Abstract
Objectives
To assess the performance of thiol-ene dental composites based on selected ester-free thiol-ene formulations. Further, to point out the benefits/drawback of having a hydrolytically stable thiol-ene matrix within a glass filled composite.
Methods
Composite samples containing 50–65 wt% of functionalized glass microparticles were prepared and photopolymerized in the presence of a suitable visible light photoinitiator. Shrinkage stress measurements were conducted as a function of the irradiation time. Degrees of conversion were measured by FT-IR analysis by comparing the double bond signals before and after photopolymerization. Mechanical tests were carried out on specimens after curing as well as after extended aging in water. Dynamic mechanical analysis was employed to track the changes in storage modulus near body temperature. The properties of the thiol-ene composites were compared with those of the BisGMA/TEGDMA control.
Results
Depending on the resin type, similar or higher conversions were achieved in thiol-ene composites when compared to the dimethacrylate controls. At comparable conversions, lower shrinkage stress values were achieved. Although exhibiting lower initial elastic moduli, the thiol-ene composite’s flexural strengths were found to be comparable with the controls. Contrary to the control, the mechanical properties of the ester-free thiol-ene composites were shown to be unaffected by extensive aging in water and at least equaled that of the control after aging in water for just five weeks.
Significance
Employing non-degradable step-growth networks as organic matrices in dental composites will provide structurally uniform, tough materials with extended service time.
Keywords: photopolymerization, dental composites, thiol-ene resins, shrinkage stress
1. Introduction
Most commercial dental restorative materials are based on dimethacrylate resins, which undergo rapid crosslinking polymerizations to form mechanically strong, functional glasses [1–5]. Such in situ formed glassy matrices are perfect to bind inorganic filling particles to result in restorative composites whose sole purpose is to match the aesthetic and mechanical characteristics of enamel. Impressively, this has not been without success since these composites have been continually commercialized for over 40 years. Despite tremendous progress made in the development of dimethacrylate dental formulations, some of the attributes of chain-growth crosslinking polymerizations that lead to the composite’s failure cannot be entirely eliminated [1,5,6]. Although it is advantageous from the clinical standpoint, the instantaneous transition from liquid to solid state as associated with early gelation and the subsequent onset of reaction diffusion controlled network formation [7] hinder the viscous flow at later stages of polymerization, which contributes to stress development at bonded interfaces [8,9]. Additionally, free volume reduction during covalent linking between monomers causes high contraction, which is proportional to the functional group conversion and strongly coupled to the desired modulus increase [10]. Ideally, low shrinkage, little or no internal stress, homogenous structure, quantitative conversions, and high elastic moduli would all be desired in a dental composite. As many of these parameters are interrelated in a contradictive manner [11], a compromise has to be identified to maximize the performance and service life of a composite restoration. It should be mentioned, that it is not just the initial mechanics that are essential but also the composite’s longterm resistance to a moist environment that will impact its suitability and performance. Swelling, leaching, and degradation over time have always been a significant concern in any composite evaluation process [12–15]. A multitude of approaches have been undertaken to improve subsequent generations of dimethacrylate composites. Urethane dimethacrylates and other low-shrinkage resins have been developed by synthesis of high molecular weight monomers and oligomers, or alternatively by employing a ring-opening polymerization mechanism, to reduce the shrinkage [16–19], . In recent years chain transfer reactions have been implemented within methacrylate polymerizations by thiol and ene inclusion [20–22] or specifically designed addition fragmentation monomers have been considered [23,24]. This study, however, focuses on neat thiol-ene composite mixtures, which have been previously considered for dental applications [25,26]. Since their first consideration as dental materials 10 years ago, significant progress has been made in the development of thiol-ene dental resins [22,27–29]. Despite all the benefits of step-growth crosslinking reactions such as high conversion, low shrinkage/shrinkage stress, and uniform network structure, pure thiol-ene materials have for the most part been impractical for dental restorative materials owing to insufficient mechanical integrity caused by the presence of soft thioether moieties.
Recently, it has been shown that the mechanics of the flexible step-growth polymer backbone containing soft sulfide linkages can be enhanced significantly in poly(thioether) networks by eliminating the presence of esters [30–33]. Achieving that, and combining other benefits of step-growth processes has led us toward revisiting the thiol-ene concept for dental composite applications. In an initial investigation [34] we aimed to elucidate the structure-property relations within ester-free thiol-ene mixtures of varied functional group concentrations and viscosities. We detailed the resin development process concentrating on resin polymerization kinetics/control over it, limiting conversions, and mechanical properties. Interestingly, we showed a three-fold reduction in water uptake of the unfilled polymers, whose apparent hydrophobicity should aid immensely in preserving the composite properties in a moist environment. We also pointed out that high functional group concentrations in the resin consisting of low molecular weight monomers of functionalities 3 and higher can indeed lead to high modulus glasses, which at the same time may exhibit high shrinkage stress. Sometimes this shrinkage stress even exceeds that of conventional the BisGMA/TEGDMA dimethacrylate control, albeit at significantly higher functional group conversions. Again a compromise has to be reached to fine-tune the resin composition and by that the final composite properties. Most importantly, out of a number of tested resins, promising candidates were preselected to be evaluated as dental composites loaded with inorganic filler particles.
Herein, in the second part of our investigation, we conducted studies on ester-free thiol-ene composite materials focusing on composite property development such as toughness, elastic modulus, shrinkage stress, limiting functional group conversion, etc. We referenced our findings to a dimethacrylate control composed with the same filler loading as the thiol-ene systems, and cured at the same curing conditions. We detailed the procedures for adequate filler silanization with thiol, and ene functionalities to enhance the bonding at the filler-resin interface in thiol-ene composites. Finally, extended water treatment studies were undertaken to assess the long-term effects of water-induced swelling on the composite’s mechanical response.
2. Materials and methods
2.1. Materials
2,2-Bis[4-(2-hydroxy-3-methacrylyloxypropoxy)phenyl] propane (Bis-GMA) and triethylene glycol dimethacrylate (TEGDMA) (Esstech, Essington, PA) were purchased from Esstech (Essington, PA) as a premixed monomer mixture in 70:30 mass ratio. Triallyl-1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (TTT), hexamethylene diisocyanate (HMDI), and triethylamine (TEA) were purchased from Sigma Aldrich. Divinyl sulfone (DVS) was purchased from Oakwood Chemicals, and Irgacure 819 (bis(2,4,6-trimethylbenzoyl )- phenylphosphineoxide) was obtained from BASF. Schott glass (mean particle size 0.4 μm) untreated as well as surface treated with a coating of γ-methacryloxypropyltrimethoxysilane, were used as the inorganic fillers (Esstech). Prior to implementation and as described later, these fillers were subsequently functionalized with thiol and/or allyl groups for inclusion and copolymerization in the composite. Tetra(2-mercaptoethyl)silane (SiTSH) and the urethane-based tetraallylether monomer (TENE) were both synthesized according to previously reported procedures [29,33,35]. The structures of DVS-activated thiol monomers as well as the other monomers and solvents used are detailed elsewhere [34].
2.2. Filler functionalization
A typical procedure for glass particles silanization is as follows: 40 g of silica particles (Schott, 4.0 μm) were first taken in a glass tube and heated at 165°C under vacuum using a Buchi heater/condenser for 3 hours. The dried microparticles were then transferred to a 250 ml bottom rounded flask containing 800 ml of dry toluene supplemented with 12 ml of either 3-mercaptopropyltrimethoxysilane or allyltrimethoxysilane pre-reacted for 2 hours with 0.8 g of n-propylamine. The reaction mixture was then left under stirring (24 hours) for silanization. After particle functionalization, the liquid suspension was centrifuged and the solid pellets collected thoroughly, and washed with toluene (4X) and methylene chloride (3X) in two separate washing/centrifugation cycles. Finally, the washed filler particles were dried under vacuum overnight at 70 °C. The thiol and allyl functionalized fillers were analyzed by FT-IR spectroscopy and thermogravimetry (TG). The 0.5 wt% mass loss difference between silanized and unfunctionalized fillers suggests successful functional group grafting on the surface of glass particles in each case (see supporting information Fig. S1). Also, the DRIFT FT-IR characterization provides evidence of silanol group disappearance around 3745 cm−1, implying successful surface modification (Fig. S2). Appropriately silanized fillers and resins were blended in a speedmixer (DAC 150 FVZ, Flakteck) to ensure homogenous formulations at 50/50 and 35/65 resin/filler wt % ratios.
2.3. Flexural tests and conversion analysis
Flexural properties of the composites were assessed in a three-point bending configuration (MTS 858 Mini Bionix II). Composite sample dimensions were 2/2.5/10 mm (n=5). Samples sandwiched between two glass slides were irradiated on both sides (5 min on each side) to ascertain uniform conversions throughout the sample thickness. All composite formulations were irradiated with visible light in the range of 400–500 nm, and at an irradiance of 30–50 mW/cm2. Conversions were analyzed in near-IR experiments by comparing the double bond peak area before and immediately after curing. In each case the curing was performed in the presence of 1 wt % of visible light photoinitiator, which was IR 819. Flexural tests were performed one week after curing, and/or after additional seven days during which time the specimen were treated with deionized water at 37–38 °C.
2.4. Shrinkage stress measurement
Polymerization shrinkage stress was measured with a tensometer device manufactured at the Paffenberger Research Center of the American Dental Association Health Foundation (ADAHF-PRC, Gaithersburg, MD). This device is based on cantilever beam theory and measures the tensile force generated by the shrinking sample, which causes the cantilever beam to deflect. Shrinkage stress is obtained by dividing the shrinkage force by the cross-sectional area of the disk-shaped sample (2.5 mm thick by 5.0 mm diameter). As the specimens tested were the particle-filled resins, the changes in opacity of the 5 mm in diameter discs during reaction did not allow for real time monitoring of the polymerization conversion. Therefore, the shrinkage stress is plotted as a function of time. The values of ultimate conversions were obtained on MTS samples analyzed before and after irradiation by utilizing near-infrared (NIR) spectroscopy coupled with a fiber optic remote sensing technique. In the methodology of shrinkage stress measurements a composite sample was placed between two cylindrical glass rods that usually are treated beforehand with a methacrylate functional silane to promote bonding at the glass surface/resin interface. However, for the thiol-ene composites the glass rods were treated with mercaptoproplytrimethoxysilane/n-propylamine mixture in a 10:1 weight ratio to promote sulfide formation and bonding at the rod surface/thiol-ene resin interface. This was necessary because methacrylate silanization did not provide sufficient bonding and delamination occurred, preventing an accurate shrinkage stress measurement from being completed. For each composition, experiments were performed in triplicate.
2.5. Dynamic mechanical analysis
Dynamic mechanical analysis (DMA) was performed on a TA Q800 instrument. The conditions for sample curing were the same as for flexural testing. The composite samples were of rectangular shape (2/2.5/6 mm). Temperature scans were performed over 10–160/200 °C with a heating rate of 3 °C/min at a frequency of 1 Hz. The loss and storage moduli were recorded as a function of temperature for the first and second heating ramp. Glass transition was taken at the maximum of tan delta versus temperature curve.
2.6. Statistical analysis
The experimental results were analyzed in a one-way analysis of variance (ANOVA) based on n-number of specimens: FTIR (n=5), shrinkage stress (n=3), flexural modulus and strength testing (n=5), DMA (n=3). Multiple pair-wise comparisons were further conducted using Tukey’s test with a significance level of 0.05.
3. Results and discussion
Here, two types of dental composites were assessed for their properties. Based on viscosity differences between sulfone-containing thiol-ene mixtures (viscosity range 0.3–2.1 Pas) and mixtures of thiol-ene neat monomers (viscosity range 45–120 mPas), composites containing either 50 or 65 wt % of inorganic microparticles were prepared. Table 1 contains detailed descriptions, along with the abbreviations subsequently used for both types of composite formulations.
Table 1.
Description of the content for the composite formulations, and the abbreviations used throughout the text.
| DVS-containing thiol-ene resins filled with 50 wt % of allylated particles. | |
| TEOC 01 | A stoichiometric mixture of 4 M SiTSH (pre-reacted with 1 M DVS) and 4.67 M TTT |
| TEOC 02 | A stoichiometric mixture of 6M SiTSH and 3 M TTTSH (pre-reacted with 3 M DVS) and 9 M TTT |
| BisGMA/TEGDMA (70/30) | Methacrylate control (50 wt% methacrylated particles) |
| Thiol-ene mixtures of neat monomers filled with 65 wt % of thiolated and allylated particles in a 50/50 weight ratio. | |
| TEC 01 | A stoichiometric mixture of SiTSH and TTT |
| TEC 02 | A stoichiometric mixture of SiTSH, TTT and TENE where the TENE content is 15 wt% |
| TEC 03 | A stoichiometric mixture of SiTSH, TTT and TENE where the TENE content is 25 wt% |
| TEC 04 | A stoichiometric mixture of SiTSH/TTTSH (1M/1M) and TTT |
| BisGMA/TEGDMA (70/30) | Methacrylate control (65 wt% methacrylated particles) |
Because of the incompatibility between the resin and the thiol-functionalized filler, oligomeric resins based on DVS were premixed with allylated fillers only, whereas the neat thiol-ene monomers were premixed with a 50/50 mixture of both types of silanized particles (Table 1). No stoichiometric adjustments associated with the filler modifications were made within the resin in either case, as the organic layer on the glass fillers was calculated to be less than 1 wt % of the thiol-ene resin loading.
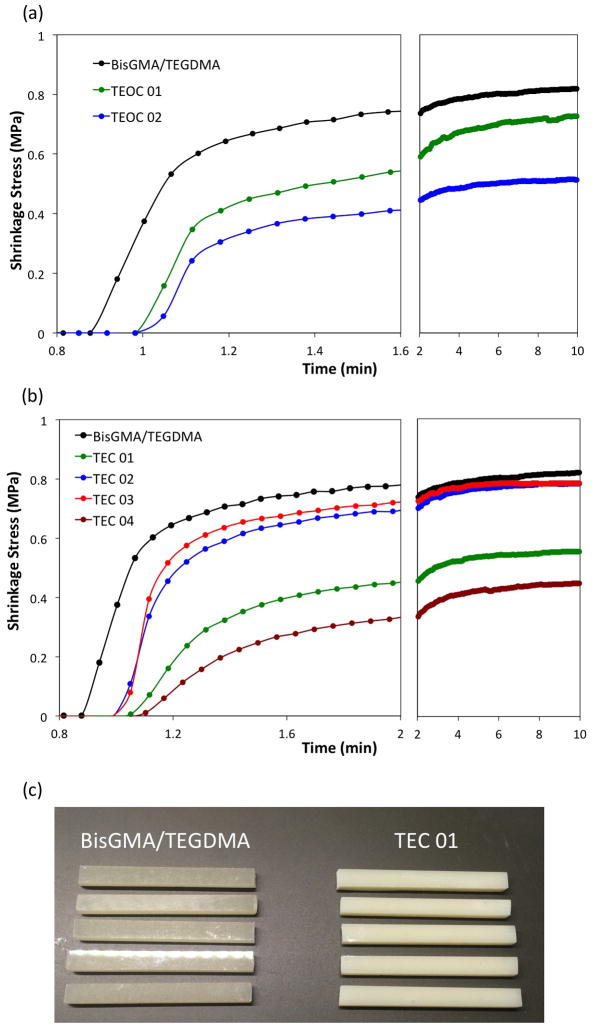
The filled thiol-ene formulations as well as two methacrylate controls (with 50 and 65 wt % particle loading) were subjected to various analyses. The evolution of shrinkage stress as a function of time is depicted for both resin types in Fig. 1.
Figure 1.
Exemplary plots of shrinkage stress evolution as a function of irradiation time: (a) DVS-containing thiol-ene resins filled with 50 wt% of allylated microparticles; (b) a mixture of thiol and ene monomers with 65 wt % microparticle loading; (c) a picture of composite samples TEC 01 and BiGMA/TEDGMA control. The composites were cured with 1 wt% IR 819, 50 mW/cm2, and 400–500 nm wavelength at ambient conditions.
Associated with the refractive index increase during photopolymerization, a loss in transparency is observed that renders the monitoring of conversion impossible in real time. Due to the high sulfur content and associated higher refractive index, the change in opacity is even more significant in thiol-ene resins as can be seen from the Fig. 1c, which depicts TEC 01 and BisGMA/TEGDMA composite samples. However, scattering of light does not hinder the photopolymerization process as indicated by the glassy nature of the composite materials as well as conversion analysis after curing of the rectangularly-shaped specimens prepared for the flexure tests. The latter analysis resulted in either similar (oligomeric resins) or higher conversions (neat monomeric mixtures) for the thiol-ene formulations as compared with the glass-filled methacrylate control composite. Interestingly, the oligomeric TEOC-based composites exhibit significantly lower shrinkage stress values than the methacrylate control (Fig. 1a and Table 2). This is not surprising because in step-growth polymerization mechanisms the Flory-Stockmayer equation predicts usually much lower gel point conversions for systems comprised of reactive oligomer species (as “pre-polymers”) than for those based exclusively on multifunctional monomers. The latter also form crosslinked polymer networks with less shrinkage stress development than the chain-growth photopolymerization of methacrylates [36].
Table 2.
Mechanical results, ultimate conversions and shrinkage stress values for DVS-based thiol-ene composites filled with 50 wt% of allylated microparticles.
| Composite | Strain at Break (%) | Young’s Modulus (GPa) | Flexural Strength (MPa) | Toughness (J·m−3·104) | Final Conv. (MTS Samples) (%) | Final Shrinkage Stress (MPa) |
|---|---|---|---|---|---|---|
| BisGMA/TEGDMA | 1.3 (0.2)A | 6.6 (0.2)A | 87 (6)A | 65 (18)A | 64 (3)A | 0.81 (0.07)A |
| TEOC 01 | 2.0 (0.2)B | 4.3 (0.3)B | 75 (2)A | 85 (17)A | 59 (1)A | 0.63 (0.02)B |
| TEOC 02 | 3.4 (0.4)C | 3.7 (0.2)C | 84 (11)A | 180 (28)B | 58 (4)A | 0.52 (0.05)B |
The results for the dimethacrylate control (filled with 50 wt % of methacrylated microparticles) are also included. Mechanical tests were performed one week after initial curing with samples aged at ambient conditions. Brackets show standard deviations. Means that do not share a superscript letter are significantly different.
Although, conversion analysis for TEOC composites revealed statistically similar conversions as in the dimethacrylate control, all the values may be subjected to a different amount of error due to the limitations associated with the non-uniform transparency changes in the glass-filled resin systems at hand. On the other hand, the restrictions in viscosities as well as increased average monomer functionalities are the known reasons for limited conversions in viscous resin composite systems [37]. The analysis of shrinkage stress in neat composite resins shows insignificant, although apparently lower, stress values for tetraallyl ether-containing monomers (composites TEC 02 and 03), which most probably have been achieved at higher conversions when compared to the control (Table 3). Further, the TEC 01 composite exhibit lower shrinkage stress than the control, but again the values of conversions from MTS samples reveal generally lower conversions. Considering the shrinkage stress results and separately measured conversions, the most promising system is the TEC 04 composite whose properties are derived from the combined effects of both thiol monomers.
Table 3.
Mechanical results, ultimate conversion and shrinkage stress values for neat thiol-ene composites filled with 65wt% of a mixture of allylated and thiolated microparticles.
| Composite | Strain at Break (%) | Young’s Modulus (GPa) | Flexural Strength (MPa) | Toughness (J·m−3·104) | Conv. (MTS Samples) (%) | Final Shrinkage Stress (MPa) |
|---|---|---|---|---|---|---|
| BisGMA/TEGDMA | 1.1 (0.3)A | 9.7 (0.3)A | 96 (21)A | 55 (28)A | 64 (3)A | 0.83 (0.04)A |
| TEC 01 | 1.6 (0.3)A,B | 7.7 (0.2)B | 100 (8)A | 99 (28)A,B | 66 (7)A | 0.56 (0.03)B |
| TEC 02 | 1.9 (0.5)B,C | 6.6 (0.1)C | 90 (11)A | 106 (50)A,B | 78 (3)B,C | 0.72 (0.11)A |
| TEC 03 | 2.5 (0.4)C | 5.6 (0.2)D | 85 (4)A | 146 (50)B | 86 (1)C | 0.76 (0.06)A |
| TEC 04 | 1.7 (0.6)A,B,C | 8.0 (0.5)B | 98 (16)A | 124 (76)A,B | 77 (6)B | 0.45 (0.02)B |
The results for the dimethacrylate control (filled with 65 wt % of methacrylated microparticles) are also included. Mechanical tests were performed one week after initial curing. Brackets show standard deviations. Means that do not share a superscript letter are significantly different.
Based on the results discussed above, it can be concluded that thiol-ene composites formed from resins with high functional group concentrations may ultimately result in high modulus materials but also with significant shrinkage stress. As was pointed out previously, the step-growth nature of thiol-ene polymerizations and the delayed gel point conversions delay the stress build up until higher conversions, but here, in high modulus systems there is significant stress generated that seems to start building up more significantly with small increments in conversions when ultimate conversions are approached. This behavior is in contrast with chain-growth reactions where shrinkage stress builds up at early stages of polymerization. Such behavior was very clearly depicted on stress-conversion curves in the resin development part of this research [33]. One possible solution to diminish the unfavorable effects of shrinkage stress without sacrificing final conversions is to employ monomers of higher molecular weights, i.e. with low functional group content. Similar approaches were quite common for successful stress reduction in methacrylate-based composites. In the present investigation, this was the reason for using the tetraallyl ether monomer, which possesses a carbamate-aliphatic backbone, conferring both toughness and flexibility properties to the final material, while having a relatively high molecular weight. Indeed, its use led to higher conversions of the thiol-ene resins with significantly reduced shrinkage stress. Conversely, the motivations for using a high density of functional groups within a thiol-ene resin was to achieve a high modulus material, preferably matching that of the BisGMA/TEDGMA control. Mechanical property data for the thiol-ene composites are also tabulated (Table 2 and 3).
From the results in Table 2 and 3, it is evident that thiol-ene composites, although all are high modulus glasses, have still lower Young’s modulus than the controls. Beneficially, the enhanced elastic character of the thiol-ene composites leads to improved strains at break, which combined with statistically insignificant differences in flexural strengths yields an apparent increase in the thiol-ene composite’s toughness. To probe the benefits of ester-free thiol-ene composites further, the TEC series of samples were immersed in deionized water for one week at 37 °C, and than subjected to flexural testing. The relevant data is summarized in Table 4.
Table 4.
Mechanical results for neat thiol-ene resin composites performed after seven days of aging in water at 37 °C.
| Composite | Strain at Break (%) | Young’s Modulus (GPa) | Flexural Strength (MPa) | Toughness (J·m−3 ·104) |
|---|---|---|---|---|
| BisGMA/TEGDMA | 1.3 (0.2)A | 9.2 (0.1)A | 110 (13)A | 82 (24)A |
| TEC 01 | 1.6 (0.2)A,B | 7.4 (0.2)B | 99 (8)A | 95 (21)A |
| TEC 02 | 2.2 (0.3)B,C | 6.5 (0.4)C | 101 (8)A | 152 (21)A,B |
| TEC 03 | 2.5 (1.3)C | 4.0 (0.2)D | 61 (16)B | 198 (74)B |
| TEC 04 | 1.6 (0.2)A,B,C | 8.1 (0.4)E | 105 (9)A | 99 (24)A |
The results for the dimethacrylate control assessed under the same conditions are also included. Brackets show standard deviations. Means in each column that do not share a superscript letter are significantly different.
Interestingly, three out of the four thiol-ene-based composites, i.e. samples designated as TEC 01, 02 and 04, show insignificant variation in the mechanical performance before and after water treatment. The TEC 03 sample evidently has higher content of hydrophilic tetra allyether monomer, hence it exhibits more significant modulus change. It is known that many commercial low-shrinkage dental composites yield composites with 20–35% lower initial values of modulus than the BisGMA-based composites [38]. The clinical conditions for these materials to function would involve a moist environment of mouth with non-acidic, neutral or slightly alkaline pH. In such conditions ester-based and hydrophilic composites will absorb some moisture which will cause an initial modulus decrease, and than start degrading slowly over time, causing further material deterioration with consequent decrease in modulus [39]. Considering this important aspect, a hydrolytically stable material would be desired, i.e. a material that an extended clinical service life would be expected from. To test this hypothesis unequivocally, the three TEC samples that showed no modulus reduction after swelling tests for one week, together with the BisGMA control were evaluated for their storage modulus values after further aging in water for five weeks (Fig. 2).
Figure 2.
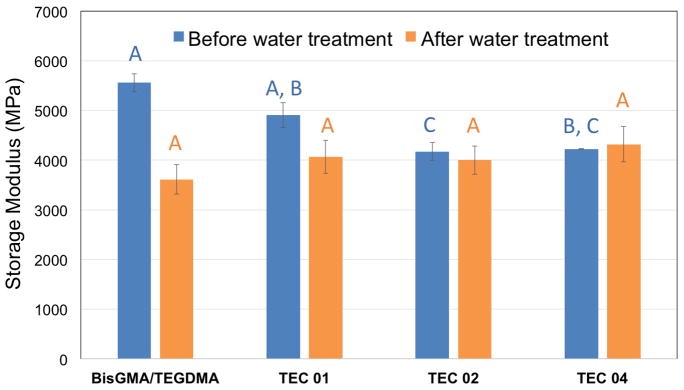
Storage moduli values for selected ester-free thiol-ene composites and the dimethacrylate control determined by DMA at 40°C. The DMA runs were performed on untreated (dry) composite samples as well as on samples after five weeks of aging in deionized water at ambient temperature. The capital letters above the columns designate the degree of significance between the untreated and water treated samples.
In Figure 2, storage moduli at 40°C are plotted before and after 5 weeks of water treatment at ambient conditions. The storage moduli determined close to body temperature show fairly similar trends as the ones found for the elastic modulus from mechanical tests, again with BisGMA/TEGDMA being the highest. However, the differences are less pronounced, and there is no statistical significance between the control and the TEC 01 composite. This distinction may mean that the drop in modulus observed with increasing temperature is more significant for the methacrylate control. More importantly, the values of storage moduli for all the composites are not statistically different after 5 weeks of water treatment. This outcome is a pivotal tendency as it points out the extraordinary stability of ester-free thiol-ene composites in moist environment. For example, swelling studies (in ethanol) revealed 25–75 % reduction in elastic modulus of commercial dental composites [40]. It is therefore presumed, that after initial water sorption the mechanical properties of thiol-ene restorations would not be subject to any further change with time which should extensively improve their service time. On the contrary, a significant reduction in elasticity recorded for the control methacrylate composite, implies a likely deterioration of its properties with time, independent of the potential for degradation of the ester-containing formulation.
4. Conclusion
In summary, ester-free thiol-ene resin composites were prepared and evaluated for their properties. The lack of ester moieties and the high concentrations of functional groups (thiol and ene) has enabled the synthesis of high modulus glasses that possess potential for dental composites. This study proves that high modulus networks, even though formed in by means of a step-growth thiol–ene polymerization mechanism, can face the same limitations as dimethacrylate-based materials such as limited conversion or significant polymerization shrinkage stress. As presented here, the proposed solutions used for thiol-ene property modification/tuning are those that have also worked for dimethacrylate networks.
However, the superiority of thiol-ene systems is evident in that the high elastic modulus (shrinkage stress) development is always accompanied by significantly higher conversions, leaving very little or no unreacted monomers. This behavior is expected to significantly improve the composite’s biocompatibility by reducing the amount of leachable species, which are common in conversion limited dimethacrylate systems. More importantly, the presented materials would not degrade over time, and once implanted, should retain their mechanical integrity unchanged for the composite’s lifetime.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the National Institutes of Health for support of this research through the 1U01DE023777-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. J Dent Res. 2011;90:402–16. doi: 10.1177/0022034510381263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stansbury JW. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent Mater. 2012;28:13–22. doi: 10.1016/j.dental.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferracane JL. Resin composite - state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Cook WD, Beech DR, Tyas MJ. Structure and properties of methaerylate based dental restorative materials. Biomaterials. 1985;6:362–8. doi: 10.1016/0142-9612(85)90094-8. [DOI] [PubMed] [Google Scholar]
- 5.Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–76. [Google Scholar]
- 6.Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25:435–40. doi: 10.1016/s0300-5712(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 7.GO. Principles of polymerization. 4. New York: Wiley-Interscience; 2004. [Google Scholar]
- 8.Lu H, Stansbury JW, Bowman CN. Towards the elucidation of shrinkage stress development and relaxation in dental composites. Dent Mater. 2004;20:979–86. doi: 10.1016/j.dental.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Ferracane JL. Developing a more complete understanding of stresses produced in dental composites during polymerization. Dent Mater. 2005;21:36–42. doi: 10.1016/j.dental.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Boaro LCC, Gonalves F, Guimarães TC, Ferracane JL, Versluis A, Braga RR. Polymerization stress, shrinkage and elastic modulus of current low-shrinkage restorative composites. Dent Mater. 2010;26:1144–50. doi: 10.1016/j.dental.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Shah PK, Stansbury JW. Role of filler and functional group conversion in the evolution of properties in polymeric dental restoratives. Dent Mater. 2014;30:586–93. doi: 10.1016/j.dental.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soderholm KJ, Zigan M, Fischlschweiger WBM. Hydrolytic degradation of dental composites. J Dent Res. 1984;63:1248–54. doi: 10.1177/00220345840630101701. [DOI] [PubMed] [Google Scholar]
- 13.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–51. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 15.Koin PJ, Kilislioglu A, Zhou M, Drummond JL, Hanley L. Analysis of the degradation of a model dental composite. J Dent Res. 2008;87:661–6. doi: 10.1177/154405910808700712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atai M, Ahmadi M, Babanzadeh S, Watts DC. Synthesis, characterization, shrinkage and curing kinetics of a new low-shrinkage urethane dimethacrylate monomer for dental applications. Dent Mater. 2007;23:1030–41. doi: 10.1016/j.dental.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Barszczewska-Rybarek IM. Characterization of urethane-dimethacrylate derivatives as alternative monomers for the restorative composite matrix. Dent Mater. 2014;30:1336–44. doi: 10.1016/j.dental.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Ge J, Trujillo M, Stansbury J. Synthesis and photopolymerization of low shrinkage methacrylate monomers containing bulky substituent groups. Dent Mater. 2005;21:1163–9. doi: 10.1016/j.dental.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Buruiana T, Melinte V, Popa ID, Buruiana EC. New urethane oligodimethacrylates with quaternary alkylammonium for formulating dental composites. J Mater Sci Mater Med. 2014;25:1183–94. doi: 10.1007/s10856-014-5141-4. [DOI] [PubMed] [Google Scholar]
- 20.Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Delayed gelation through chain-transfer reactions: Mechanism for stress reduction in methacrylate networks. Polymer (Guildf) 2011;52:3295–303. doi: 10.1016/j.polymer.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cramer NB, Couch CL, Schreck KM, Boulden JE, Wydra R, Stansbury JW, et al. Properties of methacrylate-thiol-ene formulations as dental restorative materials. Dent Mater. 2010;26:799–806. doi: 10.1016/j.dental.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beigi S, Yeganeh H, Atai M. Evaluation of fracture toughness and mechanical properties of ternary thiol-ene-methacrylate systems as resin matrix for dental restorative composites. Dent Mater. 2013;29:777–87. doi: 10.1016/j.dental.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Leung D, Bowman CN. Reducing shrinkage stress of dimethacrylate networks by reversible addition-fragmentation chain transfer. Macromol Chem Phys. 2012;213:198–204. [Google Scholar]
- 24.Park HY, Kloxin CJ, Abuelyaman AS, Oxman JD, Bowman CN. Stress Relaxation via Addition–Fragmentation Chain Transfer in High T. Macromolecules. 2012;45:5640–6. doi: 10.1021/ma300228z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu H, Carioscia JA, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater. 2005;21:1129–36. doi: 10.1016/j.dental.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Carioscia JA, Lu H, Stanbury JW, Bowman CN. Thiol-ene oligomers as dental restorative materials. Dent Mater. 2005;21:1137–43. doi: 10.1016/j.dental.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Schreck KM, Leung D, Bowman CN. Hybrid organic/inorganic thiol-ene-based photopolymerized networks. Macromolecules. 2011;44:7520–9. doi: 10.1021/ma201695x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senyurt AF, Wei H, Hoyle CE, Piland SG, Gould TE. Ternary thiol-ene/acrylate photopolymers: effect of acrylate structure on mechanical properties? Macromolecules. 2007;40:4901–9. [Google Scholar]
- 29.Senyurt AF, Hoyle CE, Wei H, Piland SG, Gould TE. Thermal and mechanical properties of cross-linked photopolymers based on multifunctional thiol-urethane ene monomers. Macromolecules. 2007;40:3174–82. [Google Scholar]
- 30.Reinelt S, Tabatabai M, Fischer UK, Moszner N, Utterodt A, Ritter H. Investigations of thiol-modified phenol derivatives for the use in thiol-ene photopolymerizations. Beilstein J Org Chem. 2014;10:1733–40. doi: 10.3762/bjoc.10.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinelt S, Tabatabai M, Moszner N, Fischer UK, Utterodt A, Ritter H. Synthesis and photopolymerization of thiol-modified triazine-based monomers and oligomers for the use in thiol-ene-based dental composites. Macromol Chem Phys. 2014;215:1415–25. [Google Scholar]
- 32.Podgórski M, Chatani S, Bowman CN. Development of Glassy Step-Growth Thiol- Vinyl Sulfone Polymer Networks. Macromol Rapid Commun. 2014;35:1497–502. doi: 10.1002/marc.201400260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Podgórski M, Becka E, Chatani S, Claudino M, Bowman CN. Ester-free thiol-X resins: new materials with enhanced mechanical behavior and solvent resistance. Polym Chem. 2015;6:2234–40. doi: 10.1039/C4PY01552E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podgórski M, Becka E, Claudino M, Shah P, Stansbury JWBC. Ester-free Thiol-ene Dental Restoratives Part A: Resin Development. Dent Mater. n.d doi: 10.1016/j.dental.2015.08.148. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podgórski M, Nair DP, Chatani S, Berg G, Bowman CN. Programmable mechanically assisted geometric deformations of glassy two-stage reactive polymeric materials. ACS Appl Mater Interfaces. 2014;6:6111–9. doi: 10.1021/am405371r. [DOI] [PubMed] [Google Scholar]
- 36.Vitale A, Hennessy MG, Matar OK, Cabral T. Interfacial Profile and Propagation of Frontal Photopolymerization Waves. Macromolecules. 2015;48:198–205. [Google Scholar]
- 37.Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23:1819–29. doi: 10.1016/s0142-9612(01)00308-8. [DOI] [PubMed] [Google Scholar]
- 38.Van Ende A, Mine AJDM, Poitevin A, Van Meerbeek B. Bonding of low-shrinking composites in high C-factor cavities. J Dent. 2012;40:295–303. doi: 10.1016/j.jdent.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Prakki A, Cilli R, Lia Mondelli RF, Kalachandra S, Pereira JC. Influence of pH environment on polymer based dental material properties. J Dent. 2005;33:91–8. doi: 10.1016/j.jdent.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Boaro LC, Gonçalves F, Guimarães TC, Ferracane JL, Pfeifer CS, Braga RR. Sorption, solubility, shrinkage and mechanical properties of “low-shrinkage” commercial resin composites. Dent Mater. 2013;29:398–404. doi: 10.1016/j.dental.2013.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.