Abstract
Insulin-producing β cells are lost or insufficient in numbers in diabetic patients, presenting the medical challenge for new β cells. Currently, there are three strategies that offer promise. One involves the generation of β cells de novo by directing the differentiation of either embryonic stem cells or induced pluripotent cells to the β cell lineage. The second is based on the conversion of another terminally differentiated cell to β cells in a process called reprogramming. The third approach is to promote the replication of existing β cells either in vivo or in vitro. Significant progress is evident for each strategy, but it remains unclear which approach will ultimately prove successful.
Introduction
The key challenge for regenerative medicine is to generate mature cells in a quantity and quality sufficient to replace destroyed or malfunctioning cells of diseased organs. Type 1 diabetes (T1D) is a chronic disease in which the β cells in the pancreas are destroyed in an autoimmune attack. Currently, the standard of care for patients with T1D involves injection of insulin and regular blood glucose monitoring. This treatment is lifesaving, but not a cure, and devastating complications often develop [1]. Clinical studies show that transplantation of islets allows temporal insulin independence and some relief from diabetes-related symptoms [2], providing a proof of principle that restoration of a sufficient functional β cells mass can be a type of cure. Nonetheless, the lack of high quality donor cells has prompted the search for alternative sources of β cells. To date, there are several approaches aimed at generating functional β cells for cell based therapies. Each exploits different biological paradigms. Among the sources of new β cells are: embryonic stem cells, endocrine progenitors, other mature cells in the pancreas, and β cell itself.
Directed differentiation of β cells from pluripotent cells: recapitulating embryonic development with ES cells
There has been significant progress in the past few years directing ES (ES= embryonic stem) cells towards the pancreatic endocrine lineage. These studies take advantage of the fact that pluripotent ES cells can generate any cell type in the body. The progress to date has come from experimental designs where ES cells are differentiated in a step-wise manner in vitro that aims to recapitulate embryonic development in vivo. The key steps of pancreatic development and signaling molecules involved have been identified based on the years of developmental biology studies (reviewed by [3],[4]). This scheme requires first moving ES cells towards definitive endoderm, then towards pancreatic progenitors, followed by endocrine progenitors and finally mature β cells. Intermediate cells at each of the steps can be distinguished by the expression of unique transcription factors. Growth factors and signaling molecules that promote the transition are identified for some, but not all, of the steps. For instance, TGF-β family members, Activin A or Nodal, can direct formation of definitive endoderm in the presence of Wnt3a from mouse or human ES cells [5], [6]. Recently, Beatge and colleagues [7], [8] showed that a series of signaling molecules and compounds can guide hES cells towards endocrine cells. This protocol for in vitro differentiation is, however somewhat inefficient and incomplete and yields immature cells with poor glucose responsiveness. The authors have improved the results using by transplanting hES-derived pre-mature clusters of cells into mice such that after 3 months incubation in vivo, the implanted cells developed into more mature endocrine cells that can regulate blood glucose levels.
Small molecules have been shown to modulate cell differentiation [9]. As an alternative to proteins or growth factors, cell permeable chemical inducers can be safer, devoid of animal-derived products. In addition, small molecules might be easy to apply and remove, inexpensive, pure, more efficient and amenable to scale-up, allowing the derivation of large quantities of replacement cells. Chemical inducers that can direct ES cell differentiation have been identified for the initial steps of β cell generation. Small molecules that direct ES differentiation towards endoderm, either without any additional growth factors [10] or in the presence of Activin A [11], were identified in high throughput screen. In a separate screen, indolactam V, an agonist of protein kinase C, was shown to induce the differentiation of hES cell derived endoderm into pancreatic progenitors [12].
The advances in studies on ES cell differentiation are encouraging, but significant hurdles remain. One is the lack of signals, either biological or chemical, that can drive differentiation to endocrine progenitors and/or mature β cells. Although transplantation of a progenitor population into patients is feasible, it may be safest to supply mature β cells. Looking ahead, signals that achieve the final phase of pancreatic endoderm specification towards endocrine cells will need to be replicated in vitro to achieve a proper specification of endocrine cells and glucose responsiveness. This may require the signals naturally received during development from interaction of pancreatic epithelium with mesenchyme and endothelial cells [13], [14],[15]. Proper specification may also require three dimensional culture condition that are more amenable for the formation of appropriate cellular connections and extra-cellular matrix interactions [16], [17].
A further complication worth noting is that, hES cell lines have a significant differences in their potential to differentiate into given cell types [18], [19]. It is therefore advisable to carefully evaluate hES cell lines and optimize the differentiation protocols. Presumably, all the established regimen of growth factors can be also applied to differentiate iPS cells into β cells although it still remains to be carefully evaluated [20]. The obvious advantage of using iPS cells would be the generation of patient-specific β cells. The same variability in differentiation potential likely exists among iPS cell lines although this has not been established.
Reprogramming other mature cell types into β cells
The development of a fertilized egg towards mature, differentiated cell types was historically considered to be a one-way process. However, recent discoveries pioneered by Yamanka’s laboratory [21–24] have demonstrated that mature cells can change their fate and re-enter a pluripotent state (iPS cells -induced pluripotent stem cells). These discoveries implied enormous potential for cell based therapy and also showed that mature cells can, indeed, change their fate. In a recent study, Zhou and colleagues [25] reprogrammed acinar cells from the exocrine pancreas of adult mice into endocrine, insulin-producing β cells. This remarkable transformation was achieved by injecting the pancreas with a pool of viruses encoding three key developmental transcription factors: Pdx1, Ngn3 and MafA. Importantly, the induced reprogramming of exocrine cells to beta cells was sufficient to ameliorate hyperglycemia in mice whose β cells had been ablated by streptozocin. Other cell types, besides exocrine tissue, especially those closely developmentally related (such as hepatocytes and intestinal cells), may be advantageous for reprogramming to β cells, as they are abundant and accessible. This potentially important new approach has several hurdles that need to be overcome before clinical use could be contemplated. One is the delivery of instructive factors; the viruses need to be replaced by safer reagents such as small molecules. The newly formed β cells stay either as single cell or small clusters and do not form structured islets which may be important for function. The crucial advantage of this so called “reprogramming strategy” to making new β cells is that it can be tailored specifically to patient. This direct lineage switching is a proof-of-principle and provides a general strategy to obtain cells of interest, whereby one uses the set of define transcription factors to turn one cell type into another. A strategy that straddles both, direct differentiation and reprogramming through the expression of key transcription factors, in pluripotent cells may be beneficial, but this combined approach has yet to be achieved.
Other mature cells have also been proposed as a source for new β cells. For example, there are reports on inducing liver cells (hepatocytes, oval cells, intra- and extra-hepatic epithelium) to trans-differentiate into β cells [26–28]. A recent example by Yechoor et al. [29] claims that transduction of Ngn3 and betacellulin in vivo rescued streptozocin induced diabetes. These authors propose that the rescue occurs in two phases: the first early phase (1–3 weeks) is mediated by activation of insulin expression in hepatocytes; in the second phase (6–12 weeks) putative adult stem cells in the liver, oval cells, transdifferentiate and form neo-islets expressing multiple endocrine hormones.
Facultative adult progenitors of β cells
During embryonic development, β cells are generated from a transient population of Ngn3 positive progenitors [30], [31]. However, during postnatal life, these progenitors disappear, and the homeostasis and maintenance of β cell mass occurs predominantly through the proliferation of existing, terminally differentiated, mature β cells [32], [33]. Moreover, all β cells have the same potential to expand [34]. There is evidence that under certain circumstances of stress, facultative progenitors of endocrine cells may be activated. Following partial duct ligation of the pancreas, Ngn3 expression is induced anew in the pancreas [35]. When these Ngn3 positive cells are sorted and injected into pancreatic transplants that are Ngn3 deficient, they give rise to insulin producing β cells along with other hormone producing cells. This study shows that the adult pancreas has a potential to reactivate an embryonic mechanism of endocrine cell generation. The source of Ngn3 positive cells, i.e. whether there is a resident stem/progenitor cell or the reactivation of Ngn3 in differentiated cell remains to be determined. And, as with all methods for β cell production, an important issue is to determine how many β cells can be generated by this procedure.
Expansion of existing β cells in vitro and in vivo
Unlike the skin, intestine or blood that have a relatively rapid turnover of cells, the β cells in pancreatic islets are a relatively quiescent population; they proliferate infrequently at a rate of about 0.1–0.3%/day [36]. However, in certain conditions, such as pregnancy [37] or an insulin-resistance state [38], [39] β cell mass increases. Thus, it may be feasible to use external stimuli to expand primary β cells ex vivo for transplantation purposes or alternatively to induce regeneration of endogenous remaining β cells or progenitors in vivo.
Recent studies suggest that when not hindered by a persistent autoimmune attack or the toxicity of high blood glucose levels, rodent and human β cells may have capacity to regenerate. Using a genetic model for conditional ablation of β cells in vivo, Nir and colleagues [40] demonstrated that normal β cell mass can be restored in a hyperglycemic state. In this model an inducible diphtheria toxin was expressed specifically in β cells, which results in the elimination of approximately 80% of β cell mass through apoptosis. The mice become frankly diabetic. Remarkably, a few weeks after expression of diphtheria toxin was ceased, β cells mass recovered and glucose homeostasis was stabilized. This demonstration of recovery from a diabetogenic injury provides an encouraging proof of concept and can be potentially used therapeutically. Lineage tracing analysis showed that enhanced proliferation of remaining β cells played the major, if not exclusive, role in the regeneration. Interestingly, Nir and colleagues also showed that common immuno-suppressants used in human islet transplantation, Sirolimus and Tacrolimus [2], had a toxic effect on replication and β cell regeneration was dramatically blunted. Naturally, this raises the question whether one can find other immuno-suppressants that are compatible with β cell regeneration.
High throughput and high content screening for small molecules that act as chemical inducers of β cell proliferation in vitro may be a rewarding and effective approach. In the study by Wang [41], several compounds were identified that induce β cell replication in vitro using growth-arrested, reversibly immortalized mouse β cells. These compounds include modulators of protein kinase C, the Wnt pathway and an activator or L-type calcium channels. It will be interesting to learn whether these compounds induce expansion of primary human β cells. Other molecules stimulating that can stimulate β cell proliferation include; HGF, human growth hormone, exendin-4, Exdenin-4 and high glucose concentration [42].
Remaining challenges
Despite these promising results, significant challenges remain for each of the strategies discussed above. First, it is important to set the bar high with respect to requirements for newly generated β cells: properly regulated insulin secretion and the capacity for cell renewal (replication) are at the top of the list. Thus, for functional β cells, one should consider the following: correct gene expression, including MafA, Nkx2.2, Glut2, PC 1/2/3, single-hormone expression (insulin positive but glucagon negative), the ability to synthesize and process insulin (judged by presence of c-peptide) at levels equivalent to those seen in human β cells; storage granules detectable by EM; glucose stimulated insulin release and after transplantation normalization of the blood glucose levels in diabetic animal model. Current protocols for the differentiation of ES cells into β cells often generate immature β cells expressing other endocrine hormones
Pancreatic islets are complex structures consisting of multiple cell types precisely arranged including several hormone-producing cells as well endothelial cells in the blood vessels. The restoration of the islet structure and also the necessity to connect β cells with blood supply may be critical for the proper response of β cells to glucose levels [43]. However, the generation of β cells, absent this complex architecture, may still have a value for cell replacement therapy [25].
Another issue is reproducibility: the protocols to generate new β cells have to be highly reproducible, safe and amenable to scale-up. Strategies to ensure uniform response from the cell population and to purify relevant intermediate and mature population will likely be needed. With a respect to hES cell derived therapies, a major concern is the persistence of undifferentiated cells competent to cause tumor formation after cell transplantation [44]. Similarly, partially reprogrammed cells might be prone to the malignant transformations. Thus, the transplantation of a purified, homogenous population of mature β cells is a reasonable target for this therapeutic approach.
And of course, a final concern that needs to be addressed is how to circumvent the recipient’s immune response. Transplantation of autologous β cells would prevent the activation of host vs. graft alloimmune reaction. In the case of diabetes type II this would eliminate the need for immune-suppression. Nevertheless, for T1D, blocking the autoimmune attack remains a if not the major hurdle. The precise mechanism of autoimmune destruction of β cells in T1D is still not well understood.
Conclusions
Advances in studies on the generation of new β cells have raised expectations aimed at generating large amounts of glucose-responsive, insulin secreting β cells for therapeutic purposes. Each of the strategies has its merits and drawbacks and at this stage it is impossible to determine with high confidence which of them will succeed. We believe that the potential of cellular therapies for diabetics is ever closer and is an achievable goal. Lastly, lessons learnt during the generation of β cells, would be beneficial for other avenues of regenerative medicine.
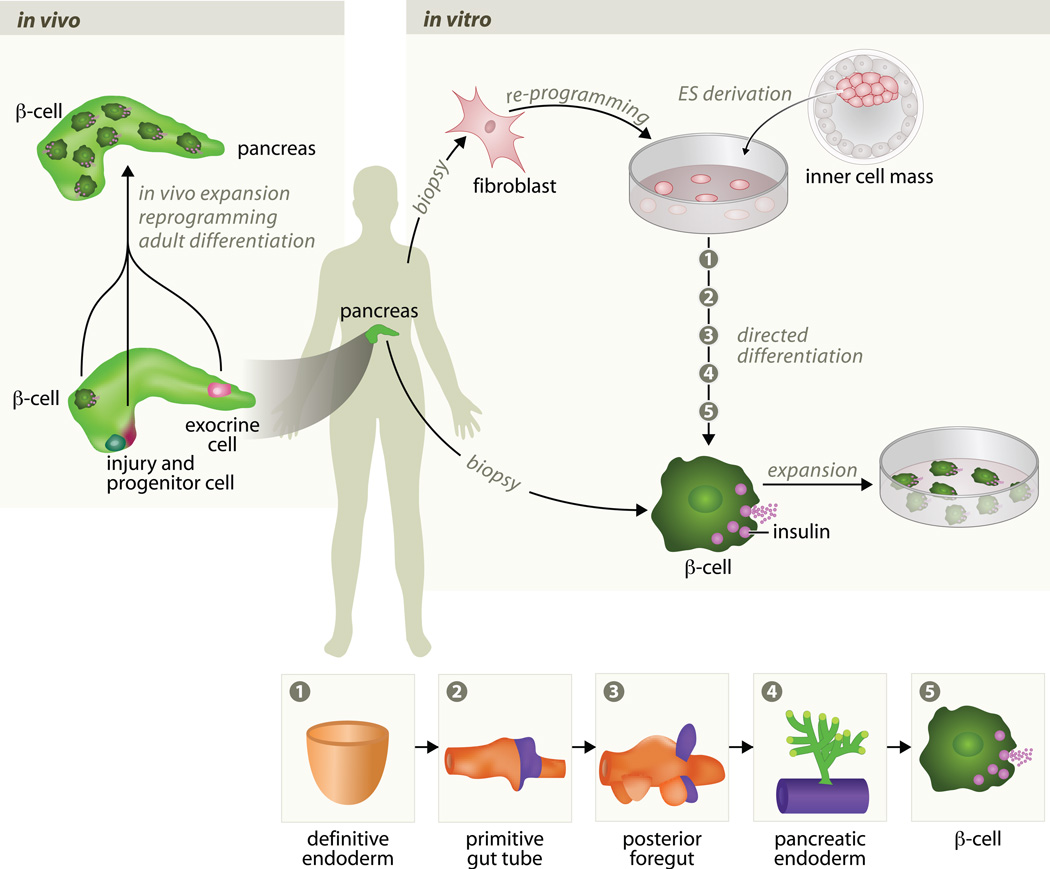
Figure 1.
Strategies to generate new β cells A. Existing β cells can be used for in vivo expansion and generation of new β cells. And other cell types in the pancreas, exocrine cells, can be reprogrammed in vivo by defined transcription factors to generate new β cells. Following duct ligation, β cells can be generated from the reactivation of Ngn3+ to form progenitor cells. An in vitro approach is to generate β cells from stem cells, either ES or iPS cells, and use those β cells for transplantation into diabetic patients. A potential single step strategy to achieve this is to find conditions to induce β cell proliferation in vitro with a high enough efficiency. A more complex approach is based on the both patient specific stem cell and recapitulate normal in vitro to generate mature β cells. B. Pancreatic organogenesis in mice (presumed to be similar in humans). During embryonic development, the pancreatic primordium is derived from definitive endoderm (2) that subsequently gives rise to the primitive gut (3) and posterior foregut (4). After induction of pancreatic epithelium (4) the endocrine cells proliferate and aggregate to form the islets of Langerhans that contain insulin-secreting β cells (5). At all stages, the epithelium is surrounded by the layer of mesenchyme that is omitted in this scheme for clarity. The mesenchyme provides essential signals for induction and survival of the epithelium, as well as the blood vessels for transport of glucose and insulin. Each of these steps is represented above, in A, for the in vitro in process of direct differentiation of ES/iPS cell into β cells.
Acknowledgments
We thank members of the Melton laboratory for discussions. We apologize to the colleagues whose work we could not cite here due to space limits. We thank Peter Carolan and Julie Sneddon for critical reading the manuscript. We also thank Renate Hellmiss for the help with the illustration preparation. D. A. M. is a Howard Hughes Medical Institute (HHMI) Work in this laboratory is supported by the Harvard Stem Cell Institute, HHMI, Juvenile Diabetes Research Foundation and National Institute of Health.
References
- 1.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Jorgensen MC, Ahnfelt-Ronne J, Hald J, Madsen OD, Serup P, Hecksher-Sorensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 4.Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes Dev. 2008;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 6.Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 7. Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. : Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393.. **A comprehensive study of the differentiation of hES cells into endocrine cells. Systematic application of regiment growth factors and signaling molecules leads to the generation of β-like cells from hES cells. Those β cell population where transplanted into animals and after months of maturation in vivo regulate blood glucose levels in streptozotocin-induced diabetic mice.
- 8.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 9.Emre N, Coleman R, Ding S. A chemical approach to stem cell biology. Curr Opin Chem Biol. 2007;11:252–258. doi: 10.1016/j.cbpa.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Borowiak M, Maehr R, Chen S, Chen AE, Tang W, Fox JL, Schreiber SL, Melton DA. Small molecules efficiently direct endodermal differentiation of mouse and human embryonic stem cells. Cell Stem Cell. 2009;4:348–358. doi: 10.1016/j.stem.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhu S, Wurdak H, Wang J, Lyssiotis CA, Peters EC, Cho CY, Wu X, Schultz PG. A small molecule primes embryonic stem cells for differentiation. Cell Stem Cell. 2009;4:416–426. doi: 10.1016/j.stem.2009.04.001.. *High throughput screen of chemical libraries led to the identification of stauprimide that primes cells for directed differentiation of ES cell and increases the efficiency of differentiation in synergy with extracellular clues.
- 12. Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, et al. : A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258–265. doi: 10.1038/nchembio.154.. **Small molecules library screen identifies the inducer, indolactam V, of pancreatic progenitors from hES derived endoderm. Indolactam V, agonist of PKC signaling acts specifically on endoderm population and increases the efficiency of pancreatic progenitor formation in presence of other growth factors. Indolactam V induced pancreatic progenitors contribute to all mature pancreatic cell types including endocrine cells.
- 13.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 14.Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DA, Kashima Y, Zaret KS. Endothelial cell promotion of early liver and pancreas development. Novartis Found Symp. 2007;283:207–216. doi: 10.1002/9780470319413.ch16. discussion 216-209, 238-241. [DOI] [PubMed] [Google Scholar]
- 16.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, et al. : The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell. 2007;129:359–370. doi: 10.1016/j.cell.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 18.Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, et al. : Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 19.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 20.Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. : Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 25. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314.. ** An elegant approach, in which authors used adenoviruses to introduce key pancreas transcription factors to ducts and thus directly convert in vivo ~ 20% of exocrine cells into insulin-secreting β cells.
- 26.Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 2007;15:255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- 27.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, et al. : Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 28.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 29.Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, Chan L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell. 2009;16:358–373. doi: 10.1016/j.devcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 32.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 33. Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011.. *The authors applied DNA-analog based multiple labeling to study the division of β cells. Long-period lineage tracing further strengthen the observation that all new β cells arise from existing β cells in adults.
- 34.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. : Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015.. **The authors showed that after bile duct ligation a population of Ngn3+ cells is activated. Those cells may be facultative stem cells in pancreas and give rise to the endocrine cells, including β cells.
- 36.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 37.Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- 38.Gupta RK, Gao N, Gorski RK, White P, Hardy OT, Rafiq K, Brestelli JE, Chen G, Stoeckert CJ, Jr, Kaestner KH. Expansion of adult beta-cell mass in response to increased metabolic demand is dependent on HNF-4alpha. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci U S A. 2001;98:7475–7480. doi: 10.1073/pnas.131192998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959.. **This study, based on genetic model of inducible β cell destruction, reveals that β cell mass can be restores in hyperglycemic state. Lineage tracing data suggest that remaining 20% β cells are origins of newly formed β cell mass.
- 41.Wang W, Walker JR, Wang X, Tremblay MS, Lee JW, Wu X, Schultz PG. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc Natl Acad Sci U S A. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YC, Nielsen JH. Regulation of beta cell replication. Mol Cell Endocrinol. 2009;297:18–27. doi: 10.1016/j.mce.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 43.Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705–714. doi: 10.1007/s00268-006-0719-8. [DOI] [PubMed] [Google Scholar]
- 44.Shih CC, Forman SJ, Chu P, Slovak M. Human embryonic stem cells are prone to generate primitive, undifferentiated tumors in engrafted human fetal tissues in severe combined immunodeficient mice. Stem Cells Dev. 2007;16:893–902. doi: 10.1089/scd.2007.0070. [DOI] [PubMed] [Google Scholar]