Abstract
Our study highlights the surveillance of Bartonella species among rodents and their associated ectoparasites (ticks, fleas, lice, and mites) in several regions across Thailand. A total of 619 rodents and 554 pooled ectoparasites (287 mite pools, 62 flea pools, 35 louse pools, and 170 tick pools) were collected from 8 provinces within 4 regions of Thailand. Bandicota indica (279), Rattus rattus (163), and R. exulans (96) were the most prevalent species of rats collected in this study. Real-time PCR assay targeting Bartonella-specific ssrA gene was used for screening and each positive sample was confirmed by PCR using nuoG gene. The prevalence of Bartonella DNA in rodent (around 17%) was recorded in all regions. The highest prevalence of Bartonella species was found in B. savilei and R. rattus with the rate of 35.7% (5/14) and 32.5% (53/163), respectively. High prevalence of Bartonella-positive rodent was also found in B. indica (15.1%, 42/279), and R. norvegicus (12.5%, 5/40). In contrast, the prevalence of Bartonella species in ectoparasites collected from the rats varied significantly according to types of ectoparasites. A high prevalence of Bartonella DNA was found in louse pools (Polyplax spp. and Hoplopleura spp., 57.1%) and flea pools (Xenopsylla cheopis, 25.8%), while a low prevalence was found in pools of mites (Leptotrombidium spp. and Ascoschoengastia spp., 1.7%) and ticks (Haemaphysalis spp., 3.5%). Prevalence of Bartonella DNA in ectoparasites collected from Bartonella-positive rodents (19.4%) was significantly higher comparing to ectoparasites from Bartonella-negative rodents (8.7%). The phylogenetic analysis of 41 gltA sequences of 16 Bartonella isolates from rodent blood and 25 Bartonella-positive ectoparasites revealed a wide range of diversity among Bartonella species with a majority of sequences (61.0%) belonging to Bartonella elizabethae complex (11 rodents, 1 mite pool, and 5 louse pools), while the remaining sequences were identical to B. phoceensis (17.1%, 1 mite pool, 5 louse pools, and 1 tick pool), B. coopersplainensis (19.5%, 5 rodents, 1 louse pool, and 2 tick pools), and one previously unidentified Bartonella species (2.4%, 1 louse pool).
Introduction
Bartonella bacteria are new emerging pathogens causing diseases in humans and animals [1, 2]. The members of genus Bartonella are rod-shaped gram negative facultative intracellular bacteria that are fastidious and slow growing at aerobic conditions. They infect human and other mammalian hosts via infected-vectors such as fleas, ticks, and lice or the bite/scratch of an infected-animal [3–5]. Moreover, the infected arthropods could transmit Bartonella bacteria to human and other mammalian hosts via feces through superficial scratches in skin [6]; for example, B. henselae and B. quintana were transmitted to hosts via contaminated feces of infected cat fleas (Ctenocephalides felis) and human body lice (Pediculus humanus), respectively [7]. Pathogenesis involves the invasion of host’s erythrocytes, endothelial cells, and dendritic cells which play an important role in the first line immune response to fight against pathogens [8, 9]. As a result of the immune system failure, a bacteremia persistent infection might occur [8, 10].
Bartonella genus comprises over 30 species and subspecies [11]. At least thirteen known or suspected species are thought to contribute to blood-borne infections in human [12]. Moreover, several studies suggested the role of Bartonella species as a potential causative agent for cases of unknown febrile illness as well as endocarditis in patients in Thailand [13]. The diversity of Bartonella species in several countries in Southeast Asia (Lao PDR, Cambodia, and Thailand) has been reported and the findings revealed that Bartonella species in rodents are much more diverse than in other animals, except bats. The species found in rodents included B. elizabethae, B. coopersplainsensis, B. phoceensis, B. queenslandensis, B. rattimassiliensis, B. tribocorum and three genotypes presumably representing new Bartonella species [14].
Bartonella transmission occurs mainly via horizontal transmission when arthropod vectors acquire Bartonella bacteria during the feeding of infected host and later they become infected and the infected vectors then transfer the bacteria to another host [5, 15]. Interestingly, some studies suggested that vertical and transstadial transmissions of Bartonella species in Ixodes ticks [16], deer ked [17], and transplacental in rodent populations [18].
High prevalence of Bartonella DNA and genotype diversity have been detected in arthropod vectors around the world. For example, ticks collected from dogs and donkeys in Peru were found to carry several Bartonella species, such as B. rochalimae, B. quintana and B. elizabethae [19]. In Taiwan, B. tribocorum, B. elizabethae, B. queenslandensis, B. rochalimae-like bacteria, B. phoceensis, and B. rattimassiliensis were detected in fleas and louse pools [20]. Several studies in Thailand have reported the detection of B. henselae, B. clarridgeiae, and B. koehlerae from cats and flea pools collected from the Thai-Myanmar border [21] and in the Bangkok area [22]. Moreover, novel species such as B. tamiae was recently isolated from whole blood of febrile patients from Thailand [23] and DNA belonging to this species was also detected from the pools of ticks and mites collected from rats in Thailand [15].
Though a number of papers on Bartonella in rodents from Thailand have been published, the comparative analysis of bartonellae between rodent hosts and ectoparasites has not been done. Our aim was to investigate the prevalence and diversity of Bartonella species in rodents and their ectoparasites, and to estimate the importance of this host-vector relationship for the transmission of Bartonella species in natural habitats of Thailand. Our results indicated a significant difference between bacterial communities recognized in mammals and arthropods.
Materials and Methods
Study sites and samples processing
The study sites were located in different regions of Thailand. Rodents and their associated ectoparasites (ticks, fleas, mites, and lice) were collected from eight provinces within four regions of Thailand during the period of December 2012 to November 2013. The regions included the Northern region (Chiang Rai and Phayao provinces), the Southern region (Chumphon and Surat Thani provinces), the Eastern region (Rayong and Trat provinces) and the Northeastern region (Loei and Nong Bua Lam Phu provinces) (Table 1). This study was carried out on private lands and the owners of the lands gave permission to conduct the study on their sites and the field studies did not involve endangered or protected species. Rodents were captured by live traps baited with bananas or dried fish. Rodents were collected from orchards, cultivated rice-fields, grassland areas, edges of dense forest, stream margins, and around houses. Traps were set for 3–5 nights and were checked early in the morning. Then, rodents were removed from the traps and later identified to species [24]. Captured rodents were killed by carbon dioxide and processed on the same day and at the site of capture. Blood and serum samples and rodent tissue samples (liver, spleen, kidney and lung) were collected and stored on dry ice, and transported to the AFRIMS laboratory. Rodent’s ears were cut and stored in 70% ethanol for mite collections and the other ectoparasites (ticks, fleas, and lice) were collected from individual rodents by combing and stored in 70% ethanol for transportation to the laboratory. Mites in their larval stage (chigger) were collected from rodent’s ears by paintbrush under the stereomicroscope and pooled by host. Three to five mites were selected from each pool and mounted on glass slides for morphological identification to genera and species if possible using taxonomic key [25]. Ectoparasites of each type (fleas, ticks, and lice) were identified morphologically [26, 27] and pooled by host, type, stage, and gender in 1.5 ml microcentrifuge tube. Pools of ectoparasites were subjected to DNA extraction procedures as described below. Louse species identification was performed following the previously published protocol [28]. Details of the ectoparasites collected from rodents in this study are provided in Table A in S1 File.
Table 1. Location coordinates of rodents and ectoparasites collection sites in Thailand (2012–2013).
| Regions | Provinces | Districts | Sub-districts | Villages | Latitude | Longitude |
|---|---|---|---|---|---|---|
| North | Chiangrai | Mae Chan | Chanchawatai | Ban Pagook | 20°15' 16.042'' N | 99°56' 2.144'' E |
| Mae Chan | Pa Sang | Rong Khi | 20°10' 47.543'' N | 99°50' 48.505'' E | ||
| Phayao | Dok Khamtai | Ban Tham | Ban Sansai | 19°6' 54.234'' N | 100°3' 44.319'' E | |
| Dok Khamtai | Ban Tham | Ban Tham Mongkol | 19°6' 2.606'' N | 100°2' 25.71'' E | ||
| Northeast | Loei | Dan Sai | Na Di | Ban Na Mue Muen | 17°19' 15.859'' N | 101°8' 58.304'' E |
| Dan Sai | Na Di | Ban Na Ho | 17°19' 30.922'' N | 101°8' 51.766'' E | ||
| Dan Sai | Pak Man | Ban Pak Man | 17°29' 42.137'' N | 101°10' 48.572'' E | ||
| NongBua Lam Phu | Si Bun Rueang | Non Sa-at | Ban Wang Khaen | 16°55' 9.883'' N | 102°8' 58.138'' E | |
| Si Bun Rueang | Na Kok | Ban Non Ngam | 16°53' 42.23'' N | 102°13' 5.375'' E | ||
| East | Rayong | Pluak Daeng | Map Yang Phon | Sapan Seeyakmahanakhorn | 13°0' 59.404'' N | 101°8' 16.778'' E |
| Pluak Daeng | Map Yang Phon | Sasithorn16 | 13°0' 48.629'' N | 101°7' 5.664'' E | ||
| Pluak Daeng | Map Yang Phon | Bo Win | 13°1' 23.012'' N | 101°6' 38.523'' E | ||
| Trat | Khlong Yai | Khlong Yai | Ban Suan Maprao | 11°46' 51.946'' N | 102°52' 36.364'' E | |
| Khlong Yai | Hat Lek | Ban Khlong Son | 11°43' 52.561'' N | 102°54' 1.699'' E | ||
| South | Chumphon | Tha Sae | Tha Kham | Ban Dinkong | 10°39' 34.463'' N | 99°6' 11.62'' E |
| Mueang | Bangluek | Ban Salaloy | 10°39' 34.650'' N | 99°6' 12.592'' E | ||
| Mueang | Bangluek | Ban Nongnean | 10°34' 23.675'' N | 99°12' 56.937'' E | ||
| Surat Thani | Mueang | Wat Pradu | Wat Ma Pring | 9°6' 59.065'' N | 99°16' 34.42'' E | |
| Phunphin | Khao Hua Khwai | Bang Or | 9°5' 16.771'' N | 99°13' 14.005'' E |
Surveillance activities were conducted in 4 regions and 8 provinces in Thailand. The Northern region (Chiangrai and Phayao provinces), the Northeastern region (Loei and Nong Bua Lam Phu provinces), and the Southern region (Chumphon and Surat Thani provinces).
Genomic DNA extraction from rodent tissue and ectoparasites
Genomic DNA was extracted from rodent livers using the Wizard® Genomic DNA purification kit (Promega, Madison, WI) according to the manufacturer’s instructions with some modifications. Briefly, the liver tissue was cut into pieces of approximately 3 millimeters in diameter and added to 600 μl of Nuclei Lysis Solution (Promega, Madison, WI). The mixture was homogenized with beads using a TissueLyser II machine (Qiagen, Hilden, Germany) at 25 Hz for 5 min twice. Subsequently, the mixture was incubated with 20 μl of Proteinase K solution (20 mg/ml) at 55°C for 1 hr, and then with 3 μl of RNase A (10 mg/ml) at 37°C for 15 min. Then 200 μl of protein precipitation solution (Promega, Madison, WI) was added and mixed vigorously by vortex. The mixture was kept on ice for 5 min. Insoluble materials were removed by centrifugation at 13,000 rpm for 4 min and the supernatant was transferred to a new tube. DNA was precipitated by adding 600 μl of isopropanol and then centrifuged at 13,000 rpm for 1 min. DNA pellet was washed using 70% ethanol and dried by SpeedVac™ concentrator (Thermo Scientific, Waltham, MA). Two hundred microliters of EB buffer (10 mM Tris Cl, pH 8.5) were used to resuspend dried DNA and stored at -20°C until further analysis.
DNA extraction from the ectoparasites was performed according to the tissue extraction protocol from QIAamp® DNA Mini Kit (Qiagen, Hilden, Germany) with some modification. Briefly, pools of ticks, fleas and lice were puncture in the presence of liquid nitrogen in a 1.5 ml microcentrifuge tube. Mites were punctured with a fine needle under microscopy. Next, ninety microliters of ATL lysis buffer were added to each sample and mixed thoroughly. Then, ten microliters of Proteinase K solution (20 mg/ml) was added and incubated at 56°C for 3 hr. One hundred microliters of AL buffer was added to the samples and mixed by pulse-vortexing for 15 sec then incubated at 70°C for 10 min. After that, 100 μl of absolute ethanol was added and the mixture was mixed by pulse-vortexing for 15 sec. Finally, the mixture was transferred to a QIAamp spin column and DNA was eluted in 50 l AE buffer. DNA solution was stored at -20°C until further analysis.
Bartonella detection in rodent tissues and ectoparasites
DNA extracts obtained from rodent tissues and ectoparasites were screened for the presence of Bartonella species using real-time PCR assay (qPCR) with TaqMan probe. A genus-specific assay targeting a transfer-mRNA gene (ssrA) of Bartonella species was used in this study following previously published protocol [29]. The primer pair, ssrA-F/ ssrA-R, and ssrA-probe were used to amplify 301 bp fragment of ssrA gene. The qPCR reaction (25 μl) consisted of 12.5 μl Platinum® Quantitative PCR SuperMix-UDG (Invitrogen, Grand Island, NY), 0.5 μM of each primer, and 0.1 μM of ssrA-Probe and 2 μl of DNA template or nuclease free water as non-template control. The qPCR conditions were performed as follows: UDG incubation at 50°C for 2 min and then initial denaturation at 95°C for 2 min followed by 45 cycles of 95°C for 15 sec and 60°C for 30 sec using the Chromo4™ Real-Time Detector (Bio-Rad, Hercules, CA). Every sample with a positive signal from the screening assay was subjected to confirmatory test. A different target gene of NADH Dehydrogenase Gamma Subunit gene (nuoG) of Bartonella species was used to confirm the positivity by conventional PCR assay. A 346 bp fragment of nuoG gene was amplified with nuoG-F and nuoG-R primer pair according to previously published protocol [30]. The PCR reaction mixture (25 μl) consisted of 1X of PCR buffer, 0.2 μM of each primer, 0.2 mM of dNTP, 1.25 U of Taq DNA Polymerase (Invitrogen, Grand Island, NY) and 5 μl of DNA template or nuclease free water as non-template control. PCR amplification was carried out using the Veriti® 96-well Thermal Cycler (Applied Biosystems, Foster City, CA) with the initial denaturation at 94°C for 3 min followed by 45 cycles of denaturation at 94°C for 45 sec, annealing at 55°C for 1 min and extension at 72°C for 1 min 30 sec, then incubated at 72°C for 10 min for the final extension step. The amplification product of 346 bp was observed with 1.5% agarose gel electrophoresis under the UV visualization.
Bartonella culture from rodent blood
Selected rodents, which were Bartonella-positive by molecular assays, were subjected to Bartonella culture following previously published protocol [31]. At the field sample site, whole blood was collected from each rodent by cardiac puncture and preserved in EDTA. Rodent whole blood was kept in -70°C until use. Briefly, whole blood was retrieved from the -70°C freezer and thawed at 4°C. Then, homogeneous whole blood was diluted 1:4 in 1X Dulbecco’s Phosphate Buffered Saline (GIBCO, Grand Island, NY) containing 5–10% Fungizone (GIBCO, Grand Island, NY). Diluted blood sample (0.1 ml) was pipetted onto Brain Heart Infusion agar plates containing 5% rabbit blood (BBL, Becton Dickinson Microbiology Systems, Cockeysville, MD). Four to five agar plates were kept in a plastic bag and incubated at 37°C, 5% CO2 for up to 4 weeks. The agar plates were monitored once a week after the initial inoculation and once per three days after sub-culturing. Sub-culturing was continued until a pure culture was obtained. Bartonella-like colonies were recognized by colony morphology and then harvested into 10% sterile glycerol and kept in -70°C freezer for further confirmation and characterization. A portion of each Bartonella-like colony was subjected to DNA extraction using QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) following manufacturer’s instruction and confirmed to be Bartonella species by citrate synthase gene (gltA) sequence identity.
Citrate synthase gene (gltA) amplification
Amplification of Bartonella gltA gene was done using published primers, BhCS.781p and BhCS.1137n [32]. The PCR reaction (50 μL) consisted of 1X of PCR buffer, 2.5 mM of MgCl2, 0.2 μM of each primer, 0.2 mM of dNTP, 2.5 U of AmpliTaq Gold® DNA Polymerase (Applied Biosystems, Foster City, CA) and 2.5 μl of DNA template or nuclease free water as non-template control. The amplification conditions were as follows: the initial denaturation at 95°C for 3 min followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 56°C for 1 min and extension at 72°C for 1 min, then the final extension step at 72°C for 10 min. The amplification product (379 bp) was observed on 1.5% agarose gel electrophoresis.
DNA sequencing
Amplification products of ssrA or gltA genes were purified using QIAquick PCR Purification Kit (QIAGEN Inc., Valencia, CA) following the manufacturer’s instruction and sent for sequencing at AITbiotech Pte. Ltd. (Singapore)
Statistical analyses
The difference in the prevalence of Bartonella DNA in ectoparasites collected from Bartonella-positive and Bartonella-negative rodents was confirmed by Chi-Square Test and the critical range (P < 0.005) was used. The statistical calculations were performed with IBM® SPSS® Statisic (version 22) software (Chicago, IL).
Sequence and Phylogenetic analysis
Sequence data were assembled using the Sequencher 5.1 software (Gene Code Corporation, Ann Arbor, MI) and the consensus sequences were used for analyses. Sequences were aligned and constructed a similarity matrix with the reference sequences of Bartonella species using Muscle algorithm implemented in MEGA 6.0 software [33]. Maximum likelihood (ML) trees based on Kimura’s 2-parameter model (K2+G+I) were constructed using molecular evolutionary genetics analysis (MEGA) 6.0 software [33] and bootstrap analyses with 1,000 resamplings performed to test the robustness of the branching.
Ethics Statement
Rodents trapping were carried out in the different locations of the provinces according to the institutional animal collection protocol entitled “Field Sampling of Small Mammal (Orders: Erinaceomorpha; Soricomorpha; Scandentia; Macroscelidea and Rodentia) Populations to Support Zoonotic Diseases Surveillance and Ectoparasite Collection” (PN# 12–06) reviewed and approved by the USAMC-AFRIMS Institutional Animal Care and Use Committee (IACUC). All sampling procedures and experimental manipulations were reviewed and approved as part of obtaining the animal collection protocol (PN# 12–06). Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
Results
Prevalence rate of Bartonella species among rodent and their associated ectoparasites in Thailand
A total of 619 rodents, 287 mite pools (3–153 mites/pool), 62 flea pools (1–14 fleas/pool), 35 louse pools (1–20 lice/pool), and 170 tick pools (1–24 ticks/pool) were collected from eight provinces within four regions of Thailand. Overall prevalence of Bartonella species in rodents was 17.6% (109/619); and prevalence in their associated ectoparasites varied substantially depending on ectoparasite type (Table 2). The highest prevalence was found in lice (57.1%, 20/35 pools) followed by fleas [25.8%, 16/62 pools (15 pools of females and 1 pool of males, Table B in S1 File)], with evident decline in ticks (3.5%, 6/170 pools of female ticks, Table C in S1 File) and mites (1.7%, 5/287 pools). A high Bartonella prevalence was found in rats collected from the North (22.9% in Phayao), the East (25.6% in Rayong), and the South (26.6% in Chumphon and 28.3% in Surat Thani), and a high prevalence in their ectoparasites was found in Eastern regions (23.3% in Rayong and 30.8% in Trat) as shown in Table 2.
Table 2. Prevalence of Bartonella DNA among wild-caught rodents and their associated ectoparasites collected from different regions and provinces in Thailand, 2012–2013.
| No. of Bartonella DNA positive/total collected (% positive) a | |||||||
|---|---|---|---|---|---|---|---|
| Rodent-associated ectoparasites | |||||||
| Region | Provinces | Rodents | Mite pool | Flea pool | Louse pool | Tick pool | Total ectoparasites |
| North | Chiangrai | 19/138 (13.8) | 0/57 (0) | - | 1/2 (50.0) | - | 1/59 (1.7) |
| North | Phayao | 14/61 (22.9) | 0/15 (0) | 0/2 (0) | - | 5/157 (3.2) # | 5/174 (2.9) |
| Northeast | Loei | 6/53 (11.3) | 0/25 (0) | 3/11 (27.3) | - | 0/7 (0) | 3/43 (7.0) |
| Northeast | NongBua Lam Phu | 3/42 (7.1) | 0/8 (0) | 2/26 (7.7) £ | 0/1 (0) | - | 2/35 (5.7) |
| East | Rayong | 10/39 (25.6) | 0/14 (0) | 1/2 (50.0) | 5/8 (62.5) | 1/6 (16.7) | 7/30 (23.3) |
| East | Trat | 6/101 (5.9) | 0/15 (0) | 9/20 (45.0) | 3/4 (75.0) | - | 12/39 (30.8) |
| South | Chumphon | 21/79 (26.6) | 5/73 (6.8) | - | 9/16 (56.3) | - | 14/89 (15.7) |
| South | Surat Thani | 30/106 (28.3) | 0/80 (0) | 1/1 (100.0) | 2/4 (50.0) | - | 3/85 (3.5) |
| Total | 109/619 (17.6) | 5/287 (1.7) | 16/62 (25.8) | 20/35 (57.1) | 6/170 (3.5) | 47/554 (8.5) | |
a Bartonella DNA was detected by ssrA and nuoG genes. The positivity for each sample was recorded only when 2 assays produced the concordant results.
# Two B. indica rats had 2 positive tick pools.
£ One R. exulans had 2 positive flea pools.
Prevalence of Bartonella DNA in rodent species and their associated ectoparasites (mite, louse, flea, and tick)
Bandicota indica (45.1%, 279/619), Rattus rattus (26.3%, 163/619) and R. exulans (15.8%, 98/619) were the rodent species with highest rates of Bartonella prevalence (Table 3). A high prevalence of Bartonella DNA was also found in B. savilei (35.7%) and R. rattus (32.5%) followed by R. sabanus (16.7%), B. indica (15.1%), R. norvegicus (12.5%), and R. exulans (3.1%). Rats of B. indica and R. rattus were heavily infested with a variety of ectoparasites (mite, tick, and louse) accounting for 84.3% of all ectoparasites collected from rodents in this study (Table 4).
Table 3. Distribution of Bartonella DNA among rodent species in different regions and provinces of Thailand, 2012–2013.
| No. of Bartonella DNA positive/total collected (% positive) a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| North | Northeast | East | South | ||||||
| Rodent species | Chiangrai | Phayao | Loei | Nong Bua Lam Phu | Rayong | Trat | Chumphon | Surat Thani | Total |
| B. indica | 17/119(14.3) | 11/56(19.6) | 1/4(25.0) | 1/1(100) | 0/2(0) | 0/13(0) | 0/20(0) | 12/64(18.8) | 42/279(15.1) |
| B. savilei | 2/9(22.2) | - | - | 2/4(50.0) | - | - | 1/1(100) | - | 5/14(35.7) |
| Mus caroli | - | - | 0/1(0) | - | - | - | - | - | 0/1(0) |
| M. cervicolor | 0/1(0) | - | - | - | 0/2(0) | - | - | - | 0/3(0) |
| R. berdmorei | - | - | - | - | - | - | 0/1(0) | - | 0/1(0) |
| R. bukit | - | - | 0/3(0) | - | - | - | - | - | 0/3(0) |
| R.exulans | 0/3(0) | 2/2(100) | 1/26 (3.8) | 0/33(0) | 0/1(0) | 0/28(0) | 0/1(0) | 0/2(0) | 3/96(3.1) |
| R. losea | 0/3(0) | - | - | - | - | - | - | - | 0/3(0) |
| R. norvegicus | - | - | - | - | 3/17(17.6) | 2/23(8.7) | - | - | 5/40(12.5) |
| R. rattus | 0/3(0) | 1/3(33.3) | 3/3(100) | 0/4(0) | 7/17(41.2) | 4/37(10.8) | 20/56(35.7) | 18/40(45.0) | 53/163(32.5) |
| R. sabanus | - | - | 1/6(16.7) | - | - | - | - | - | 1/6(16.7) |
| R. surifer | - | - | 0/10(0) | - | - | - | - | - | 0/10(0) |
| Total | 19/138(13.8) | 14/61(22.9) | 6/53(11.3) | 3/42(7.1) | 10/39(25.6) | 6/101(5.9) | 21/79(26.6) | 30/106(28.3) | 109/619(17.6) |
a Bartonella DNA was detected by ssrA and nuoG genes. The positivity for each sample was recorded only when 2 assays produced the concordant results.
Table 4. Prevalence of Bartonella DNA-positive ectoparasites by rodent species based on detection of the ssrA gene fragment.
| No. of Bartonella DNA-positive pools/total collected (% positive) | |||||
|---|---|---|---|---|---|
| Host species | Mites | Fleas | Lice | Ticks | All ectoparasites |
| B. indica | 0/153 (0) | 0/5 (0) | 1/3 (33.3) | 5/153 (3.3) | 6/314 (1.9) |
| B. savilei | 0/9 (0) | - | 1/1 (100) | - | 1/10 (10.0) |
| Mus. caroli | - | - | - | - | - |
| M. cervicolor | - | - | - | - | - |
| R. berdmorei | 0/1 (0) | - | - | - | 0/1 (0) |
| R. bukit | 0/2 (0) | - | - | 0/1 (0) | 0/3 (0) |
| R. exulans | - | 13/45 (28.9) | 0/1 (0) | 0/1 (0) | 13/47 (27.7) |
| R. losea | 0/1 (0) | - | - | - | 0/1 (0) |
| R. norvegicus | 0/1 (0) | 1/1 (100) | 1/2 (50.0) | 0/2 (0) | 2/6 (33.3) |
| R. rattus | 5/104 (4.8) | 2/11 (18.2) | 17/28 (60.7) | 1/10 (10.0) | 25/153 (16.3) |
| R. sabanus | 0/6 (0) | - | - | 0/2 (0) | 0/8 (0) |
| R. surifer | 0/10 (0) | - | - | 0/1 (0) | 0/11 (0) |
| Total | 5/287 (1.7) | 16/62 (25.8) | 20/35 (57.1) | 6/170 (3.5) | 47/554 (8.5) |
The lice were identified as Polyplax spp. (61.1%) and Hoplopleura spp. (38.9%), fleas were identified as Xenopsylla cheopis, and ticks were identified as Haemaphysalis spp. Mites collected from rats were more diverse than other types of ectoparasites. Three to five mites were selected from each pool and morphologically identified to genus (subgenus), and species if possible. They were all identified as trombiculid mites (Trombiculidae family, Trombiculinae subfamily). The most predominant genera were as follows: Gahrliepia (39.9%), Leptotrombidium (34.3%), Ascoschoengastia (14.6%), Blankaartia (5.4%), Schoengastia (4.0%), Helenicula (1.5%) and Lorillatum (0.3%). Two major genera collected from rats were further identified to subgenus. The results showed that within the genus Gahrliepia, subgenus Walchia was the most prevalent followed by Schoengastiella, and then Gahrliepia. Almost all mites in Leptotrombidium genus belonged to subgenus Leptotrombidium.
Among the tested ectoparasites, a high prevalence of Bartonella DNA was detected in lice (57.1%, 20/35) and fleas (25.8%, 16/62). High prevalence of Bartonella DNA was detected in 17/28 louse pools (60.7%) collected from R. rattus and 13/45 flea pools collected from R. exulans. Mites and ticks were mostly collected from B. indica and R. rattus and only 1.7% (5/287) of mite pools and 3.5% (6/170) of tick pools were positive for Bartonella DNA. Among Bartonella-positive trombiculid mites, 3 pools were Leptotrombidium genus, 1 pool was Ascoschoengastia genus, and the last pool did not have slide for morphological identification.
Identification of Bartonella species in rodent hosts and their associated ectoparasites based on gltA sequence variations
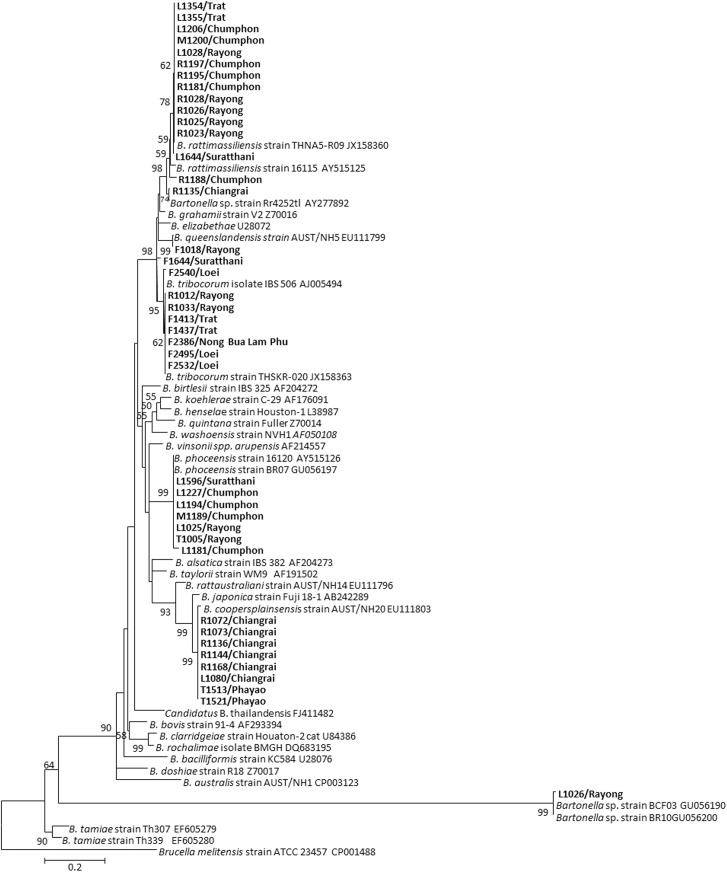
A total of 16 Bartonella isolates were successfully cultured from 26 individual rodent blood samples. Bartonella species detected from rats and ectoparasites based on sequences and phylogenetic analyses of 318 bp gltA amplicon are presented in Table 5 and Fig 1. Percent identity to the reference Bartonella gltA sequences of Bartonella species detected in this study are summarized in Table 6. Maximum-likelihood (ML) tree (Fig 1) shows the relationship between sequences generated from Bartonella-positive samples clustered into 8 different cladograms as described below. The majority of identified sequences (25/41 sequences) fell within B. elizabethae species complex. Within this B. elizabethae complex group (25 sequences), 15 B. rattimassiliensis were detected from 9 rats, 5 louse pools, and 1 mite pool sharing 96.5–100% identity with strain 16115 (AY515125) and strain THNA5-R09 (JX158360). Eight sequences of B. tribocorum were detected from 2 rats and 6 flea pools sharing 99.3–100% identity with strain IBS506 (AJ005494) and strain THSKR-020 (JX158363). One sequence of B. queenslandensis was detected from a flea pool sharing 99.6% identity with strain AUST/NH5 (EU111799). One sequence of undescribed Bartonella species within B. elizabethae complex was detected from a flea pool (#F1644) with a percent identity ranging from 96.8 to 97.1% to B. tribocorum strain IBS506 (AJ005494) and strain THSKR-020 (JX158363). The rest of the detected Bartonella sequences (16 sequences) were identified into eight B. coopersplainsensis which were detected from 5 rats, 2 tick pools, and 1 louse pool sharing 99.0–100% identity with strain AUST/NH20 (EU111803). Seven B. phoceensis were detected solely from ectoparasites: 5 in louse pools, 1 in a mite pool, and 1 in a tick pool sharing 98.4–100% identity with B. phoceensis strain 16120 (AY515126) and B. phoceensis strain BR07 (GU056197). Interestingly, one sequence obtained from Bartonella-positive louse pool (#L1026) was distantly related to the rest of the Bartonella species (66.3–68.2% identity) detected in this study, however, it was similar to some new strains of Bartonella species strain BCF03 (GU056190) and strain BR10 (GU056200) obtained from Taiwan with 99.3% identity (Table 6).
Table 5. Bartonella species identified in rodents and their associated ectoparasites based on gltA gene sequence similarities.
| Bartonella species (gltA) | Rodents | Mites | Fleas | Lice c | Ticks | Total |
|---|---|---|---|---|---|---|
| B. rattimassiliensis | 9 | 1 a | - | 5 | - | 15 (36.6%) |
| B. tribocorum | 2 | - | 6 | - | - | 8 (19.5%) |
| B. elizabethae | - | - | - | - | - | - |
| B. queenslandensis | - | - | 1 | - | - | 1 (2.4%) |
| Other Bartonella of B. elizabethae species complex | - | - | 1 | - | - | 1 (2.4%) |
| B. phoceensis | - | 1 b | 5 | 1 | 7 (17.1%) | |
| B. coopersplainsensis | 5 | - | - | 1 | 2 | 8 (19.5%) |
| New Bartonella species (GU056190) | - | - | - | 1 d | - | 1 (2.4%) |
| Total | 16 (39.0%) | 2 (4.9%) | 8 (19.5%) | 12 (29.3%) | 3 (7.3%) | 41 (100%) |
a L. deliense.
b Mite species was not available since there were only 3 mites collected from this host and no slide was made for species identification.
c Bartonella DNA was equally detected in Polyplax and Hoplopleura lice.
d Polyplax spp.
Fig 1. Phylogenetic relationship between gltA sequences of Bartonella species.
Bartonella species detected from rodents and their associated ectoparasites; mite (M), flea (F),tick (T), and louse (L), along with reference sequences (GenBank accession numbers are noted after each sequence). Only bootstrap replicates of >50% are shown. The Bartonella species detected in this study are indicated in bold letters.
Table 6. Percent similarity of gltA sequence for Bartonella identification detected from rodents (R) and their associated ectoparasites; Mite (M), flea (F), tick (T), and louse (L).
| % Similarity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Names | B. elizabethae | B. rattimassiliensis strain 16115 | B. rattimassiliensis strain THNA5-R09 | B. queenslandensis strain AUST/NH5 | B. tribocorum isolate IBS506 | B. tribocorum strain THSKR-020 | B. phoceensis strain 16120 | B. phoceensis strain BR07 | B. coopersplainsensis strain AUST/NH20 | B.grahamii strain V2 | Bartonella species strain BCF03 | Bartonella species strain BR10 |
| F1644 | 95.9 | 93.4 | 94 | 95.6 | 97.1 | 96.8 | 88.4 | 88.4 | 85.9 | 96.5 | 67.2 | 67.2 |
| R1135 | 94 | 97.1 | 97.1 | 93.1 | 94.7 | 94.3 | 87.8 | 87.8 | 85.3 | 95.3 | 67.2 | 67.2 |
| R1188 | 92.8 | 96.5 | 96.5 | 92.2 | 92.8 | 92.5 | 87.8 | 87.8 | 85 | 94.3 | 67.2 | 67.2 |
| Group 1 a | 93.1 | 97.5 | 100 | 92.2 | 93.4 | 93.1 | 87.2 | 87.2 | 84.1 | 94.3 | 66.6 | 66.6 |
| L1644 | 93.4 | 97.8 | 99.6 | 92.5 | 93.7 | 93.4 | 87.5 | 87.5 | 84.4 | 94.7 | 66.3 | 66.3 |
| F1018 | 93.7 | 92.2 | 92.5 | 99.6 | 94 | 93.7 | 87.5 | 87.5 | 83.4 | 95.3 | 67.9 | 67.9 |
| F2540 | 94.7 | 92.2 | 92.8 | 94.3 | 99.3 | 99 | 88.1 | 88.1 | 85.6 | 95.3 | 67.2 | 67.2 |
| Group 2 b | 94.3 | 92.5 | 93.1 | 93.4 | 99.6 | 100 | 89 | 89 | 85.9 | 95.6 | 67.2 | 67.2 |
| Group 3 c | 88.1 | 87.2 | 87.2 | 87.8 | 88.7 | 89 | 100 | 100 | 88.7 | 90.3 | 67.6 | 67.6 |
| L1181 | 87.5 | 86.2 | 86.2 | 87.2 | 87.8 | 88.1 | 98.4 | 98.4 | 87.8 | 89.4 | 67.6 | 67.6 |
| Group 4 d | 85.6 | 85.6 | 85 | 84.4 | 86.9 | 86.6 | 89.7 | 89.7 | 99 | 87.5 | 66.6 | 66.6 |
| L1026 | 66.6 | 65.4 | 66.3 | 67.6 | 67.2 | 66.9 | 67.2 | 67.2 | 66.3 | 68.2 | 99.3 | 99.3 |
a Group 1 consists of sample no. R1023, R1025, R1026, R1028, R1181, R1195, R1197, L1028, M1200, L1206, L1354, and L1355.
b Group 2 consists of sample no. R1012, R1033, F1413, F1437, F2386, F2495, and F2532.
c Group 3 consists of sample no. T1005, L1025, M1189, L1194, L1227, and L1596.
d Group 4 consists of sample no. R1072, R1073, R1136, R1144, R1168, L1080, T1513, and T1521.
The distribution of Bartonella species found in the North and northeast seems to be a region-specific distribution. Four samples of B. tribocorum were found in the Northeast and 8 samples of B. coopersplainsensis were detected in the North, even though one B. rattimassiliensis was also detected in the North (Fig 1 and Table 5). In contrast, Bartonella species found in samples collected from the East and the South are quite diverse consisting of six different species; B. rattimassiliensis, B. queenslandensis, B. tribocorum, Bartonella species within B. elizabethae species complex, and a presumably new strain of Bartonella species.
In this study, we found that the distribution of Bartonella species among rodents supports a host-specific pattern. Thus, five B. coopersplainsensis were solely detected from B. indica. Almost all B. rattimassiliensis (8/9) were detected from R. rattus, while two B. tribocorum were detected from R. norvegicus only. However, no specific pattern has been observed among ectoparasites. For example, two different Bartonella species (B. rattimassiliensis and B. phoceensis) were detected in mite pools (Table 5), 3 species (B. tribocorum, B. queenslandensis and other Bartonella of B. elizabethae species complex) were detected in flea pools (Xenopsylla cheopis), 4 species (B. rattimassiliensis, B. phoceensis, B. coopersplainsensis and new Bartonella species (GU056190)) were detected in louse pools (Polyplax spp. and Hoplopleura spp.), and 2 species (B. phoceensis and B. coopersplainsensis) were detected in tick pools (Haemaphysalis spp.).
Prevalence and distribution of Bartonella species detected in ectoparasites collected from Bartonella-positive and Bartonella-negative rats
The difference of Bartonella DNA prevalence in ectoparasites collected from Bartonella-positive and Bartonella-negative rats was investigated and presented in Table 7. Among Bartonella-positive rats, 20/103 (19.4%) ectoparasite pools were positive for Bartonella species. In contrast, only 27/309 (8.7%) ectoparasite pools were positive from Bartonella-negative rats. Of these Bartonella-positive ectoparasites, louse pools possessed the highest prevalence of 65% (13/20) and 46.7% (7/15) for positive and negative rats, respectively (Table 7). In general, Bartonella DNA prevalence in ectoparasites collected from positive rats (19.4%) were higher significantly (Chi-Square Tests, P = 0.003) comparing to ectoparasites from negative rats (8.7%).
Table 7. Comparison of Bartonella prevalence in ectoparasites collected from Bartonella-positive and Bartonella-negative rodents.
| Prevalence of Bartonella species in vectors among Bartonella-positive rodent | Prevalence of Bartonella species in vectors among Bartonella-negative rodents | |||||
|---|---|---|---|---|---|---|
| Ectoparasites | No. of positive | No. of infested rodent host | % positive | No. of positive | No. of infested rodent host | % positive |
| Mites | 4 | 69 | 5.8 | 1 | 218 | 0.5 |
| Fleas a | 1 | 4 | 25 | 15 | 50 | 30 |
| Ticks a | 2 | 10 | 20 | 4 | 26 | 15.4 |
| Lice | 13 | 20 | 65 | 7 | 15 | 46.7 |
| Total | 20 | 103 | 19.4 b | 27 | 309 | 8.7 b |
a There are 2 samples where Bartonella was detected in host (R. rattus), mite, and lice; 1–4 pools of ticks and fleas can be collected from one rodent.
b Bartonella DNA prevalence in ectoparasites collected from positive rats (19.4%) were higher significantly (Chi-Square Tests, P = 0.003) comparing to ectoparasites from negative rats (8.7%).
Discussion
Our study highlights the surveillance of Bartonella species among rodents and their associated ectoparasites (ticks, fleas, lice, and mites) in several regions across Thailand. The data demonstrated the high prevalence of Bartonella DNA in rats and their associated ectoparasites, especially in lice and fleas, as well as the finding of a diverse range of Bartonella species circulated among them. Previous studies have shown the high prevalence of Bartonella species among rats captured from Chiang Rai province (8.7%) [34] and from several regions across the country (41.5%) [35]. Although the prevalence in the latter study by Bai et al. was higher (41.5%, 137/330) than what we found (17.6%, 109/619), the proportion of rodent species are relatively the same except that B. indica was the highest number trapped in our study (45.1%, 279/619), while Bai et al. reported R. rattus as the most prevalent species investigated in their study (65.2%, 88/135). Given the higher rate of Bartonella-positive detected in R. rattus comparing to B. indica rat, the high Bartonella prevalence reported in Bai’s study can be explain by rodent species composition. Similarly, high prevalence (41.3%) of Bartonella was reported in rodents and shrews in Taiwan with the highest prevalence was found in R. norvegicus (52.7%) [36].
Although Bartonella DNA was detected in all types of ectoparasites (ticks, lice, fleas, and mites) collected from rats in this study, the prevalence varied substantially between ectoparasite types. The highest rate was found in lice and fleas that were in agreement with the study conducted in Taiwan [20]. Though Bartonella DNA was found in lower prevalence in ticks and mite pools in both studies, Kabeya et al. reported high prevalence in mites (82.9%) collected from rats in Thailand and all Bartonella species detected from mites were identified into B. tamiae based on their DNA sequences [15]. Although we do not believe that rat-associated lice can transmit pathogens to humans because of their high specificity to the host, for example, Polyplax and Hoplopleura lice are more likely specific to Rattus rats or to some other relative rat species [37, 38], the circulation of bartonellae via different ectoparasites, including lice, can influence the diversity of a rat-associated Bartonella community.
A diverse range of Bartonella species were isolated from whole blood of rats and shrews, including B. rattimassiliensis, B. grahamii, B. elizabethae, B. tribocorum, B. coopersplainensis, B. phoceensis, B. queenslandensis, and unknown genogroup [20, 32, 35, 36]. While only three of these species, B. rattimassiliensis, B. tribocorum, B. coopersplainensis, were isolated from rats in our study. Bartonella species found in rodent-associated ectoparasites were more diverse with seven different species found, including species isolated from their rat hosts. In this study, gltA gene sequence was used to identify Bartonella species, although ssrA gene was also sequenced and analyzed. We found that both genes were effective to identify Bartonella at the genus level; however, gltA gene sequence was found to be more suitable for species identification than ssrA gene sequence since the gltA sequence has expressed a higher range of variation comparing to the ssrA. Moreover, the availability of gltA reference sequences for Bartonella species in available databases is much higher than for ssrA genes that makes it a reliable and accurate tool for Bartonella species identification. Additionally, phylogenetic trees constructed from Bartonella culture isolates using both ssrA and gltA genes created quite similar phylogenetic tree topologies as shown in S1 Fig.
Kabeya et al. [15] reported the detection of B. tamiae, previously isolated from Thai febrile illness patients, in mites and tick pools collected from rats in Thailand. Two dominant mite genus most infected with B. tamiae were Leptotrombidium (66.7%) and Schoengastia (78.6%), therefore, the author suggested the role of mite as potential vector for B. tamiae transmission to human patients. In this study, Bartonella DNA was also detected from mites of Leptotrombidium and Ascoschoengastia genera, although B. tamiae was not found in our study. Our finding supports the role of trombiculid mite as a vector for Bartonella transmission. Because of the difficulty of mite species identification, the mite species in each pool was determined based on 3–5 mites selected from each pool and mounted on a glass slide as mentioned in materials and methods.
From our study, we found that Bartonella positive ectoparasites from Bartonella-positive rats was higher than in Bartonella-negative rats suggesting that this environment promotes the occurrence of horizontal transmission of Bartonella bacteria during the bite/feeding of vectors on rats. However, in order to prove infection route, transmission studies from an infected vector to naïve hosts/rats should be done in a controlled laboratory environment. Seasonal dynamics of Bartonella infection in natural populations of rats can also obscure a correlation between prevalence in rats and ectoparasites.
Bandicota and Rattus rats are the most common reservoir hosts for Bartonella infection in Southeast Asia which raises a question about the potentially important role of these rodent-borne agents as sources of febrile illness in human populations in Thailand. Detection of Bartonella DNA similar to B. tamiae, isolated from three febrile patients [23], and from mites collected from rodents in Thailand [15] imply a potential connection role for transmission of the disease to humans. Data from Kosoy et al. 2010, support this potential transmission route came from isolation of Bartonella species, previously identified from rodent hosts, from Thai patients’ blood and supported by analysis of history of the patients having an exposure to rats during the 2 weeks before the illness [35]. Bartonella species identified in these human cases included B. elizabethae, B. rattimassiliensis, and B. tribocorum, and the last two of these species were also detected in rodents, mites, fleas, and lice reported in this study.
Disclaimer
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Supporting Information
The GenBank accession numbers are shown for each reference sequences. Trees constructed from ssrA and gltA genes were able to discriminate all samples into two major clusters (C1 and C2), although some different branching patterns of sequences in C1 group (R1012, R1033, R1135, and R1188) were noticed.
(TIF)
Detail information of ectoparasites collected from rodents in this study (Table A).Bartonella DNA detection in flea pools classified by gender (Table B) and in tick pools classified by stage and gender (Table C)
(DOCX)
Acknowledgments
We wish to thank Taweesak Monkanna and Opas Thachin (AFRIMS) for their assistances in the field work, Ms. Achareeya Korkusol for her technical support and analysis on louse identification by DNA sequencing and analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been supported by the Department of Defense Global Emerging Infections Surveillance and Response System (GEIS, https://www.afhsc.mil/Home/Divisions/GEIS). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Angelakis E, Raoult D. Pathogenicity and treatment of Bartonella infections. Int J Antimicrob Agents. 2014;44(1):16–25. 10.1016/j.ijantimicag.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 2. Mogollon-Pasapera E, Otvos L J, Giordano A, Cassone M. Bartonella: emerging pathogen or emerging awareness? Int J Infect Dis. 2009;13(1):3–8. 10.1016/j.ijid.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 3. Jacomo V, Kelly PJ, Raoult D. Natural History of Bartonella Infections (an Exception to Koch’s Postulate). Clin Diagn Lab Immunol. 2002;9(1):8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu XY, Bonnet SI. Hard Tick Factors Implicated in Pathogen Transmission. PLoS Negl Trop Dis. 2014;8(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsai YL, Chang CC, Chuang ST, Chomel BB. Bartonella species and their ectoparasites: selective host adaptation or strain selection between the vector and the mammalian host? Comp Immunol Microbiol Infect Dis. 2011;34(4):299–314. 10.1016/j.cimid.2011.04.005 [DOI] [PubMed] [Google Scholar]
- 6. Pulliainen AT, Dehio C. Persistence of Bartonella spp. stealth pathogens: from subclinical infections to vasoproliferative tumor formation. FEMS Microbiol Rev. 2012;36(3):563–99. 10.1111/j.1574-6976.2012.00324.x [DOI] [PubMed] [Google Scholar]
- 7. Seki N, Kasai S, Saito N, Komagata O, Mihara M, Sasaki T, et al. Quantitative analysis of proliferation and excretion of Bartonella quintana in body lice, Pediculus humanus L . Am J Trop Med Hyg. 2007;77(3):562–6. [PubMed] [Google Scholar]
- 8. Dehio C. Bartonella interactions with endothelial cells and erythrocytes. Trends Microbiol. 2001;9(6):279–85. [DOI] [PubMed] [Google Scholar]
- 9. Harms A, Dehio C. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin Microbiol Rev. 2012;25(1):42–78. 10.1128/CMR.05009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Minnick MF, Battisti JM. Pestilence, persistence and pathogenicity: infection strategies of Bartonella . Future Microbiol. 2009;4:743–58. 10.2217/fmb.09.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kosoy MY. Ecological Associations between Bacteria of the Genus Bartonella and Mammals. Biology Bulletin. 2009;37(7):716–24. [Google Scholar]
- 12. Chomel BB, Kasten RW, Williams C, Wey AC, Henn JB, Maggi R, et al. Bartonella endocarditis: a pathology shared by animal reservoirsand patients. Ann N Y Acad Sci. 2009;1166:120–6. 10.1111/j.1749-6632.2009.04523.x [DOI] [PubMed] [Google Scholar]
- 13. Watt G, Pachirat O, Baggett H, Maloney S, Lulitanond V, Raoult D, et al. Infective Endocarditis in Northeastern Thailand Emerg Infect Dis. 2014;20(3):473–6. 10.3201/eid2003.131059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiyipong T, Jittapalapong S, Morand S, Raoult D, Rolain JM. Prevalence and genetic diversity of Bartonella spp. in small mammals from Southeastern Asia. Appl Environ Microbiol. 2012;78(23):8463–6. 10.1128/AEM.02008-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kabeya H, Colborn JM, Bai Y, Lerdthusnee K, Richardson JH, Maruyama S, et al. Detection of Bartonella tamiae DNA in Ectoparasites from Rodents in Thailand and Their Sequence Similarity with Bacterial Cultures from Thai Patients. Vector Borne Zoonotic Dis. 2010;10:429–34. 10.1089/vbz.2009.0124 [DOI] [PubMed] [Google Scholar]
- 16. Cotté V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis HJ, et al. Transmission of Bartonella henselae by Ixodes ricinus . Emerg Infect Dis. 2008;14(7):1074–80. 10.3201/eid1407.071110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korhonen EM, Pérez Vera C, Pulliainen AT, Sironen T, Aaltonen K, Kortet R, et al. Molecular detection of Bartonella spp. in deer ked pupae, adult keds and moose blood in Finland. Epidemiol Infect 2015. February;143(3):578–85. 2015;143(3):578–85. 10.1017/S0950268814001411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kosoy MY, Regnery RL, Kosaya OI, Jones DC, Marston EL, Childs JE. Isolation of Bartonella spp. from embryos and neonates of naturally infected rodents. J Wildl Dis. 1998;34(2):305–9. [DOI] [PubMed] [Google Scholar]
- 19. Billeter SA, Cáceres AG, Gonzales-Hidalgo J, Luna-Caypo D, Kosoy MY. Molecular detection of Bartonella species in ticks from Peru. J Med Entomol. 2011;48(6):1257–60. [DOI] [PubMed] [Google Scholar]
- 20. Tsai YL, Chuang ST, Chang CC, Kass PH, Chomel BB. Bartonella species in small mammals and their ectoparasites in Taiwan. Am J Trop Med Hyg. 2010;83(4):917–23. 10.4269/ajtmh.2010.10-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parola P, Sanogo OY, Lerdthusnee K, Zeaiter Z, Chauvancy G, Gonzalez J, P, et al. Identification of Rickettsia spp. and Bartonella spp. in Fleas from the Thai-Myanmar Border. Ann NY Acad Sci. 2003;990:173–81. [DOI] [PubMed] [Google Scholar]
- 22. Assarasakorn S, Veir J, Hawley J, Brewer M, Morris A, Hill A, et al. Prevalence of Bartonella species, hemoplasmas, and Rickettsia felis DNA in blood and fleas of cats in Bangkok, Thailand. Res Vet Sci. 2012;93:1213–6. 10.1016/j.rvsc.2012.03.015 [DOI] [PubMed] [Google Scholar]
- 23. Kosoy MY, Morway C, Sheff KW, Bai Y, Colborn J, Chalcraft L, et al. Bartonella tamiae sp. nov., a Newly Recognized Pathogen Isolated from Three Human Patients from Thailand. J Clin Microbiol. 2008;46(2):772–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MacKinnon K. Review of Boonsong Lekagul, and Jeffrey A. McNeely 'Mammals of Thailand'. Oryx, 1978;14(3), 262–262. [Google Scholar]
- 25. Nadchatram M, Dohany AL. A Pictorial key to the subfamilies, genera and subgenera of Southeast Asian chiggers (Acari, Prostigmata, Trombiculidae) Kuala Lumpur: Institute for Medical Research; 1974. [Google Scholar]
- 26. Tanskull P, Inlao I. Keys to the adult ticks of Haemaphysalis Koch, 1844, in Thailand with notes on changes in taxonomy (Acari: Ixodoidea: Ixodidae). J Med Entomol. 1989; 26: 573–601. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Disease Control Prevention. Pictorial keys to arthropods, reptiles, birds, and mammals of public health significance: Department of Health & Human Services, Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 28. Hafner MS, Sudman PD, Villablanca FX, Spradling TA, Demastes JW, Nadler SA. Disparate rates of molecular evolution in cospeciating hosts and parasites. Science. 1994;265(5175):1087–90. [DOI] [PubMed] [Google Scholar]
- 29. Diaz MH, Bai Y, Malania L, Winchell JM, Kosoy MY. Development of a Novel Genus-Specific Real-Time PCR Assay for Detection and Differentiation of Bartonella Species and Genotypes. J Clin Microbiol. 2012;50(5):1645–9. 10.1128/JCM.06621-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Colborn JM, Kosoy MY, Motin VL, Telepnev MV, Valbuena G, Myint KS, et al. Improved Detection of Bartonella DNA in Mammalian Hosts and Arthropod Vectors by Real-Time PCR Using the NADH Dehydrogenase Gamma Subunit (nuoG). J Clin Microbiol. 2010;48(12):4630–3. 10.1128/JCM.00470-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bai Y, Kosoy MY, Lerdthusnee K, Peruski LF, Richardson JH. Prevalence and Genetic Heterogeneity of Bartonella Strains Cultured from Rodents from 17 Provinces in Thailand. Am J Trop Med Hyg. 2009;81(5):811–6. 10.4269/ajtmh.2009.09-0294 [DOI] [PubMed] [Google Scholar]
- 32. Norman AF, Regnery R, Jameson P, Greene C, Krause DC. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J Clin Microbiol. 1995;33(7):1797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9.34. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castle KT, Kosoy M, Lerdthusnee K, Phelan L, Bai Y, Gage KL, et al. Prevalence and diversity of Bartonella in rodents of northern Thailand: a comparison with Bartonella in rodents from southern China. Am J Trop Med Hyg. 2004;70(4):429–33. [PubMed] [Google Scholar]
- 35. Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney S, et al. Identification of Bartonella Infections in Febrile Human Patients from Thailand and Their Potential Animal Reservoirs. Am J Trop Med Hyg. 2010;82(6):1140–5. 10.4269/ajtmh.2010.09-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsieh JW, Tung KC, Chen WC, Lin JW, Chien LJ, Hsu YM, et al. Epidemiology of Bartonella infection in rodents and shrews in Taiwan. Zoonoses Public Health. 2010;57(6):439–60. 10.1111/j.1863-2378.2009.01234.x [DOI] [PubMed] [Google Scholar]
- 37. Durden LA, Musser GG. The Sucking Lice (Insecta, Anoplura) of the World—a Taxonomic Checklist with Records of Mammalian Hosts and Geographical Distributions. B Am Mus Nat Hist. 1994(218):1–90. [Google Scholar]
- 38. Durden LA. The Spiny Rat Louse, Polyplax spinulosa, as a Parasite of the Rice Rat, Oryzomys palustris, in North-America. J Parasitol. 1988;74(5):900–1. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The GenBank accession numbers are shown for each reference sequences. Trees constructed from ssrA and gltA genes were able to discriminate all samples into two major clusters (C1 and C2), although some different branching patterns of sequences in C1 group (R1012, R1033, R1135, and R1188) were noticed.
(TIF)
Detail information of ectoparasites collected from rodents in this study (Table A).Bartonella DNA detection in flea pools classified by gender (Table B) and in tick pools classified by stage and gender (Table C)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.