Abstract
BACKGROUND
The arrhythmic role of the left atrial appendage (LAA) has been implicated in the maintenance of persistent atrial fibrillation. LAA isolation with catheter ablation has been successful but is limited by the risk of tamponade and electromechanical dissociation with the potential for LAA thrombus formation.
OBJECTIVE
To assess whether LAA ligation results in LAA electrical isolation.
METHODS
A total of 68 patients with contraindication or intolerance to oral anticoagulation therapy underwent LAA ligation with the LARIAT suture delivery device. Patients had unipolar [n = 30 (44%)] or bipolar [n = 38(56%)] voltage measurements pre- and post-LAA ligation.
RESULTS
All 68 patients underwent successful LAA ligation. There was a statistically significant reduction in the mean LAA voltage from pre-ligation (unipolar pre-ligation voltage 1.1 ± 0.53 mV; bipolar pre-ligation voltage 4.7 ± 2.83 mV) to post-ligation (unipolar post-ligation voltage 0.3 ± 0.38 mV; bipolar post-ligation voltage 0.6 ± 0.27 mV). Ninety-four percent of the patients had a reduction in the LAA voltage after the closure of the snare, with 10 of 30 (33%) of the patients having complete elimination of LAA voltage with the initial tightening of the suture. Pacing from the LAA after the closure of the snare resulted in lack of capture of the left atrium in 28 of 31 patients.
CONCLUSIONS
The snare closure of the LAA using the LARIAT device produces an acute reduction in the LAA voltage and inhibits the capture of the left atrium during LAA pacing. Future studies are needed to determine whether LAA ligation affects atrial fibrillation burden.
Keywords: Atrial fibrillation, Left atrial appendage, Left atrial appendage ligation
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in clinical practice. Pulmonary vein isolation for persistent AF has a 20% single procedure and 45% multiple procedure success rate at 5 years.1 The arrhythmic role of the LAA has been implicated in the maintenance of persistent AF.2,3 Left atrial appendage (LAA) isolation with catheter ablation decreases potential triggers for recurrent AF, but is limited by the risk of tamponade and electromechanical dissociation with the potential for LAA thrombus formation.2 Preclinical studies and isolated clinical reports of epicardial LAA exclusion has been shown to result in LAA electrical isolation and successful therapy for LAA tachycardias.3–6 LAA isolation and exclusion may contribute to the success of the Cox-Maze IV surgical ablation procedure for AF by eliminating focal LAA triggers.2,3,7 LAA occlusion with a percutaneous suture ligation method has been shown to be safe and effective for LAA closure in humans.8–10 The objective of this study was to test the hypothesis that LAA ligation would acutely lower LAA electrical voltage.
Methods
Patient population
This was an observational study of patients who had an LAA ligation performed between July 2010 and April 2013. The protocol was conducted with the approval of the Polish Ministry of Health and the ethics committee at John Paul II Hospital, Krakow, Poland, the Institutional Review Board at Methodist Hospital in Houston, TX, and Kansas University Medical Center, Kansas City, KS. Forty consecutive patients undergoing LAA ligation performed at John Paul II Hospital had endocardial unipolar voltage recordings pre- and post-ligation. Of the 40 patients, 30 patients had acceptable unipolar voltage measurements for analysis. Forty-three consecutive patients with an LAA ligation at Methodist Hospital and Kansas University Medical Center had bipolar LAA voltage measurements pre- and post-ligation. Of these 43 patients, 38 patients had acceptable bipolar voltage measurements for analysis. The final cohort consisted of 68 patients (age range 31–96 years).
Patients were eligible for LAA ligation if they met the following criteria: (1) 18 years or older, (2) history of nonvalvular AF, (3) presence of at least 1 risk factor of embolic stroke (CHADS2 score ≥1), (4) classified as a poor candidate or ineligible for warfarin therapy (eg, labile international normalized ratio level, noncompliant, contraindicated) and/or a warfarin failure (ie, transient ischemic attack or stroke while receiving warfarin therapy), and (5) a life expectancy of at least 1 year (Table 1).
Table 1.
Baseline characteristics (N = 68)
| Age (y) | 66 ± 12.7 |
| Sex | |
| Male | 43 (63) |
| Female | 25 (37) |
| Atrial fibrillation | |
| Paroxysmal | 30 (44) |
| Persistent | 33 (49) |
| Permanent | 5 (7) |
| Congestive heart failure | 15 (22) |
| Hypertension | 63 (93) |
| Age ≥ 75 y | 18 (26) |
| Diabetes mellitus | 21 (31) |
| Stroke/transient ischemic attack while receiving OAC | 23 (34) |
| Failure/complication* while receiving OAC | 2 (3) |
| Contraindication to OAC | 4 (5.9) |
| Labile INR | 17 (25) |
| Coronary artery disease or peripheral vascular disease | 25 (37) |
| CHADS2 score | 2.3 ± 1.23 |
| CHADS2-VASc score | 3.3 ± 1.70 |
| HAS-BLED | 2.4 ± 1.20 |
Values are presented as mean ± SD or as n (%).
CHADS2 = congestive heart failure; hypertension; age > 75 years; diabetes mellitus; stroke, transient ischemic attack, thromboembolic event; CHA2DS2-VASc = congestive heart failure; hypertension; age > 75 years; diabetes mellitus; stroke, transient ischemic attack, thromboembolic event; vascular disease; age 65–74 years; sex category (female); HAS-BLED = hypertension; abnormal renal/liver function; stroke; bleeding history or predisposition; labile INR; elderly; drugs (including aspirin, nonsteroidal anti-inflammatory drugs)/alcohol concomitantly; INR = international normalized ratio; OAC = oral anticoagulation.
Failure of OAC: history of left atrial/left atrial appendage thrombus despite OAC. Complication of OAC: history of bleeding complication with OAC.
Patients were excluded from LAA ligation if they met any of the following exclusion criteria9: (1) history of pericarditis, (2) history of cardiac surgery, (3) pectus excavatum, (4) myocardial infarction within the last 3 months, (5) embolic event within the last 30 days, (6) New York Heart Association class IV heart failure symptoms, (7) left ventricular function less than 30%, and (8) thoracic radiation. Patients meeting the criteria for the study enrollment underwent a screening contrast cardiac computed tomographic scan. Additional exclusion criteria based on LAA anatomy included (1) an LAA width greater than 40 mm, (2) a superiorly oriented LAA with the LAA apex directed behind the pulmonary trunk, (3) bilobed LAA or multilobed LAA in which lobes were oriented in different planes exceeding 40 mm, and (4) a posteriorly rotated heart.
Percutaneous suture ligation of LAA
The LARIAT suture delivery device and accessories (SentreHEART, Inc, Redwood City, CA) used for the exclusion of the LAA were previously described and consisted of 3 components3: (1) a 15-mm compliant occlusion balloon catheter (EndoCATH, SentreHEART, Inc, Redwood City, CA), (2) 0.025- and 0.035-in magnet-tipped guidewires (FindrWRZ, SentreHEART, Inc, Redwood City, CA), and (3) a 12-F suture delivery device (LARIAT).8,9
LAA ligation using the LARIAT suture delivery device has been described previously.9,10 Briefly, patients were prepped and draped with sterile preparation of the subxyphoid and bilateral groin regions. Once the patient was anesthetized, a transesophageal echocardiogram (TEE) was performed to rule out LAA thrombus. Pericardial access using a 17-G epidural needle was performed as described previously.11 The epicardial puncture was performed to access the anterior surface of the heart. Anterior-posterior and lateral fluoroscopic views were used to guide the needle for epicardial puncture. Once epicardial access was confirmed, a 0.035-in guidewire was left in the pericardial space while a transseptal catheterization was performed. Five thousand units of heparin was administered intravenously once the transseptal catheterization was completed. At John Paul II Hospital, an additional 2000 units of heparin was administered intravenously every 45 minutes for prolonged procedures. At John Paul II Hospital, an activated clotting time was not obtained owing to the lack of available equipment. At John Paul II Hospital, the transseptal sheath was flushed with heparinized saline every 5 minutes. At Methodist Hospital, additional heparin boluses and a heparin infusion were titrated for a goal activated clotting time of 300–350 seconds. The transseptal sheath was attached to a heparinzed saline flush solution under constant pressure. An 8.5-F SL1 catheter (St Jude Medical, St Paul, MN) was directed anteriorly in the left atrium toward the LAA. A left atriagram was performed in the right anterior oblique view to delineate the ostium and body of the LAA. The 15-mm occlusion balloon catheter (EndoCATH) was back loaded with a 0.025-in endocardial magnet-tipped guidewire (FindrWIRZ) and inserted into the end of the SL1 transseptal sheath. The endocardial magnet-tipped guidewire was advanced to the apex of the LAA under fluoroscopic guidance. The epicardial access site was then sequentially dilated over the guidewire for the placement of the 14-F soft-tipped epicardial guide cannula (SentreHEART, Inc). The 0.035-in epicardial magnet-tipped guidewire was placed through the epicardial sheath to achieve an end-to-end magnetic union with the endocardial guidewire.
Both unipolar and bipolar LAA voltage measurements were performed after the endocardial magnet-tipped guidewire was connected to the epicardial magnet-tipped guidewire at the LAA apex. An LAA angiogram was performed through the lumen of the balloon catheter to verify the position of the endocardial magnet-tipped guidewire at the LAA apex. A unipolar voltage measurement was recorded using the left leg electrocardiographic (ECG) electrode attached to the endocardial unipolar guidewire. After printing the ECG recording, the balloon catheter was advanced over the wire and positioned at the LAA ostium.
Once the LARIAT suture delivery device was positioned over the LAA, the EndoCATH balloon was used to position the snare at the LAA ostium. The TEE was used to verify the anatomic position of the EndoCATH balloon at the ostium of the LAA. After confirmation of the balloon catheter at the LAA ostium, the snare was closed. A left atriogram was performed to confirm complete capture of the LAA and rule out the existence of a remnant trabeculated LAA. After verifying LAA capture, the preloaded suture was released from the snare and tightened to exclude the LAA. A repeat unipolar voltage recording was performed for the assessment of the post-ligation endocardial voltage recording. Unipolar recordings were performed with the endocardial magnet-tipped guidewire connected to the epicardial magnet-tipped guidewire pre- and post-LAA ligation, thus assuring the location and contact was consistent. To ensure probe interface issues did not falsely overestimate the reduction in voltage recordings, patients were eliminated from the analysis if the magnets detached and could not be reattached after the release of the suture. The endocardial guidewire and EndoCATH balloon were then removed from the LAA. Tightening of the suture knot was completed using a suture tightening device (TenSURE, SentreHEART, Inc).
For the patients who had a bipolar voltage recording, bipolar LAA electrograms were obtained by peeling the back end of the endocardial and epicardial magnet-tipped guidewires, connecting them to anode and cathode alligator clips, and sampling the signal with a pacemaker analyzer (St Jude Medical). Bipolar LAA electrogram amplitudes and capture of the atrium via pacing from the LAA were measured before and after the closure of the LARIAT suture delivery device at the base of the appendage. Pacing from the LAA was performed in 31 patients, starting at 1 V and increasing to 20 V at a pulse width of 0.4 ms. Care was taken to ensure that the endocardial and epicardial magnets remained connected to each other after the closure of the snare. Repeat signals and pacing within the LAA were then recorded.
After verifying complete capture of the LAA with a TEE and a left atriagram, the EndoCATH occlusion balloon and the endocardial guidewire were removed and the preloaded suture was released from the snare. The suture knot was completely tightened using the TenSURE device. The LARIAT snare device was removed from the pericardial space. The suture was cut near the LAA with a suture cutter (SentreHEART, Inc) that was passed over the suture.
Clinical follow-up
Sixty-seven of 68 patients had their initial follow-up TEEs ranging from 1 to 6 months post-LAA ligation with the LARIAT device. One patient declined to have a follow-up TEE. If a patient had a leak detected using color flow Doppler imaging, a repeat TEE was performed between 6 and 12 months after the initial TEE. After the procedure, the patients were observed overnight in the intensive care unit. The patients were then monitored on a telemetry floor for an additional 24–48 hours before discharge. Clinical follow-up via phone contact was performed by the investigators at 1 month, and outpatient appointments were made at 6 months and 1 year post-ligation. Patients with a contraindication to warfarin continue to not use warfarin. It was recommended that patients with a CHADS2 score of 2 or higher who could tolerate warfarin (ie, noncompliant or labile international normalized ratio level) continue warfarin. Warfarin use in patients with a CHADS2 score of 1 was left to the discretion of the referring physician. For patients not receiving warfarin, aspirin therapy was recommended. Twenty-four-hour Holter monitoring ECG (Philips Healthcare, Andover, MA, and Rozinn Electronics, Glendale, NY) was obtained at 1 year post-ligation to assess the presence of AF post-ligation in patients at John Paul II Hospital. In patients with a dual-chamber pacemaker (n = 12), AF burden 3 months pre- and post-LAA ligation were determined by interrogation of the device.
Statistical analysis
Normally distributed continuous variables are expressed as mean ± SD. Continuous variables that were not normally distributed are expressed as median (interquartile range).
Results
Baseline data
The baseline characteristics of the 68 patients who had acceptable unipolar or bipolar electrograms recorded pre- and post-ligation are summarized in Table 1. The mean CHADS2 score was 2.3 ± 1.23, and the mean CHA2DS2-VASc score was 3.3 ± 1.70. The mean HAS-BLED score was 2.4 ± 1.20.
Before LAA ligation, 25 (37%) patients were in AF, 6 (9%) were in atrial flutter, and 37 (54%) patients were in sinus rhythm. After LAA ligation, 29 (43%) patients were in AF, 5 (7%) were in atrial flutter, and 34 (50%) patients were in sinus rhythm.
LAA ligation
Ligation success was defined as the successful closure of the LAA with the absence of a contrast leak on left atriogram and 1 mm or less jet as visualized using color flow Doppler imaging on the TEE. At the 1–3-month follow-up TEE, 63 of 68 (93%) patients had a complete closure of the LAA, only 1 patient (1%) had a less than 2-mm leak detected using color flow Doppler imaging, and 4 patients (6%) had a 3–5-mm leak detected using color flow Doppler imaging. A subsequent 1-year follow-up TEE demonstrated a complete closure of the LAA using color flow Doppler imaging in the patient with the 2-mm leak while 2 patients with a 3–5-mm leak remained the same. Two patients with a 3-mm leak are still awaiting their 1-year follow-up TEE. One complication occurred in the periprocedural period: this patient had a perforation secondary to the transseptal puncture when the Brockenbrough needle went through the lateral wall of the left atrium after the successful puncture of the fossa ovalis. There was no hemodynamically significant pericardial effusion associated with this perforation, and the LAA ligation was completed successfully in this patient. After LAA ligation, 4 patients had pericardial drainage of less than 300 cm3 within 24 hours of the ligation without cardiac tamponade or need for intervention. Adverse events included 2 cases of pericarditis that persisted for more than 2 days, requiring a course of nonsteroidal anti-inflammatory drugs; 2 patients developed plural effusions that resolved with no long-term sequelae; and 1 patient developed acute renal failure that did resolve. There was 1 patient who had a nonmobile thrombus next to the site of closure extending toward the left lateral ridge (colloquially termed coumadin ridge) that resolved with warfarin therapy. There were no embolic events or deaths noted during the follow-up period.
LAA voltage measurements pre- and post-ligation
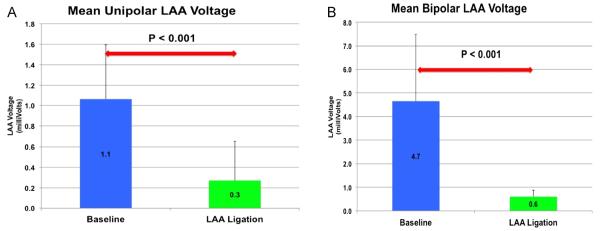
Of the 68 patients in this study, 64 (94%) had a reduction in their LAA voltage. In the unipolar voltage group, 28 of 30 (93%) patients had a reduction in their LAA voltage with ligation, 1 patient had no change in the unipolar voltage, and 1 patient had an increase in the unipolar voltage. In the bipolar voltage group, 36 of 38 (95%) patients had a reduction in their LAA voltage with ligation and 2 patients had no change in the pre- and post-ligation voltages. There was a statistically significant reduction in the unipolar voltage post-LAA ligation (P < .001; Figure 1). The mean pre-ligation unipolar voltage was 1.1 ± 0.53 mV, and the mean post-ligation unipolar voltage was 0.3 ± 0.38 mV. There was also a statistically significant difference between the mean pre-ligation bipolar voltage of 4.7 ± 2.83 mV and the mean post-ligation bipolar voltage of 0.6 ± 0.27 mV (P = .001; Figure 2). The 4 patients with leaks between 3 and 5 mm demonstrated a reduction in the LAA voltage similarly to those with complete LAA closure. For the 30 patients who had unipolar voltage recordings obtained pre- and post-ligation, 10 patients had a post-ligation unipolar voltage of 0 mV. One patient in the bipolar group had a complete reduction in the bipolar voltage to 0 mV. Figure 3 shows an example of a patient who underwent successful ligation with a complete reduction in the unipolar LAA voltage amplitude from a baseline unipolar voltage in leads aVR, aVL, and aVF (1.8, 0.9, and 0.9 mV, respectively) to 0 mV for all 3 leads. In 28 of 31 patients with pre- and post-LAA occlusion pacing, pacing from the LAA did not result in left atrial capture.
Figure 1.
(A) Mean unipolar and (B) mean bipolar left atrial appendage (LAA) voltage pre- and post-LAA closure.
Figure 2.
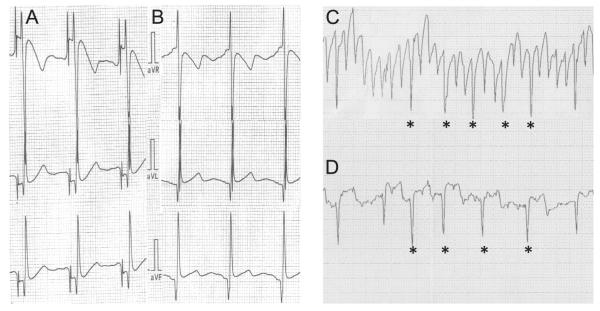
Left atrial appendage (LAA) voltage recordings pre- and post-LAA closure. Pre- and post-LAA unipolar voltage recordings (A and B) from leads aVR, aVL, and aVF are displayed. Pre-ligation (C) and post-ligation (D) LAA bipolar voltage recordings.
Figure 3.
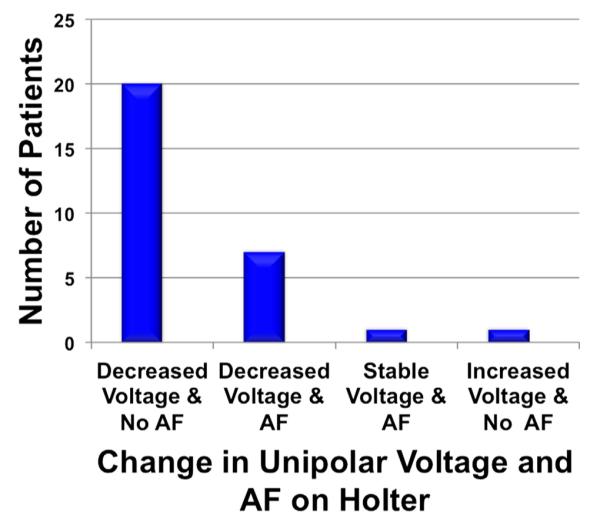
Change in unipolar voltage and presence of atrial fibrillation on the Holter electrocardiogram.
Arrhythmia follow-up
Of the 30 patients with unipolar LAA voltage data, 1 patient was lost to follow-up. Twenty-nine patients completed 1-year post-ligation Holter monitoring, and 8 patients had AF detected on the Holter ECG. Twenty-one patients were in sinus rhythm on their 1-year Holter ECG. When the 29 patients with 1-year Holter ECG data were separated according to pre-ligation paroxysmal (12 patients) and persistent (17 patients) AF, AF was detected on the Holter ECG more frequently in the persistent AF group (6 of 17 [35%] patients) than in the paroxysmal AF group (2 of 12 [17%] patients). Twenty of 30 (63%) patients had reduced unipolar voltage at ligation, with no AF detected on the Holter ECG. Seven of 30 (23%) patients had reduced unipolar voltage, with recurrent AF detected on the Holter ECG. One of 29 (3%) had stable voltage, and no AF was detected on the Holter ECG; 1 patient had increased unipolar voltage, and no AF recurrence was detected on the Holter ECG at 1 year (Figure 3).
Of the 12 patients with a dual-chamber pacemaker, LAA closure did not produce an increase in AF burden in any of the patients. There was a reduction in AF burden post-LAA ligation in 8 of 12 patients. There was a significant average reduction in AF burden pre- and post-LAA ligation (85.2% ± 28.3% pre-LAA ligation and 52.0% ± 38.6% post-LAA ligation).
Postligation anticoagulation and antiplatelet therapy
Before LAA ligation, 25 patients received warfarin for thromboembolic event prophylaxis. After LAA ligation, 13 patients continued anticoagulation therapy with warfarin. By 6 months post-ligation, all 68 patients had their warfarin discontinued and received aspirin. One patient had a non-embolic stroke 3 months post-LAA ligation. No deaths were noted during the follow-up period.
Discussion
The main finding of this study is that with successful percutaneous LAA closure, there are acute reductions in LAA unipolar and bipolar voltages and lack of left atrial capture during bipolar pacing from the occluded LAA in 90% of the patients tested. With closure of the snare seen in the patients during bipolar recordings, there is an acute reduction in the LAA voltage. The acute reduction in the LAA voltage during LAA closure is similar to the decrease in voltage mapping in regions of left atrial scar as defined by left atrial delayed enhancement on MRI.12 These regions of reduced bipolar voltage correlated with atrial delayed enhancement used to assess the presence of left atrial scar. The release of the suture from the snare resulted in the complete elimination of unipolar voltage recording from the LAA in 33% of the unipolar voltage measurement cohort. This observation is consistent with more extensive LAA injury caused by the suture tightening around the neck of the LAA.
LAA ligation most likely leads to an ischemic necrosis. The observation of PR segment elevation with the closure of the snare suggests ischemia. A preclinical study demonstrated that LAA ligation with the LARIAT device produced scar formation.13 Furthermore, endothelization at the LAA ostium at 1 week post-ligation and atrophy of the LAA with incorporation into the visceral pericardium at 3 months were observed. An autopsy analysis post-LAA ligation of a patient using the LARIAT suture delivery device revealed extensive fibrosis and scar tissue of the LAA with obliteration of the LAA cavity and atrophy of the LAA.14 Replacement fibrosis (scar) is the hallmark of ischemic damage.
Percutaneous ligation of canine LAA results in electrical modification of the LAA.5 There has been 1 case report of an LAA atrial tachycardia treated with LAA exclusion using the AtriClip device (Atricure, Inc, West Chester, Ohio).6 This patient had an incessant atrial tachycardia with previous failed radiofrequency catheter ablation. Termination of the atrial tachycardia occurred immediately after the placement of the AtriClip device. Subsequent testing of exit block from the LAA to the left atrium was confirmed with failure to capture the left atrium during bipolar surgical stimulator pacing at the distal LAA.6 Similar results were found in the cohort of patients who underwent LAA pacing after the closure of the snare using the LARIAT device. Lack of left atrial capture during LAA pacing was seen in 90% of the patients tested. Other investigators have reported successful radiofrequency catheter ablation of atrial tachycardias originating from the LAA.15–18 Catheter ablation of LAA triggers for persistent AF has been reported to improve the success of AF ablation.2 One concern with complete electrical isolation of the LAA using radiofrequency catheter ablation is an increased incidence of LAA thrombus with subsequent embolic events. Although our study was not designed to assess the risk of thromboembolic events in this cohort of patients, Cox-Maze III surgery data have demonstrated a low incidence of thromboembolic events with successful LAA exclusion/excision over long-term follow-up and a 90% success rate at maintaining sinus rhythm.19,22,23
For patients who had unipolar voltage measurements post-LAA ligation on a 1-year follow-up Holter ECG, an acute reduction in the LAA voltage was associated with a reduced incidence of AF on a 1-year post-ligation Holter ECG (Figure 3). These results are corroborated in the 12 patients who had a dual-chamber pacemaker, demonstrating reduced AF burden in 8 of 12 patients with a significant average reduction in AF burden in these patients. The strength of these observations is strictly hypothesis generating, as the incidence of postablation AF has been shown to increase with an increased frequency and longer duration of post-ablation ambulatory monitoring.20,21 Despite the limitations of a lack of long-term monitoring to define each patient’s AF burden and a defined antiarrhythmic drug regimen post-LAA ligation, the finding that LAA ligation is correlated with a reduced incidence of AF at 1 year and in the patients with a pacemaker is intriguing and is deserving of further study in a large cohort with systematic monitoring follow-up and multivariable analysis. This concept is supported by data from patients who underwent catheter ablation for the isolation of the LAA during ablation for recurrent AF, and the finding that LAA electrical isolation is associated with an increased probability of maintaining sinus rhythm.2,3 In order to assess the true contribution of the LAA to recurrent and persistent AF, a randomized trial assigning patients to LAA ligation before AF ablation vs standard of care AF ablation would be useful for addressing this question.
The adverse events noted in this study are similar to those described in previous reports.9,10 All adverse events resolved without further consequences. However, the observed adverse events highlight the need for post-LAA ligation care in the management and prevention of pericarditis and effusions resulting from Dressler’s syndrome. Pain management post-LAA ligation should be supplemented with anti-inflammatory medications such as colchicine and nonsteroidal anti-inflammatory drugs. In recurrent or persistent pericarditis and/or Dressler’s syndrome that are unresponsive to colchicine and nonsteroidal anti-inflammatory drugs, oral steroids could be considered. The inflammatory response at the site of the closure may also lead to endothelial dysfunction and contribute to the potential of thrombus formation. The 1.5% incidence of thrombus formation in this study is consistent with the 1% incidence reported by Bartus et al.9 Although thrombus formation appears to occur infrequently, acetylsalicyclic acid or oral anticoagulation therapy post-LAA ligation until a 6-week follow-up TEE to assess leaks and thrombus may be beneficial in preventing the thrombus formation. The acute renal failure seen in 1 patient emphasizes the need to be aware of contrast-induced nephropathy in the elderly patients with preprocedural renal insufficiency.
Study limitations
The lack of systematic and serial ambulatory monitoring of AF post-ligation does not allow us to make a definitive conclusion regarding the effect of LAA ligation on the recurrence of AF. However, this study was not designed to assess the incidence of AF pre- and post-LAA ligation. In addition, a significant proportion of our population had minimally symptomatic or asymptomatic AF and thus AF recurrence was not a prespecified outcome variable. Nevertheless, the findings of our study deserve further study in patients at risk of thromboembolic events with symptomatic AF.
Another limitation of our study is that we did not assess the long-term effects of LAA ligation on LAA electrical isolation and LAA exit block. However, by the nature of the procedure, achieving endocardial access to the distal LAA is not possible post-ligation. Given the acute reduction in the LAA voltage with ligation of the LAA and the course of LAA ischemic necrosis post-ligation, it is unlikely that the improvement in LAA electrical activity would be restored.22,23
Conclusions
LAA closure produces acute reductions in LAA unipolar and bipolar voltages, which is consistent with LAA ischemic necrosis. These changes are a likely marker of long-term electrical LAA isolation. The correlation between LAA isolation and long-term AF burden should be studied further to assess whether LAA ligation improves the efficacy of catheter ablation for AF.
ABBREVIATIONS
- AF
atrial fibrillation
- ECG
electrocardiogram/electrocardiographic
- LAA
left atrial appendage
- TEE
transesophageal echocardiogram
References
- 1.Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60:1921–1929. doi: 10.1016/j.jacc.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 2.Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–118. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 3.Hocini M, Shah AJ, Nault I, et al. Localized reentry within the left atrial appendage: arrhythmogenic role in patients undergoing ablation of persistent atrial fibrillation. Heart Rhythm. 2011;8:1853–1861. doi: 10.1016/j.hrthm.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komahara, et al. A novel device for LAA exclusion (AtriaClip) J Thorac Cardiovasc Surg. 2005;130:16-39–16-44. [Google Scholar]
- 5.Bruce CJ, Stanton CM, Asirvatham SJ, et al. Percutaneous epicardial left atrial appendage closure: intermediate-term results. J Cardiovasc Electrophysiol. 2011;22(1):64–70. doi: 10.1111/j.1540-8167.2010.01855.x. [DOI] [PubMed] [Google Scholar]
- 6.Benussi S, Mazzone P, Maccabelli G, et al. Thoracoscopic appendage exclusion with an atriclip device as a solo treatment for focal atrial tachycardia. Circulation. 2011;123:1575–1578. doi: 10.1161/CIRCULATIONAHA.110.005652. [DOI] [PubMed] [Google Scholar]
- 7.Weimar T, Schena S, Bailey MS, et al. The Cox-Maze procedure for lone atrial fibrillation: a single-center experience over 2 decades. Circ Arrhythm Electrophysiol. 2012;5:8–14. doi: 10.1161/CIRCEP.111.963819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartus K, Bednarek J, Myc J, et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011;8:188–193. doi: 10.1016/j.hrthm.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Bartus K, Han F, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. 2013;62:108–118. doi: 10.1016/j.jacc.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 10.Massumi A, Chelu MG, Nazeri A, et al. Initial experience with a novel percutaneous left atrial appendage exclusion device in patients with atrial fibrillation, increased stroke risk, and contraindications to anticoagulation. Am J Cardiol. 2013;111:869–873. doi: 10.1016/j.amjcard.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Sosa E, Scanavacca M, d’Avila A, et al. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 12.Spragg DD, Khurram I, Zimmerman SL, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012;9:2003–2009. doi: 10.1016/j.hrthm.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Lee RJ, Bartus K, Yakubov SJ. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: initial experience in a canine model. Circ Cardiovasc Interv. 2010;3:224–229. doi: 10.1161/CIRCINTERVENTIONS.109.914978. [DOI] [PubMed] [Google Scholar]
- 14.Bartus K, Morelli RL, Szczepanski W, et al. Anatomic analysis of the LAA following closure with the LARIAT device. Circ Arrhythmia Electrophysiol. 2014 doi: 10.1161/CIRCEP.113.001084. (In Press) [DOI] [PubMed] [Google Scholar]
- 15.Hillock RJ, Singarayar S, Kalman JM, et al. Tale of two tails: the tip of the atrial appendages is an unusual site for focal atrial tachycardia. Heart Rhythm. 2006;3:467–469. doi: 10.1016/j.hrthm.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Ma J, Zhang S, et al. Focal atrial tachycardia originating from the distal portion of the left atrial appendage: characteristics and long-term outcomes of radiofrequency ablation. Europace. 2012;14:254–260. doi: 10.1093/europace/eur302. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T, Murakami Y, Yoshida Y, et al. Electrophysiologic and electrocardiographic characteristics and radiofrequency catheter ablation of focal atrial tachycardia originating from the left atrial appendage. Heart Rhythm. 2007;4:1284–1291. doi: 10.1016/j.hrthm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Wang YL, Li XB, Quan X, et al. Focal atrial tachycardia originating from the left atrial appendage: electrocardiographic and electrophysiologic characterization and long-term outcomes of radiofrequency ablation. J Cardiovasc Electrophysiol. 2007;18:459–464. doi: 10.1111/j.1540-8167.2007.00808.x. [DOI] [PubMed] [Google Scholar]
- 19.Schaff HV, Dearani JA, Daly RC, et al. Cox-Maze procedure for atrial fibrillation: Mayo Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:30–37. doi: 10.1016/s1043-0679(00)70014-1. [DOI] [PubMed] [Google Scholar]
- 20.Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–313. doi: 10.1161/CIRCULATIONAHA.104.518837. [DOI] [PubMed] [Google Scholar]
- 21.Kottkamp H, Tanner H, Kobza R, et al. Time course and quantitative analysis of atrial fibrillation episodes number and duration after circular plus linear left atrial lesions: trigger elimination or substrate modification; early or delayed cure? J Am Coll Cardiol. 2004;44:869–877. doi: 10.1016/j.jacc.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy PM, Gillinov AM, Castle L, et al. The Cox-Maze procedure: the Cleveland Clinic experience. Semin Thorac Cardiovasc Surg. 2000;12:25–29. doi: 10.1016/s1043-0679(00)70013-x. [DOI] [PubMed] [Google Scholar]
- 23.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox Maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]