miRNA-specific regulation of gene expression has now been implicated in the development of a number of pathophysiologic processes that underlie diseases of the cardiovascular system1. As such, miRNAs have become attractive targets for the design of potential therapies aimed at atherosclerosis, myocardial infarction and cardiomyopathy. However, their ability to control multiple pathways in different tissue types leads to the possibility that their inhibition will result in unwanted effects. One challenge has therefore been the development of tissue or cell-specific miRNA therapeutic approaches that avoid these “off target” effects. In this issue of ATVB, Wang et al report the targeted delivery of miR-21 inhibitors for the treatment of arterial in-stent restenosis (ISR), and demonstrate how using an anti-miR-21-coated stent approach overcomes untoward side effects of systemic anti-miR-21 delivery2.
Despite advances in the prevention and management of coronary artery disease (CAD) risk factors, a substantial number of patients still require revascularization therapy to open obstructed vessels. Conventionally, metal stents were deployed in diseased coronary arteries to dilate the lumen and restore blood flow that is otherwise blocked by large atherosclerotic lesion(s). However, the risk of re-narrowing or re-stenosis of the diseased vessel caused by the proliferation of vascular smooth muscle cells and increased synthesis of extracellular matrix is high with this approach3, and stents that elute anti-proliferative drugs are now widely used to prevent ISR due to myointimal hyperplasia4. Current drug-eluting stents use small molecules that broadly inhibit the cell cycle to block proliferation, but do not specifically target the underlying mechanisms that accompany ISR, nor do they address mechanisms that prevail at later stages following stent implantation4. To address this challenge, Wang and colleagues investigated the regulatory role of microRNAs in ISR, using a humanized animal model in which balloon-injured human internal mammary arteries (hMA), with and without stenting, are transplanted into RNU rats2. The authors identified miR-21 as being highly upregulated in stented hMA in this model and confirmed the increase of miR-21 expression in human ISR tissue samples compared to CAD specimens. Indeed this miRNA has previously been associated with increased cell proliferation and decreased apoptosis in the vessel wall5, 6, and was also found to be upregulated in murine, pig and human vein graft failure models7., Furthermore, genetic deletion of miR-217 or antisense-mediated inhibition of miR-21 using pluronic gel8 limited the proliferative response and reduced myointima formation in murine models, further underscoring its promise as a therapeutic target for ISR.
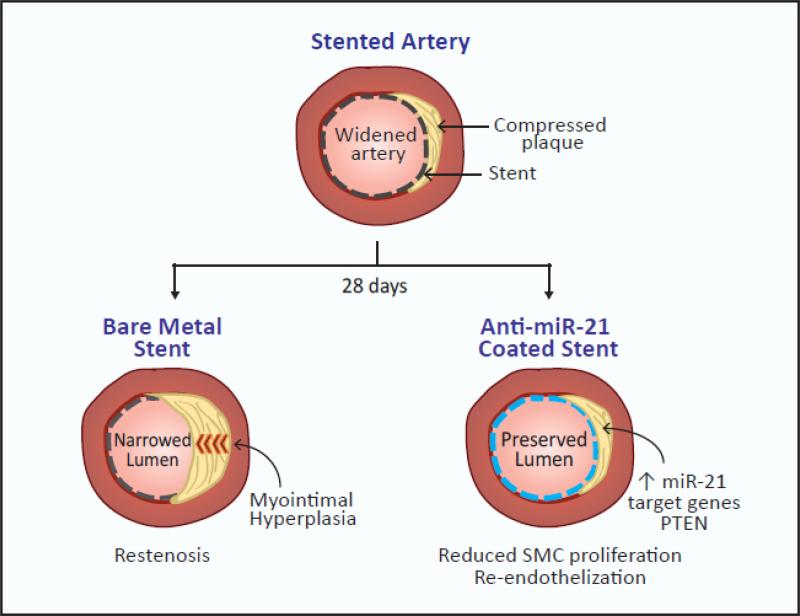
Wang et al. first attempted to inhibit miR-21 systemically in the hMA-RNU rat model by delivering a single intravenous dose of a fluorescently-labeled anti-miR-21 locked nucleic acid (LNA) one day after hMA implantation. The retrieval of the stented vessels 28 days later showed that systemic miR-21 inhibition markedly reduced luminal obliteration compared to untreated mice, demonstrating the effectiveness of miR-21 inhibition in reducing smooth muscle cell proliferation. However, despite these promising effects on myointimal hyperplasia, systemic anti-miR-21-LNA delivery also resulted in significant reductions of miR-21 expression in liver, heart, lung and kidneys, and was accompanied by an increase in serum creatinine levels suggesting a negative impact on renal function. To overcome these unwanted side effects, the investigators next tried a targeted approach in which stents were coated with the anti-miR-21 LNA to permit local delivery to the hMA vessels. After 28 days, vessels retrieved from the RNU rats showed that compared to otherwise identical bare metal stents, the anti-miR-21 coated stents markedly reduced ISR. Furthermore, no accumulation of the LNA was observed in the kidney, and anti-miR-21 did not delay vessel re-endothelization, a side effect of current drug-eluting stent medications such as rapamycin, which is believed to be a major contributor to stent thrombosis and late ISR9, 10. Although previous studies have suggested that miR-21 may regulate EC proliferation, anti-miR-21 treatment was not found to alter EC proliferation in the current study, which the authors suggest may be due to low endogenous miR-21 expression in the endothelium compared to the vascular smooth muscle cells that predominate in the myointimal response to injury.
An emerging risk following the placement of current generation drug-eluting stents in diseased arteries is the development of late thrombosis and neoatherosclerosis10, which is believed to result from a different mechanism than the initial proliferative response of early phase ISR. While the anti-proliferative drugs currently used in drug-eluting stents are unlikely to combat this problem, a study of genetic miR-21 ablation in mice reported that reductions in neointimal formation following stent implantation were accompanied by a decrease in macrophage inflammatory activation11. Although more work needs to be done to address this possibility, these data suggest that anti-miR-21-eluting stents may offer anti-inflammatory as well as anti-proliferative effects that would be advantageous for combating late-thrombosis in ISR.
This study is the first to employ anti-miRs for the treatment of ISR, and opens the door to the incorporation of anti-miRs into the arsenal of drugs available for use with drug-eluting stents. With the continuing discovery of miRNAs that modulate the vascular response to stent implantation, the potential for anti-miR combinations and timed-released formulations will no doubt offer interesting possibilities for the future. Finally, although this study represents an advance in targeting miRNAs in coronary arteries, we will need similarly creative solutions that allow anti-miR-based therapeutic modulation in a tissue- or cell-specific manner for the treatment of other disease conditions.
Figure 1.
Targeted delivery of miR-21 inhibitors for the treatment of arterial in-stent restenosis (ISR)
Acknowledgements
This work was supported by funding from the Canadian Institutes of Health Research (K.J.R: MOP130365, MSH130157) and from the National Institutes of Health (R01HL108182 to K.J.M; R01HL119047 to K.J.M. and K.J.R.)
References
- 1.Romaine SP, Tomaszewski M, Condorelli G, Samani NJ. Micrornas in cardiovascular disease: An introduction for clinicians. Heart. 2015;101:921–928. doi: 10.1136/heartjnl-2013-305402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Deuse T, Stubbendorff M, Chernogubova E, Erben R, Eken S, Jin H, Li Y, Heeger C, Behnisch B, Reichenspurner H, Robbins R, Spin J, Tsao P, Schrepfer S, Maegdefessel L. Local microrna modulation using a novel anti-mir-21-eluting stent effectively prevents experimental in-stent restenosis. Arteriosclerosis, thrombosis, and vascular biology. 2015 doi: 10.1161/ATVBAHA.115.305597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol. 2014;63:2659–2673. doi: 10.1016/j.jacc.2014.02.545. [DOI] [PubMed] [Google Scholar]
- 4.Alfonso F. Treatment of drug-eluting stent restenosis the new pilgrimage: Quo vadis? J Am Coll Cardiol. 2010;55:2717–2720. doi: 10.1016/j.jacc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell MV, Dalman RL, Spin JM, Tsao PS. Microrna-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Science translational medicine. 2012;4:122ra122. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li XX, Liu Y, Cheang TY, Huang XL, Wang SM. Microrna-21 regulates vascular smooth muscle cell function via targeting tropomyosin 1 in arteriosclerosis obliterans of lower extremities. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2044–2053. doi: 10.1161/ATVBAHA.111.229559. [DOI] [PubMed] [Google Scholar]
- 7.McDonald RA, White KM, Wu J, Cooley BC, Robertson KE, Halliday CA, McClure JD, Francis S, Lu R, Kennedy S, George SJ, Wan S, van Rooij E, Baker AH. Mirna-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. European heart journal. 2013;34:1636–1643. doi: 10.1093/eurheartj/eht105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. Microrna expression signature and antisense-mediated depletion reveal an essential role of microrna in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 9.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka F, Nakano M, Ladich E, Kolodgie FD, Virmani R. Pathologic etiologies of late and very late stent thrombosis following first-generation drug-eluting stent placement. Thrombosis. 2012;2012:608593. doi: 10.1155/2012/608593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald RA, Halliday CA, Miller AM, Diver LA, Dakin RS, Montgomery J, McBride MW, Kennedy S, McClure JD, Robertson KE, Douglas G, Channon KM, Oldroyd KG, Baker AH. Reducing in-stent restenosis: Therapeutic manipulation of mirna in vascular remodeling and inflammation. J Am Coll Cardiol. 2015;65:2314–2327. doi: 10.1016/j.jacc.2015.03.549. [DOI] [PMC free article] [PubMed] [Google Scholar]