Abstract
The microphthalmia (MiT) family of transcription factors is an important mediator of metabolism. Family members Mitf and Tfeb directly regulate the expression of the master regulator of metabolism, peroxisome-proliferator activated receptor gamma coactivator-1 alpha (Pgc-1alpha), in melanomas and in the liver, respectively. Pgc-1alpha is enriched in tissues with high oxidative capacity and plays an important role in the regulation of mitochondrial biogenesis and cellular metabolism. In skeletal muscle, Pgc-1alpha affects many aspects of muscle functionally such as endurance, fiber-type switching, and insulin sensitivity. Tfe3 also regulates muscle metabolic genes that enhance insulin sensitivity in skeletal muscle. Tfe3 has not yet been shown to regulate Pgc-1alpha expression. Our results reported here show that Tfe3 directly regulates Pgc-1alpha expression in myotubes. Tfe3 ectopic expression induces Pgc-1alpha, and Tfe3 silencing suppresses Pgc-1alpha expression. This regulation is direct, as shown by Tfe3’s binding to E-boxes on the Pgc-1alpha proximal promoter. We conclude that Tfe3 is a critical transcription factor that regulates Pgc-1alpha gene expression in myotubes. Since Pgc-1alpha coactivates numerous biological programs in diverse tissues, the regulation of its expression by upstream transcription factors such Tfe3 implies potential opportunities for the treatment of diseases where modulation of Pgc-1alpha expression may have important clinical outcomes.
The microphthalmia (MiT) family of basic helix-loop-helix leucine zipper transcription factors includes Mitf, Tfe3, Tfeb, and Tfec family members. These factors regulate gene expression by binding in a homo- or hetero-dimeric manner to a specific consensus sequence called E-boxes that resides in promoter regions of target genes (Beckmann and Kadesch, 1991; Zhao et al., 1993; Steingrimsson et al., 2004). A significant number of biological and pathological processes are regulated by MiT factors. Recently MiT factors have been recognized as major players controlling the expression of key metabolic genes. Tfe3 mediates metabolism through the regulation of genes that participate directly in the insulin-signaling pathway (Nakagawa et al., 2006), through the regulation of lipolysis in adipose tissue (Fujimoto et al., 2013), and through the regulation of glucose metabolism in skeletal muscle (Iwasaki et al., 2012). Mitf directly regulates the expression of the master coactivator PGC-1alpha in melanomas (Haq et al., 2013; Vazquez et al., 2013), while Tfeb participates in lipid metabolism through direct regulation of Pgc-1alpha and Ppar-alpha complex in liver (Settembre et al., 2013) and functions as a key transcriptional regulator of autophagy (Settembre et al., 2011).
The Pgc1 family of coactivators has three members: Pgc-1alpha, Pgc-1beta, and PRC. Pgc-1alpha is a coregulator of cell metabolism that induces mitochondrial biogenesis, mitochondrial remodeling, respiration, gluconeogenesis and glucose transport, fatty acid oxidation, peroxisomal remodeling, and detoxification of reactive oxygen species (ROS) (Puigserver, 2005; Handschin and Spiegelman, 2006; Austin and St-Pierre, 2012). Pgc-1alpha and Pgc-1beta are expressed in mitochondrial rich tissues such as skeletal muscle, and they overlap functionally (St-Pierre et al., 2003; Handschin and Spiegelman, 2006; Rowe et al., 2010). Many physiological stimuli that require a demanding metabolic response induce expression of Pgc-1alpha including: cold, exercise, oxidative stress, and fasting (Ventura-Clapier et al., 2008). During cold shock, Pgc-1alpha stimulates the expression of uncoupling protein 1 (Ucp1) in brown adipose tissue (BAT) and, consequently, adaptive thermogenesis (Puigserver et al., 1998; Lin et al., 2004); in skeletal muscle Pgc-1alpha stimulates glucose uptake and is involved in the expression of muscle fiber type I and IIa (Michael et al., 2001a; Lin et al., 2002b). Endurance training has been shown to activate Pgc-1alpha reinforcing mitochondrial biogenesis, fatty acid oxidation and angiogenesis (Arany, 2008). Following fasting the expression of Pgc-1alpha is elevated in the liver, thus mediating metabolic programs through the coactivation of hepatic transcription factors (Lin et al., 2005).
Based on previous investigations that show that Mitf and Tfeb regulate Pgc-1alpha expression by directly binding to the Pgc-1alpha promoter (Lin et al., 2002b; Settembre et al., 2011, 2013; Strub et al., 2011; Haq et al., 2013; Vazquez et al., 2013), we predicted that Tfe3 might also function as an important regulator of Pgc-1alpha expression. We demonstrate here that overexpression or downregulation of TFe3 in primary differentiated myoblasts and in differentiated C2C12 cells resulted in a significant increase or reduction in levels of Pgc-1alpha, respectively. The mouse promoter region of the Pgc-1alpha gene was evaluated, and three E-boxes were found. We confirmed by ChIP and luciferase assays that Tfe3 transactivates two of the three consensus sequences.
Due to the role of Pgc-1alpha in modulating energy metabolism, tuning its expression pharmacologically has been under study for the treatment of metabolic maladies. Specifically targeting transcription factors like Tfe3 involved in the regulation of Pgc-1alpha can effectively modulate Pgc-1alpha expression at physiological levels. MiT factors are interesting targets since recently they have been used for the treatment of human neurological and lysosomal diseases and in cancer (Kauffman et al., 2014; Martina et al., 2014).
Materials and Methods
Animals
All animal experiments were performed according to procedures approved by the Ethics Committee for Animal Care and the Subcommittee on Research Animal Care and were carried out in accordance with the approved guidelines of the Institutional Animal Care and Use Committee (IACUC) of Massachusetts General Hospital. C57BL/6J mice used in this study were obtained from Jackson Laboratory, Bar Harbor, ME.
Cells
C2C12 myoblastic cell line (acquired from ATCC) was grown in DMEM 10% fetal bovine serum (FBS). Mouse primary myoblasts originated from neonatal C57BL/6 (B6) mice were obtained following an adapted protocol from Springer et al. (2002) and grown in DMEM 10% FBS.
Overexpression experiments
C2C12 and the mouse primary myoblasts were stably transduced with the mouse Tfe3 cDNA expression vector. After antibiotic selection, cells were treated with differentiation media (DMEM 2% horse serum (HS)) for 4 days and total RNA and protein were harvested.
Knockdown experiments
Tfe3, Mitf, and Tfeb were knocked down independently by small interference RNA (siRNA) transfected into differentiated C2C12 and primary B6 cells. These experiments were performed using four pools of siRNA (ON-TARGET plus SMART pool siRNA), and as the control non-targeting siRNA pool (Thermo Scientific, Waltham, MA). After 72 h, RNA and protein was obtained for RT-PCR and Western blot analysis.
Of note, Tfe3, Mitf, and Tfeb knockdown vectors did not exhibit any sequence cross reactivity.
RNA analysis
RNA from cell cultures was isolated using RNeasy (Qiagen, Hilden, Germany) and reverse transcribed with quantiTect reverse transcription reagent (Qiagen). cDNA was amplified by RT-PCR with QuantiFast SYBR Green PCR master mix (Qiagen). The sequences of the primers used were: 5′-GAACGACGCAGGCGATTCAACATT-3′ and 5′-ATCCACAGATGC CTTCAGGATGGT-3′ for Tfe3; 5′-CCACCCCAGCCATCAACAC-3′ and 5′-CAGACAGATACTCCCGAACCTT-3′ for Tfeb; 5′-AAGGTCCCCAGGCAGTAGAT-3′ and 5′-GGCTGTAGGGTGACCTTGAA-3′ for Pgc-1alpha; 5′-TGCATAAGCACCTCACTTCG-3′ and 5′-CACCTCCTCCTCCTCCTCTT-3′ for Pgc-1beta 5′-GGGAGGCAAGCATAAGACTG-3′ and 5′-CCAGGTGATGCCTTTGTTCT-3′ for Cycs; 5′-GATGAGGAGCAGGCTATGG-3′ and 5′-GTCTTCCTTGGTGCCTGAAG-3′ for Cox5b; 5′-ACGCTCGCTTCTTGAGGTTA-3′ and 5′-CTTCTGGGATTTCTGCAAGC-3′ for Ndu5b; 5′-AGGTTCTGGCCAACGGTCTAG-3′ and 5′-CCCTCTATGGGCTCGAATTTT-3′ for the mouse 18S. The murine Tfeb was obtained from the primer bank at http://pga.mgh.harvard.edu/primerbank/index.html. Mouse primers for 36b4 and common Mitf were previously described (Mikkelsen et al., 2010; Ooishi et al., 2012).
Protein extracts and Western analysis
Isolation of protein was performed using M-PER mammalian protein extraction reagent (Thermo Scientific). Protein concentration was determined using Coomassie Plus (Bradford) Assay Kit (Thermo Scientific). Sixty micrograms of each supernatant sample of protein was separated by electrophoresis through an 8% SDS–polyacrylamide gels and transferred to a 0.2-μm Trans-Blot transfer medium nitrocellulose (Bio-Rad, Helcules, CA). Following transfer, immunoblottings were performed. Membranes were first blocked with 5% milk in Tris–EDTA–Tween (TBST) buffer and probed with the following primary antibodies: Tfe3 (Calbiochem, San Diego, CA) and tubulin (Sigma-Aldrich, St. Louis, MO). Individual proteins were detected by incubation of the membranes with horseradish peroxidase-conjugated secondary antibodies (GE, Fairfield, CT and Thermo Scientific) followed by treatment with enhanced chemiluminescence (Thermo Scientific) according to manufacture directions.
Chromatin Immunoprecipitation (ChIPs)
The procedure was adopted from the Upstate protocol. C2C12 cells and primary B6 cells were differentiated for 4 days and fixed with 1% formaldehyde. Cross-linking was stopped by adding glycine. Myoblasts were washed with PBS and collected in PBS containing protease and phosphatases inhibitors (Roche, Penzberg, Upper Bavaria, Germany). After centrifugation, the pellets of cells were re-suspended in SDS–lysis buffer (containing protease and phosphatase inhibitors) and sheared by sonication (BioruptorTm UCD-200, Diagenode) at 4°C to obtain fragments from 200 to 500 bp. Soluble chromatin was diluted 10 times with Immunoprecipitation Buffer containing protease and phosphatase inhibitors and precleared at 4°C with 50% slurry ultraLink Immobilized Protein A/G. After incubation, the beads were pelleted, and the supernatant was separately immunoprecipitated with Tfe3 antibodies (Santa Cruz Biotechnology), with RNA polymerase II (Phospho Serine 2) (Abcam) and with control IgG (Santa Cruz Biotechnology) at 4°C overnight. Immune complexes were collected with a 50% slurry of ultraLink Immobilized Protein A/G in Tris–EDTA buffer (TE) by incubating at 4°C for 1 h. UltraLink Immobilized Protein A/G beads were washed sequentially at 4°C with increased stringency buffers and finally twice with TE. Immune complexes were eluted from the beads with 1% SDS in TE, and protein–DNA cross-links were reversed by adding NaCl and heating at 65°C overnight. After treatment with proteinase K, the samples were purified with QIAquick PCR purification Kit (Qiagen) and analyzed by real-time PCR.
Real-time PCRs were performed with KAPA SYBR qPCR kit (KAPA Biosystems, Cape Town, South Africa).
Primers used were: 5′-GGAACTGAAGCAAGCGCTCC′ and 5′-ATCGGGGGTGTTGCCTTCAA-3′ for the Pgc-1alpha E-box 1; 5′-ACACACAGCACACACTCATG-3′ and 5′-GACAGCCAGCCTACTTTTT-3′ for the Pgc-1alpha E-box 2; 5′-CTCCATACAGAGTCTTGGCT-3′ and 5′-CATGAGTGTGTGCTGTGTGT-3′ for the Pgc-1alpha E-box 3; and the control albumin primers, described in BIO-RAD tech note 2916. ChIP analyses were performed in triplicate, four times.
Luciferase reporter
Vector construction
The murine Pgc-1alpha proximal promoter (~1,000 bp) containing two E-boxes was amplified by PCR using murine genomic DNA as a template. After digestion with Kpn and NcoI the PCR fragment was run in an agarose gel and the band was purified by QIAquick gel extraction kit (Qiagen) and the purified fragment was cloned into the pGl3 basic vector (Promega, Madison, WI).
The primer sequences used were: 5″-GGGGGTACCGAAATAAGGGGTGGGGGCAGGTGA-3′ and 5″-GGGCCATGGGCAGGCAACCAGCCCCTTACTGAG-3′. The purified PCR fragment was cloned into the linearized vector pGL3-basic (Promega) by incubation with ligase enzyme (NEB). The sequence was confirmed and mutagenesis was performed using Quick-Change Site-Directed mutagenesis Kit (Stratagene, La Jolla, CA) according to manufacturer instructions. Primers used were: 5′-CTCATGAAAATGTATCACTTCAGGAGCGCTTGCTTCAGTTC-3′ for E-box 1 and 5′-CTTTTTAATAGCTTTGTGAAGTGACTGGGGACTGTAGTAGTAAG-3′ for E-box 2. Mutated sites in the above are indicated by bold underlined text.
Cell culture co-transfections
Each experiment was performed in triplicate, four times. Differentiated C2C12 cells were split and plated in 24-well plates at a density of 6 × 104 cells/cm2. Each well received 1 ng of the Renilla luciferase vector to follow transfection efficiency and was co-transfected with different combinations of reporter and Tfe3 cDNA containing vectors. A total of 50 ng of pGl3-Pgc-1alpha (or of each mutated construct), 50 ng of empty pGl3, and 240 ng of mouse Tfe3 expression construct in the pcDNA3.1+ vector were used. The total amount of DNA transfected per well was 0.8 μg. To maintain this concentration per well, pcDNA3.1+ empty vector was used. The cells were transfected using TransIT/LT1 transfection reagent (Mirus, Madison, WI) according to manufacturer instructions. The transfections were performed for 24 h, and then the cells were washed and lysed with passive lysis buffer (Promega). Aliquots of the supernatant were assayed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega). Luciferase activity was normalized relative to the Renilla activity to the control (empty vector).
Results
Tfe3 induces Pgc-1alpha expression
To assess whether Tfe3 is required for Pgc-1alpha expression, we first evaluated if Tfe3 ectopic overexpression modulates Pgc-1alpha levels. C2C12 myoblastic cell line (which expresses low levels of Pgc-1alpha) and mouse primary myoblasts from B6 mice were stably transduced with the mouse Tfe3 cDNA expression vector. Cells were differentiated into myotubes by incubation with HS for 4 days. Levels of mRNA and protein from myotubes overexpressing Tfe3 are shown in Figure 1A,B,E.
Fig. 1.
Effects of Tfe3 modulation on Pgc-1alpha expression. C2C12 cell line or primary myoblasts from B6 mice were stably transduced with retrovirus containing cDNA for the mouse Tfe3. After confluence, cells were exposed to the differentiation media and total RNA was harvested and reverse transcribed. Gene expression was analyzed by real-time PCR. A,B: Pgc-1alpha mRNA levels in C2C12 cell line and B6 myotubes, respectively. C,D: Pgc-1alpha target genes mRNA levels. E: Tfe3 protein levels in cells ectopically expressing Tfe3. F,G: Pgc-1alpha and target genes mRNA levels after knocking down Tfe3 in B6 myotubes. H: Protein levels of Tfe3 after knocking down Tfe3. I: Tfeb and Mitf mRNA levels after knocking down Tfe3. Experiments were performed in triplicates and the analysis of the qPCRs were obtained using the 2ΔΔCt method. Data are average ± SD relative to empty vector (n = 3) and statistics were performed using Student’s t-test (* P < 0.05).
Ectopic expression of Tfe3 caused a significant induction of Pgc-1alpha mRNA levels (Fig. 1A,B) as well as inducing the expression of the well-known Pgc-1alpha target genes Nduf5b, Cox5b, and Cycs (Fig. 1C,D) in myotubes. Additionally, we found that Pgc1b, the closest homolog of Pgc-1alpha (Lin et al., 2002a), was upregulated at lower levels when Tfe3 was overexpressed in B6 differentiated myoblasts (Fig. 1B). Two E-box elements were found in the promoter of Pgc-1beta (CACGTG at −278 from TSS, CATGTG at +818 from TSS) that may be occupied by MiT factors. Hence, higher levels of Pgc-1alpha mRNA are linked to ectopic expression of Tfe3.
Knocking down Tfe3 reduces Pgc-1alpha expression in primary myoblasts
We next investigated if the loss of function of Tfe3 affected Pgc-1alpha expression in primary differentiated myoblasts. These experiments were performed using pools of siRNA that knocked down Tfe3. A non-targeting pool siRNA was used as a negative control. Mouse primary myoblasts were differentiated, and then transfected with the corresponding siRNA pools. After 72 h, RNA and protein were obtained for RT-PCR and Western blot analysis. Figure 1F shows that individually knocking down Tfe3 in B6 differentiated myoblasts caused a significant downregulation (about 50%) of Pgc-1alpha expression and downregulation of some of its mitochondrial target genes (Fig. 1G). The efficiency of the knockdown was confirmed by Western blots (Fig. 1H). Interestingly, we found that mRNA levels of Pgc-1beta were also significantly affected by knocking down Tfe3. Due to the functional interconnection between MiT factors and Pgc1 coactivators it is not surprising to find that Pgc1-1beta may also be regulated by Tfe3. Recently it was shown that PGC-1alpha and PGC-1beta are key components in the production of pigments in melanocytes through the activation of MITF via alpha-MSH signaling pathway (Shoag et al., 2013). These results taken together demonstrate that Tfe3 is involved in Pgc-1alpha regulation in myotubes.
After knocking down Tfe3, some Pgc-1alpha mRNA levels remained. Therefore, we next investigated if other members of the MiT family potentially contribute to Pgc-1alpha expression upon knockdown of Tfe3. Functional redundancy within MiT members has been previously described (Weilbaecher et al., 1998; Motyckova et al., 2001; Steingrimsson et al., 2002; Huan et al., 2005; Davis et al., 2006). We examined levels of Tfeb and Mitf and found that Mitf mRNA significantly increased ~2-fold in myotubes upon suppression of Tfe3, while Tfeb levels remained unchanged (Fig. 1I).
Tfe3 occupies the endogenous Pgc-1alpha promoter
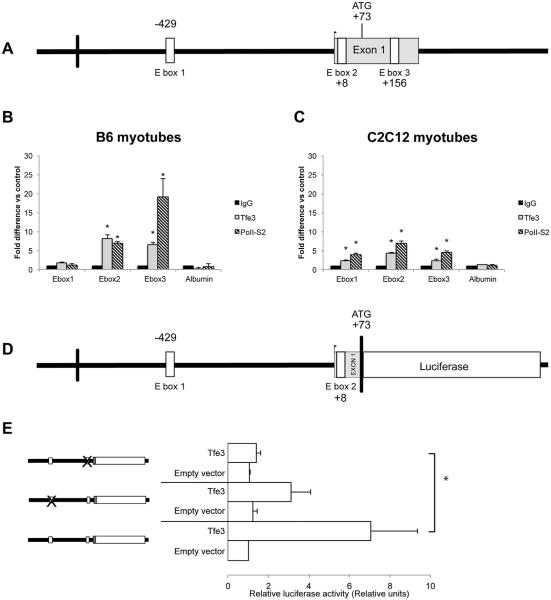
We expected that Tfe3, as a transcription factor, would directly regulate the expression of Pgc-1alpha by binding and transactivating Pgc-1alpha’s regulatory region. The in silico analysis of the mouse Pgc-1alpha proximal promoter showed three E-boxes with the consensus CA(C/T) GTG and CA(CA/TG)TG located at: −429, +8, and +156 relative to TSS (−568, −131, and +11 of ATG), denominated here: E-box 1, 2, and 3, respectively (Fig. 2A). These sites are conserved between species (Haq et al., 2013). The E-box at −429 CATGTG in mouse is homologous to another E-box CACATG in human; +8–131 CACATG is conserved in human; and, +156 CACATG is conserved in human.
Fig. 2.
Tfe3 is recruited to and transactivates the Pgc-1alpha promoter region in differentiated myoblasts. A: Structure of the murine Pgc-1alpha promoter showing the location of E-boxes. B: ChIP analysis of primary B6 myotubes and (C) differentiated C2C12 cells. Cells were induced to differentiate for 4 days in 2% HS containing media and cells were processed for ChIP assays. Soluble chromatin was immunprecipitated with pre-immune IgG, anti-Tfe3, and the RNA polymerase II (phospho S2) antibodies. Immunoprecipitates were subjected to qPCR using primers flanking the regions of the E-boxes on the Pgc-1alpha promoter (denominated here: E-box1, E-box 2, and E-box 3). Albumin was used as a control. Values were normalized to the input relative over the IgG control. Values are the average ± SD (n = 3). D: The murine Pgc-1alpha promoter region construct. An approximately 1,000-base pair fragment of the murine Pgc-1alpha promoter (upstream from the initiation site) was subcloned into the pGl3-luciferase vector. The positions of the E-boxes are: 429 and +8 from TSS. E: Mutations of E-boxes diminish luciferase activity. Luciferase activity was measured after co-transfecting Tfe3 with Pgc-1alpha WT or mutated individually in each construct into C2C12. A control renila plasmid was co-transfected for normalization purposes. The level of luciferase activity of the empty vector in the absence of Tfe3 was defined as 1. Fold activation was estimated to this level of activity. Values are the average ± SD (n = 3). Statistics were performed using Student’s t-test (* P < 0.05).
ChIPs performed in differentiated C2C12 and primary differentiated myoblasts showed that endogenous Tfe3 and polII-S2 were recruited to the Pgc-1alpha promoter, predominantly on E-box 2 and 3 (Fig. 2B,C). The ChIP signal from C2C12 myotubes is lower than in primary myotubes, and this correlates with lower levels of Pgc-1alpha mRNA.
Because of the DNA fragment size obtained after sonication (~500 bp), it is not possible to ascertain whether the ChIP signal came from E-box 2 or E-box3 or both.
The results discussed thus far show that Tfe3 binds to the Pgc1-1alpha promoter and thus appears to directly regulate Pgc-1alpha expression in skeletal muscle cells.
Tfe3 activates transcription from the Pgc-1alpha promoter
To evaluate whether the Pgc-1alpha promoter region could mediate transcriptional activation by Tfe3, a luciferase reporter construct was generated. The upstream regulatory promoter region of the mouse Pgc-1alpha gene containing two of the E-boxes (located upstream the ATG) was cloned into a luciferase reporter and analyzed for responsiveness to Tfe3 (Fig. 2D).
The Pgc-1alpha promoter-luciferase construct was co-transfected into C2C12 cells together with the full-length coding cDNA of the murine Tfe3 with the addition of differentiation media. Co-transfection with Tfe3 augmented luciferase activity by ~7-fold (Fig. 2E). Site-directed mutagenesis was performed to evaluate the requirement of both E-box sequences. Mutation of each E-box reduced the binding of Tfe3 and attenuated Pgc-1alpha promoter activity in C2C12, with the most suppression occurring for the E-box located +8 to the TSS (−131 upstream the ATG) (Fig. 2E).
Thus, transactivation of Pgc-1alpha by Tfe3 is significant and dependent on E box sites.
Discussion
In muscle, mitochondrial function, endurance, induction of angiogenesis, glucose, and lipid metabolism are connected to Pgc-1alpha’s coactivation activity (Arany, 2008). While a decrease in Pgc-1alpha expression in skeletal muscle has been linked to the pathogenesis of type II diabetes (Mootha et al., 2003; Patti et al., 2003; Handschin et al., 2007), its induction by exercise training protects from stress-induced depression (Agudelo et al., 2014). Therefore, modulation of Pgc-1alpha expression has been considered as a valuable potential therapeutic approach. However, surpassing physiological levels has been shown to be counter-productive by inducing insulin resistance (Miura et al., 2003; Choi et al., 2008). Nonetheless, fine-tuning Pgc-1alpha expression may still enable therapeutic benefits. Indeed, modest increases of Pgc-1alpha expression have been shown to improve insulin resistance in muscle of obese Zucker rats (Benton et al., 2010). In general, modulation of upstream signals or transcription factors that influence Pgc-1alpha regulation is of pivotal interest.
The regulation of Pgc-1alpha expression is mediated by environmental and cell-specific signals. These signals stimulate a number of transcription factors including: the myocyte enhancer factor 2 (MEF2), the forkhead box class-O (FoxO1), the activation transcription factor (ATF2), the cAMP response element-binding protein (CREB) (Fernandez-Marcos and Auwerx, 2011), Parkin-interacting substrate (PARIS) (Shin et al., 2011), and the MiT members Tfeb and MITF (Haq et al., 2013; Settembre et al., 2013; Vazquez et al., 2013).
In this report, we show that Tfe3, another member of the MiT family, transcriptionally regulates expression of Pgc-1alpha in myotubes. Tfe3 knockdown decreases and Tfe3 ectopic overexpression in myotubes induces expression of Pgc-1alpha and subsequently the expression of Pgc-1alpha’s target genes. Tfe3 directly binds and regulates Pgc-1alpha promoter consensus E-boxes within the Pgc-1alpha transcriptional regulatory region.
The role of Tfe3 in skeletal muscle was shown in a previous study using transgenic mice that selectively express Tfe3 in skeletal muscle (Iwasaki et al., 2012). The study showed that Tfe3 is involved in muscle metabolism by upregulation of hexokinase II (HkII), glucose transporter 4 (Glut4) and glycogen synthase (Gys) expression. The Tfe3 transgenic mice increased glucose uptake and glycogen synthesis in muscle, which consequently increased exercise endurance capacity. The upregulation of Glut4 and muscle Gys is not mediated directly by Tfe3. Based on our findings, we suspect that Tfe3 may mediate the activation of these genes indirectly through the regulation of Pgc-1alpha. Pgc-1alpha induces Glut4 expression by direct regulation of this gene (Michael et al., 2001b). Although direct regulation of muscle Gys by Pgc-1alpha has not been proven, there is a rapid depletion of glycogen in skeletal muscle in KO Pgc-1alpha mice (Zechner et al., 2010). Further studies using Tfe3 KO mice should help to determine the functional contribution of Tfe3 on those tissues where regulation of Pgc-1alpha expression relies on TFe3 transcriptional activity. Since the Tfe3 KO mouse is normal, it is likely that functional redundancy occurs between MiT members in certain tissues, therefore the impact of Tfe3 function can be determined by combined inactivation of other members (Weilbaecher et al., 1998; Steingrimsson et al., 2002; Yasumoto et al., 2002; Davis et al., 2003).
In summary our study demonstrates the direct regulation of Pgc-1alpha expression by Tfe3 and, together with previous studies, broadens the recognition of Pgc-1alpha as a transcriptional target of the MiT transcription factor family.
Acknowledgments
We thank Kyle Nitzsche and Rajini Mudhasani for important discussion and comments. This study was supported by the American Heart Association Fellowship (AHA) (nos. AHA-0726015T, R01 CA163336, RO1-AR043369, P01 CA163222, R01 HL094499), the PhRMA Foundation, the National Cancer Institute, the Melanoma Research Alliance, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the National Institutes of Health, the US-Israel Binational Science Foundation, and the Doris Duke Medical Research Foundation.
Contract grant sponsor: The American Heart Association Fellowship (AHA);
Contract grant numbers: AHA-0726015T, R01 CA163336, RO1-AR043369, P01 CA163222, R01 HL094499.
Contract grant sponsor: PhRMA Foundation. Contract grant sponsor: National Cancer Institute.
Contract grant sponsor: Melanoma Research Alliance. Contract grant sponsor: Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Contract grant sponsor: National Institutes of Health.
Contract grant sponsor: US-Israel Binational Science Foundation. Contract grant sponsor: Doris Duke Medical Research Foundation.
Literature Cited
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Petterson AJ, Ferreina DM, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Rual JL. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Arany Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr Opin Genet Dev. 2008;18:426–434. doi: 10.1016/j.gde.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S, St-Pierre J. PGC1alpha and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci. 2012;125:4963–4971. doi: 10.1242/jcs.113662. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Kadesch T. The leucine zipper of TFE3 dictates helix-loop-helix dimerization specificity. Genes Dev. 1991;5:1057–1066. doi: 10.1101/gad.5.6.1057. [DOI] [PubMed] [Google Scholar]
- Benton CR, Holloway GP, Han XX, Yoshida Y, Snook LA, Lally J, Glatz JF, Luiken JJ, Chabowski A, Bonen A. Increased levels of peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PGC-1alpha) improve lipid utilisation, insulin signalling and glucose transport in skeletal muscle of lean and insulin-resistant obese Zucker rats. Diabetologia. 2010;53:2008–2019. doi: 10.1007/s00125-010-1773-1. [DOI] [PubMed] [Google Scholar]
- Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang YJ, Liu ZX, Lee HY, Distefano A, Samuel VT, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IJ, Hsi BL, Arroyo JD, Vargas SO, Yeh YA, Motyckova G, Valencia P, Perez-Atayde AR, Argani P, Ladanyi M, et al. Cloning of an Alpha-TFEB fusion in renal tumors harboring the t(6;11)(p21;q13) chromosome translocation. Proc Natl Acad Sci USA. 2003;100:6051–6056. doi: 10.1073/pnas.0931430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IJ, Kim JJ, Ozsolak F, Widlund HR, Rozenblatt-Rosen O, Granter SR, Du J, Fletcher JA, Denny CT, Lessnick SL, et al. Oncogenic MITF dysregulation in clear cell sarcoma: Defining the MiT family of human cancers. Cancer Cell. 2006;9:473–484. doi: 10.1016/j.ccr.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S–890. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Nakagawa Y, Satoh A, Okuda K, Shingyouchi A, Naka A, Matsuzaka T, Iwasaki H, Kobayashi K, Yahagi N, et al. TFE3 controls lipid metabolism in adipose tissue of male mice by suppressing lipolysis and thermogenesis. Endocrinology. 2013;154:3577–3588. doi: 10.1210/en.2013-1203. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, Frederick DT, Hurley AD, Nellore A, Kung AL, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell. 2013;23:302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan C, Sashital D, Hailemariam T, Kelly ML, Roman CA. Renal carcinoma-associated transcription factors TFE3 and TFEB are leukemia inhibitory factor-responsive transcription activators of E-cadherin. J Biol Chem. 2005;280:30225–30235. doi: 10.1074/jbc.M502380200. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Naka A, Iida KT, Nakagawa Y, Matsuzaka T, Ishii KA, Kobayashi K, Takahashi A, Yatoh S, Yahagi N, et al. TFE3 regulates muscle metabolic gene expression, increases glycogen stores, and enhances insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2012;302:E896–E902. doi: 10.1152/ajpendo.00204.2011. [DOI] [PubMed] [Google Scholar]
- Kauffman EC, Ricketts CJ, Rais-Bahrami S, Yang Y, Merino MJ, Bottaro DP, Srinivasan R, Linehan WM. Molecular genetics and cellular features of TFE3 and TFEB fusion kidney cancers. Nat Rev Urol. 2014;11:465–475. doi: 10.1038/nrurol.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. The Journal of biological chemistry. 2002a;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002b;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Martina JA, Diab HI, Li H, Puertollano R. Novel roles for the MiTF/TFE family of transcription factors in organelle biogenesis, nutrient sensing, and energy homeostasis. Cell Mol Life Sci. 2014;71:2483–2497. doi: 10.1007/s00018-014-1565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA. 2001a;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci US. 2001b;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Xu Z, Zhang X, Wang L, Gimble JM, Lander ES, Rosen ED. Comparative epigenomic analysis of murine and human adipogenesis. Cell. 2010;143:156–169. doi: 10.1016/j.cell.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Kai Y, Ono M, Ezaki O. Overexpression of peroxisome proliferator-activated receptor gamma coactivator-1alpha down-regulates GLUT4 mRNA in skeletal muscles. J Biol Chem. 2003;278:31385–31390. doi: 10.1074/jbc.M304312200. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Motyckova G, Weilbaecher KN, Horstmann M, Rieman DJ, Fisher DZ, Fisher DE. Linking osteopetrosis and pycnodysostosis: Regulation of cathepsin K expression by the microphthalmia transcription factor family. Proc Natl Acad Sci USA. 2001;98:5798–5803. doi: 10.1073/pnas.091479298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Shimano H, Yoshikawa T, Ide T, Tamura M, Furusawa M, Yamamoto T, Inoue N, Matsuzaka T, Takahashi A, et al. TFE3 transcriptionally activates hepatic IRS-2, participates in insulin signaling and ameliorates diabetes. Nat Med. 2006;12:107–113. doi: 10.1038/nm1334. [DOI] [PubMed] [Google Scholar]
- Ooishi R, Shirai M, Funaba M, Murakami M. Microphthalmia-associated transcription factor is required for mature myotube formation. Biochim Biophys Acta. 2012;1820:76–83. doi: 10.1016/j.bbagen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 2005;29:S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rowe GC, Jiang A, Arany Z. PGC-1 coactivators in cardiac development and disease. Circ Res. 2010;107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Jurgen Klisch T, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoag J, Haq R, Zhang M, Liu L, Rowe GC, Jiang A, Koulisis N, Farrel C, Amos CI, Wei Q, et al. PGC-1 coactivators regulate MITF and the tanning response. Mol Cell. 2013;49:145–157. doi: 10.1016/j.molcel.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer ML, Rando TA, Blau HM. Gene delivery to muscle. Curr Protoc Hum Genet. 2002 doi: 10.1002/0471142905.hg1304s31. Chapter 13: Unit 13.4. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Tessarollo L, Pathak B, Hou L, Arnheiter H, Copeland NG, Jenkins NA. Mitf and Tfe3, two members of the Mitf-Tfe family of bHLH-Zip transcription factors, have important but functionally redundant roles in osteoclast development. Proc Natl Acad Sci USA. 2002;99:4477–4482. doi: 10.1073/pnas.072071099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Lin J, Krauss S, Tarr PT, Yang R, Newgard CB, Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- Strub T, Giuliano S, Ye T, Bonet C, Keime C, Kobi D, Le Gras S, Cormont M, Ballotti R, Bertolotto C, et al. Essential role of microphthalmia transcription factor for DNA replication, mitosis and genomic stability in melanoma. Oncogene. 2011;30:2319–2332. doi: 10.1038/onc.2010.612. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Lim JH, Chim H, Bhalla K, Girnun G, Pierce K, Clish CB, Granter SR, Widlund HR, Spiegelman BM, et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- Weilbaecher KN, Hershey CL, Takemoto CM, Horstmann MA, Hemesath TJ, Tashjian AH, Fisher DE. Age-resolving osteopetrosis: A rat model implicating microphthalmia and the related transcription factor TFE3. J Exp Med. 1998;187:775–785. doi: 10.1084/jem.187.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto K, Takeda K, Saito H, Watanabe K, Takahashi K, Shibahara S. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. Embo J. 2002;21:2703–2714. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, Collia D, Chen Z, Wozniak DF, Leone TC, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell metabolism. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhao Q, Zhou X, Mattei MG, de Crombrugghe B. TFEC, a basic helix-loop-helix protein, forms heterodimers with TFE3 and inhibits TFE3-dependent transcription activation. Mol Cell Biol. 1993;13:4505–4512. doi: 10.1128/mcb.13.8.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]