/jnumed.114.148437. [DOI] [PubMed] [Google Scholar]
- 36.Barone R, Borson-Chazot F, Valkema R, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med. 2005 Jan;46( Suppl 1):99S–106S. [PubMed] [Google Scholar]
- 37.Walrand S, Jamar F, Mathieu I, et al. Quantitation in PET using isotopes emitting prompt single gammas: application to yttrium-86. Eur J Nucl Med Mol Imaging. 2003 Mar;30(3):354–361. doi: 10.1007/s00259-002-1068-y. [DOI] [PubMed] [Google Scholar]
- 38.Walrand S, Flux GD, Konijnenberg MW, et al. Dosimetry of yttrium-labelled radiopharmaceuticals for internal therapy: 86Y or 90Y imaging? Eur J Nucl Med Mol Imaging. 2011 May;38( Suppl 1):S57–68. doi: 10.1007/s00259-011-1771-7. [DOI] [PubMed] [Google Scholar]
- 39.Wessels BW, Konijnenberg MW, Dale RG, et al. MIRD pamphlet No. 20: the effect of model assumptions on kidney dosimetry and response--implications for radionuclide therapy. J Nucl Med. 2008 Nov;49(11):1884–1899. doi: 10.2967/jnumed.108.053173. [DOI] [PubMed] [Google Scholar]
- 40.Konijnenberg M, Melis M, Valkema R, Krenning E, de Jong M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nucl Med. 2007 Jan;48(1):134–142. [PubMed] [Google Scholar]
- 41.Flux GD, Chittenden SJ, Saran F, Gaze MN. Clinical applications of dosimetry for mIBG therapy. Q J Nucl Med Mol Imaging. 2011 Apr;55(2):116–125. [PubMed] [Google Scholar]
- 42.Cremonesi M, Ferrari M, Di Dia A, et al. Recent issues on dosimetry and radiobiology for peptide receptor radionuclide therapy. Q J Nucl Med Mol Imaging. 2011 Apr;55(2):155–167. [PubMed] [Google Scholar]
- 43.Guerriero F, Ferrari ME, Botta F, et al. Kidney dosimetry in (1)(7)(7)Lu and (9)(0)Y peptide receptor radionuclide therapy: influence of image timing, time-activity integration method, and risk factors. Biomed Res Int. 2013;2013:935351. doi: 10.1155/2013/935351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kratochwil C, Stefanova M, Mavriopoulou E, et al. SUV of [(68)Ga]DOTATOC-PET/CT Predicts Response Probability of PRRT in Neuroendocrine Tumors. Mol Imaging Biol. 2015 Jun;17(3):313–318. doi: 10.1007/s11307-014-0795-3. [DOI] [PubMed] [Google Scholar]
- 45.Bodei L, Kidd M, Paganelli G, et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015 Jan;42(1):5–19. doi: 10.1007/s00259-014-2893-5. [DOI] [PubMed] [Google Scholar]
- 46.Wild D, Fani M, Fischer R, et al. Comparison of somatostatin receptor agonist and antagonist for Peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014 Aug;55(8):1248–1252. doi: 10.2967/jnumed.114.138834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.