Abstract
Spinal cord injuries (SCI) are associated with altered cardiovascular autonomic control. Sleep is characterized by modifications of autonomic control across sleep stages; however, no data are available on the effects of SCI on CAC during sleep. The aim of our study was to assess cariac autonomic modulation during sleep in SCI patients.
Overnight polysomnographic recordings were obtained in 27 patients with cervical (Cerv) and thoracic (Thor) SCI and in healthy subjects (Controls). ECG and respiration were extracted from PSG, divided into sleep stages (W, N2, N3 and REM) for assessment of CAC, using symbolic analysis and Corrected Conditional Entropy. SA identifies three main indices, 0V%, index of sympathetic modulation, and 2LV% and 2UV%, markers of vagal modulation. CCE evaluates the complexity of heart period time series.
Symbolic analysis revealed a reduction of 0V% in N2 and N3 compared to W and REM and an increase of 2LV% and 2UV% in N2 and N3 compared to W and REM in SCI patients, independent of the level of the lesion, and similar to Controls. Corrected Conditional Entropy was higher in N2 and N3 compared to W and REM in all three groups.
In SCI patients, cardiac autonomic control changed across sleep stages, with a reduction of sympathetic and an increase of parasympathetic modulation during NREM compared to W and REM and a parallel increase of complexity during NREM, similar to Controls. Cardiac autonomic dynamics during sleep are maintained in SCI, independent of the level of the lesion.
Keywords: Sleep, spinal cord injury, autonomic nervous system, symbolic analysis, entropy measures
1. Introduction
Spinal cord injuries (SCI) are one of the major causes of disabilities among young populations all over the world, with more than 200 million individuals living with chronic neurological disabilities due to SCI [Wyndaele and Wyndaele 2006].
Over the past decade, several studies have demonstrated that SCI patients have an increased cardiovascular risk [Wahaman et al. 2010; Grigorean et al. 2009; Myers et al. 2007]. In fact, abnormalities in heart rate (HR) and blood pressure (BP) control together with increased prevalences of obesity, dyslipidemia and altered glucose metabolism have been described in these patients, and are responsible for a worse cardiovascular risk profile. It has also been shown that alterations of cardiovascular autonomic control could play an important role in this setting. However it is not known whether this autonomic dysregulation may affect cardiovascular and autonomic dynamics occurring during sleep.
Analysis of heart rate variability (HRV), a non-invasive tool to evaluate autonomic cardiovascular regulation in health and disease [Montano et al. 2009; Malliani et al. 1998], revealed significant changes of sympatethic and parasympathetic control in SCI patients. In fact, several studies described important modifications of rhythmical components of HRV representing sympathetic and parasympatehtic control in patients with both cervical and thoracic SCI, sometimes reporting contrasting findings [Guzzetti et al. 1994; Inoue et al. 1995; Inoue et al. 1991; Grimm et al. 1997; Wang et al. 2000; Wecht et al. 2006]. To our knowledge, no studies have evaluated cardiac autonomic control in SCI patients during sleep.
Recently, new non-linear methods, such as symbolic dynamics and entropy-derived measures, have been proposed as valid tools able to overcome important technical limitation of spectral analysis and capable of providing complementary information on the neural mechanisms which control and regulate cardiac sinus node function [Tobaldini et al. 2009; Tobaldini et al. 2013; Viola et al. 2011; Porta et al. 2007; Porta et al. 2007].
Hence, the aim of the present study was to assess cardiac autonomic modulation using new non-linear tools (i.e. symbolic and entropy analysis) during wakefulness and through sleep stages in SCI patients, taking into consideration the site of the lesion.
2. Methods
2.1 Study population and Experimental Protocol
The study was approved by the IRB of the Niguarda Ca’ Granda Hospital and an informed consent was signed by all participants.
From November 2010 to December 2011, we consecutively enrolled 27 patients with a neurological and radiological diagnosis of cervical (Cerv, n=12, i.e. tetraplegic) and thoracic (Thor, n=15, i.e.paraplegic) SCI. A group of normal healthy subjects matched for age, gender and BMI with the SCI group was enrolled as a control group at Mayo Clinic, Rochester, Minnesota (Controls, n=8). The unique exclusion criterion was the absence of sinus rhythm due to atrial fibrillation, excessive supra or ventricular premature beats (more than 5% of the entire recording) or pacemaker rhythm.
Each subject underwent a complete polysomnographic study (PSG) during the hospitalization at the Department of Neurosciences, “Niguarda Ca’ Granda” Hospital, Milan, within a mean time of 65 days from the injury.
Standard overnight full polysomnography was performed in the ward in an attended setting (AURA®;PSG Ambulatory Systems GRASS Technologies). In accordance with standard criteria 18, the recording included: electroencephalography (at least three channels, frontal, central and occipital), bilateral electro-oculography, chin and bilateral tibial electromyography, electrocardiography, oronasal airflow, chest and abdominal effort, pulse oximetry, sensor of body position. All studies were reviewed by a medical doctor specialized in sleep medicine, certified by the Italian Association of Sleep Medicine. Sleep was staged and respiratory and motor events were visually scored according to current standard criteria [Iber et al. 2007].
Motor and sensory examinations were performed and all patients were classified using the ASIA impairment scale A–D.
2.2 Data analysis
From PSG recordings, ECG and respiratory traces were extracted using an ad hoc off-line program and then divided into wakefulness (W), non-REM sleep 2 (N2), non-REM sleep 3 (N3) and REM. The first two complete sleep cycles of the night for each subject (NREM and REM sleep) were selected for the analysis.
The respiratory traces were carefully checked and any period with apneas/hypopneas or irregular breathing were excluded from the analysis, thus considering only those ECG segments associated with stable and regular breathing.
The QRS complexes were identified and parabolic interpolation was used to locate the apex of R waves. All the ECG traces were linearly detrended and carefully checked to avoid any missing beats and any incorrect detection of QRS complexes.
Samples of consecutive 250–300 beats were identified according to the sleep stages (W, N2, N3 and REM) for each group and off-line algorithms were applied for the assessment of HRV (i.e. symbolic analysis and corrected conditional entropy).
Respiratory traces were resampled at 512 Hz and the respiratory rate was assessed from the respiratory signal.
2.3 Non linear analysis of HRV
a) Symbolic analysis (SA)
SA is a novel non-linear tool for the assessment of HRV which has been validated in health and pathological conditions [Tobaldini et al. 2009; Porta et al 2007; Guzzetti et al. 2005]. With respect to classical power spectral tools, SA is capable of detecting non-reciprocal changes of the two autonomic branches and is more reliable than other classical linear tools in conditions characterized by low total variability.
A full description of the mathematical details of SA algorithms is furnished elsewhere [Porta et al. 2007]. Briefly, SA is based on 1) the transformation of time series into a sequence of symbols; 2) the construction of patterns using these symbols (i.e. words); 3) the reduction of the number of patterns into four families; and, 4) the evaluation of their rate of occurrence expressed as percentages. Therefore, all patterns are grouped without any loss into four categories: 0V, pattern with no variation (i.e. all the symbols are in the same level), 1V category, pattern with one variation (2 consecutive symbols are equal and the remaining one is different), 2LV, patterns with two like variations (the 3 symbols form an ascending or descending ramp), and 2UV, pattern with two unlike variations (the three symbols are organized forming a peak or a valley). As stated above, the rate of occurrence of the patterns is expressed as a percentage (i.e. 0V%, 1V%, 2LV%, and 2UV%). Experimental and pharmacological studies have shown that category 0V is marker of sympathetic modulation while the categories 2LV and 2ULV are considered markers of parasympatehtic modulation [Tobaldini et al. 2009; Porta et al 2007; Guzzetti et al. 2005].
b) Corrected Conditional Entropy
There has been growing interest in the complexity of heart period time series and several measures have been proposed [Porta et al 2007; Porta et al. 2001]. Of these, we applied Corrected Conditional Entropy (CCE), which has already been used to assess autonomic cardiac complexity during wake and sleep. CCE can be derived from Conditional Entropy, which evaluates the amount of information carried by the current RR sample (i.e., RR(i)) when L-1 past samples of RR are known (i.e., RRL-1(i-1) =(RR(i-1), …, RR(i-L+1)). In other words, CE represents the difficulty in predicting future values of a time series when the past values are known.
For the CE estimation, RR series are uniformly quantized over ξ=6 bins (number optimized to deal with short data sequences)17. However, because the calculation of CE is biased, CCE was designed to overcome these mathematical limits. CCE is decreases to 0 when the new sample is fully predictable, reaches its maximum value when the new sample is completely unpredictable, and reaches its minimum value when the knowledge of past values is helpful to reduce the uncertainty associated with future values.
2.4 Statistical analysis
Data are presented as mean ± SD. SigmaPlot 12 (Systat Software Inc., Chicago, IL, USA) was used for statistical analysis. A two-way ANOVA analysis for variance was used to check the differences between the groups within the sleep stages. The normality test (Shapiro-Wilk test) was applied to check the normality of the distribution; if this was not the case, an equal variance test was performed. A Holm-Sidak method for all pair wise multiple comparison procedures was used. A p<0.05 was considered statistically significant.
3. Results
Mean age was 41.9±17 yr in the Cerv group, 34.5±14 yr in the Thor group and 40±15 yr in the Control group. There were no sex differences between the groups as well as no differences in BMI (23.5±2.5, 22.8±3.1 and 25±3 kg/m2 in Cerv,Thor and Control respectively).
The lesion was complete (ASIA A) in 14 patients, while incomplete (ASIA B, C and D) in 13 patients. After injury the majority of patients were under poly pharmacotherapy, including benzodiazepines, antidepressant, opioids and muscle relaxants.
As shown in Figure 1, comparing the three groups (Cerv, Thor and Controls), HR was significantly higher in Cerv and Thor compared to Controls during W and sleep.
Figure 1.
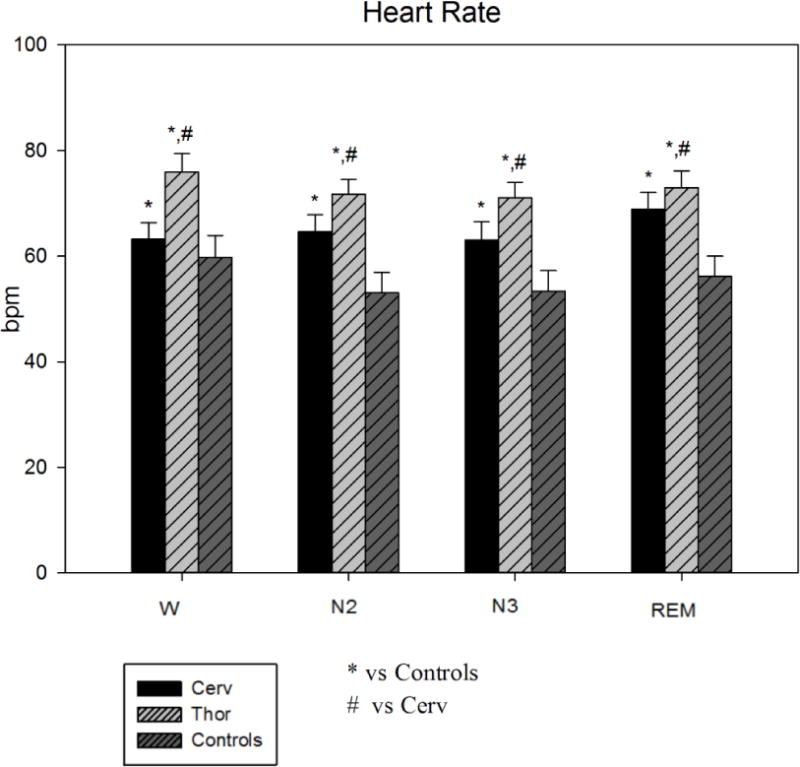
Heart Rate during sleep stages in the three groups (Cerv, Thor, Controls). HR is significantly higher in Cerv and Thor compared to Controls during W and sleep. * = p<0.05 vs Controls; # = p<0.05 vs Cerv.
Total power was significantly reduced in Cerv and Thor compared to Controls during W and REM. No differences were observed comparing the different sleep stages.
SA showed no differences within all groups across the sleep stages, while it revealed significant differences among the sleep stages. Namely, 0V%, an index of sympathetic modulation, was significantly reduced in N2 and N3 compared to W and REM in the three groups (see Table 1). 2LV%, a marker of parasympathetic modulation, increased in N2 and N3 compared to W and REM in the three groups (see Table 1), as did 2UV%, which was significantly higher in N3 compared to W and REM).
Table 1.
Non linear analysis of HRV in Spinal Cord Injury patients (Cerv and Thor) and in control subjects (Controls).
| W | N2 | N3 | REM | |
|---|---|---|---|---|
| Heart rate (bpm) | ||||
| Controls (n=8) | 60±4 | 53±3.8 | 53±3.8 | 56±3.8 |
| Cerv (n=12) | 63±3.1* | 65±3.1* | 63±3.4* | 69±3.1* |
| Thor (n=15) | 76±3*,# | 72±2.8*,# | 71±2.9*,# | 73±3.1*,# |
| Variance (ms2) | ||||
| Controls (n=8) | 7993± 1087 | 6704±1016 | 3525±1016 | 7782±1016ϒ |
| Cerv (n=12) | 1928±830* | 2337±830* | 2137±909* | 4556±830*ϒ |
| Thor (n=15) | 1047±909* | 1749±742* | 1363±768* | 2146±830*,#,ϒ |
| 0V% | ||||
| Controls (n=8) | 29.9±5.1 | 23.5±4.8 | 15.8±4.8§ | 39.4±4.8δ,ϒ |
| Cerv (n=12) | 27.7±3.9 | 24.1±3.9 | 18.3±4.3§ | 37.7±3.9δ,ϒ |
| Thor (n=15) | 35.6±4.3 | 26.4±3.5 | 19.2±3.6§ | 34.9±3.9δ,ϒ |
| 1LV% | ||||
| Controls (n=8) | 47.0±2.1 | 47.1±1.9 | 46.4±1.9 | 43.6±1.9 |
| Cerv (n=12) | 46.2±1.5 | 44.2±1.5 | 43.8±1.7 | 42.4±1.5 |
| Thor (n=15) | 43.2±1.7 | 45.1±1.4 | 44.7±1.4 | 42.7±1.5 |
| 2LV% | ||||
| Controls (n=8) | 8.3±2.2 | 12.50±2.1§ | 16.0±2.1§ | 6.4±2.1δ,ϒ |
| Cerv (n=12) | 8.3±1.6 | 12.4±1.6§ | 14.3±1.8§ | 6.6±1.6δ,ϒ |
| Thor (n=15) | 6.0±1.8 | 9.1±1.5§ | 11.9±1.5§ | 5.9±1.6δ,ϒ |
| 2UV% | ||||
| Controls (n=8) | 14.6±3.6 | 16.7±3.4 | 21.7±3.4§ | 10.5±3.4ϒ |
| Cerv (n=12) | 17.4±2.8 | 19.1±2.8 | 23.4±3.1§ | 13.1±2.8ϒ |
| Thor (n=15) | 15.1±3.1 | 19.4±2.5 | 23.9±2.6§ | 16.3±2.8ϒ |
| CCE | ||||
| Controls (n=8) | 0.93±0.05 | 1.03±0.04§ | 1.13±0.05§ | 0.88±0.05δ,ϒ |
| Cerv (n=12) | 0.92± 0.04 | 1.00± 0.04§ | 1.13± 0.05§ | 0.88 ±0.04δ,ϒ |
| Thor (n=15) | 0.87±0.04 | 0.93±0.05§ | 1.07±0.04§ | 0.90±0.04δ,ϒ |
Data are expressed as mean ± standard deviation.
= p<0.05 vs Controls;
= p<0.05 vs Cerv;
p<0.05 vs W;
p<0.05 vs N2;
p<0.05 vs N3.
CCE was similar in the three groups while, comparing the different sleep stages, it was increased in N2 and N3 compared to W and REM (see Figure 2).
Figure 2.
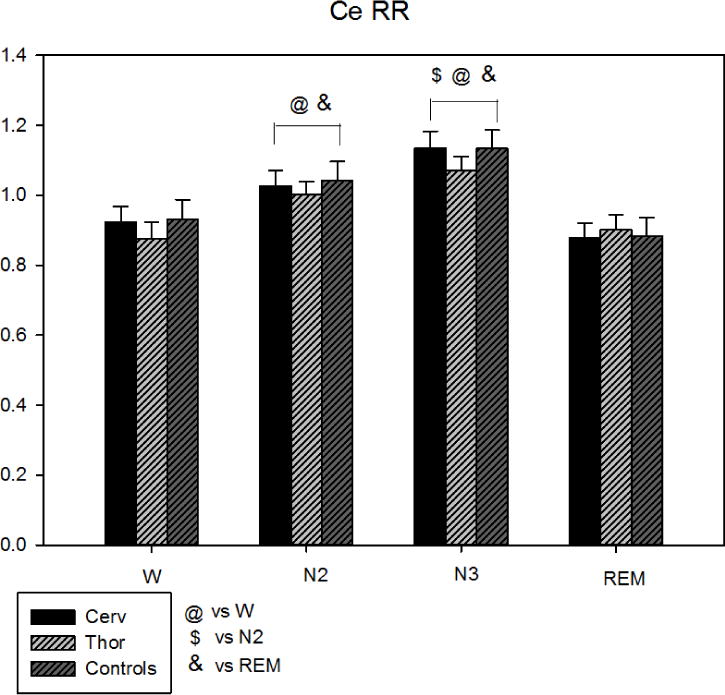
Corrected Conditional Entropy during sleep stages in the three groups (Cerv, Thor, Controls). CCE was similar in the three groups while, comparing the different sleep stages, it was significantly higher in N2 and N3 compared to W and REM. @ = p<0.05 vs W, $ = p<0.05 vs N2, & = p<0.05 vs REM.
Comparing complete and incomplete lesions, we found no different results than the comparison between Cerv, Thor and Controls. These data are reported in Table 2.
Table 2.
Non linear analysis of HRV in complete and incomplete lesions groups.
| W | N2 | N3 | REM | |
|---|---|---|---|---|
| Heart rate (bpm) | ||||
| Complete (n=14) | 69±3.8 | 68±3.4 | 67±3.5 | 72±3.5 |
| Incomplete (n=13) | 68±3.6 | 68±3.5 | 68±3.8 | 69±3.8 |
| Total power (ms2) | ||||
| Complete (n=14) | 1564±920 | 1976±816 | 1465±847 | 2113±847 |
| Incomplete (n=13) | 1607±880 | 2047±847 | 1946±920 | 4814±920 |
| 0V% | ||||
| Complete (n=14) | 32.8±4.5 | 24.4±4.0 | 19.3±4.1# | 36.1±4.1 |
| Incomplete (n=13) | 30.1±4.3 | 26.5±4.1 | 18.3±4.5# | 36.6±4.5 |
| 1V% | ||||
| Complete (n=14) | 45.1±1.8 | 46.5±1.60 | 46.1±1.7 | 43.3±1.6 |
| Incomplete (n=13) | 44.7±1.7 | 42.7±1.7 | 42.3±1.8 | 41.7±1.8 |
| 2LV% | ||||
| Complete (n=14) | 6.8± 1.9 | 11.9±1.7 | 13.7±1.7*,# | 7.1±1.7 |
| Incomplete (n=13) | 7.5±1.8 | 9.1±1.7 | 12.1±1.9*,# | 5.4±1.9 |
| 2UV% | ||||
| Complete (n=14) | 15.2±3.1 | 17.1±2.7 | 20.8±2.8# | 13.4±2.8 |
| Incomplete (n=13) | 17.4±2.9 | 21.7±2.8 | 27.2±3.1# | 16.2±3.1 |
| CCE | ||||
| Complete (n=14) | 0.92±0.05 | 1.03±0.04 | 1.08±0.06# | 0.89±0.04 |
| Incomplete (n=13) | 0.89±0.05 | 0.99±0.04 | 1.12±0.05*,# | 0.884±0.05 |
Data are expressed as mean ± standard deviation.
= p<0.05 vs W;
= p<0.05 vs REM.
4. Discussion
The most relevant findings of this study are the following: 1) SCI patients differ in terms of mean HR and total variability from normal subjects during wake and sleep, with SCI patients showing a lower total variability compared to healthy subjects; 2) non-linear symbolic analysis detects consistent autonomic fluctuations across sleep stages, with a gradual shift of the sympatho-vagal balance towards parasympathetic predominance during NREM compared to REM and wakefulness; these modifications are similar in SCI and normal subjects; 3) entropy-derived tools show an increase of CCE during NREM compared to REM and wakefulness in both healthy and SCI patients, suggesting a change in cardiac autonomic complexity during sleep but independent of the presence or absence of SCI; 4) non-linear cardiac autonomic dynamics across sleep stages seem to be similar in presence of complete or incomplete SCI lesions. Ultimately, these data demonstrate that both partial or complete peripheral sympathetic denervation does not affect the central regulation of the sympathovagal balance during sleep.
SCI is one of the major world-wide causes of neurological disabilities [Wyndaele and Wyndaele 2006; Ackery et al. 2004]. Patients with SCI have an increased cardiovascular risk and death from cardiac problems, due to the presence of abnormalities in HR and BP regulation, hypertension, obesity, disorders of lipid and glycemic metabolism [Grigorean et al. 2009; Myers et al. 2007; Bravo et al. 2004; Wahman et al. 2010] and chronic inflammation [Beck et al. 2010]. Experimental and clinical studies have established that derangement of the autonomic nervous system may play a key role in the pathophysiology of the cardiovascular consequences that occur in patients with acute and chronic SCI. During sleep, in normal subjects, autonomic cardiac control is highly dynamic and active, with a predominant vagal modulation during NREM sleep and a shift of sympatho-vagal balance towards sympathetic predominance during REM sleep [Trinder et al. 2001; Crasset et al. 2001; Beharav et al. 1995; Busek et al. 2005; Tobaldini et al. 2013]. However, no studies have so far investigated the autonomic cardiac changes during sleep in SCI patients.
An expected finding of our study is that HR during wakefulness and sleep tends to be higher in SCI compared to healthy subjects, as previously reported [Guzzetti et al. 1994; Inoue et al. 1995; Grimm et al 1997], but it is lower in cervical compared to thoracic SCI. This is likely to be due to the fact that most of the sympathetic efferent fibers to the heart emerge from the last two cervical and the first thoracic spinal segments. Furthermore, the differences in HR appear to increase in N2 and N3 sleep, with slowing of HR in normal, but attenuated slowing in SCI (Table 1).
Total variability is significantly reduced in SCI compared to healthy subjects with no significant differences between cervical and thoracic SCI. Total variability can be considered a global index of the ability of the cardiovascular system to adapt to endogenous and exogenous stimuli [Malliani et al. 1981], thus SCI patients seem to be less capable of counteracting stressor challenges, likely being more susceptible to acute cardiac events. This phenomenon could be possibly related to typical SCI clinical features such as autonomic dysreflexia, orthostatic hypotension and cardiovascular deconditioning [Krassioukov 2009; Teasell et al. 2000]. On the other hand, a novel finding of our study is that total variability was reduced not only in wakefulness but also during N2 and REM, while during N3 this difference was not significant. Even though the reduction of total variability has been already described in healthy subjects during NREM sleep [Beharav et al. 1995; Busek et al. 2005], this is the first time that such changes are described in SCI patients during the different sleep stages.
In order to evaluate cardiac autonomic control across sleep stages, we applied two new non-linear methods, SA and CCE, which have been already reported to be able to detect autonomic dynamics in health and disease [Tobaldini et al. 2009; Viola et al. 2011; Porta et al. 2007; Tobaldini et al. 2009] and have some important advantages compared to classical linear tools. In fact, SA is able to detect changes of autonomic cardiac control on time series characterized by non-linearities and in conditions characterized by non-reciprocal changes of the two branches of ANS (i.e. sympathetic and parasympathetic controls); on the other hand, entropy measures provide information on the complexity of cardiovascular regulation. SA revealed no differences between healthy and SCI patients; however, it has been able to detect similar changes across sleep stages within both groups. Results in healthy subjects are in agreement with those found using linear tools such as spectral analysis of HRV [Beharav et al. 1995; Busek et al. 2005], which showed a predominant vagal modulation during NREM and, on the contrary, a sympathetic predominance during REM sleep. Entropy analysis also revealed interesting results. In fact, CCE was significantly higher in N2 and N3 compared to W and REM in all the three groups. This result is in agreement with a previous finding by Viola and colleagues [Viola et al. 2011] showing that CCE is higher during NREM and lower during REM sleep. Physiologically, an increase of CCE means a higher degree of complexity over different temporal scales, suggesting that the different control systems involved in the regulation of sinus node function (i.e. cortical inputs, hypothalamic and brainstem autonomic oscillators, peripheral reflex circuits and autonomic reflexes) interact in a complex way in order to regulate beat-to-beat cardiac function. A decrease of this index, as we observed in REM sleep compared to N2 and N3, revealed a simplification of the beat-to-beat control, with the dominance of one mechanism over the others, thus reducing the plasticity of cardiovascular responses to stressor stimuli [Porta et al. 2007]. It is worth noting that no differences were observed in cardiac autonomic dynamics across sleep stages when comparing SCI and healthy subjects. From these results, we speculate that, although SCI is characterized by a complete or incomplete lesion of the descending sympathetic neural pathway in the spinal cord, leading to altered autonomic responses such as orthostatic hypotension and cardiovascular deconditioning [Guzzetti et al. 1994; Mathias et al. 1983; Taylor et al. 1974], this does not modify the central push-pull between adrenergic and cholinergic pathways regulating cardiovascular function during sleep.
The present study has some limitations, such as the relatively small sample size and the fact that we did not performed a follow up with a new sleep recording. However, strengths of the present studies are first, the larger and homogeneous sample size of patients with both cervical and thoracic SCI compared to previous studies; second, all patients were studied in a relatively acute phase, considering that PSG studies were performed within 9 months from the injury; third, for the first time autonomic cardiac modulation has been assessed using new non-linear tools able to provide complementary information on the regulation of cardiac sinus node function; fourth, to our knowledge, this is the first study assessing autonomic control as a dynamic process across sleep stages in SCI patients.
Acknowledgments
Dr. Somers was supported by NIH HL65176. This publication was also supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Dr. Montano was supported by a European Regional Development Fund—Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123).
Katrina Sambusida has received financial support from Vivisol s.r.l., SOL group.
Footnotes
Conflict of Interest: Dr. Somers has served as a consultant for Respicardia, Neu Pro, and ResMed and is an investigator on studies funded with grants from the Phillips Respironics Foundation. Other authors have no disclosures.
Author contributorship: Tobaldini E.: study design, data analysis, manuscript writing. Proserpio P., Sambusida K., Lanza A., Redaelli T., Frigerio P., Fratticci L., Rosa S.: data collection, data analysis. Casali K.R., Somers V.K.: study design, manuscript supervision. Nobili L., Nicola Montano: study design, data interpretation, manuscript supervision.
Reference List
- Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–70. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- Baharav A, Kotagal S, Gibbons V, et al. Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology. 1995;45:1183–7. doi: 10.1212/wnl.45.6.1183. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–47. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo G, Guizar-Sahagun G, Ibarra A, Centurion D, Villalon CM. Cardiovascular alterations after spinal cord injury: an overview. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:133–48. doi: 10.2174/1568016043477242. [DOI] [PubMed] [Google Scholar]
- Busek P, Vankova J, Opavsky J, Salinger J, Nevsimalova S. Spectral analysis of the heart rate variability in sleep. Physiol Res. 2005;54:369–76. [PubMed] [Google Scholar]
- Crasset V, Mezzetti S, Antoine M, Linkowski P, Degaute JP, van de Borne P. Effects of aging and cardiac denervation on heart rate variability during sleep. Circulation. 2001;103:84–8. doi: 10.1161/01.cir.103.1.84. [DOI] [PubMed] [Google Scholar]
- Grigorean VT, Sandu AM, Popescu M, et al. Cardiac dysfunctions following spinal cord injury. J Med Life. 2009;2:133–45. [PMC free article] [PubMed] [Google Scholar]
- Grimm DR, de Meersman RE, Almenoff PL, Spungen AM, Bauman WA. Sympathovagal balance of the heart in subjects with spinal cord injury. Am J Physiol. 1997;272:H835–H842. doi: 10.1152/ajpheart.1997.272.2.H835. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Cogliati C, Broggi C, et al. Influences of neural mechanisms on heart period and arterial pressure variabilities in quadriplegic patients. Am J Physiol. 1994;266:H1112–H1120. doi: 10.1152/ajpheart.1994.266.3.H1112. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Borroni E, Garbelli PE, et al. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation. 2005;112:465–70. doi: 10.1161/CIRCULATIONAHA.104.518449. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, et al. for the American Academy of Sleep Medicine . The MSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 15th. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Inoue K, Miyake S, Kumashiro M, Ogata H, Ueta T, Akatsu T. Power spectral analysis of blood pressure variability in traumatic quadriplegic humans. Am J Physiol. 1991;260:H842–H847. doi: 10.1152/ajpheart.1991.260.3.H842. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ogata H, Hayano J, et al. Assessment of autonomic function in traumatic quadriplegic and paraplegic patients by spectral analysis of heart rate variability. J Auton Nerv Syst. 1995;54:225–34. doi: 10.1016/0165-1838(95)00012-m. [DOI] [PubMed] [Google Scholar]
- Krassioukov A. Autonomic function following cervical spinal cord injury. Respir Physiol Neurobiol. 2009;169:157–64. doi: 10.1016/j.resp.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Malliani A, Lombardi F, Pagani M. Functions of afferents in cardiovascular sympathetic nerves. J Auton Nerv Syst. 1981;3:231–6. doi: 10.1016/0165-1838(81)90065-5. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Montano N, Mela GS. Sympathovagal balance: a reappraisal. Circulation. 1998;98:2640–3. [PubMed] [Google Scholar]
- Mathias CJ, Frankel HL. Clinical manifestations of malfunctioning sympathetic mechanisms in tetraplegia. J Auton Nerv Syst. 1983;7:303–12. doi: 10.1016/0165-1838(83)90083-8. [DOI] [PubMed] [Google Scholar]
- Montano N, Porta A, Cogliati C, et al. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci Biobehav Rev. 2009;33:71–80. doi: 10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007;86:142–52. doi: 10.1097/PHM.0b013e31802f0247. [DOI] [PubMed] [Google Scholar]
- Porta A, Guzzetti S, Montano N, et al. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans Biomed Eng. 2001;48:1282–91. doi: 10.1109/10.959324. [DOI] [PubMed] [Google Scholar]
- Porta A, Tobaldini E, Guzzetti S, Furlan R, Montano N, Gnecchi-Ruscone T. Assessment of cardiac autonomic modulation during graded head-up tilt by symbolic analysis of heart rate variability. Am J Physiol Heart Circ Physiol. 2007;293:H702–H708. doi: 10.1152/ajpheart.00006.2007. [DOI] [PubMed] [Google Scholar]
- Porta A, Gnecchi-Ruscone T, Tobaldini E, Guzzetti S, Furlan R, Montano N. Progressive decrease of heart period variability entropy-based complexity during graded head-up tilt. J Appl Physiol. 2007;103:1143–9. doi: 10.1152/japplphysiol.00293.2007. [DOI] [PubMed] [Google Scholar]
- Taylor AG. Autonomic dysreflexia in spinal cord injury. Nurs Clin North Am. 1974;9(4):717–25. [PubMed] [Google Scholar]
- Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–16. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Porta A, Wei SG, et al. Symbolic analysis detects alterations of cardiac autonomic modulation in congestive heart failure rats. Auton Neurosci. 2009;150:21–6. doi: 10.1016/j.autneu.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaldini E, Montano N, Wei SG, et al. Autonomic cardiovascular modulation. IEEE Eng Med Biol Mag. 2009;28:79–85. doi: 10.1109/MEMB.2009.934620. [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Brugada J, Benito B, et al. Cardiac autonomic control in Brugada syndrome patients during sleep: The effects of sleep disordered breathing. Int J Cardiol. 2013;168:3267–72. doi: 10.1016/j.ijcard.2013.04.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N. Heart rate variability in normal and pathological sleep. Front Physiol. 2013;4:294. doi: 10.3389/fphys.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- Viola AU, Tobaldini E, Chellappa SL, Casali KR, Porta A, Montano N. Short-term complexity of cardiac autonomic control during sleep: REM as a potential risk factor for cardiovascular system in aging. PLoS One. 2011;6:e19002. doi: 10.1371/journal.pone.0019002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk factors in persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med. 2010;42:272–8. doi: 10.2340/16501977-0510. [DOI] [PubMed] [Google Scholar]
- Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Increased cardiovascular disease risk in Swedish persons with paraplegia: The Stockholm spinal cord injury study. J Rehabil Med. 2010;42:489–92. doi: 10.2340/16501977-0541. [DOI] [PubMed] [Google Scholar]
- Wang YH, Huang TS, Lin JL, et al. Decreased autonomic nervous system activity as assessed by heart rate variability in patients with chronic tetraplegia. Arch Phys Med Rehabil. 2000;81:1181–4. doi: 10.1053/apmr.2000.6300. [DOI] [PubMed] [Google Scholar]
- Wecht JM, Weir JP, Bauman WA. Blunted heart rate response to vagal withdrawal in persons with tetraplegia. Clin Auton Res. 2006;16:378–83. doi: 10.1007/s10286-006-0367-y. [DOI] [PubMed] [Google Scholar]
- Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523–9. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]


