Abstract
BACKGROUND
In toxicology studies, perfluorinated compounds affect fetal growth, development, viability, and postnatal growth. There are limited epidemiologic studies on child development.
METHODS
We recruited and evaluated 321 children who participated in the C8 Health Project, a 2005–2006 survey in a mid-Ohio-Valley community highly exposed to perfluorooctanoate (PFOA) through contaminated drinking water. We examined associations between measured childhood PFOA serum concentration and mother and teacher reports of executive function (Behavior Rating Inventory of Executive Function), ADHD-like behavior (Conner’s ADHD DSM-IV Scales), and behavioral problems (Behavior Assessment System for Children) assessed 3–4 years later at ages 6–12 years.
RESULTS
Overall, neither reports from mothers nor teachers provided clear associations between exposure and child behavior. Mother reports, however, did suggest favorable associations between exposure and behavior among boys and adverse associations among girls. On the composite scale from the Behavior Rating Inventory of Executive Function (n=318), PFOA exposure had a favorable association among boys (highest vs. lowest quartile β = −6.39; 95% CI −11.43, −1.35) and an adverse association among girls (highest vs. lowest quartile β = 4.42; 95% CI −0.03, 8.87; interaction p=0.01). Teacher reports (n=189) replicated some but not all of the sex-interactions observed in mothers’ reports.
CONCLUSIONS
Aggregate results did not suggest adverse effects of PFOA on behavior, but sex-specific results raise the possibility of differing patterns by sex. Results are not consistent between mothers’ and teachers’ reports. Effect modification by sex may warrant further investigation.
Keywords: attention deficit disorders, child behavior, epidemiology, fluorocarbons, perfluorooctanoic acid
Perfluorooctanoate (PFOA) is a perfluorinated compound (PFC) that has been widely used in numerous consumer products since the 1950s.1–3 Human exposure typically occurs through transfer from food packaging, bioaccumulation in the food chain, and inhalation of household dust 4. PFOA is almost always detectable in serum5 and has been found in amniotic fluid,6, 7 maternal and umbilical cord blood,8, 9 and breast milk.10–13
Epidemiologic evidence on PFOA exposure and child development is limited, with variation in exposure timing and specific endpoints. In the Danish National Birth Cohort (n=1,400), prenatal PFOA levels were unrelated to maternal reports of child development through age 7.14, 15 Three cross-sectional studies observed divergent associations among measures of attention in school-age children. In the National Health and Nutrition Examination Survey (NHANES; n=571), higher PFOA levels were associated with increased odds of parent-reported Attention Deficit/Hyperactivity Disorder (ADHD).16 In New York State, higher levels of several PFCs – although not PFOA – were associated with impulsivity (n=79).17 Using C8 Health Project data (n=10,546), the parent study of the present report, Stein and Savitz reported reduced odds of ADHD at the highest PFOA exposure level.18 We recently examined the children in the present report in a prospective study (n=320) of PFOA exposure and performance on neuropsychological tests.19 With increased childhood PFOA exposure there was a modest increase in intelligence quotient (IQ), decrease in behaviors typical of ADHD, and null associations with reading skills, math skills, and neuropsychological functioning. The current study complements the existing literature by providing a prospective examination of childhood PFOA concentration and mother and teacher report of child behavior assessed 3 – 4 years later at ages 6 – 12.
In 2001, a group of residents from the West Virginia and Ohio communities surrounding a chemical plant near Parkersburg, West Virginia filed a class action lawsuit alleging health damage from drinking water supplies drawing from PFOA-contaminated groundwater.20 Geometric mean PFOA levels in this population were approximately 5 times the national average; exposure to most other PFCs reflected typical background levels. We invited a subset of children in the C8 Health Project to participate in a follow-up study assessing neurobehavioral development.
METHODS
Study Population
The C8 Health Project enrolled 69,030 people from 2005 – 2006.20 Individuals were eligible to participate if they could prove they had consumed PFOA-contaminated water for at least 1 year since 1950 in 1 of 6 PFOA-contaminated water districts or private wells within the area of documented contamination.
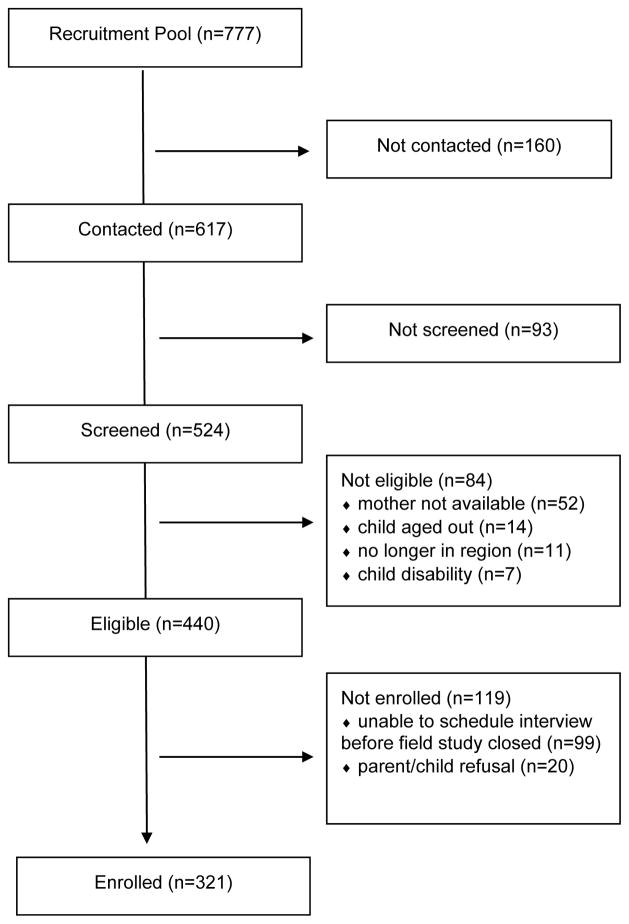
From 2009 – 2010 we conducted a follow-up to the C8 Health Project among children aged 6 – 12 years. We identified children who had lived in the same PFOA-contaminated water district from the time of the mother’s pregnancy until C8 Health Project enrollment, had serum PFC measurements, and whose parents permitted contact for additional studies. Only one child (birthday closest to interview) per family was eligible. Of 777 identified children, 617 were successfully contacted, 524 of the contacted agreed to be screened, 440 of the screened met eligibility criteria, and 321 of the screened children and their biological mothers participated in the follow-up study (Figure 1). There was no difference between enrolled and not enrolled children in measured serum PFOA concentration (p=0.69), age (p=0.79), sex (p=0.32), or maternal smoking (p=0.99).
Figure 1.
Enrollment scheme, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009 – 2010.
During the mother’s interview we requested contact information for the child’s teacher. We mailed 312 survey packets to teachers and received 188 (60%) completed surveys. The Mount Sinai Program for the Protection of Human Subjects and the Battelle Centers for Public Health Research & Evaluation Institutional Review Board approved all study procedures.
Exposure Assessment
PFOA was measured in serum collected at C8 Health Project enrollment. Laboratory analyses (Exygen Research Inc., State College, PA, USA) used protein precipitation extraction with reverse-phase high-performance liquid chromatography/tandem mass spectrometry.20, 21 This measured serum PFOA concentration corresponds to a time point 3 – 4 years prior to the behavioral assessment when the children were aged 2 – 8 years.
We calculated effect estimates representing a 1 natural log unit increase in exposure because the transformation provided a better model fit, and as quartiles of exposure with the lowest quartile as the referent.
Outcome Assessment
Mothers and teachers completed 3 surveys to elicit information on the child’s behavior. The Behavior Rating Inventory of Executive Function (BRIEF) assesses executive function, which helps guide, direct, and manage cognition, emotion, and behavior.22 The BRIEF has 8 clinical scales; we focused on 3 measures. The Global Executive Composite is a summary score that incorporates 8 clinical scales. The Behavioral Regulation Index focuses on inhibitory control – the ability to manage transitions and regulate emotions. The Metacognition Index reflects the ability to plan, organize, and monitor performance. To ensure validity, we excluded mother (n=1) and teacher (n=1) reports with negativity scores greater than 6 or inconsistency scores greater than 8. Age-standardized T-scores have mean = 50 and standard deviation (SD) = 10; lower scores are favorable.
The Conners’ ADHD DSM-IV Scales-Revised (CADS) reports on ADHD-like behaviors.23 The ADHD Index score is based on responses to 12 items that best discriminate children who have been diagnosed with ADHD from those who have not. There are also 3 DSM-IV Symptoms subscales: Combined, Inattentive, and Hyperactive-Impulsive. Age-standardized T-scores have mean = 50 and SD = 10; lower scores are favorable.
The Behavior Assessment System for Children-2 (BASC) evaluates child personality, behavioral problems, and emotional disturbances.24 We focus on the Behavioral Symptom Index and 3 composite scales: Adaptive Skills, Internalizing Problems, and Externalizing Problems. For Adaptive Skills higher scores are favorable; for the Behavioral Symptom Index, Internalizing, and Externalizing Problems, lower scores are favorable. Age-standardized T-scores have mean = 50 and SD = 10. To ensure validity, we excluded mother reports (n=6) with F-index greater than 6, Pattern Response Index greater than 12, or Consistency Index greater than 17. We excluded teacher reports (n=6) with F-index greater than 3, Pattern Response Index greater than 111, or Consistency Index greater than 15.
Covariate Assessment
We interviewed the mother to collect information to address confounding, including family demographics, pregnancy and delivery complications, breastfeeding history, and presence of co-morbid health conditions in the child. The Home Observation for Measurement of the Environment-Short Form Mother Supplement (HOME) measured the quality and extent of stimulation in the home.25 Maternal Full Scale IQ was measured using the Wechsler Abbreviated Scale of Intelligence.26
Statistical Analysis
We used linear regression to determine the crude association between PFOA exposure and mother and teacher reports of child behavior. Based on their influence on neurobehavioral assessment, we identified an a priori set of covariates to be included in all models for mother reports (child’s age in years at neuropsychological assessment, child sex, continuous cognitive and emotional HOME scores, continuous maternal Full Scale IQ) and teacher reports (child’s age in years at neuropsychological assessment, child sex). Separately for mothers and teachers, we determined the potential for confounding from additional covariates by examining the covariate-exposure and covariate-outcome associations for over 60 variables collected through the maternal interview,27 including race/ethnicity, educational attainment, employment, smoking, and income. Only covariates with p<0.20 for both associations were included. Using a backwards elimination strategy we built separate models for each combination of mother, teacher and outcome. Variables were deemed confounders if their removal changed the β for PFOA by more than 10%.27 To attain comparability across models, final models included only those empirically-derived covariates found to confound at least half of the models. Including the a priori covariates noted above and screening for other candidates, final models of mother-reported outcomes were adjusted for child age, child sex, HOME score, maternal full scale IQ, maternal age at interview (continuous), and maternal employment at interview (none, part-time, full-time). Final models of teacher-reported outcomes were adjusted only for child age and child sex. Generalized Estimating Equations accounted for within-teacher correlations because some teachers reported on multiple children, although 75% reported on just 1 child. Analysis used SAS Version 9.2 (Cary, NC).
Given the concern that PFOA may be hormonally active,28 we examined the potential for sex to modify the association by comparing the effect estimates of stratified and unstratified models and by examining the likelihood ratio p-value for the PFOA-sex interaction term.27
We performed several secondary analyses. (1) To assess the potential for low-dose exposure effects that may have been obscured in this highly exposed population, we restricted the analysis to the children with PFOA concentrations below the median. (2) To ensure that our findings were not an artifact of treatment for ADHD, we restricted the analysis to the children with no reported ADHD diagnosis. (3) To incorporate the co-exposure to other PFCs, we adjusted for measured perfluorooctane sulfonate (PFOS), perfluorohexane sulfonate (PFHxS), and perfluorononanoate (PFNA) levels.
RESULTS
The average age of the children at behavioral assessment was 9.9 (SD 1.8; range 6 – 12) years and 54 percent were female (Table 1). Only 10 (3.1 percent) children were non-white. On average, the mothers were 38.4 (SD 5.9) years old at the time of follow-up and 65 percent had at least some college education.
Table 1.
Characteristics of children 6 to 12 years of age by quartile of serum PFOA measured at 2 to 8 years of age, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009 – 2010 (n=321)
| Characteristic | PFOA, ng/mL | Quartile 1: 0.7 – <15.8 | Quartile 2: 15.8 – <35.1 | Quartile 3: 35.1 – <94.1 | Quartile 4: 94.1 – 838.6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| N | Mean (%) | SD | N | Mean (%) | SD | N | Mean (%) | SD | N | Mean (%) | SD | ||
| PFOS, ng/mL | 80 | 15.5 | 8.1 | 80 | 19.5 | 12.7 | 80 | 27.1 | 15.4 | 81 | 22.2 | 13.1 | |
| PFHxS, ng/mL | 80 | 6.1 | 5.7 | 80 | 10.2 | 16.4 | 80 | 13.1 | 19.7 | 81 | 9.7 | 9.2 | |
| PFNA, ng/mL | 80 | 2.3 | 1.7 | 80 | 1.7 | 1.0 | 79 | 2.0 | 0.9 | 80 | 1.6 | 0.6 | |
| BRIEF – mother | |||||||||||||
| Global Executive Composite | 80 | 51.9 | 10.9 | 80 | 51.1 | 10.9 | 79 | 49.6 | 11.6 | 79 | 50.5 | 11.9 | |
| Behavioral Regulation Index | 80 | 51.5 | 10.9 | 80 | 50.8 | 11.5 | 79 | 48.7 | 10.8 | 80 | 50.2 | 11.1 | |
| Metacognition Index | 80 | 51.9 | 10.6 | 80 | 51.0 | 10.5 | 79 | 50.1 | 11.8 | 79 | 50.6 | 12.1 | |
| BRIEF – teacher | |||||||||||||
| Global Executive Composite | 42 | 57.0 | 16.3 | 50 | 58.8 | 15.2 | 49 | 55.7 | 12.9 | 47 | 55.1 | 13.3 | |
| Behavioral Regulation Index | 42 | 56.2 | 14.5 | 50 | 56.6 | 16.3 | 49 | 52.7 | 10.7 | 47 | 53.8 | 12.9 | |
| Metacognition Index | 42 | 56.8 | 16.5 | 50 | 59.2 | 14.6 | 49 | 56.8 | 13.9 | 47 | 55. 2 | 13.4 | |
| CADS – mother | |||||||||||||
| ADHD Index | 80 | 53.6 | 9.9 | 80 | 53.6 | 10.5 | 80 | 53.7 | 11.9 | 80 | 54.5 | 13.3 | |
| Combined Type | 80 | 53.8 | 9.4 | 80 | 53.6 | 9.7 | 80 | 53.4 | 10.8 | 80 | 54.5 | 12.5 | |
| Inattentive Type | 80 | 51.5 | 8.7 | 80 | 51.5 | 9.8 | 80 | 51.9 | 10.9 | 80 | 52.4 | 12.3 | |
| Hyperactive-Impulsive Type | 80 | 56.2 | 10.6 | 80 | 55.8 | 9.8 | 80 | 55.1 | 11.3 | 80 | 56.4 | 12.1 | |
| CADS – teacher | |||||||||||||
| ADHD Index | 41 | 57.3 | 14.2 | 50 | 53.7 | 12.2 | 49 | 53.5 | 12.4 | 47 | 51.2 | 12.2 | |
| Combined Type | 41 | 53.9 | 13.1 | 50 | 51.9 | 10.7 | 49 | 52.1 | 11.4 | 47 | 50. 1 | 11.7 | |
| Inattentive Type | 41 | 49.9 | 10.9 | 50 | 49.7 | 10.4 | 49 | 49.9 | 11.1 | 47 | 46.7 | 9.5 | |
| Hyperactive-Impulsive Type | 41 | 54.1 | 12.7 | 50 | 50.1 | 10.3 | 49 | 51.4 | 11.2 | 47 | 51.0 | 11.1 | |
| BASC – mother | |||||||||||||
| Behavioral Symptom Index | 78 | 50.4 | 8.9 | 80 | 51.4 | 10.2 | 76 | 49.5 | 10.6 | 79 | 50.7 | 10.4 | |
| Adaptive Skills | 78 | 49.3 | 9.3 | 80 | 49.4 | 9.8 | 76 | 52.1 | 10.5 | 79 | 49.8 | 10.3 | |
| Internalizing Problems | 78 | 53.2 | 10.0 | 80 | 54.1 | 11.0 | 76 | 50.9 | 12.2 | 79 | 53.9 | 14.0 | |
| Externalizing Problems | 78 | 50.93 | 8.3 | 80 | 50.8 | 9.9 | 76 | 49.52 | 9.4 | 79 | 50.7 | 9.0 | |
| BASC – teacher | |||||||||||||
| Behavioral Symptom Index | 32 | 49.73 | 9.4 | 36 | 48.99 | 8.8 | 33 | 48.24 | 8.4 | 36 | 48.63 | 8.8 | |
| Adaptive Skills | 32 | 51.7 | 10.3 | 36 | 52.4 | 10.6 | 33 | 51.8 | 9.1 | 36 | 52.9 | 9.9 | |
| Internalizing Problems | 32 | 52.1 | 12.4 | 36 | 51.9 | 11.3 | 33 | 50. 7 | 12.2 | 36 | 49. 9 | 10.7 | |
| Externalizing Problems | 32 | 49.2 | 8.4 | 36 | 47.2 | 7.5 | 33 | 47.1 | 5.9 | 36 | 48.5 | 8.4 | |
| Child age at PFOA measurement, years | 80 | 5.9 | 2.0 | 80 | 5.7 | 1.7 | 80 | 5.7 | 1.8 | 81 | 5.6 | 1.8 | |
| Child age at neuropsychological assessment, years | 80 | 10.0 | 1.9 | 80 | 9.8 | 1.7 | 80 | 9.9 | 1.7 | 81 | 9.8 | 1.7 | |
| Child sex | |||||||||||||
| Male | 34 | (42.5) | 41 | (51.3) | 38 | (47.5) | 36 | (44.4) | |||||
| Female | 46 | (57.5) | 39 | (48.7) | 42 | (52.5) | 45 | (55.6) | |||||
| Child race | |||||||||||||
| Non-white | 1 | (1.3) | 3 | (3.8) | 4 | (5.0) | 2 | (2.5) | |||||
| White | 78 | (98.7) | 76 | (96.2) | 76 | (95.0) | 79 | (97.5) | |||||
| Reported ADHD diagnosis | |||||||||||||
| Yes | 10 | (12.5) | 11 | (13.7) | 8 | (10.0) | 7 | (8.6) | |||||
| No | 70 | (87.5) | 69 | (86.3) | 72 | (90.0) | 74 | (91.4) | |||||
| Maternal Full-scale IQ | 80 | 96.1 | 12.4 | 80 | 96.8 | 12.5 | 80 | 102.5 | 11.0 | 81 | 101.5 | 12.4 | |
| HOME score – maternal section only | |||||||||||||
| Cognitive scale | 80 | 6.6 | 1.7 | 80 | 6.8 | 1.7 | 80 | 6.8 | 1.7 | 81 | 6.9 | 2.0 | |
| Emotional scale | 80 | 10.0 | 1.5 | 80 | 9.6 | 1.6 | 80 | 9.8 | 1.5 | 81 | 9.7 | 1.5 | |
| Maternal age, years | 80 | 37.6 | 5.4 | 80 | 37.3 | 6.1 | 80 | 39.4 | 5.9 | 80 | 39.2 | 5.8 | |
| Maternal employment | |||||||||||||
| None | 41 | (51.3) | 33 | (41.3) | 22 | (27.5) | 25 | (30.9) | |||||
| Part-time | 9 | (11.2) | 11 | (13.7) | 15 | (18.8) | 14 | (17.3) | |||||
| Full-time | 30 | (37.5) | 36 | (45.0) | 43 | (53.7) | 42 | (51.8) | |||||
BRIEF = Behavior Rating Scale of Executive Function; CADS = Conner’s ADHD/DSM-IV Scales; BASC = Behavior Assessment System for Children-2; ADHD = Attention Deficit/Hyperactivity Disorder
There were few differences by child sex or teacher participation based on T or Chi-Square tests. On average, girls as compared to boys had better HOME cognitive scores (6.99 vs. 6.53, p=0.02), worse mother CADS Inattentive scores (53.22 vs. 50.26, p=0.01), better teacher CADS Inattentive scores (46.84 vs. 51.73, p=0.001), and more unemployed fathers (24.3% vs. 18.6%, p=0.03). Children with participating teachers had more mothers who worked in the manufacturing facility (5.8% vs. 0%, p=0.01) and more unemployed fathers (27.3% vs. 13.5%, p=0.01) as compared to children without participating teachers.
The median PFOA concentration was 35.1 ng/mL (interquartile range (IQR) 15.8 – 94.1. There was no difference in exposure by child sex (p=0.50) or teacher participation (p=1.00). Correlations were weak between PFOA concentration and PFOS (R=0.15, p=0.008), PFHxS (R=0.03, p=0.53), and PFNA (R=−0.03, p=0.65) concentrations.
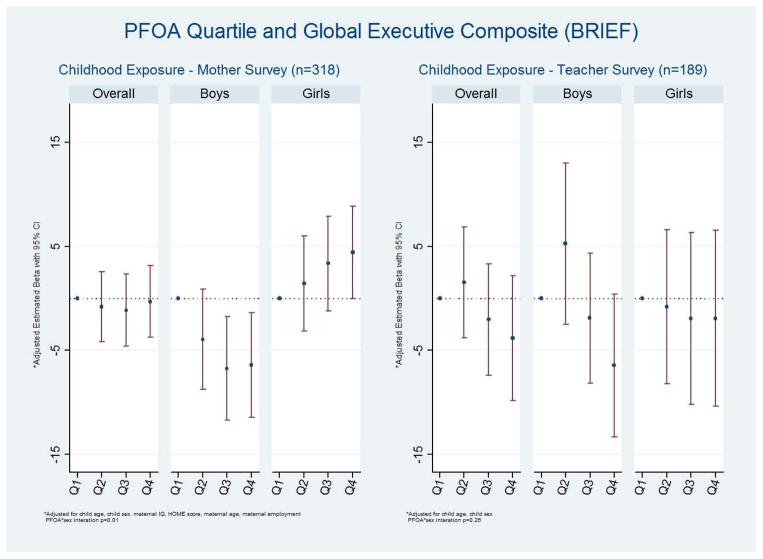
We present adjusted results for the composite scale from each instrument, and then note when the results for the individual scales differ from those for the composite scale. On the mother BRIEF, in the primary analyses there was no clear association between PFOA exposure and the Global Executive Composite (Figure 2, Supplemental Table 1). When stratified by sex, however, comparing the 4th to 1st quartiles PFOA had a favorable association among boys (β = −6.39; 95% CI −11.43, −1.35) and an adverse association among girls (β = 4.42; 95% CI −0.03, 8.87; interaction p=0.01).
Figure 2.
Multivariable association between quartiles of PFOA exposure and the Behavioral Rating Inventory of Executive Function (BRIEF) Global Executive Composite among 6 to 12 year old children, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009 – 2010. Lower scores are better.
On the teacher BRIEF, the βs for the 2nd as compared to 1st quartile of PFOA were elevated on the Global Executive Composite (Figure 2, Supplemental Table 1). The βs for the 3rd and 4th quartiles were below the null with wide confidence intervals. There was little evidence of a sex interaction.
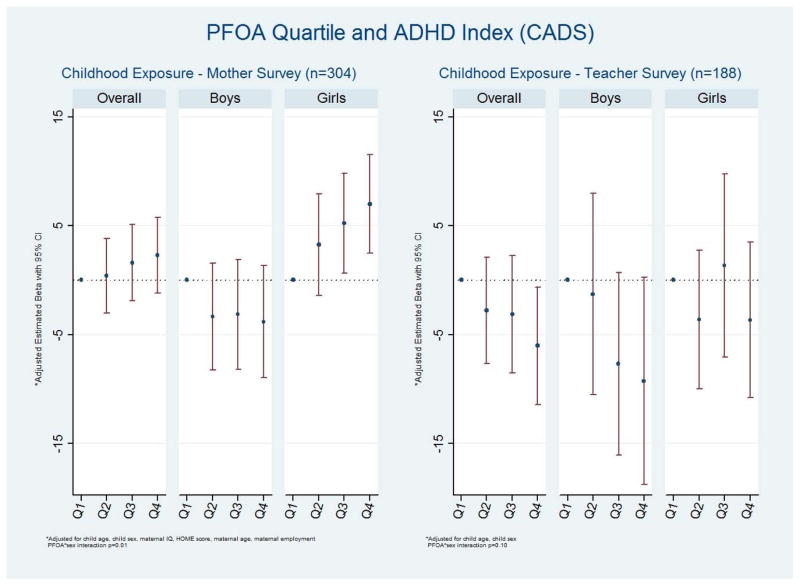
On the mother CADS, there was minimal evidence for an overall association between PFOA and the ADHD Index (Figure 3, Supplemental Table 2). For the overall teacher reports, increased exposure was generally associated with a (favorable) decrease in scores. When stratified by sex, for the mothers, there again appeared to be a favorable association between exposure and outcome among boys and an adverse association among girls. For the association between PFOA and the ADHD Index, the β for a 1 natural log unit increase was −1.15 (95% CI −2.64, 0.35) for boys and 1.79 (95% CI 0.54, 3.03) for girls (interaction p=0.003). For the teacher reports, the associations among boys appeared to be stronger than the mother reports; the effect among girls was not monotonic.
Figure 3.
Multivariable association between quartiles of PFOA exposure and Conners’ ADHD DSM-IV Scales-Revised (CADS) ADHD Index among 6 to 12 year old children, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009 – 2010. Lower scores are better.
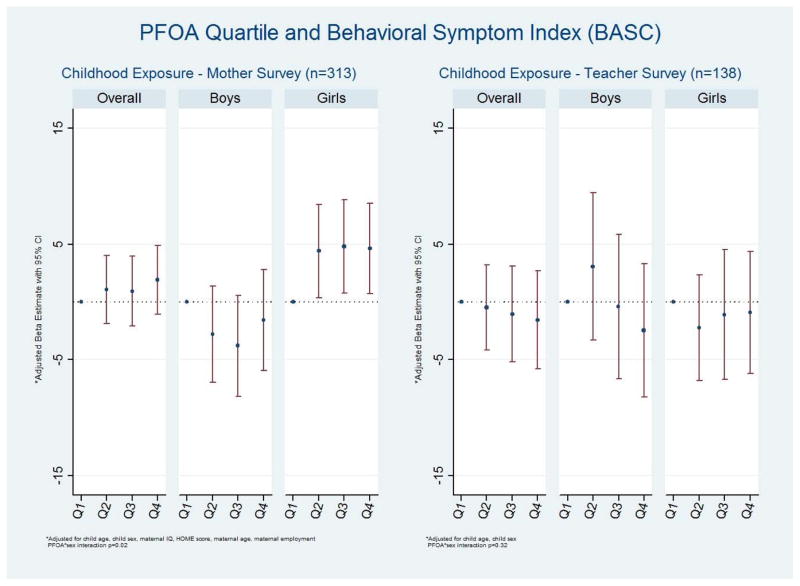
On the mother BASC, in the primary analyses there was no substantive association between PFOA exposure and the Behavioral Symptom Index (Figure 4, Supplemental Table 3). A sex interaction was not universally present across the BASC scales, although the Adaptive Skills and Externalizing scales exhibited favorable associations among boys and adverse associations among girls. On the teacher reports there was little evidence of a similar sex interaction.
Figure 4.
Multivariable association between quartiles of PFOA exposure and the Behavior Assessment System for Children-2 (BASC) Behavioral Symptom Index exposure among 6 to 12 year old children, C8 Health Project Neurobehavioral Follow-up Study, Mid-Ohio Valley, 2009 – 2010. Lower scores are better.
Restricting to the population with PFOA exposure below the median tended to negate the statistically significant sex interactions (data not shown). None of the other sensitivity analyses materially altered the primary study findings (data not shown). Since only 60% of children had teacher reports, as an additional analysis we restricted the mother reports to children with participating teachers. This restriction did not change the pattern of results (data not shown).
COMMENTS
While the overall findings were largely null, in sex-stratified analyses, the mother BRIEF, CADS, and to lesser extent BASC suggested favorable associations between exposure and outcome among boys and adverse associations among girls. Teacher reports replicated some of this sex interaction, tending towards favorable effects among boys and no effect among girls. The disparate findings between parents and teachers are unlikely to be explained by demographics or participation because there were few differences in exposure or outcome by child sex or teacher participation.
Different informants differentially report on child behavior, with parents typically reporting more problems than teachers.29, 30 While parents observe their child’s behavior across a range of situations and teachers primarily observe behavior only in the classroom, parental report of child behavior is colored by their emotional connection to the child as well as their own psychological health.29, 31 Teachers, however, are able to gauge a child’s behavior in relation to the child’s peers, and comment on difficulties related to academic or social success that parents may never see. Reports on boys tend to have more agreement across raters than do reports on girls.29, 30
In the present study teachers generally reported more problems than mothers. When we dichotomized the rating scales at clinical range cutoffs, on the Global Executive Composite and ADHD Index larger proportions of children were in the clinical range based on teacher report than mother report.
The observed inverse association among boys is intriguing and not entirely implausible. One study suggested that PFOA may be neuroprotective.32 Protective associations between PFCs and cognition in older adults was observed in NHANES.32 PFOA activates human in vitro peroxisome proliferator-activated receptor (PPAR) alpha and to a lesser extent gamma, functioning as agonists of these nuclear receptors.33 PPAR-gamma agonists appear to have neuroprotective and central nervous system anti-inflammatory properties.34, 35 These neuroprotective properties may hypothetically manifest in children as better executive function and improved attention skills, although it is unclear why this affect may be evident only in boys. The statistically significant sex interactions were diminished when restricted to the exposure range of the general population,.
There are limited existing epidemiological data with which to compare these results given the paucity of studies and disparate outcome measures. The most relevant comparison is with the present report’s companion study examining PFOA exposure and child performance on neuropsychological tests, suggesting no adverse association between exposure and neuropsychological development, an inverse association between exposure and attention, and no interaction with sex.19 Our earlier cross-sectional study of PFOA concentration and parent-reported ADHD in the full C8 Health Project population observed sporadic inverse associations between PFOA and ADHD.18 In contrast, in NHANES, PFOA concentration was associated with increased prevalence of parent-reported ADHD even at considerably lower median exposure.16 Aside from these two cross-sectional studies of PFOA and ADHD, no published studies replicate either exposure timing or comparable neurodevelopmental outcome assessments.
Multiple comparisons of exposure and outcome measures are pertinent to the interpretation of our results. With little a priori guidance on the kinds of deficits that may result from PFOA exposure we chose to include multiple neurodevelopmental assessments and 2 raters. While this choice allowed us to explore different domains of neurodevelopment it also necessitated a large number of statistical tests; random variation may have resulted in spurious associations, but all results were included regardless of direction of association or p-values. Additionally, what appears to be replication across instruments, such as the common pattern of adverse effects of PFOA on executive function and ADHD-like behaviors among girls, may be due in part to the instruments measuring related behaviors. The BRIEF may be used during ADHD diagnosis to help differentiate between ADHD types.22
In utero or early childhood may be a more relevant window of susceptibility than the ages at our exposure measurement. An estimation of in utero PFOA concentration based on an exposure reconstruction model is available for this population; 19 the correlation with the measured childhood PFOA concentration was high (r=0.69; p<0.0001). This strong correlation underscores how measured childhood exposure is a reflection of total lifetime exposure, including maternal transfer during gestation and lactation. Relatedly, the disparate ages of the children at exposure and outcome assessments limits our ability to investigate the importance of exposure timing. The study design, however, required that children resided in the same water district from the time of their mother’s pregnancy through exposure measurement at C8 Health Project enrollment. When water districts are ranked by drinking water PFOA levels, the relative ranking of the water districts was consistent over time even as the absolute levels of PFOA contamination fluctuated. Consequently the relative ranking of children in our study is largely a function of water district, which we held stable. While exposure from gestation and lactation plays a larger role the younger the child is at exposure measurement, because the children continued to reside in the same water district as they aged may have helped maintain their relative exposure ranking.
Socioeconomic status is a frequent confounder of studies examining neurobehavioral development. We collected extensive covariate data on household demographics, as well as pregnancy and child health. Although many of these variables were associated with the outcome, few were associated with exposure. The shared PFOA exposure source from public drinking water systems makes this study less susceptible to confounding by socioeconomic surrogates that could be related to this exposure in other settings, such as diet and use of stain resistant carpeting or water-repellent clothing. Confounding from unmeasured factors, however, remains possible.
Even with these limitations, this study is the most comprehensive investigation to date of PFOA exposure and child neurobehavioral development. We included a measure of PFOA exposure that pre-dated the outcome assessment. To best characterize the outcomes, we assessed the children with standardized, validated, normed, neuropsychological tests. We solicited reports of child behavior from the biological mother and teacher and amassed extensive covariate details from the mother. The exposure, outcome, and covariate data were all of high quality. It is also important to note that in this study we focused on PFOA because the population was exposed to high levels of PFOA through contaminated drinking water. The results we report for PFOA are not applicable to other PFCs. Additional studies may clarify our internally inconsistent findings, in particular the modification by sex observed in the mother and some of the teacher reports.
Supplementary Material
Acknowledgments
We would like to thank Lisa John and Christopher Lyu for their assistance collecting the data for the study.
This research was funded by the C8 class action settlement agreement [Jack W. Leach, et al. v. E.I. du Pont de Nemours & Company (no. 01-C-608 W.Va., Wood County Circuit Court, West Virginia, USA] between DuPont and plaintiffs. Funds were administered by the Garden City Group (Melville, New York) that reports to the court. Our work and conclusions are independent of either party to the lawsuit. Cheryl Stein was supported by the National Institute of Environmental Health Sciences (K01 ES019156).
Footnotes
The authors declare no competing financial interests.
References
- 1.US Environmental Protection Agency. Perfluorooctanoic Acid (PFOA) and Fluorinated Telomers. Washington, DC: U.S. Environmental Protection Agency; 2009. [updated January 16, 2013; cited 2013 March 1]; Available from: http://www.epa.gov/oppt/pfoa/pubs/pfoainfo.html. [Google Scholar]
- 2.US Environmental Protection Agency. Perfluorooctanoic Acid (PFOA) Washington, DC: U.S. Environmental Protection Agency; 2009. [updated January 16, 2013; cited 2013 March 1]; Available from: http://www.epa.gov/oppt/pfoa/index.html. [Google Scholar]
- 3.Stahl T, Mattern D, Brunn H. Toxicology of perfluorinated compounds. Environ Sci Europe. 2011:23. [Google Scholar]
- 4.D’Eon JC, Mabury SA. Is Indirect Exposure a Significant Contributor to the Burden of Perfluorinated Acids Observed in Humans? Environ Sci Technol. 2011;45:7974–7984. doi: 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in Exposure to Polyfluoroalkyl Chemicals in the U.S. Population: 1999–2008 (dagger) Environ Sci Technol. 2011 doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MS, Norgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, et al. Phthalates and Perfluorooctanesulfonic Acid in Human Amniotic Fluid: Temporal Trends and Timing of Amniocentesis in Pregnancy. Environ Health Perspect. 2012 doi: 10.1289/ehp.1104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein CR, Wolff MS, Calafat AM, Kato K, Engel SM. Comparison of polyfluoroalkyl compound concentrations in maternal serum and amniotic fluid: A pilot study. Reprod Toxicol. 2012 doi: 10.1016/j.reprotox.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, et al. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspect. 2004;112:1204–1207. doi: 10.1289/ehp.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midasch O, Drexler H, Hart N, Beckmann MW, Angerer J. Transplacental exposure of neonates to perfluorooctanesulfonate and perfluorooctanoate: a pilot study. Int Arch Occup Environ Health. 2007;80:643–648. doi: 10.1007/s00420-006-0165-9. [DOI] [PubMed] [Google Scholar]
- 10.Kuklenyik Z, Reich JA, Tully JS, Needham LL, Calafat AM. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol. 2004;38:3698–3704. doi: 10.1021/es040332u. [DOI] [PubMed] [Google Scholar]
- 11.So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, et al. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ Sci Technol. 2006;40:2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- 12.Tao L, Kannan K, Wong CM, Arcaro KF, Butenhoff JL. Perfluorinated compounds in human milk from Massachusetts, U.S.A. Environ Sci Technol. 2008;42:3096–3101. doi: 10.1021/es702789k. [DOI] [PubMed] [Google Scholar]
- 13.Volkel W, Genzel-Boroviczeny O, Demmelmair H, Gebauer C, Koletzko B, Twardella D, et al. Perfluorooctane sulphonate (PFOS) and perfluorooctanoic acid (PFOA) in human breast milk: results of a pilot study. Int J Hyg Environ Health. 2008;211:440–446. doi: 10.1016/j.ijheh.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ Health Perspect. 2008;116:1391–1395. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect. 2011;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect. 2010;118:1762–1767. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45:8151–8159. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein CR, Savitz DA. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ Health Perspect. 2011;119:1466–1471. doi: 10.1289/ehp.1003538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein CR, Savitz DA, Bellinger DC. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology. 2013;24:590–599. doi: 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisbee SJ, Brooks AP, Jr, Maher A, Flensborg P, Arnold S, Fletcher T, et al. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117:1873–1882. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaherty JM, Connolly PD, Decker ER, Kennedy SM, Ellefson ME, Reagen WK, et al. Quantitative determination of perfluorooctanoic acid in serum and plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;819:329–338. doi: 10.1016/j.jchromb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Gioia GA. BRIEF : behavior rating inventory of executive function : professional manual. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 23.Conners CK. Conners’ Rating Scales-Revised. North Tonawanda, NY: Multi-Health Systems, Inc; 2001. [Google Scholar]
- 24.Reynolds CR, Kamphaus RW. BASC-2 : Behavior Assessment System for Children : manual. Minneapolis, MN: Pearson; 2004. [Google Scholar]
- 25.National Longitudinal Survey Program. U.S. Bureau of Labor Statistics; [cited 2012 May 25]; Available from: http://www.bls.gov/nls/nlsy97.htm. [Google Scholar]
- 26.The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Harcourt Brace and Company; 1999. [Google Scholar]
- 27.Rothman KJ, Greenland S. Modern epidemiology. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 28.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J Steroid Biochem Mol Biol. 2011;127:16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briggs-Gowan MJ. Discrepancies among mother, child, and teacher reports: examining the contributions of maternal depression and anxiety. Journal of abnormal child psychology. 1996;24:749–765. doi: 10.1007/BF01664738. [DOI] [PubMed] [Google Scholar]
- 30.Touliatos J. Congruence of parents’ and teachers’ ratings of children’s behavior problems. Journal of abnormal child psychology. 1981;9:347–354. doi: 10.1007/BF00916839. [DOI] [PubMed] [Google Scholar]
- 31.Phares V. Perspectives on child behavior problems: Comparisons of children’s self-reports with parent and teacher reports. Psychological assessment. 1989;1:68–71. [Google Scholar]
- 32.Power MC, Webster TF, Baccarelli AA, Weisskopf MG. Cross-Sectional Association between Polyfluoroalkyl Chemicals and Cognitive Limitation in the National Health and Nutrition Examination Survey. Neuroepidemiology. 2012;40:125–132. doi: 10.1159/000342310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 34.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–1826. doi: 10.2741/2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaundal RK, Sharma SS. Peroxisome proliferator-activated receptor gamma agonists as neuroprotective agents. Drug News Perspect. 2010;23:241–256. doi: 10.1358/dnp.2010.23.4.1437710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.