Abstract
Insulin expressing cells that have been differentiated from human pluripotent stem cells in vitro lack the glucose responsiveness characteristic of mature β-cells. β-cell maturation in mice was studied to find genetic markers that enable screens for factors that induce bona fide β-cells in vitro. We find that functional β-cell maturation is marked by an increase in the glucose threshold for insulin secretion and by expression of the gene urocortin 3.
The directed differentiation of human pluripotent stem cells (HPSCs) has the potential to produce β-cells for transplantation into diabetics. However, the available protocols for in vitro differentiation produce only “β-like” cells. These “β-like” cells do not perform the accurate glucose-stimulated-insulin-secretion (GSIS) found in mature β cells unless they are transplanted into mice and allowed to further differentiate for many weeks1. During normal development, insulin-expressing β-cells appear around embryonic day 13.5 in mice or week 8–9 post-conception in humans2, 3, but regulated GSIS has been observed only days after birth. The signals and mechanisms governing β-cell maturation, either during postnatal development or after transplantation, are unknown.
We aim to define functional β-cell maturation based on GSIS parameters, and to identify markers of functionally mature β-cells that could be used to make functional HPSC-derived β-cells in culture.
Traditionally, GSIS is measured by the fold change in insulin secretion between low (2.8–5mM) and high (>10mM) glucose concentrations4. In this assay, neonatal β-cells display a high basal insulin secretion at low glucose concentrations, and stimulation with a high concentration of glucose results in a small fold increase in insulin secretion. These data could be explained if neonatal β-cells have uncontrolled insulin “leakiness” at low glucose concentrations, or alternatively, if they have a lower glucose concentration threshold at which they secrete insulin. To distinguish between these two hypotheses, we performed dynamic GSIS on neonatal (P1) and older (P15) mouse islets using a very low baseline glucose level of 0.5mM. The data show that neonatal P1 islets execute a full GSIS response (both first and second phases of insulin secretion) at low (2.8mM) glucose concentrations, whereas P15 islets show no response (no insulin secretion) at this concentration (Figure 1A). These results show that immature β-cells are not “leaky”, but rather have a reduced threshold for GSIS, secreting insulin in response to a lower glucose concentration than mature β-cells.
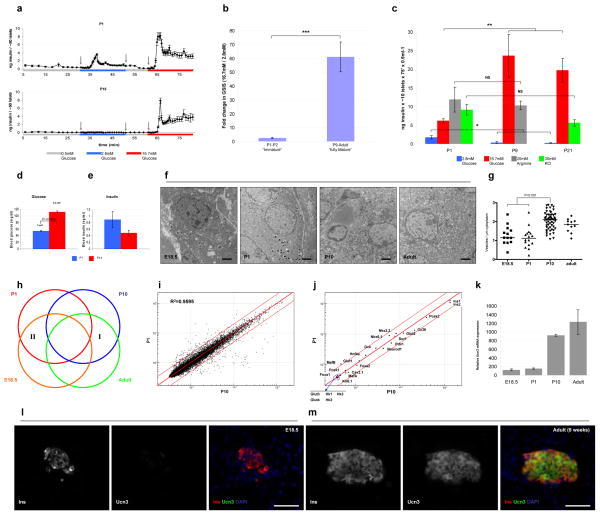
Figure 1. β-cell maturation is defined by a decrease in GSIS sensitivity to low glucose levels and by the expression of Ucn3.
(A) Three independent sets of 50 islets each, from P1 or P15 mice, were sequentially perfused with basal (0.5mM, gray), low (2.8mM, blue) or high (16.7mM, red) glucose in a dynamic GSIS assay. Arrows indicate the time points at which solutions were changed. P1 islets display complete first and second phases of GSIS in response to low glucose, whereas P15 islets do not secrete insulin at this glucose concentration. (B) Triplicates of 10 islets from P1 to adult were assayed for GSIS using low glucose (2.8mM) and high glucose (16.7mM). Two age groups can be distinguished according to their stimulation index (fold change in GSIS). ***, P<5×10−5. (C) Three independent sets of ten islets each from P1, P9 or P21 were assayed for GSIS using low glucose (2.8mM, blue), high glucose (16.7mM, red), 20mM arginine (gray) or 30mM KCl (green). The difference in the amount of insulin secreted between mature and immature islets is specific to glucose. *, P<0.05; **, P<0.001; NS, not significant). (D) Blood glucose and (E) insulin levels in immature (P1, blue) and mature (P14, red) mouse pups. Insulin levels in the immature pups are higher than in the mature ones, although their blood glucose levels are lower. (F) Electron micrograph of insulin vesicles in β-cells at various ages. Scale bars = 2μm. (G) Quantification of the number of insulin vesicles vs. β-cell area of the data shown in F. (H) A scheme representing the microarray approach. Genes differentially expressed in both mature age groups compared to both immature age groups (I and II) are chosen as candidate markers. (I) Representative scattered plot from the microarray. Note high similarity (R2) in gene expression between the mature (P10) and immature (P1) samples. (J) The expression levels of most β-cell markers are unchanged during GSIS maturation. Scatter plots of global gene expression from microarrays on FACS-sorted immature (P1) and mature (P10) β-cells. Red lines mark a 2-fold difference in expression and, with the exception of MafB, gene expression is not significantly different between these stages. (K) The expression of Ucn3 mRNA at various ages as detected in the microarray. (L) Immunostaining of Ucn3 (green) and insulin (red) on pancreata from E18.5 and adult mice. Nuclei are stained with DAPI (blue). Scale bars = 50μm (M) Ucn3 is undetectable in E18.5 embryo. Ucn3 is detected at high levels and co-localizes with adult β-cells.
To determine when β-cells acquire a mature GSIS capacity, we tested mouse islets isolated from P1 to adult for their response to low (2.8mM) and high (16.7mM) glucose concentrations. Islets from neonatal mice, ages P1 and P2, secreted 2.6±0.5 fold more insulin in high glucose than in low glucose, whereas islets from P9 to adult secreted, on average, 60.9±10.7 fold more insulin in high glucose than in low glucose (Figure 1B). Thus the dramatic change in GSIS response between low and high glucose that characterizes β-cell maturation occurs between P2 and P9. Islets of mice younger than P2 display an “immature” response, whereas islets from mice older than P9 respond as “mature” β-cells. Between P3 to P8, a mixed (intermediate) GSIS phenotype was observed.
The differences in insulin secretion between mature and immature β-cells is specific for glucose. The amount of insulin secreted by P1 and P9 islets in response to 30mM KCl was 11.9±3.5ng and 10.3±1.1ng, respectively. The amount of insulin secreted from P1 and P21 islets in response to 20mM arginine was 9.17±1.4ng and 5.66±0.9ng, respectively. These differences are not statistically significant (Figure 1C). In contrast, islets from P1 mice secreted only 6.2±0.6ng insulin, during 75min in 0.5ml high (17.7mM) glucose, while the same number of islets from P9 or P21 secreted 23.7±5.7ng and 19.7±3.2ng insulin, respectively (P<0.001). At low glucose levels, the opposite trend was observed: P1 islets secrete 1.8±0.5ng insulin at 2.8mM glucose, whereas P9 and P21 islets secrete only 0.4±0.3ng and 0.3±0.1ng insulin, respectively (P<0.05) (Figure 1C).
We examined the physiological consequences in vivo of the differences observed in vitro between mature and immature β-cells’ response to glucose. In agreement with previous reports4, P1 pups had significantly lower blood glucose levels than P14 pups. The average blood glucose concentration at P1 is 3mM whereas blood glucose at P14 averages 6.2mM (P<2.5×10−24) (Figure 1D). Notably, the average blood glucose level in P1 pups is higher than the glucose concentration that causes insulin secretion in vitro in P1 islets. If the in vitro observation that immature β-cells secrete insulin at low glucose levels (Figure 1A) holds true in vivo, one should see higher insulin in the blood of neonates. Consistent with this prediction, the P1 pups had nearly two-fold higher levels of insulin in their blood than P14 animals (Figure 1E), although we note that there is a high variability of blood insulin in non-fasted animals. We also examined insulin granules in β-cells at each stage using electron microscopy (Figure 1F–G). P1 β-cells contained approximately 2-fold fewer insulin granules compared to P10 β-cells, suggesting a mechanistic difference in insulin secretion.
To find molecular markers whose expression pattern correlates with β-cell maturation, β-cells expressing Pdx1-EGFP from P1 or P10 animals were sorted by FACS and their global gene expression patterns were compared using transcriptional arrays. The Pdx1-EGFP strain was used instead of the insulin-EGFP strain due to the slightly diabetic phenotype observed in the latter animals. We also analyzed β-cells from E18.5 embryos and adult mice (Figure 1H) to further reduce the number of genes whose transcriptional differences are not related to GSIS maturation. Remarkably, the gene expression profiles of functionally mature and immature β-cells tested this way is very similar (Figure 1I).
Various molecular mechanisms have been proposed to explain the ineffective GSIS observed in fetal and neonatal β-cells as compared to adult β-cells. These include insensitivity of the ATP-regulated K+ channel5, 6, reduced expression of glucose transporters6, low activity of glucokinase7 or low levels of the β-cell selective gap junction protein Connexin368. Recently, genetic ablation of the transcription factors NeuroD1 in adult mouse β-cells9 or the combined deletion of Foxa1 and Foxa210 resulted in β-cells with an immature-like GSIS phenotype. We thus first assessed known β-cell genes whose expression levels could explain the functional difference between mature and immature β-cells. We examined expression levels of β-cell transcription factors (Pdx1, Nkx2.2, Nkx6.1, NeuroD1, Foxa1, Foxa2, MafA, MafB and Hnf4a), key proteins involved in glucose sensing and insulin secretion (Glucokinase, Glut2, Cav6.1, Kir6.1, Sur1, Pcsk1 and Pcsk2), the β-cell-selective gap junction gene Connexin36 and the genes for Insulin1 and Insulin2. We also looked at tissue specific glucose transporters (Glut 1, 3 and 4) and hexokinases (Hexokinase 1, 2 and 3) (Figure 1J). The RNA expression levels of most of these genes does not change significantly between immature and mature cells (or change expression by less than two-fold making them unsuitable for on/off detection of mature β-cells). One exception is the transcription factor MafB which is expressed at 2.5-fold higher levels in immature β-cells, consistent with previous reports11.
We next examined all genes for which expression changes by more than two-fold between immature and mature cells. We excluded genes for which a significant change in expression also occurred between E18.5 and P1 or P10 and adult, thereby focusing on genes that change expression specifically within the time window of β-cell maturation (groups I and II in Figure 1H). We found 71 genes (81 probes) that were upregulated and 66 genes (72 probes) that were downregulated during β-cell maturation (Supplementary Table 1). Of the former group, 36 genes were acinar-related genes (marked with asterisks in supplementary table 1) which is best explained by the rapid expansion of exocrine tissue at this stage, thereby increasing the probability of a small acinar cell contamination during FACS sorting and resulting in the misleading indication that acinar genes are up-regulated. We chose 16 genes (underlined in supplementary table 1) for which β-cell expression had previously been reported and analyzed their protein expression levels using western blotting and immunohistochemistry. From all these analyses, one strong candidate emerged: the gene Urocortin 3 (Ucn3).
The levels of Ucn3 mRNA increase more than 7 fold between immature and mature β-cells, and nearly 10 fold between E18.5 and adult (Figure 1K and supplementary table 1). Immunofluorescence staining showed that Ucn3 is highly expressed in all adult β-cells, but is undetectable in islets from E18.5 embryos (Figure 1L–M). As with insulin, the signal intensity of Ucn3 protein varies from cell to cell in the adult islet. This variation does not correlate with the variation in insulin intensity as cells that show high staining intensity for insulin show both high and low staining intensities for Ucn3, and vice versa. No co-localization of Ucn3 with glucagon, somatostatin or pancreatic polypeptide was observed (supplementary figure 1).
Ucn3 is a secreted protein expressed in regions of the brain and in the pancreas, and was reported to be exclusively expressed by β-cells but not other endocrine cells in the islet12. Li et al.12, 13 found that secretion of Ucn3 from β-cells is induced by high glucose in adult mice, and that the gene has a positive effect on GSIS at high glucose concentrations. We tried to mature fetal β-cells in vitro by culturing them in the presence of recombinant Ucn3 protein for several days, but did not observe any effect of the recombinant protein on the GSIS profile of the cells, suggesting that Ucn3 by itself can not induce β-cell maturation (data not shown). It remains to be determined whether Ucn3 knockout mice have β-cell maturation defects.
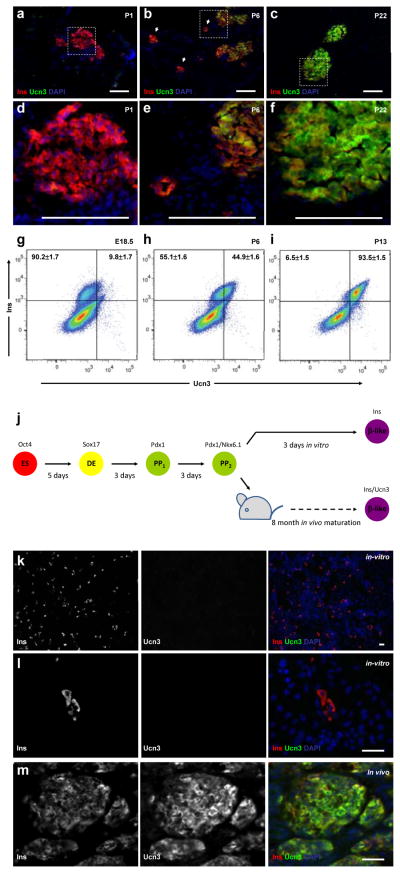
We next examined the patterns of Ucn3 expression at additional time points during the period of β-cell maturation. Ucn3 protein was not detected in any islets of P1 pups (Figure 2A and D). At P6, Ucn3 expression is found primarily in large islets, not in small β-cell aggregates (Figure 2B and E). By P22, Ucn3 protein is strongly detected in all β-cells (Figure 2C and F). Intra-cellular FACS analysis with antibodies against Ucn3 and insulin was performed to quantify the percentage and levels of Ucn3 expression in β-cells. At E18.5, 90.2±1.7% of β-cells express insulin alone while 9.8±1.7% also stain weakly for Ucn3 (Figure 2G). The low expression level of Ucn3 in the small population detected by FACS at this age is probably too low to be detected by conventional immunofluorescence on tissue sections. At P6, 55.1±1.6% of β-cells are either negative for Ucn3 or express low levels of the protein, while 44.9±1.6% of β-cells express high levels of both Ucn3 and insulin (Figure 2H). By P13, just at the end of the maturation window, 93.5±1.5% of the β-cells express high levels of Ucn3 and only 6.5±1.5% express insulin alone (Figure 2I). The increase in Ucn3 in β-cells during maturation is gradual, as can be seen by the shift in the mean Ucn3 signal intensity (supplementary figure 2A). The signal intensity of insulin is unchanged, indicating that expression of insulin protein remains constant throughout this time period (supplementary figure 2B). This mixed pattern of Ucn3 expression may explain why a “marginally mature” phenotype was observed between P2 and P9.
Figure 2. Ucn3 expression gradually increases during the course of mouse β-cell maturation in vivo and is expressed in HESC-derived β-like cells after differentiation and maturation in vivo, but not after differentiation in vitro.
(A–C) Immunostaining of Ucn3 (green) and insulin (red) on pancreata from P1, P6 and P22 mice. (D–F) Enlargement insets shown in A–C, respectively. Nuclei are stained with DAPI (blue). Scale bars = 50μm. (A, D) Ucn3 in not detected at P1 even in large islets. (B, E) At P6, some large islets express Ucn3, but small aggregates do not express the peptide (arrows). (D, F) At P22, Ucn3 is highly expressed in all islets. (G–I) Intra-cellular FACS analysis of insulin and Ucn3 at E18.5, P6 and P13. Numbers in upper quadrants represent the percentage of insulin only (left) or insulin and Ucn3 co-expressing cells (right) of all insulin-expressing cells (two upper quadrants), calculated as average±sem of three independent biological repeats (three separate litters) for each age group. (J) An outline of the experimental approach on HESCs differentiation. HESCs (ES, red) marked by Oct4 were differentiated in vitro into definitive endoderm (DE, yellow) marked by Sox17 and subsequently to pancreatic progenitors (PP, green), marked by the expression of Pdx1 and NKX6.1. The cells were transplanted into SCID-beige mice to complete maturation in vivo. (K, L) Immunostaining for Ucn3 (green) and insulin (red) on the in vitro differentiated cells shown at two magnifications (K, low magnification; L, high magnification). In vivo differentiated (transplanted) cells are shown in (M). Nuclei are stained with DAPI (blue). Scale bars = 50μm. Ucn3 is expressed in the in vivo matured cells, but not in in vitro differentiated insulin-positive β-like cells.
Finally, we wished to determine if Ucn3 could serve as a marker for functionally mature β-cells derived from human pluripotent stem cells (HPSCs). Immunoassaying with antibodies against Ucn3 on pancreatic sections obtained from an adult human donor revealed that the gene is expressed by all insulin-positive β-cells, and is excluded from glucagon-expressing α-cells. A small fraction of somatostatin- and PPT-expressing cells also express Ucn3 (Supplementary fig. 3). To see whether Ucn3 is induced during the maturation of human ESC-derived β-cells following transplantation, human embryonic stem cells were differentiated using a 4-step protocol to Pdx1 and NKX6.1-positive pancreatic progenitors14. These cells were then differentiated in vitro for 3 more days to become insulin-positive β-like cells (see figure 2J and supplementary material and methods for details). Separately, stage 4 clusters of Pdx+ Nkx6.1+ pancreatic progenitors, containing a few insulin-positive β-like cells, were transplanted to the kidney capsule of SCID-beige mice where they differentiate further and mature in vivo (Figure 2J). A glucose tolerance test, performed on transplanted animals, showed fasting human C-peptide levels above background 12 weeks after transplantation (Supplementary fig. 4). Despite high variation in fasting human C-peptide between the transplanted mice, all but one animal (6/7) showed an increase in blood human C-peptide between 1.7-fold to 7.6-fold (average 2.8±0.9-fold), demonstrating that the transplanted human embryonic stem cell- (HESC)-derived cells matured to glucose-responsive β-cells. Immunostaining showed that while the in vitro differentiated β-like cells express insulin, they are negative for Ucn3 staining (Figure 2K–L). Conversely, the in vivo matured cells stained positive for both insulin and Ucn3 proteins (Figure 2M). This expression of human Ucn3 in the transplant is exclusive to the β-cells; the Ucn3 protein is not detected in any glucagon-, somatostatin- or PPY-expressing cells (Supplementary fig. 5).
In summary, we propose an operational definition for mature β-cells based on changing glucose thresholds for GSIS response during development. We also identify a molecular marker, Ucn3, that distinguishes mature from immature β-cells. Notably, we find that Ucn3 is induced in HESC-derived β-cells following maturation in vivo. High-throughput screening can now utilize the difference in GSIS and the expression of Ucn3 as benchmarks in studies aimed at finding conditions to induce functional β-cell maturation in vitro.
Supplementary Material
Acknowledgments
We thank Prof Wylie Vale (Salk Institute) for providing anti-human Ucn3 antibody, Kfir Blum for writing the MATLAB BioGUI for analysis of GSIS, Anastasie Kweudjeu for help with transcriptional arrays, Christian Honore for help with FACS analyses, Adam Roose, Allen Cheng and Francis Deng for excellent technical assistance and Dena Cohen, Yoav Mayshar and Felicia Pagliuca for critical reading of the manuscript. We are grateful to all members of the Melton lab and the HMS Critical Discussion Group for helpful discussions and experimental advice. B.B. was supported by EMBO and JDRF postdoctoral fellowships. D.A.M. is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 2.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 3.Slack JM. Developmental biology of the pancreas. Development. 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- 4.Rozzo A, Meneghel-Rozzo T, Delakorda SL, Yang SB, Rupnik M. Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Ann N Y Acad Sci. 2009;1152:53–62. doi: 10.1111/j.1749-6632.2008.04003.x. [DOI] [PubMed] [Google Scholar]
- 5.Rorsman P, et al. Failure of glucose to elicit a normal secretory response in fetal pancreatic beta cells results from glucose insensitivity of the ATP-regulated K+ channels. Proc Natl Acad Sci USA. 1989;86:4505–4509. doi: 10.1073/pnas.86.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson CC, et al. Low levels of glucose transporters and K+ATP channels in human pancreatic beta cells early in development. Diabetologia. 2007;50:1000–1005. doi: 10.1007/s00125-007-0644-x. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi S, Tanigawa K, Miwa I. Immaturity of glucose-induced insulin secretion in fetal rat islets is due to low glucokinase activity. Horm Metab Res. 2000;32:97–102. doi: 10.1055/s-2007-978598. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho CP, et al. Beta cell coupling and connexin expression change during the functional maturation of rat pancreatic islets. Diabetologia. 53:1428–1437. doi: 10.1007/s00125-010-1726-8. [DOI] [PubMed] [Google Scholar]
- 9.Gu C, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao N, et al. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol Endocrinol. 2010;24:1594–1604. doi: 10.1210/me.2009-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artner I, et al. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, et al. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Chen P, Vaughan J, Lee KF, Vale W. Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA. 2007;104:4206–4211. doi: 10.1073/pnas.0611641104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezania A, et al. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes. 60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.