Abstract
Objectives
To determine the neuroprotective efficacy of the inert gas xenon following traumatic brain injury, and to determine whether application of xenon has a clinically relevant therapeutic time window.
Design
Controlled animal study.
Setting
University research laboratory.
Subjects
Male C57BL/6N mice (n=196)
Interventions
75% xenon, 50% xenon or 30% xenon, with 25% oxygen (balance nitrogen) treatment following mechanical brain lesion by controlled cortical impact.
Measurements & Main Results
Outcome following trauma was measured using: 1) functional neurological outcome score, 2) histological measurement of contusion volume, 3) analysis of locomotor function and gait. Our study shows that xenon-treatment improves outcome following traumatic brain injury. Neurological outcome scores were significantly (p<0.05) better in xenon-treated groups in the early phase (24 hours) and up to 4 days after injury. Contusion volume was significantly (p<0.05) reduced in the xenon-treated groups. Xenon treatment significantly (p<0.05) reduced contusion volume when xenon was given 15 minutes after injury or when treatment was delayed 1 hour or 3 hours after injury. Neurological outcome was significantly (p<0.05) improved when xenon treatment was given 15 minutes or 1 hour after injury. Improvements in locomotor function (p<0.05) were observed in the xenon-treated group, 1 month after trauma.
Conclusions
These results show for the first time that xenon improves neurological outcome and reduces contusion volume following traumatic brain injury in mice. In this model, xenon application has a therapeutic time window of up to at least 3 hours. These findings support the idea that xenon may be of benefit as a neuroprotective treatment in brain trauma patients.
Keywords: xenon, neuroprotection, inert gases, head trauma, brain injury
INTRODUCTION
Traumatic brain injury (TBI) affects both young and elderly populations throughout the world and results in a significant global healthcare burden. In developed countries TBI is the main cause of death and disability in those aged under 45 [1] with falls and motor-vehicle crashes being the leading causes [2, 3]. In the United States, between the years 2002 and 2006, on average 1.7 million people per year suffered a TBI [4]. Of these, 1.4 million were treated in hospital Emergency Departments, 275,000 required hospitalization and survived, but 52,000 died [4]. TBI causes 30% of all injury-related deaths in the United States [4]. Current clinical practice for TBI patients is largely supportive, centred on non-specific endpoints such as management of tissue oxygenation, cerebral perfusion pressure and intracranial pressure [5-8]. At present there are no specific pharmacological neuroprotective treatments for TBI [9-12]. Given the high economic costs associated with TBI, there is a need for neuroprotective treatments that can minimize or attenuate the brain damage following TBI and promote a faster and more complete recovery. The current study evaluates the neuroprotective efficacy of xenon in the rodent controlled cortical impact brain trauma model.
Blunt-trauma TBI is characterized by a “primary injury” determined by the initial mechanical force, followed by a “secondary injury” which begins soon after the initial trauma and continues to develop in the following hours and days. The biological processes involved in development of secondary injury are complex and involve multiple injury cascades [13, 14], however glutamate excitotoxicity, involving overactivation of N-methyl-D-aspartate(NMDA)-receptors, is thought to play a key role [15-17].
The noble gas xenon has been used as a general anesthetic since the 1950s [18, 19] but its molecular targets were unknown [20]. Following the discovery that xenon is an NMDA receptor antagonist [21-24], xenon was shown to be neuroprotective in a number of in vitro and in vivo models of ischemic injury [25-34] and xenon is currently undergoing clinical trials as a treatment for ischemic brain injury [35, 36] and post-operative delirum [37]. Information on xenon neuroprotection against TBI is much more limited. In the present study we use the rodent controlled cortical impact brain trauma model to test the following hypotheses: that xenon protects against secondary injury development; that xenon improves neurological outcome after trauma; and that xenon has a clinically relevant therapeutic time window.
MATERIALS AND METHODS
Adult male C57BL/6N mice aged 2.5 months, mean weight 24 ± 3 g (SEM, n=196), were obtained from Charles River Laboratory, Sulzfeld, Germany. Animals were cared for in compliance with the institutional guidelines of the Johanes Gutenberg University, Mainz. All experiments were approved by the Animal Ethics Committee of the Landestuntersuchungsamt Rheinland-Pfalz (protocol number: G12-1-010).
Traumatic brain injury
Animals were anesthetized with 3.5% sevoflurane in an air/oxygen mixture (40% O2 / 60% N2) supplied via a facemask in spontaneously breathing animals. Core temperature was monitored and maintained at 37°C for the duration of the surgery by means of a rectal probe and feedback-controlled heating pad (Hugo Sachs, March-Hugstetten, Germany). Traumatic injury was performed using the controlled cortical impact (CCI) model, as described previously [38], by an experimenter blinded to the treatment groups. Animals were fixed in a stereotactic frame (Kopf Instruments, Tujunga, USA) and a 4mm × 5mm craniotomy window was created using a saline-cooled high-speed drill, along the coronal (anterior) and lambdoid (posterior) sutures and laterally as close as possible to the temporalis muscle insertion. The bone flap was lifted exposing the dura above the right parietal cortex, between the sagittal, lambdoid, and coronal sutures. The tip of a custom-built controlled cortical impact device (L. Kopacz, Mainz, Germany) was positioned above the intact dura in the centre of the craniotomy window (1mm from saggital suture and 1mm from lambdoid suture). The angle of the impactor, typically 25 degrees from the sagittal plane, was adjusted such that the tip was perpendicular to the dural surface. An impactor tip of diameter 3 mm, impact velocity of 8 m/s and impact duration of 150 ms, were used. In the xenon pre-exposure & post-exposure experiments shown in Figure 1 (n=12 animals) a penetration depth of 1.5 mm was used, in all other experiments (n=184 animals) the penetration depth was 1.0 mm. Our pneumatically controlled CCI device is similar in design to that used by Smith et al [39]] and Fox et al [40] who developed the CCI model in mice and our impact parameters are similar to those found by those workers to result in moderate injury. Following CCI-injury, the craniotomy was closed with the bone flap, fixed with tissue glue (Histoacryl, Braun-Melsungen, Melsungen, Germany), and the skin sutured. Animals were returned to their individual home cages in a heated incubator (33°C, 35% humidity; IC8000, Draeger, Germany) and allowed to recover for 15 minutes before treatment; during this time they were breathing room air.
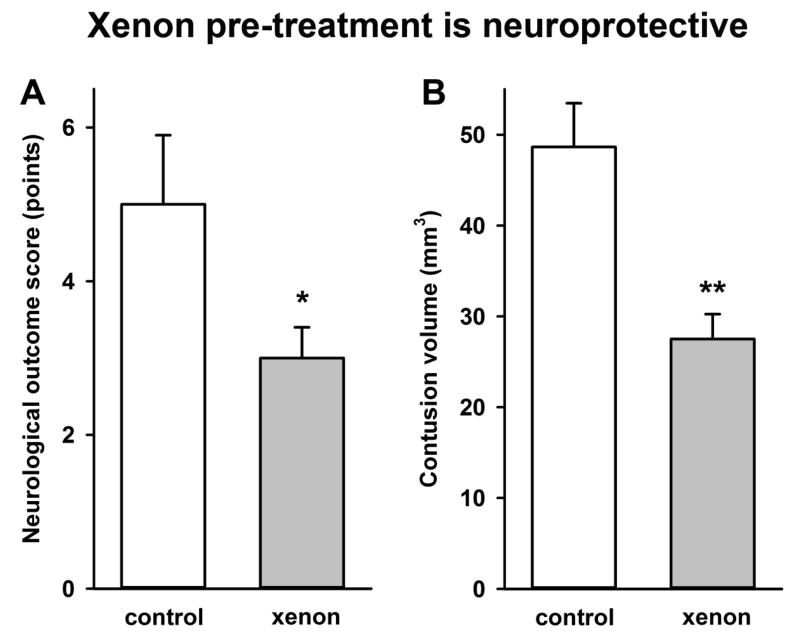
Figure 1.
Treatment with xenon before and after injury (A) improves neurological outcome and (B) reduces contusion volume following trauma. Xenon-treated animals (grey bars) received 75% xenon : 25% oxygen for 2 hours before injury and for 2 hours after injury. Control animals (white bars) received 75% nitrogen : 25% oxygen. Neurological outcome and contusion volume were measured 24 hours after trauma. Bars represent mean values and error bars are standard errors (control n=6, xenon n=6). * = p<0.05; ** = p<0.01, compared to control.
Experimental groups, randomizing and blinding
Animals were randomly assigned to receive xenon (75%, 50% or 30%) with 25% oxygen (balance nitrogen) or 75% nitrogen : 25% oxygen (control gas). The experimenter performing the CCI surgery was blinded to the treatment. A separate experimenter, also blinded to treatment, performed the behavioral tests. Treatment was begun 15 minutes, 1 hour, 3 hours or 6 hours after injury. Animals were allowed to survive 24 hours, 5 days or 1 month. In the short-term (24 hr) survival experiments we aimed to have 9-10 animals in each xenon-treatment group. In some experiments (eg time delay experiments) it was not possible to perform all experiments on the same day. In these cases we had additional control groups that were pooled resulting in larger numbers of mice in the control groups. In the 5-day survival experiment we had 12 animals in each group; at 24 hours neuroscore data from 24 hour survival and 5 day survival experiments was pooled. Three animals died shortly after CCI injury and post-mortem showed evidence of intra-cerebral hemorrhage. One animal in the 5 day survival group became unresponsive and immobile on day 4 and was euthanized, post-mortem indicated intra-cerebral hemorrhage. Due to technical issues with the impact device (movement of the impactor during CCI, affecting impact parameters) 4 animals were excluded from the dose-response analysis (1 in control group, 1 in 30% xenon-group & 2 in 50% xenon-group). In our long-term (1 month) survival experiments we had 20 animals each in the xenon and control-treatment groups and 10 animals in the sham-surgery group, all animals survived and performed Rotarod test, 3 animals (one in each group) failed to perform the CatWalk®-XT test.
Xenon or control gas administration
Gas treatments were administered to spontaneously breathing animals in a series of custom made xenon exposure chambers linked in a closed circuit, for a total duration of three hours. Gas concentrations inside the circuit were monitored continuously, via a xenon meter (model 439 EX, Nyquist Ltd, UK) and an oxygen meter (Oxydig, Draeger, Luebeck, Germany) included in the circuit. Carbon dioxide was removed from the system by soda lime pellets. Additional volumes of gases were added into the system as necessary to maintain their respective concentrations. Gases were circulated at 700 ml/min by a small animal ventilator (Inspira ASV, Harvard Apparatus, Holliston, MA, USA). Chambers were housed inside an incubator heated to 33°C (Model IC8000, Draeger, Lubeck, Germany). In the therapeutic time window experiments, the start of the 3 hours of xenon-treatment was delayed 15 minutes, 1 hour, 3 hours, and 6 hours after CCI injury.
Physiological measurements
Systolic and diastolic blood pressure and heart rate were measured using a Kent Coda non-invasive blood pressure monitor (EMKA Technologies, Paris, France). Temperature measurements were made using an infra-red thermometer (Thermoworks model TW2).
Histological evaluation
At the end of the observation period (24 hours), and immediately following the final neuroscore, animals were anesthetized with sevoflurane and sacrificed by cervical dislocation. The brain was carefully removed, frozen on powdered dry ice, and stored at −80°C. Frozen brains were embedded in Optimal Cutting Temperature mounting media (Cell Path Ltd, Newton, Powys, UK) and cut in the coronal plane with a cryostat tissue slicer (Leica CM3050, Leica Microsystems, Milton Keynes, UK). For each brain a total of 16 – 18 sections (10 μm thickness) were collected on Superfrost® Plus microscope slides (Thermo Fisher Scientific, Loughborough, UK) every 500 μm. For the quantification of contusion volume, slices were stained with cresyl violet (Thermo Fisher Scientific, Loughborough, UK). Slices were imaged with a digital camera (Scopetek DCM510, Scopetek Opto-Eletric Co., Hangzhou, China) attached to a stereomicroscope (Wild model M8, Heerbrugg, Switzerland). The contusion was evident from a clear difference in the intensity of the cresyl-staining. The area of the contusion was measured using image-analysis software (Scopephoto 3.1, Scopetek Opto-Eletric Co., Hangzhou, China) by an investigator blinded to the experimental groups. Contusion volume was calculated by multiplying contusion areas, A, by the distance between brain sections, d, (500 μm), according to the following formula:
Quantification of functional outcome
(i) Neuroscore. Functional outcome before and after CCI injury was determined using a 15-point neurological outcome score [41, 42]. The neuroscore consists of tasks evaluating locomotor ability, vestibulomotor function and general behaviour (Table 1). The neuroscore was performed in real time, before surgery and repeated at 24-hour intervals following CCI-injury, with the final test applied immediately before the animals were sacrificed (either 24 hours or 5 days after injury). Long term outcomes after 1 month were assessed using RotaRod and CatWalk-XT tests. (ii) RotaRod. Mice were placed on the RotaRod (Ugo Basile, Comerio, Italy). The RotaRod was accelerated linearly from 4 to 40 rev/minute over 5 minutes and the time the mouse remained on the drum recorded. Three trials were performed with the longest time on the drum used. (iii) CatWalk-XT is an automated gait analysis system (Noldus Information Technology, Wageningen, The Netherlands), consisting of a glass plate with dim light illuminating the glass from the side. In a darkened environment (< 1 lux of illumination), light is reflected downward when the animal’s paws contact the glass surface. Images of the footprints are recorded by a video camera under the walkway. The images from each trial are processed and analysed on a PC by CatWalk-XT software. Three consecutive runs were performed and the mean values for the gait parameters for each animal were obtained.
Table 1.
Criteria for the neurological outcome score. Points are scored for the failure to successfully perform a task, up to a maximum of 15 points for severe impairment. Healthy animals scored 0 points. Modified from Tsenter et al [42]
| Task | Points |
|---|---|
| Failure to exit a 25 cm diameter circle in 30 sec | 1 |
| Failure to exit a 25 cm diameter circle in 60 sec | 1 |
| Failure to exit a 25 cm diameter circle in 120 sec | 1 |
| Inability/impaired ability to traverse a 3 cm wide beam | 2/1 |
| Inability/impaired ability to traverse a 2 cm wide beam | 2/1 |
| Inability/impaired ability to traverse a 1 cm wide beam | 2/1 |
| Inability to balance on a 0.5 cm diameter round stick | 1 |
| Inability to balance on a 0.5 cm square stick | 1 |
| Presence of hemiparesis | 1 |
| Inability to walk in a straight line | 1 |
| Loss of startle reflex * | 1 |
| Lack of exploratory behaviour # | 1 |
no response to handclap
does not explore environment
Statistics
We assessed significance using either a Student’s t-test or one-way ANOVA, with Bonferroni’s post-hoc test. In the 5-day survival experiments we used two-way ANOVA with repeated measures with Bonferroni’s post-hoc test. Factor 1 was treatment (Xe, or control) and factor 2 was the time after injury (e.g. 1 day, 2 days, 3 days 4 days or 5 days). We therefore used repeated measures ANOVA with factor 1 as the repeated factor. We used two-tailed hypothesis testing with p-values of less than 0.05 taken to indicate a significant difference between groups. The sample sizes (n) are indicated in the figure legends. From preliminary experiments with short-term outcomes (24 hour) we estimated that a sample size of ~10 animals in each treatment group would give a statistical power of ~0.80 (α=0.05). A post-hoc power analysis of the results indicate that at a level of 0.05, the power of the experiments is on average 0.83 (range 0.55 to 0.98). Sample sizes for long-term behavioral experiments were estimated from previous experience with these tests. Where error bars are shown these are the standard error of the mean. Statistical tests were implemented using SigmaPlot software (Systat Inc., Point Richmond, CA).
RESULTS
Xenon pre-treatment and post-treatment is neuroprotective
We investigated the effect of xenon administration on neurological outcome and contusion volume when xenon was given both before & after, but not during, brain trauma (Figure 1). Treatment with 75% xenon:25% oxygen for 2 hours immediately before the trauma and then for another 2 hours after the trauma resulted in a significant improvement (40 ± 11%, p<0.05) in functional neurological outcome 24 hours after trauma (Figure 1A). Contusion volume was reduced by 43 ± 7% (p<0.01) following xenon treatment (Figure 1B).
Xenon post-treatment is neuroprotective
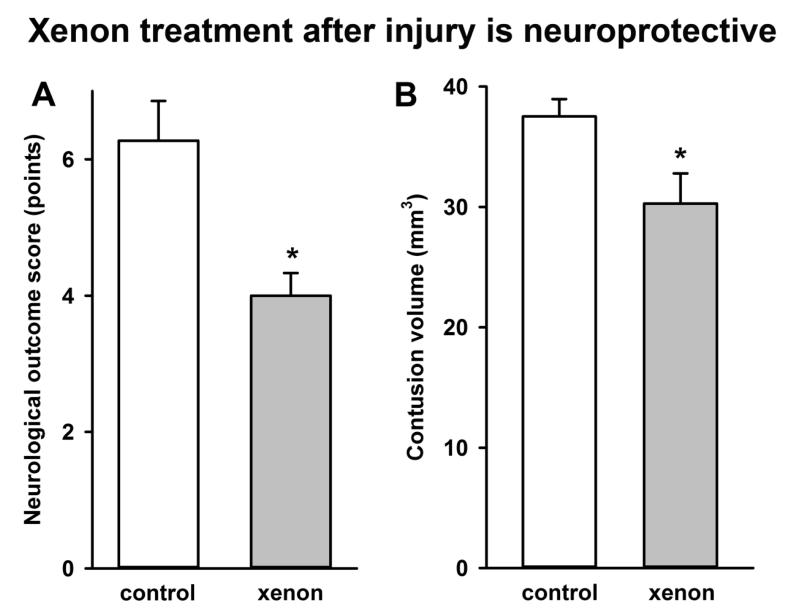
We next determined whether xenon was effective when given only after traumatic brain injury. We found that 75% xenon:25% oxygen administered 15 minutes after injury for a period of 3 hours duration resulted in a 36 ± 6% (p<0.05) improvement in neurological outcome score 24 hours after injury (Figure 2A). We found that xenon treatment after injury significantly reduced contusion volume, by 19 ± 7% (p<0.05), compared to untreated controls (Figure 2B).
Figure 2.
Treatment with xenon after injury (A) improves neurological outcome and (B) reduces contusion volume following trauma. Xenon-treated animals (grey bars) received 75% xenon : 25% oxygen for 3 hours duration, 15 minutes after trauma. Control animals (white bars) received 75% nitrogen : 25% oxygen. Neurological outcome and contusion volume were measured 24 hours after trauma. Bars represent mean values and error bars are standard errors (Neurological outcome: n=22, control; n=21, xenon; Contusion volume: n=22, control; n=9 xenon) * = p<0.05 compared to control.
Delayed xenon treatment is neuroprotective
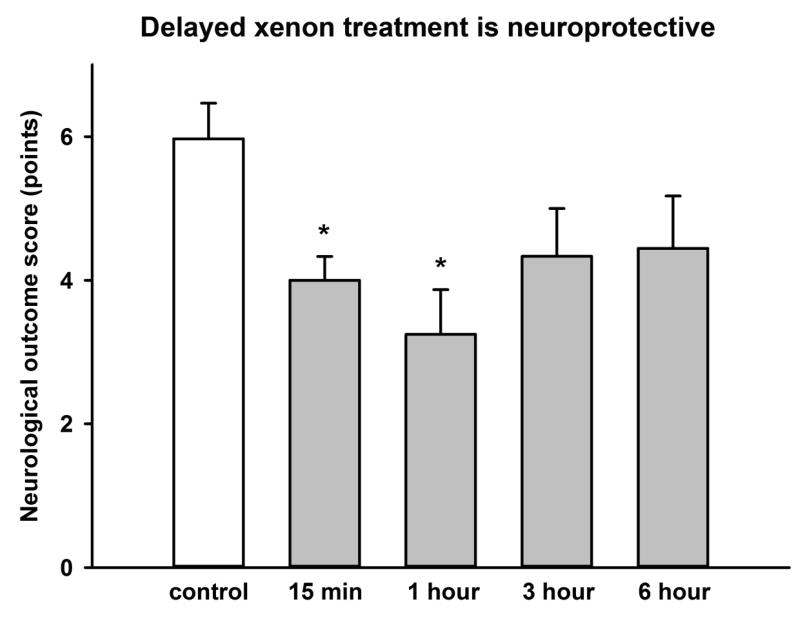
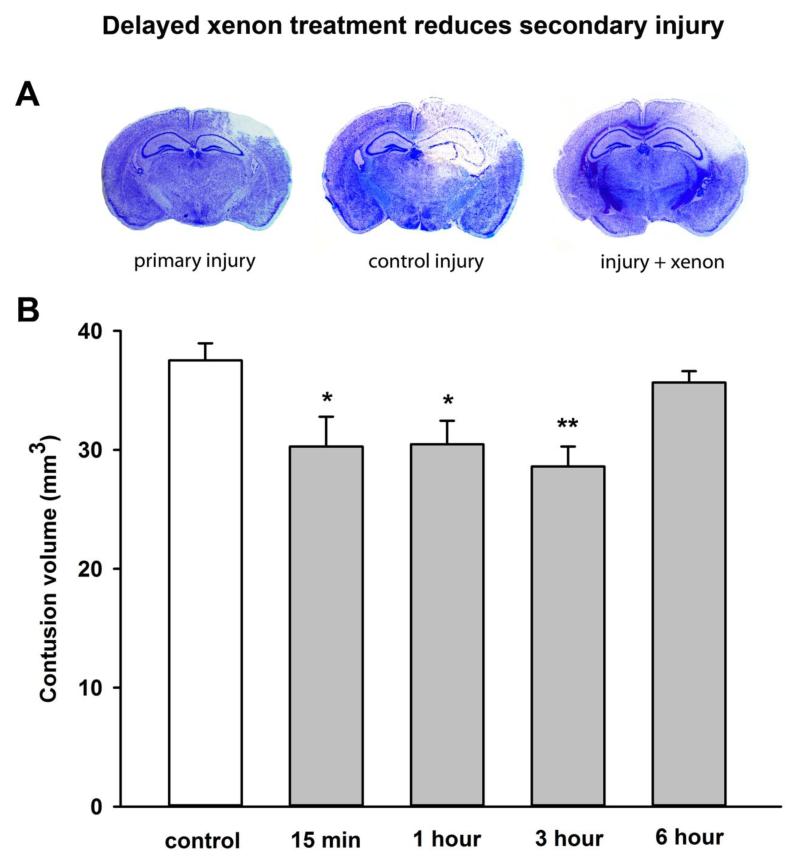
Having shown that xenon was effective when given immediately after injury, we determined the therapeutic time window in which xenon remained effective. We investigated the effect of delaying the start of the xenon treatment until 1 hour, 3 hours or 6 hours after injury. We found xenon-treatment begun 15 minutes, or 1 hour after injury significantly (p<0.05) improved the neurological outcome score 24 hours after injury by 33% and 46% respectively (Figure 3). In the groups where xenon-treatment was delayed for 3 hours or 6 hours there was a trend to improved neuroscore (27% and 26% respectively), but this did not reach statistical significance. We investigated the effect of delayed xenon treatment on contusion volume. We found xenon treatment starting 15 minutes, 1 hour or 3 hours after injury significantly (p<0.05) reduced total injury by ~20% (Figure 4), but delaying treatment until 6 hours after injury made the xenon-treatment ineffective (5.0 ± 0.2% reduction).
Figure 3.
Delayed xenon treatment following trauma improves neurological outcome 24 hours after trauma. Xenon-treated animals (grey bars) received 75% xenon : 25% oxygen for 3 hours duration, starting at 15 minutes, 1 hour, 3 hours or 6 hours after trauma. Control animals (white bar) received 75% nitrogen : 25% oxygen. Neurological outcome was measured 24 hours after trauma. Bars represent mean values and error bars are standard errors (n=34, control; n=21, xenon 15 min; n=8, xenon 1 hr; n=9, xenon 3 hr; n=9, xenon 6 hr) * = p<0.05 compared to control.
Figure 4.
Delayed xenon treatment following trauma reduces injury 24 hours after trauma. (A) Typical cresyl-violet stained slices, 2.2 mm posterior to Bregma, showing primary injury 15 min after trauma, control injury and xenon-treated injury at 24 hours after trauma. In example shown xenon treatment was delayed until 1 hour after trauma. (B) Xenon-treated animals (grey bars) received 75% xenon : 25% oxygen for 3 hours duration starting at 15 minutes, 1 hour, 3 hours or 6 hours after trauma. Control animals (white bar) received 75% nitrogen:25% oxygen. Contusion volume was measured 24 hours after trauma. Bars represent mean values and error bars are standard errors (n=22, control; n=9, xenon 15 min; n=8, xenon 1 hr; n=9, xenon 3 hr; n=9, xenon 6 hr) * = p<0.05; ** = p<0.01 compared to control.
Concentration dependence of xenon’s neuroprotective effect
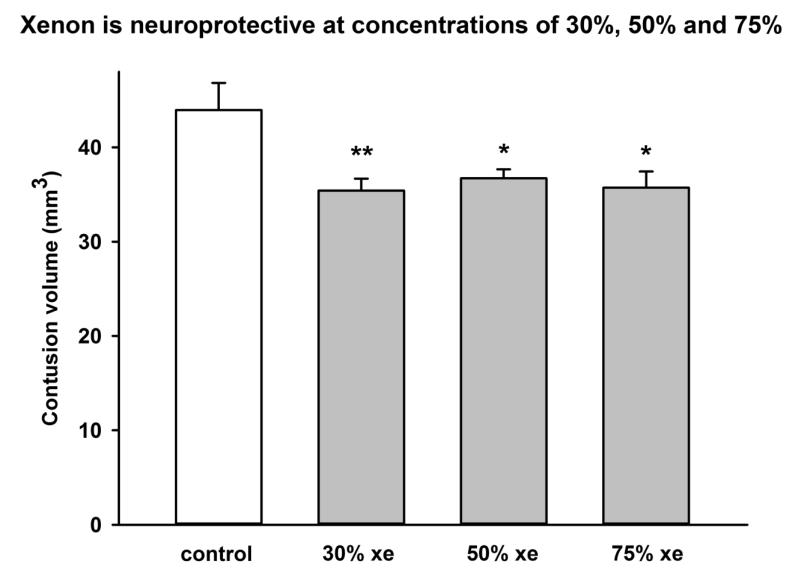
We performed a series of experiments where animals were treated with 30% xenon:25% oxygen (balance nitrogen), 50% xenon:25% oxygen (balance nitrogen), 75% xenon:25% oxygen, or 75% nitrogen:25% oxygen, for 3 hours, starting 15 minutes after injury. Xenon reduced injury significantly (p<0.05) at all concentrations tested (Figure 5), by 19 ± 1%, at a concentration of 30%, by 17 ± 1% at a concentration of 50% and by 19 ± 2% at a concentration of 75% xenon. Neurological outcome (data not shown) was significantly improved by treatment with 30% xenon or 75% xenon, by 45 ± 10 % (p<0.05) and 67 ± 15 % (p<0.001) respectively. Treatment with 50% xenon resulted in a trend to reduction in neuroscore (24 ± 5 %) but this did not reach significance.
Figure 5.
Xenon is neuroprotective at concentrations of 30%, 50% and 75%. Xenon-treated animals (grey bars) received 75% xenon : 25% oxygen, 50% xenon, 25% oxygen, 25% nitrogen or 30% xenon, 25% oxygen, 45% nitrogen for 3 hours duration starting at 15 minutes after trauma. Control animals (white bar) received 75% nitrogen : 25% oxygen. Contusion volume was measured 24 hours after trauma. Bars represent mean values and error bars are standard errors (n=9, control; n=9, xenon 75% ; n=7, xenon 50% ; n=9, xenon 30%;) * = p<0.05, ** = p<0.01 compared to control.
Effect of xenon treatment on physiological parameters
In order to determine whether xenon treatment affected the physiology of the animals we measured blood pressure, heart rate and temperature in a cohort of animals that received CCI injury followed by 3 hours of treatment with either 75% xenon:25% oxygen or control gas, 75% nitrogen:25% oxygen, starting 15 minutes after injury. There was no significant difference in physiological parameters between the xenon-treated or control-gas treated animals (Table 2).
Table 2. Physiological measurements.
Treatment with 75% xenon:25% oxygen for 3 hours, starting 15 minutes after trauma had no effect on blood pressure, heart rate or temperature compared to 75% nitrogen:25% oxygen control. Data shown below are individual values for each animal (n=16) together with mean and SEM for each group. There were no significant differences between control or xenon treatment groups for systolic or diastolic blood pressure (p=0.49, p=0.43), heart rate (p=0.36) or temperature (p=0.53).
| treatment | Systolic BP (mm Hg) | Diastolic BP (mm Hg) | Heart Rate min−1 | Temperature °C |
|---|---|---|---|---|
| control | 179 | 150 | 493 | 36.7 |
| control | 162 | 129 | 487 | n.d. |
| control | 165 | 135 | 482 | n.d. |
| control | 157 | 124 | 540 | 36.6 |
| control | 146 | 114 | 462 | 35.9 |
| control | 132 | 106 | 344 | 37.3 |
| control | 171 | 149 | 452 | 37.7 |
| control | 170 | 130 | 400 | 37.1 |
| mean ± SEM | 160 ± 5 | 130 ± 5 | 458 ± 21 | 36.9 ± 0.2 |
| 75% xenon | 171 | 133 | 424 | 37.4 |
| 75% xenon | 143 | 101 | 431 | 36.5 |
| 75% xenon | 166 | 140 | 389 | 36.8 |
| 75% xenon | 143 | 120 | 552 | 36.7 |
| 75% xenon | 162 | 137 | 484 | 37.4 |
| 75% xenon | 145 | 112 | 425 | 36.8 |
| 75% xenon | 164 | 136 | 401 | 37.3 |
| 75% xenon | 150 | 109 | 310 | 37.6 |
| mean ± SEM | 156 ± 4 | 124 ± 5 | 427 ± 25 | 37.0 ± 0.1 |
Effect of xenon up to 5 days post-injury
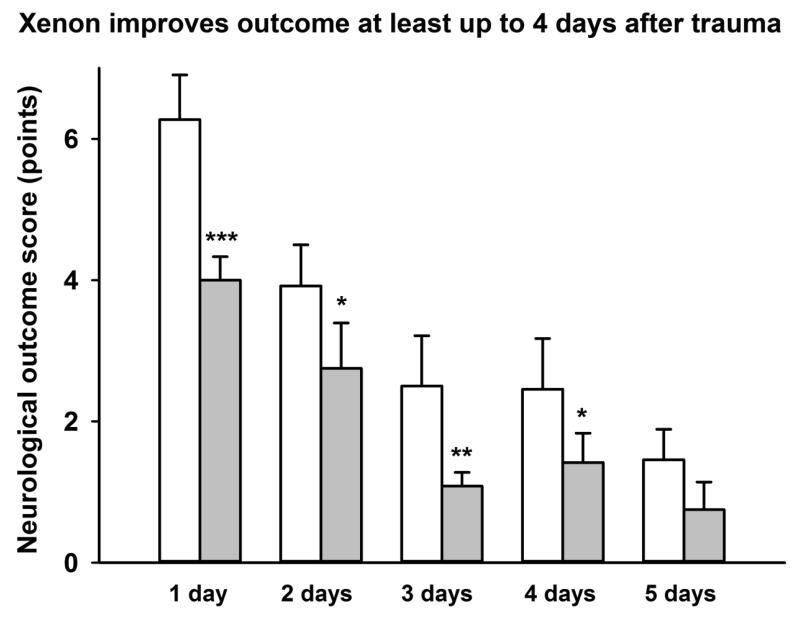
Neurological function was measured at 1 day, 2 days, 3 days, 4 days and 5 days after injury (Figure 6). Neurological outcome in the xenon-treated groups improved significantly (p<0.05) compared to the control group, up to 4 days after injury. On day 5, there was a trend for improvement in neurological outcome score in the xenon-treated group compared to the control group but this difference was not significant (p=0.06).
Figure 6.
Xenon treatment improves neurological outcome up to 4 days after trauma. Xenon-treated animals (grey bars) received 75% xenon:25% oxygen, for 3 hours duration, starting 15 minutes after trauma. Control animals (white bars) received 75% nitrogen:25% oxygen. Neurological outcome was measured at 1 day intervals for 5 days after trauma. Bars represent mean values and error bars are standard errors (day 1: n=22, control; n=21, xenon; day 2-3: n=12, control; n=12, xenon; day 4-5: n=11, control; n=12, xenon) * = p<0.05; ** = p<0.01; *** = p<0.001 compared to control.
Effect of xenon 1 month after injury
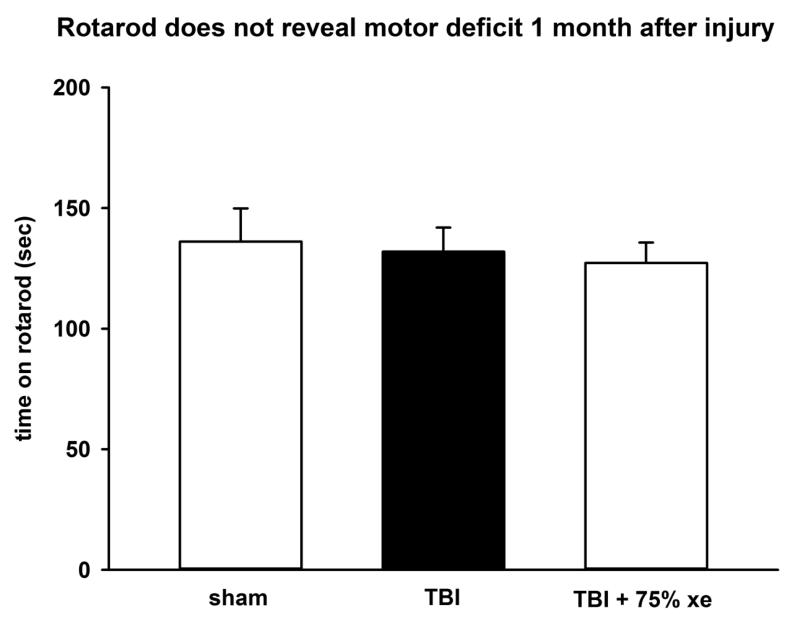
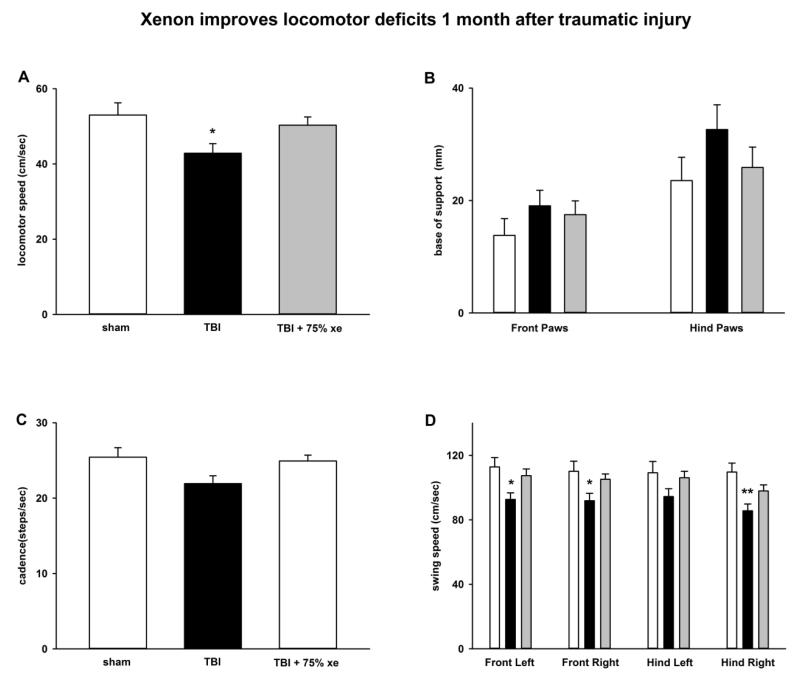
In order to establish whether xenon’s protective effect persisted, a cohort of animals (N=50) were allowed to survive 1 month after traumatic injury. Two groups underwent CCI surgery followed by treatment with either 75% xenon:25 % oxygen or 75% nitrogen:25 % oxygen, starting 15 minutes after injury, for 3 hours duration, a third group underwent sham-surgery including anesthesia, skin-incision with exposure of skull, but no craniotomy or TBI impact. The duration of anaesthesia and time between skin incision and suturing was identical to the CCI-surgery group. The sham group received treatment with 75% nitrogen:25 % oxygen for 3 hours. One month after injury we examined performance on the RotaRod to determine whether this revealed a locomotor deficit (Figure 7). There was no difference in Rota-Rod performance between the control-gas treated TBI group or the sham-surgery group or the xenon-treated TBI group, indicating that the RotaRod is not a suitable outcome at this timepoint. We tested the animals using the CatWalk-XT gait-analysis system in order to determine whether more subtle measures of locomotor function would reveal a deficit. There was a significant (p<0.05) deficit in locomotor running-speed in the control TBI-group (43 ± 3 cm/sec) compared to the sham-surgery group (53 ± 3 cm/sec). Xenon-treatment improved the deficit in running speed (50 ± 2 cm/sec); there was no significant difference (p=0.65) between the xenon-treated TBI group and the sham-surgery group (see Figure 8A). We next examined the base-of-support for front and hind paws (Figure 8B). The base-of-support (BOS) measures distance between mid-points of the front paws or rear paws during locomotion. There was no change in the BOS of the front paws. However, there was a trend to increase in the BOS of the rear paws in control-TBI group (33 ± 4 mm) compared to sham-operated animals (24 ± 4 mm), but this was not significant. Xenon-treatment prevented the increase in BOS; there was no significant difference between the xenon-treated TBI group (25 ± 4 mm) and the sham-surgery group. The increase in BOS observed in the rear paws is consistent with the observation in the short-term neuroscore results that faults in rear-paw placement occur. We observed a trend towards a reduction in cadence (Figure 8C) in the TBI-control group compared to sham-surgery group (p=0.06). This trend was absent in the xenon-treated TBI group. To determine whether there were specific locomotor deficits in particular limbs we examined the swing speed for each limb (Figure 8D). Swing speed is calculated from stride-length and time between paw placements for each limb. We found there was a locomotor deficit for each paw in the TBI-control group compared to sham-surgery group. This was significant (p<0.05) for all paws except the hind left which showed a trend (p=0.06). Interestingly, the deficit in the right hind paw was the largest (22 ± 2% reduction) and this was highly significant (p<0.01). Xenon-treatment prevented the locomotor deficit; in all cases the xenon-treated TBI group was not significantly different to the sham-surgery group.
Figure 7.
Performance on RotaRod does not reveal motor deficit. There was no difference in Rotarod performance between sham-operated animals, CCI injury or CCI injury with 75% xenon treatment for 3 hours duration, starting at 15 minutes after trauma. The test was performed 1 month after injury or sham-surgery. Bars represent mean values and error bars are standard errors (n=10, sham; n=20, TBI; n=20, TBI + xenon).
Figure 8.
Xenon improves locomotor deficits 1 month after injury. CatWalk-XT gait-analysis tests reveals deficits in locomotor speed, base of support and swing speed that are prevented by treatment with 75% xenon for 3 hours duration, starting at 15 minutes after trauma. A) There is a significant reduction in locomotor speed in TBI-injured animals compared to sham-operated controls. Xenon-treated TBI-injured animals are not significantly different to sham-operated controls. B) Base of support (average separation) of the hind paws was increased in the TBI group compared to sham-operated controls or xenon-treated TBI group, but this did not reach significance. C) Cadence was reduced in the TBI group compared to sham-surgery group, but this did not reach significance (p=0.06). D) Analysis of individual limb speed shows a significant reduction in TBI-injured animals in front left, front right and right hind limb speed compared to sham-operated animals. Xenon-treated TBI-injured animals are not significantly different to sham-operated controls. Bars represent mean values of sham (white), TBI-injury (black) and xenon-treated TBI injury groups (grey). The error bars are standard errors (n=9 sham, n=19, TBI, n=19 TBI + xenon). * = p<0.05, ** = p<0.01 compared to sham-operated control.
DISCUSSION
The findings presented here are the first to show that xenon is neuroprotective in an animal model of traumatic brain injury. We show that xenon treatment improves functional neurological outcome and reduces secondary injury development, even when commencement of the xenon treatment is delayed 1-3 hours following trauma. Our findings are consistent with previous in vitro studies showing xenon is neuroprotective in an in vitro model of traumatic brain injury [43, 44], using mouse organotypic hippocampal slice cultures subjected to focal mechanical injury and with cell-injury quantified by propidium-iodide fluorescence. Such in vitro models are useful in screening putative neuroprotectants and understanding their mechanism of action [44], but cannot model behavioral neurological outcome. Hence, it is important to test putative neuroprotectants such as xenon in pre-clinical models that allow assessment of improvements in functional neurological outcome at clinically relevant timepoints.
Experimental model
We investigated the neuroprotective efficacy of the noble gas xenon in an in vivo mouse model of traumatic brain injury. We used male animals to exclude effects of the estrous cycle and any potential confounding neuroprotective effects of female sex hormones. The rodent CCI model we used has been widely used in pre-clinical TBI studies [45-52] and is highly reproducible. In our experiments with xenon we chose to investigate the effects of xenon on both acute outcome 1-5 days after injury and long term outcome 1 month after injury.
Xenon pre-treatment and post-treatment
In both in vitro and in vivo models of ischemic injury there is evidence that xenon treatment before injury protects neuronal and cardiac tissue [53-61]. We reasoned that giving xenon both before and after traumatic injury would be protective. Our results show that 75% xenon:25% oxygen given for 2 hours immediately before injury and then for a further 2 hours after trauma significantly improves neurological outcome (p<0.05) and reduces contusion volume (p<0.01) 24 hours after injury. It is possible that xenon could be used before traumatic injury, e.g. in neurosurgical procedures, to minimize damage to neighboring healthy tissue. Nevertheless, in an accidental TBI, such as a motor-vehicle crash, xenon pre-treatment would obviously not be possible.
Xenon post-treatment
The main objective of our study was to evaluate xenon’s potential as a treatment for TBI in clinically relevant settings. We therefore determined the effect of xenon treatment, when xenon was given only after the trauma. We chose a xenon treatment time of 3 hours based on previous studies with xenon in in vivo ischemic injury models [30, 62]. We chose 15 minutes post-injury as the earliest start time for xenon treatment, based on a scenario that a TBI patient could receive medical attention at the scene of injury within 15-20 minutes. Our results show that giving xenon for 3 hours after injury results in a significant (p<0.05) reduction in contusion volume when xenon was given at concentrations of 30%, 50% or 75%.
Therapeutic time-window for xenon treatment
One of the most important considerations for a potential treatment for TBI is whether there is a clinically relevant time-window within which treatment is effective. We investigated the effect of delaying xenon treatment until 1 hour, 3 hours or 6 hours after injury. When xenon treatment was delayed up to 3 hours after injury, there was a significant (p<0.05) reduction in contusion volume, but delaying treatment for 6 hours did not result in reduction of contusion. Our findings also show a significant (p<0.05) improvement in neurological outcome when treatment is delayed up to at least 1 hour after injury.
Effect of xenon up to 5 days after trauma
The untreated-TBI animals showed an improvement in neurological outcome over the course of 5 days. This underlying improvement in control neuroscore may reflect both an recovery in locomotor function and a component of learning on successive test days. Nevertheless, on each measurement day, the xenon-treated group had an better neurological score, by 42% on average, compared to the un-treated group. The improvement with xenon-treatment was significant (p<0.05) up to day 4 after injury. The neurological severity score measurement we used has been shown to be a good predictor of long-term outcome and to correlate with injury severity assessed by MRI [42].
Locomotor outcomes 1 month after injury
In human TBI patients long-term impairments have the greatest financial and social cost. In order that our results have clinical relevance we examined the effect of xenon-treatment on behavioral outcomes 1 month after traumatic injury. TBI patients frequently report postural instability that persists years after the trauma [63, 64]. Studies using analysis of gait in TBI patients have revealed deficits compared to healthy controls. The CatWalk-XT gait-analysis system has been used to measure deficits in a variety of rodent brain-injury models [65-68]. Gait analysis one month after injury revealed significant (p<0.05) deficits in the TBI group, in locomotor speed and in the swing speed of all four limbs. Remarkably, none of these deficits were present in the xenon-treated TBI group. We also observed a trend to increase in base of support of hind limbs in the TBI group that was absent in the xenon treated group. These findings are of particular clinical relevance because deficits in walking speed and base of support are observed in TBI patients [69-71]. A recent systematic review of studies in TBI patients found that in all studies that reported speed, TBI patients walked more slowly than healthy controls [72]. Our results indicate that xenon-treatment may prevent these persistent locomotor deficits in TBI patients.
Clinical relevance of these findings
Xenon has many properties of an ideal general anesthetic [20], but its widespread use has been limited, in part due to its cost. Since the discovery that xenon was an NMDA receptor antagonist [24] and the subsequent demonstration that xenon has neuroprotective properties in models of ischemic injury [25-34] there has been a resurgence of interest in the use of xenon as a neuroprotectant. Clinical trials are currently evaluating xenon in different types of ischemic injury (e.g. neonatal asphyxia, brain damage after cardiac arrest [35, 36]). In this study we have shown for the first time that xenon is protective against blunt-traumatic brain injury, in the controlled cortical impact rodent model. Our results show that xenon treatment reduces secondary injury, improves neurological outcome both acutely and 1 month after injury, and that xenon has a clinically relevant therapeutic time-window of up to 3 hours. The fact that xenon is effective at a concentration of 30% is particularly relevant to the treatment of patients with severe TBI where it may be necessary to give increased concentrations of oxygen. The duration of xenon treatment we used is relatively short (3 hours) and it is possible, indeed likely, that extending this treatment time would result in a greater degree of neuroprotection and/or a longer therapeutic time window. A recent clinical study in cardiac-arrest patients has shown that it is practical to give xenon to patients in the ITU for up to 24 hours [36]. Our findings support the idea that xenon could provide a realistic first-line treatment for brain-trauma patients and further research in this area is warranted.
ACKNOWLEGEMENTS
We thank Frida Kornes, laboratory manager, Department of Anesthesiology, Medical Center of Johannes Gutenberg University, Mainz, Germany, for technical support, Prof. William Wisden, Professor of molecular neuroscience, Department of Life Sciences, Imperial College London, for the use of the Leica cryostat, Carina Freidrich, medical student, Johannes Gutenberg University, Mainz, Germany, and Dr Valentina Ferretti, postdoctoral researcher, Imperial College London for advice on cryo-sectioning and histology.
This work was supported by: European Society for Anaesthesiology, Brussels, Belgium, The Association of Anaesthetists of Great Britain & Ireland, London, United Kingdom. The Royal College of Anaesthetists, London, United Kingdom. Royal Centre for Defence Medicine, Birmingham, United Kingdom, Medical Research Council, London, United Kingdom. Deutscher Akademischer Austauschdienst (DAAD), German Academic Exchange Service, Bonn, Germany. Rita Campos-Pires is the recipient of a doctoral training award from the Fundação para a Ciência e a Tecnologia, Lisbon, Portugal. Scott Armstrong is the recipient of a studentship from the Medical Research Council, London, United Kingdom (G G1000390-1/1).
Footnotes
Conflict of interest statement: Professor Franks has an interest as a named inventor on patents covering the use of xenon as a neuroprotectant.
REFERENCES
- 1.Bruns J., Jr. Hauser, WA: The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–6. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 3.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002 - 2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 5.Martin D, Smith M. Medical management of severe traumatic brain injury. Hosp Med. 2004;65:674–80. doi: 10.12968/hosp.2004.65.11.17047. [DOI] [PubMed] [Google Scholar]
- 6.Smith M. Monitoring intracranial pressure in traumatic brain injury. Anesth Analg. 2008;106:240–8. doi: 10.1213/01.ane.0000297296.52006.8e. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson PJ, Kolias AG, Czosnyka M, Kirkpatrick PJ, Pickard JD, Menon DK. Intracranial pressure monitoring in severe traumatic brain injury. Bmj. 2013;346:f1000. doi: 10.1136/bmj.f1000. [DOI] [PubMed] [Google Scholar]
- 8.Teig M, Smith M. Where should patients with severe traumatic brain injury be managed? All patient should be managed in a neurocritical care unit. J Neurosurg Anesthesiol. 2010;22:357–9. doi: 10.1097/ANA.0b013e3181f0dada. [DOI] [PubMed] [Google Scholar]
- 9.Janowitz T, Menon DK. Exploring new routes for neuroprotective drug development in traumatic brain injury. Sci Transl Med. 2010;2:27rv1. doi: 10.1126/scitranslmed.3000330. [DOI] [PubMed] [Google Scholar]
- 10.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–9. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KK, Larner SF, Robinson G, Hayes RL. Neuroprotection targets after traumatic brain injury. Curr Opin Neurol. 2006;19:514–9. doi: 10.1097/WCO.0b013e3280102b10. [DOI] [PubMed] [Google Scholar]
- 12.Warner DS, Borel CO. Treatment of traumatic brain injury: one size does not fit all. Anesth Analg. 2004;99:1208–10. doi: 10.1213/01.ANE.0000139930.04010.0D. [DOI] [PubMed] [Google Scholar]
- 13.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–22. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teasdale GM, Graham DI. Craniocerebral trauma: protection and retrieval of the neuronal population after injury. Neurosurgery. 1998;43:723–37. doi: 10.1097/00006123-199810000-00001. discussion 737-8. [DOI] [PubMed] [Google Scholar]
- 15.Bullock R, Zauner A, Myseros JS, Marmarou A, Woodward JJ, Young HF. Evidence for prolonged release of excitatory amino acids in severe human head trauma. Relationship to clinical events. Ann N Y Acad Sci. 1995;765:290–7. doi: 10.1111/j.1749-6632.1995.tb16586.x. [DOI] [PubMed] [Google Scholar]
- 16.Kochanek PM, Clark RS, Ruppel RA, Adelson PD, Bell MJ, Whalen MJ, Robertson CL, Satchell MA, Seidberg NA, Marion DW, Jenkins LW. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr Crit Care Med. 2000;1:4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson P, Hillered L, Ponten U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10:631–7. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 18.Cullen SC, Gross EG. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951;113:580–582. doi: 10.1126/science.113.2942.580. [DOI] [PubMed] [Google Scholar]
- 19.Pittinger CB, Moyers J, Cullen SC, Featherstone RM, Gross EG. Clinicopathologic studies associated with xenon anesthesia. Anesthesiology. 1953;14:10–17. doi: 10.1097/00000542-195301000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson R, Franks NP. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit Care. 2010;14:229. doi: 10.1186/cc9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong SP, Banks P, McKitrick TJW, Geldart CH, Babla R, Simillis C, Franks NP, Dickinson R. Identification of two mutations (F758W & F758Y) in the N-Methyl-D-Aspartate receptor glycine-binding site that selectively prevent competitive inhibition by xenon without affecting glycine binding. Anesthesiology. 2012;117:38–47. doi: 10.1097/ALN.0b013e31825ada2e. [DOI] [PubMed] [Google Scholar]
- 22.de Sousa SLM, Dickinson R, Lieb WR, Franks NP. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–67. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 24.Franks NP, Dickinson R, de Sousa SLM, Hall AC, Lieb WR. How does xenon produce anaesthesia? Nature. 1998;396:324. doi: 10.1038/24525. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm S, Ma D, Maze M, Franks NP. Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology. 2002;96:1485–91. doi: 10.1097/00000542-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 26.Ma D, Wilhelm S, Maze M, Franks NP. Neuroprotective and neurotoxic properties of the ‘inert’ gas xenon. Br J Anaesth. 2002;89:739–46. [PubMed] [Google Scholar]
- 27.Banks P, Franks NP, Dickinson R. Competitive Inhibition at the Glycine Site of the N-methyl-D-aspartate Receptor Mediates Xenon Neuroprotection Against Hypoxia-Ischemia. Anesthesiology. 2010;112:614–22. doi: 10.1097/ALN.0b013e3181cea398. [DOI] [PubMed] [Google Scholar]
- 28.Dingley J, Tooley J, Porter H, Thoresen M. Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxia-ischemia. Stroke. 2006;37:501–6. doi: 10.1161/01.STR.0000198867.31134.ac. [DOI] [PubMed] [Google Scholar]
- 29.Fries M, Nolte KW, Coburn M, Rex S, Timper A, Kottmann K, Siepmann K, Hausler M, Weis J, Rossaint R. Xenon reduces neurohistopathological damage and improves the early neurological deficit after cardiac arrest in pigs. Crit Care Med. 2008;36:2420–6. doi: 10.1097/CCM.0b013e3181802874. [DOI] [PubMed] [Google Scholar]
- 30.Ma D, Hossain M, Chow A, Arshad M, Battson RM, Sanders RD, Mehmet H, Edwards AD, Franks NP, Maze M. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol. 2005;58:182–93. doi: 10.1002/ana.20547. [DOI] [PubMed] [Google Scholar]
- 31.Homi HM, Yokoo N, Ma D, Warner DS, Franks NP, Maze M, Grocott HP. The neuroprotective effect of xenon administration during transient middle cerebral artery occlusion in mice. Anesthesiology. 2003;99:876–81. doi: 10.1097/00000542-200310000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Sheng SP, Lei B, James ML, Lascola CD, Venkatraman TN, Jung JY, Maze M, Franks NP, Pearlstein RD, Sheng H, Warner DS. Xenon neuroprotection in experimental stroke: interactions with hypothermia and intracerebral hemorrhage. Anesthesiology. 2012;117:1262–75. doi: 10.1097/ALN.0b013e3182746b81. [DOI] [PubMed] [Google Scholar]
- 33.Bickler PE, Warren DE, Clark JP, Gabatto P, Gregersen M, Brosnan H. Anesthetic protection of neurons injured by hypothermia and rewarming: roles of intracellular Ca2+ and excitotoxicity. Anesthesiology. 2012;117:280–92. doi: 10.1097/ALN.0b013e318260a7b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preckel B, Weber NC, Sanders RD, Maze M, Schlack W. Molecular mechanisms transducing the anesthetic, analgesic, and organ-protective actions of xenon. Anesthesiology. 2006;105:187–97. doi: 10.1097/00000542-200607000-00029. [DOI] [PubMed] [Google Scholar]
- 35.Azzopardi D, N.J. R, Kapetanakis A, Griffiths J, Rennie JM, Mathieson SR, Edwards AD. Anticonvulsant effect of xenon on neonatal asphyxial seizures. Arch Dis Child Fetal Neonatal Ed. 2013;98:437–9. doi: 10.1136/archdischild-2013-303786. [DOI] [PubMed] [Google Scholar]
- 36.Arola OJ, Laitio RM, Roine RO, Gronlund J, Saraste A, Pietila M, Airaksinen J, Perttila J, Scheinin H, Olkkola KT, Maze M, Laitio TT. Feasibility and Cardiac Safety of Inhaled Xenon in Combination With Therapeutic Hypothermia Following Out-of-Hospital Cardiac Arrest. Critical Care Medicine. 2013 doi: 10.1097/CCM.0b013e31828a4337. July 26 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Coburn M, Sanders RD, Maze M, Rossaint R, Investigators H. The Hip Fracture Surgery in Elderly Patients (HIPELD) study: protocol for a randomized, multicenter controlled trial evaluating the effect of xenon on postoperative delirium in older patients undergoing hip fracture surgery. Trials. 2012;13:180. doi: 10.1186/1745-6215-13-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thal SC, Schaible EV, Neuhaus W, Scheffer D, Brandstetter M, Engelhard K, Wunder C, Forster CY. Inhibition of proteasomal glucocorticoid receptor degradation restores dexamethasone-mediated stabilization of the blood-brain barrier after traumatic brain injury. Crit Care Med. 2013;41:1305–15. doi: 10.1097/CCM.0b013e31827ca494. [DOI] [PubMed] [Google Scholar]
- 39.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–78. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 40.Fox GB, Fan L, Levasseur RA, Faden AI. Sustained sensory/motor and cognitive deficits with neuronal apoptosis following controlled cortical impact brain injury in the mouse. J Neurotrauma. 1998;15:599–614. doi: 10.1089/neu.1998.15.599. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y, Constantini S, Trembovler V, Weinstock M, Shohami E. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma. 1996;13:557–68. doi: 10.1089/neu.1996.13.557. [DOI] [PubMed] [Google Scholar]
- 42.Tsenter J, Beni-Adani L, Assaf Y, Alexandrovich AG, Trembovler V, Shohami E. Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J Neurotrauma. 2008;25:324–33. doi: 10.1089/neu.2007.0452. [DOI] [PubMed] [Google Scholar]
- 43.Coburn M, Maze M, Franks NP. The neuroprotective effects of xenon and helium in an in vitro model of traumatic brain injury. Critical Care Medicine. 2008;36:588–595. doi: 10.1097/01.CCM.0B013E3181611F8A6. [DOI] [PubMed] [Google Scholar]
- 44.Harris K, Armstrong SP, Campos-Pires R, Kiru L, Franks NP, Dickinson R. Neuroprotection against Traumatic Brain Injury by Xenon, but Not Argon, Is Mediated by Inhibition at the N-Methyl-D-Aspartate Receptor Glycine Site. Anesthesiology. 2013 doi: 10.1097/ALN.0b013e3182a2a265. Jul 17 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Faden AI, Fox GB, Di X, Knoblach SM, Cernak I, Mullins P, Nikolaeva M, Kozikowski AP. Neuroprotective and nootropic actions of a novel cyclized dipeptide after controlled cortical impact injury in mice. J Cereb Blood Flow Metab. 2003;23:355–63. doi: 10.1097/01.WCB.0000046144.31247.33. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan PG, Thompson M, Scheff SW. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp Neurol. 2000;161:631–7. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- 47.Dempsey RJ, Baskaya MK, Dogan A. Attenuation of brain edema, blood-brain barrier breakdown, and injury volume by ifenprodil, a polyamine-site N-methyl-D-aspartate receptor antagonist, after experimental traumatic brain injury in rats. Neurosurgery. 2000;47:399–404. doi: 10.1097/00006123-200008000-00024. discussion 404-6. [DOI] [PubMed] [Google Scholar]
- 48.Kroppenstedt SN, Stroop R, Kern M, Thomale UW, Schneider GH, Unterberg AW. Lubeluzole following traumatic brain injury in the rat. J Neurotrauma. 1999;16:629–37. doi: 10.1089/neu.1999.16.629. [DOI] [PubMed] [Google Scholar]
- 49.Faden AI, Fox GB, Fan L, Araldi GL, Qiao L, Wang S, Kozikowski AP. Novel TRH analog improves motor and cognitive recovery after traumatic brain injury in rodents. Am J Physiol. 1999;277:R1196–204. doi: 10.1152/ajpregu.1999.277.4.R1196. [DOI] [PubMed] [Google Scholar]
- 50.Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol Res. 1997;19:334–9. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- 51.Kawamata T, Katayama Y, Maeda T, Mori T, Aoyama N, Kikuchi T, Uwahodo Y. Antioxidant, OPC-14117, attenuates edema formation and behavioral deficits following cortical contusion in rats. Acta Neurochir Suppl. 1997;70:191–3. doi: 10.1007/978-3-7091-6837-0_59. [DOI] [PubMed] [Google Scholar]
- 52.Thal SC, Timaru-Kast R, Wilde F, Merk P, Johnson F, Frauenknecht K, Sebastiani A, Sommer C, Staib-Lasarzik I, Werner C, Engelhard K. Propofol impairs neurogenesis and neurologic recovery and increases mortality rate in adult rats after traumatic brain injury. Crit Care Med. 2014;42:129–41. doi: 10.1097/CCM.0b013e3182a639fd. [DOI] [PubMed] [Google Scholar]
- 53.Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–95. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baumert JH, Hein M, Gerets C, Baltus T, Hecker KE, Rossaint R. The effect of xenon anesthesia on the size of experimental myocardial infarction. Anesth Analg. 2007;105:1200–6. doi: 10.1213/01.ane.0000284697.73471.9c. table of contents. [DOI] [PubMed] [Google Scholar]
- 55.Baumert JH, Hein M, Gerets C, Baltus T, Hecker KE, Rossaint R. The effect of xenon on isoflurane protection against experimental myocardial infarction. J Cardiothorac Vasc Anesth. 2009;23:614–8. doi: 10.1053/j.jvca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 56.Limatola V, Ward P, Cattano D, Gu J, Giunta F, Maze M, Ma D. Xenon preconditioning confers neuroprotection regardless of gender in a mouse model of transient middle cerebral artery occlusion. Neuroscience. 2010;165:874–81. doi: 10.1016/j.neuroscience.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 57.Mio Y, Shim YH, Richards E, Bosnjak ZJ, Pagel PS, Bienengraeber M. Xenon preconditioning: the role of prosurvival signaling, mitochondrial permeability transition and bioenergetics in rats. Anesth Analg. 2009;108:858–66. doi: 10.1213/ane.0b013e318192a520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shu Y, Patel SM, Pac-Soo C, Fidalgo AR, Wan Y, Maze M, Ma D. Xenon pretreatment attenuates anesthetic-induced apoptosis in the developing brain in comparison with nitrous oxide and hypoxia. Anesthesiology. 2010;113:360–8. doi: 10.1097/ALN.0b013e3181d960d7. [DOI] [PubMed] [Google Scholar]
- 59.Weber NC, Frassdorf J, Ratajczak C, Grueber Y, Schlack W, Hollmann MW, Preckel B. Xenon induces late cardiac preconditioning in vivo: a role for cyclooxygenase 2? Anesth Analg. 2008;107:1807–13. doi: 10.1213/ane.Ob013e31818874bf. [DOI] [PubMed] [Google Scholar]
- 60.Weber NC, Toma O, Damla H, Wolter JI, Schlack W, Preckel B. Upstream signaling of protein kinase C-epsilon in xenon-induced pharmacological preconditioning. Implication of mitochondrial adenosine triphosphate dependent potassium channels and phosphatidylinositol-dependent kinase-1. Eur J Pharmacol. 2006;539:1–9. doi: 10.1016/j.ejphar.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 61.Yang T, Zhuang L, Rei Fidalgo AM, Petrides E, Terrando N, Wu X, Sanders RD, Robertson NJ, Johnson MR, Maze M, Ma D. Xenon and sevoflurane provide analgesia during labor and fetal brain protection in a perinatal rat model of hypoxia-ischemia. PLoS One. 2012;7:e37020. doi: 10.1371/journal.pone.0037020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin JL, Ma D, Hossain M, Xu J, Sanders RD, Franks NP, Maze M. Asynchronous administration of xenon and hypothermia significantly reduces brain infarction in the neonatal rat. Br J Anaesth. 2007;98:236–40. doi: 10.1093/bja/ael340. [DOI] [PubMed] [Google Scholar]
- 63.Anderson SI, Wilson CL, McDowell IP, Pentland B, Gray JM, Robertson IH. Late rehabilitation for closed head injury: a follow-up study of patients 1 year from time of discharge. Brain Inj. 1996;10:115–24. doi: 10.1080/026990596124601. [DOI] [PubMed] [Google Scholar]
- 64.Masson F, Maurette P, Salmi LR, Dartigues JF, Vecsey J, Destaillats JM, Erny P. Prevalence of impairments 5 years after a head injury, and their relationship with disabilities and outcome. Brain Inj. 1996;10:487–97. doi: 10.1080/026990596124205. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Ao LJ, Lu G, Leong E, Liu Q, Wang XH, Zhu XL, Sun TF, Fei Z, Jiu T, Hu X, Poon WS. Quantitative gait analysis of long-term locomotion deficits in classical unilateral striatal intracerebral hemorrhage rat model. Behav Brain Res. 2013;257:166–77. doi: 10.1016/j.bbr.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Mountney A, Leung LY, Pedersen R, Shear D, Tortella F. Longitudinal assessment of gait abnormalities following penetrating ballistic-like brain injury in rats. J Neurosci Methods. 2013;212:1–16. doi: 10.1016/j.jneumeth.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 67.Neumann M, Wang Y, Kim S, Hong SM, Jeng L, Bilgen M, Liu J. Assessing gait impairment following experimental traumatic brain injury in mice. J Neurosci Methods. 2009;176:34–44. doi: 10.1016/j.jneumeth.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang XH, Lu G, Hu X, Tsang KS, Kwong WH, Wu FX, Meng HW, Jiang S, Liu SW, Ng HK, Poon WS. Quantitative assessment of gait and neurochemical correlation in a classical murine model of Parkinson’s disease. BMC Neurosci. 2012;13:142. doi: 10.1186/1471-2202-13-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basford JR, Chou LS, Kaufman KR, Brey RH, Walker A, Malec JF, Moessner AM, Brown AW. An assessment of gait and balance deficits after traumatic brain injury. Arch Phys Med Rehabil. 2003;84:343–9. doi: 10.1053/apmr.2003.50034. [DOI] [PubMed] [Google Scholar]
- 70.Niechwiej-Szwedo E, Inness EL, Howe JA, Jaglal S, McIlroy WE, Verrier MC. Changes in gait variability during different challenges to mobility in patients with traumatic brain injury. Gait Posture. 2007;25:70–7. doi: 10.1016/j.gaitpost.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Williams G, Morris ME, Schache A, McCrory PR. Incidence of gait abnormalities after traumatic brain injury. Arch Phys Med Rehabil. 2009;90:587–93. doi: 10.1016/j.apmr.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Williams G, Galna B, Morris ME, Olver J. Spatiotemporal deficits and kinematic classification of gait following a traumatic brain injury: a systematic review. J Head Trauma Rehabil. 2010;25:366–74. doi: 10.1097/HTR.0b013e3181cd3600. [DOI] [PubMed] [Google Scholar]