Abstract
The effect of a pharmacologic increase in serotonin concentrations on striatal dopamine (D2) receptor availability has been measured in several studies using positron emission tomography (PET) and the radiotracer [11C]-raclopride as a method for the in vivo imaging of serotonin modulation of striatal dopamine in human subjects. These studies have shown that an acute increase in serotonin concentrations produced a decrease in striatal D2 receptor availability. The current study was undertaken to measure the effects of a more pharmacologically selective serotonergic agent compared to previous studies, the serotonin reuptake inhibitor, citalopram, on striatal D2 receptor availability. Twelve healthy control subjects underwent two PET scans performed on the same day following i.v. administration of saline (Scan 1) and citalopram (Scan 2, 40 mg, i.v.). The [11C]-raclopride data were analyzed with a graphical analysis method using the cerebellum as the input function. Plasma levels of citalopram, cortisol, and prolactin were measured. The citalopram concentrations peaked at the end of infusion (EOI) and remained relatively consistent from 30 min to 3 h postinfusion. An increase in cortisol and prolactin concentrations was observed from the EOI until 60 min after the EOI. A significant decrease in striatal D2 receptor availability was observed after citalopram infusion (−5%), presumably due to an increase in endogenous dopamine concentrations. In summary, i.v. administration of the selective serotonin reuptake inhibitor, citalopram, produced modest reductions in striatal D2 receptor availability, consistent with other human [11C]-raclopride studies using less pharmacologically selective serotonergic agents.
Keywords: selective serotonin reuptake inhibitors, citalopram, serotonin, positron emission tomography (PET), dopamine, [11C]-raclopride
INTRODUCTION
The demonstration that endogenous neurotransmitter concentrations could be measured in vivo by combining neurotransmitter receptor binding measures with acute pharmacologic interventions has been an important development in neurochemical brain imaging methodology, particularly with respect to the dopamine system. Specifically, radiotracer binding to the dopamine (D2) receptor has been shown repeatedly to be altered by administration of agents that increased or decreased endogenous dopamine concentrations by direct or indirect pharmacologic mechanisms, using positron emission tomography (PET) or single photon emission computed tomography methods [single photon emission computed tomography (SPECT)] (e.g., Dewey et al., 1991, 1993a,b; Innis et al., 1992; Smith et al., 1997; Volkow et al., 1994). Acute administration of agents that directly (d-amphetamine, methylphenidate or cocaine) or indirectly increase dopamine concentrations through interactions with other neurotransmitter systems (e.g., fenfluramine, scopolamine, ketamine for the serotonergic, cholinergic, and glutamatergic systems, respectively) resulted in a decrease in radio-tracer binding, presumably due to increased competition between dopamine and the radiotracer for binding to the D2 receptor (Breier et al., 1998; Dewey et al., 1990, 1995; Smith et al., 1997, 1998). Conversely, agents that decreased dopamine concentrations directly (reserpine, α-methyl p-tyrosine) or indirectly (lorazepam, γ-vinyl GABA) resulted in an increase in receptor binding due to reduced competition between dopamine and the radiotracer for binding to the D2 receptor (e.g., Dewey et al., 1992; Laruelle et al., 1997). This research strategy continues to represent the most direct, noninvasive method available to measure endogenous dopamine modulation in the living brain.
The development and application of methods to evaluate dopamine modulation by other neurotransmitter systems is an opportunity to test alternative hypotheses regarding pathophysiology and drug mechanisms of action. Serotonin modulation of dopamine function is relevant to several neuropsychiatric conditions including schizophrenia, obsessive compulsive disorder, major depression and drug abuse and thus, has been a particular focus of [11C]-raclopride studies in nonhuman primates and humans (Bantick et al., 2005; Rosa-Neto et al., 2004; Smith et al., 1997; Vollenweider et al., 1999). Several human studies have observed that a pharmacologic increase in serotonin concentrations produced a reduction in striatal D2 receptor availability (Smith et al., 1997; Tiihonen et al., 1996; Vollenweider et al., 1999).
With respect to the mechanisms underlying serotonin modulation of dopamine, interactions between the two systems have been demonstrated at the level of the cell bodies of origin (dorsal and medial raphe for the serotonin systems and substantia nigra and ventral tegmental area for the dopamine system), as well as the dopamine terminals in the striatum, limbic structures, and cerebral cortex (Azmitia and Segal, 1978; Dray et al., 1976; Herve et al., 1987; Nedergaard et al., 1988; Parent et al., 1981; Schmidt et al., 1992, as reviewed by Alex and Pehek, 2007). The evidence is most consistent for a role of 5-HT1B, 5-HT2A/C, and 5-HT4 receptors mediating dopamine release in the striatum (as reviewed by Alex and Pehek, 2007). As pharmacologic agents are administered systemically in the human neuroimaging studies, the observed results represent the net effect of interactions in the cortex, striatum, and cell bodies of origin on striatal dopamine.
The purpose of the present study was to evaluate the effects of the most selective of the serotonin reuptake inhibitors (SSRI’s), citalopram, on dopamine receptor availability using PET and the D2/3 antagonist radiotracer [11C]-raclopride. The use of citalopram in this study addresses two potential limitations of prior studies as citalopram is more selective than other agents used (e.g., fenfluramine psilocybin, methylene-dioxymethamphetamine (MDMA) and is administered i.v. The same dose and route of administration of citalopram has been evaluated with respect to neuroendocrine and cerebral metabolic effects (Smith et al., 2002a,b). It was hypothesized that citalopram administration would decrease striatal D2 receptor availability, consistent with an increase in endogenous dopamine concentrations, as observed in prior studies using less pharmacologically selective agents.
MATERIALS AND METHODS
Subject screening and selection
Subjects underwent medical (physical and laboratory testing, toxicology screening, physical examination) and psychiatric evaluation and Magnetic Resonance Imaging Scan (General Electric 1.5T). Subjects were excluded based upon a history of or current significant medical, psychiatric or neurological illness, substance abuse, family history (first or second degree relatives) of psychiatric or neurological illness or substance abuse or use of prescription or over the counter medications with central nervous system effects (e.g., antihistamines, cold medications) within the past month. Twelve subjects (seven male and five female) with a mean age of 27.8 ± 5.6 years (range 20–42 years) were enrolled in the study. After a complete description of the study to the subjects, written informed consent was obtained. The consent form was approved by the North Shore-Long Island Jewish Health System Institutional Review Board.
Study design
For each subject, two [11C]-raclopride studies were performed on the same day after infusion of a saline placebo (250 ml, Scan 1) or citalopram (40 mg of the drug diluted in 250-ml saline, Scan 2; mean pretreatment interval 54.6 ± 53.5, range 14–189 min). Both infusions lasted 60 min. The study was single blind in that the subjects were told that they would receive either citalopram or placebo (saline) prior to each study, but the investigator knew the identity of the infusion. Citalopram and neuroendocrine (cortisol and prolactin) samples were obtained at predetermined intervals (baseline, end of infusion (EOI), 15, 30, 60, 90, 120, 180 min post-citalopram infusion). The citalopram and neuroendocrine assays were performed in the Geriatric Psychopharmacology Laboratory, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, as described previously (Smith et al., 2002a,b).
Pharmacologic intervention
The dose and route of administration of citalopram (40 mg, i.v.) used in this study is consistent with the clinical use of i.v. citalopram in Europe and has been used in previous neuroimaging and neuroendocrine studies in normal volunteers and in depressed patients (Geday et al., 2005; Kapitany et al., 1999; Seifritz et al., 1996; Smith et al., 2002a,b). Several items from a visual analog scale (Guy, 1976) were administered prior to and following the placebo/citalopram infusions and after the PET scan were completed to determine the effects of citalopram on mood and anxiety.
PET procedures
[11C]-raclopride PET scans were performed in the Functional Brain Imaging Laboratory at North Shore University Hospital. [11C]-raclopride was synthesized by the reaction of carbon-11 labeled methyl iodide with nor-raclopride (Farde et al., 1988). The GE Advance tomograph was used for the PET scans. The performance characteristics of the scanner have been determined in the laboratory (Dhawan et al., 1998). The PET studies were performed in three-dimensional acquisition mode. Before each [11C]-raclopride injection, the subject was positioned in the GE Advance scanner. A 10-min transmission scan was obtained. Then, 15 mCi ± 10% of [11C]-raclopride was injected. Upon i.v. injection of [11C]-raclopride, a 60-min dynamic PET data acquisition began. The scan protocol for [11C]-raclopride involved 5 scans of 60 s, followed by 7 scans of 5 min and 2 scans of 10 min. Subjects were scanned in a quiet, dimly lit room, with eyes open and ears unoccluded.
Image analysis
Image processing of the [11C]-raclopride scans was performed using Statistical Parametric Mapping (SPM99; Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab (Math-works, Sherborn, MA), as described previously (Asanuma et al., 2005). For each scan acquisition (baseline and post-citalopram), the individual scan frames were aligned to frame 10 (30 min) and then for the baseline, a mean image was created from the first 10 frames (0–30 min). The frames from the post-citalopram scan were coregistered to those from the baseline scan by using the corresponding mean images. After the PET coregistration, regions of interest (ROIs) were identified on the mean image. The ROI’s for the caudate and putamen and cerebellum were drawn on three consecutive slices. The ROIs were drawn on the mean image for Scan 1, copied onto the mean image for Scan 2 and then, copied onto the individual frames to generate time activity curves from both scans. The time activity curves from the PET scan were analyzed and a receptor binding parameter (Distribution Volume Ratio, DVR) was calculated over a time window of 30–60 min postinjection using the cerebellar time activity curve as the input function as described by Logan et al. (1996). The use of the cerebellar time activity curve as the input function has been validated in many studies (e.g., Lammertsma et al., 1996; Logan et al., 1996). The PET scanning session was designed for the estimation of receptor-specific radiotracer binding on a regional basis. The use of the DVR allows such a measurement with good test–retest characteristics for [11C]-raclopride (Volkow et al., 1993). Repeated measures analysis of variance (ANOVA) was used to compare changes over time in plasma levels of citalopram, cortisol, prolactin, and the caudate and putamen DVR measurements for [11C]-raclopride.
RESULTS
The results of the plasma analyses for levels of citalopram, cortisol, and prolactin are shown in Table I. A significant main effect of time was obtained for the plasma levels of citalopram (F[4,40] = 25.26, P < 0.001), cortisol (F[5,40] = 6.90, P < 0.001), and prolactin (F[5,40] = 6.70, P < 0.001). The citalopram levels were increased at the EOI and remained at relatively steady state for up to 3 h after the EOI.
TABLE I.
Plasma levels of citalopram (ng/ml), prolactin (ng/ml), and cortisol (μg/dl)
| Mean ± standard deviation
|
|||
|---|---|---|---|
| Citalopram | Prolactin | Cortisol | |
| Preinfusion | 12.4 ± 17.1 | 16.4 ± 13.2 | |
| End of infusion (EOI) | 64.3 ± 20.7 | 16.3 ± 13.6* | 20.6 ± 9.9* |
| 15 min post-EOI | 44.6 ± 7.4 | 19.1 ± 13.1* | 23.2 ± 13.5** |
| 30 min post-EOI | 42.3 ± 7.6 | 21.3 ± 15.0* | 23.5 ± 11.0 |
| 60 min post-EOI | 43.2 ± 7.2 | 18.5 ± 16.9* | 21.9 ± 11.5 |
| 120 min post-EOI | 40.9 ± 8.6 | 19.6 ± 19.0** | 18.8 ± 8.3 |
| 180 min post-EOI | 42.0 ± 4.2 | 11.1 ± 6.2 | 21.4 ± 10.1 |
Significantly different from baseline (P < 0.05).
Significantly different from baseline (P < 0.01).
Posthoc ANOVA’s revealed a significant increase in cortisol levels from the EOI until 15-min postinfusion (EOI: F[1,11] = 9.16, P = 0.02, 15 min post-EOI: F[1,11] = 15.9, P = 0.004, 30 min post-EOI: F[1,11] = 2.19, P = 0.177, 60 min post-EOI: F[1,11] = 2.16, P = 0.175, 120 min post-EOI: F[1,11] = 3.42, P = 0.107, and 180 min post-EOI: F[1,11] = 0.112, P = 0.759). Prolactin levels were significantly elevated from the EOI until 2 h postinfusion (EOI: F[1,11] = 9.16, P = 0.02, 15 min post-EOI: F[1,11] = 3.21, P = 0.116, 30 min post-EOI: F[1,11] = 5.00, P = 0.044, 60 min post-EOI: F[1,11] = 10.80, P = 0. 011, 120 min post-EOI: F[1,11] = 18.89, P = 0.003, and 180 min post-EOI: F[1,11] = 6.16, P = 0.089).
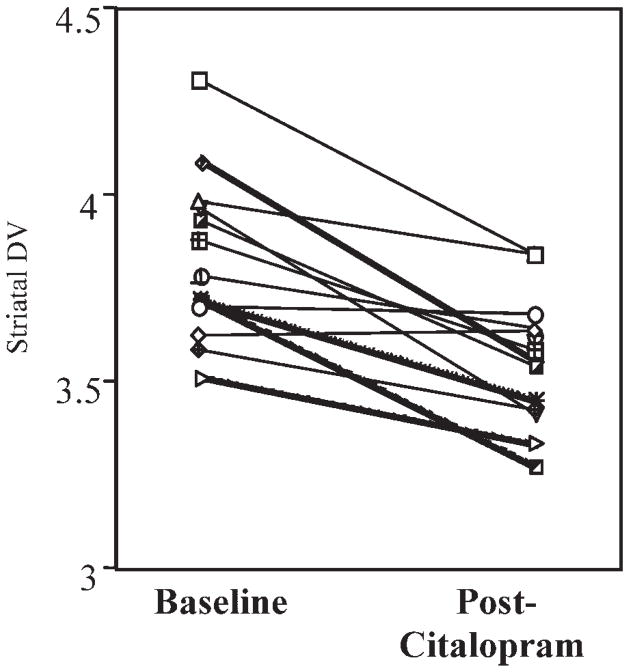
The DV’s for the caudate and putamen are shown in Table II and the data for individual subjects in Figure 1. The DV’s for the caudate (left: F[1,11] = 9.77, P = 0.01, right: F[1,11] = 36.53, P = 0.02) and the putamen (left: F[1,11] = 8.72, P = 0.01, right: F[1,11] = 12.57, P = 0.005) were significantly decreased after citalopram treatment. The visual analog scale scores were not significantly different between saline and citalopram conditions (P > 0.1; data not shown).
TABLE II.
The effects of citalopram on striatal dopamine (D2) receptor availability
| Distribution Volumes (DV)
|
% Change [Post- Citalopram-Baseline)/Baseline] × 100 | ||
|---|---|---|---|
| Baseline | Post-Citalopram | ||
| Left Caudate | 3.72 ± 0.30 | 3.53 ± 0.23 | −4.71 ± 5.23 |
| Right Caudate | 3.64 ± 0.31 | 3.47 ± 0.20 | −4.30 ± 5.44 |
| Left Putamen | 3.81 ± 0.29 | 3.64 ± 0.27 | −5.29 ± 5.01 |
| Right Putamen | 3.91 ± 0.28 | 3.69 ± 0.24 | −4.37 ± 4.98 |
Fig. 1.
The effects of citalopram (40 mg, i.v.) on dopamine (D2) receptor availability measured with PET and [11C]raclopride: Results shown for left putamen mean change—5.29 ± 5.01.
Finally, correlations were performed between the receptor availability measures and the plasma measures. No significant correlations were obtained between the baseline or post-treatment [11C]-raclopride values or magnitude of change in D2 receptor availability and any of the plasma measurements including citalopram, prolactin, and cortisol or pre-treatment interval (P > 0.05).
DISCUSSION
The results of the present study demonstrated that in normal human subjects, i.v. administration of the most selective of the SSRI’s citalopram, produced an increase in cortisol and prolactin concentrations as observed previously (Smith et al., 2002b). A decrease in striatal D2 receptor availability was observed (an average of 5% across regions). As shown in Figure 1, variability in the magnitude of response was observed between subjects, consistent with other acute pharmacological challenge studies using PET and [11C]-raclopride (e.g., Smith et al., 1997, 1998). The variability observed was not explained by differences between subjects in the plasma concentration of citalopram or the magnitude of the neuroendocrine effect. The lack of correlation between the neuroendocrine effects and change in striatal D2 receptor availability may be attributable to the fact that the acute increase in cortisol and prolactin reflects a direct serotonergic effect (the increase in serotonin concentrations activating postsynaptic, hypothalamic serotonin receptors 5-HT1A and 5-HT2A to increase cortisol levels and 5-HT1A, 5-HT2A, and 5-HT2C receptors for prolactin; as reviewed by Raap and Van de Kar, 1999), rather than an effect mediated by dopamine. The citalopram infusion did not produce a significant change in ratings of mood or anxiety that is consistent with our prior study in normal volunteers (Smith et al., 2002b).
Based on the results of simultaneous microdialysis and [11C]-raclopride PET studies, it has been estimated that an 8% increase in striatal dopamine concentrations produced a corresponding 1% decrease in striatal dopamine (Endres et al., 1997). Thus, the results of the present study suggest that the reductions in striatal D2 receptor availability are associated with approximately a 35% increase in striatal dopamine. This magnitude of change is consistent with reports of microdialysis studies in rats of the effects of serotonergic agents on extracellular dopamine concentrations (e.g., Lucas et al., 2000). It is noteworthy that the change in striatal [11C]-raclopride binding in the present study is less in magnitude than our previous study of the effects of fenfluramine. A potential explanation is that the net increase in extracellular serotonin is greater for fenfluramine than citalopram, because in addition to blocking serotonin reuptake, fenfluramine also releases serotonin directly from vesicular stores (Kreiss et al., 1993). Thus, fenfluramine may have a greater effect on dopamine than citalopram because the greater magnitude of increase of serotonin may result in a greater increase in dopamine. The results of the present study in comparison to the earlier fenfluramine challenge study suggest that in the design of studies to evaluate serotonin modulation of dopamine, the pharmacologic agents that work by a dual mechanism of action (reuptake inhibition and direct neurotransmitter release) may be needed to sufficiently increase serotonin levels and significantly affect striatal D2 receptor availability.
Previous studies have shown that acute administration of such agents as fenfluramine, psilocybin, MDMA, LSD, and fenfluramine produced a decrease in striatal D2 receptor availability using [11C]-raclopride (Minuzzi et al., 2005; Rosa-Neto et al., 2004; Smith et al., 1997; Vollenweider et al., 1999). The results of the present study are also consistent with an earlier demonstration of the chronic effects of oral citalopram (Tiihonen et al., 1996). Other human studies using such agents as the selective 5-HT1A receptor agonist, flesinoxan or single oral doses of the anti-depressants nefazodone or paroxetine did not report significant alterations in striatal [11C]-raclopride receptor availability (Bantick et al., 2005; Fowler et al., 1999). Although the results of some human [11C]-raclopride studies suggest that pharmacologic-induced increase in serotonin produces a secondary increase in dopamine concentrations, these findings are in contrast to previous PET studies in anesthetized nonhuman primates that show an inhibitory role of serotonin with respect to striatal dopamine (Dewey et al., 1995). Both methodological factors (e.g., the lack of selective ligands used to distinguish among the 14 serotonin receptor subtypes) and physiological factors (e.g., the baseline status of dopamine and serotonin systems with respect to resting or activated states, activation of serotonin receptor subtypes that might facilitate or inhibit dopamine) may explain the controversial nature of interactions between serotonin and dopamine (as reviewed by Alex and Pehek, 2007).
In interpreting the results of the [11C]-raclopride PET studies, it is important to note that the results observed are the effects of a systemic increase in serotonin concentrations in cortical and subcortical areas. Striatal D2 receptor availability is the final common pathways of these changes. As described in the introduction, serotonin and dopamine systems have been shown to interact in both the nigrostriatal and mesolimbic dopamine systems, at the level of the cell bodies as well as the dopamine terminals (Aghajanian and Bunney, 1974; Azmitia and Segal, 1978; Dray et al., 1976; Herve et al., 1987; Parent et al., 1981; Van Bockstaele et al., 1993). Thus, the changes in striatal D2 receptor availability may be attributable to effects in the nigrostriatal pathway as well as secondary cortical effects. The present study demonstrated a modest effect of i.v. administration of the most selective of the SSRIs, citalopram on striatal dopamine receptor availability as measured with PET and [11C]-raclopride. These findings are consistent with some previous studies using less pharmacologically selective agents (Smith et al., 1997; Vollenweider et al., 1999). Further optimizing the experimental parameters for studying serotonin–dopamine interactions in vivo, either by identifying radiotracers more sensitive to changes in endogenous dopamine or more safe and potent compounds to increase serotonin concentrations is an important area for future study given the importance of studying this interaction in neuropsychiatric disorders.
Acknowledgments
David Bjelke, CNMT, and Claude Margouleff, B.S. are gratefully acknowledged for their contribution to the conduct of the PET studies. Bruce G. Pollock, M. D., Ph.D., Margaret Kirshner, B.S., Denise Soriso, B. S., and Kimberly A. Huber, M.P.H., Geriatric Psychopharmacology Laboratory, Department of Psychiatry, University of Pittsburgh School of Medicine are gratefully acknowledged for providing the citalopram and analyzing the citalopram and neuroendocrine samples.
Contract grant sponsor: National Institute of Health; Contract grant numbers: MH49936, MH57078, MH64823, MH01621.
References
- Aghajanian GK, Bunney BS. Pre and post synaptic feedback mechanisms in central dopaminergic neurons. In: Seeman P, Brown GM, editors. Frontiers in Neurology and Neuroscience Research. Vol. 4. Toronto: Neuroscience Institute; 1974. pp. 11–24. [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol. 1978;179:641–668. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Bantick RA, De Vries MH, Grasby PM. The effect of a 5-HT1A receptor agonist on striatal dopamine release. Synapse. 2005;57:67–75. doi: 10.1002/syn.20156. [DOI] [PubMed] [Google Scholar]
- Breier A, Adler CM, Weisenfeld N, Su TP, Elman I, Picken L, Malhotra AK, Pickar D. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: Application of a novel PET approach. Synapse. 1998;29:142–147. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dewey S, Smith G, Logan J, Brodie J, Yu D, Ferrieri R, King P, MacGregor R, Martin T, Wolf A, Volkow N, Fowler J. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Simkowitz P, MaeGregor RR, Fowler JS, Volkow ND, Wolf AP. Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography (PET) in normal human subjects. Proc Natl Acad Sci USA. 1993a;90:11816–11820. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993b;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- Dewey S, Smith G, Logan J, Alexoff D, Ding Y, King P, Pappas N, Brodie J, Ashby C. Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in vivo microdialysis. J Neurosci. 1995;15(1 Part 2):821–829. doi: 10.1523/JNEUROSCI.15-01-00821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Brodie JD, Fowler JS, MacGregor RR, Schlyer DJ, King PT, Alexoff DL, Volkow ND, Shiue CY, Wolf AP. Positron emission tomography (PET) studies of dopaminergic/cholinergic interactions in the baboon brain. Synapse. 1990;6:321–327. doi: 10.1002/syn.890060403. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Logan J, Wolf AP, Brodie JD, Angrist B, Fowler JS, Volkow ND. Amphetamine induced decreases in (18F)-N-methylspiroperidol binding in the baboon brain using positron emission tomography (PET) Synapse. 1991;7:324–327. doi: 10.1002/syn.890070409. [DOI] [PubMed] [Google Scholar]
- Dhawan V, Kazumata K, Robeson W, Belakhlef A, Margouleff C, Chaly T, Nakamura T, Dahl R, Margouleff D, Eidelberg D. Quantitative brain PET: Comparison of 2D and 3D acquisition on the GE Advance Scanner. Clin Positron Imaging. 1998;1:135–144. doi: 10.1016/s1095-0397(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Dray A, Gonye TJ, Oakley NR, Tanner T. Evidence for the existence of a raphe projection to the substantia nigra in rat. Brain Res. 1976;113:45–57. doi: 10.1016/0006-8993(76)90005-6. [DOI] [PubMed] [Google Scholar]
- Endres CJ, Kolachana BS, Saunders RC, Su T, Weinberger D, Breier A, Eckelman WC, Carson RE. Kinetic modeling of [11C]raclopride: Combined PET-microdialysis studies. J Cereb Blood Flow Metab. 1997;17:932–942. doi: 10.1097/00004647-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Farde L, Pauli S, Hall H. Stereoselective binding of 11C-raclopride in the living human brain-a search for extrastriatal central D2 dopamine receptors by PET. Psychopharmacology. 1988;94:471–478. doi: 10.1007/BF00212840. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Wang GJ, Volkow ND, Ieni J, Logan J, Pappas N, Dewey SL. PET studies of the effect of the antidepressant drugs Nefazodone or Paroxetine on [11C]raclopride binding in human brain. Clin Positron Imaging. 1999;2:205–209. doi: 10.1016/s1095-0397(99)00028-x. [DOI] [PubMed] [Google Scholar]
- Geday J, Hermansen F, Rosenberg R, Smith DF. Serotonin modulation of cerebral blood flow measured with positron emission tomography (PET) in humans. Synapse. 2005;55:224–229. doi: 10.1002/syn.20112. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU: An assessment manual for psychopharmacology. Vol. 76. Rockville, MD: US Department of Health Education and Welfare Publication (ADM); 1976. p. 336. [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: Fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Innis R, Mallison R, Al-Tikriti M, Hoffer M, Sybirska E, Seibyl J, Zoghbi S, Baldwin R, Laruelle M, Smith E, Charney D, Henninger G, Elsworth J, Roth R. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- Kapitany T, Schindl M, Schindler S, Helmann B, Fureder T, Barnas C, Sieghart, Kasper S. The citalopram challenge test in patients with major depression and in healthy controls. Psychiatry Res. 1999;88:75–88. doi: 10.1016/s0165-1781(99)00082-7. [DOI] [PubMed] [Google Scholar]
- Kreiss D, Wieland S, Lucki I. The presence of a serotonin uptake inhibitor alters pharmacological manipulations of serotonin release. Neuroscience. 1993;52:295–301. doi: 10.1016/0306-4522(93)90157-b. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Laruelle M, D’Souza CD, Baldwin RM, Abi-Dargham A, Kanes SJ, Fingado CL, Seibyl JP, Zoghbi SS, Bowers MB, Jatlow P, Charney DS, Innis RB. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17:162–174. doi: 10.1016/S0893-133X(97)00043-2. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lucas G, De Deurwaerdere P, Porras G, Spampinato U. Endogenous serotonin enhances the release of dopamine in the striatum only when nigrostriatal dopaminergic transmission is activated. Neuropharmacology. 2000;39:1984–1995. doi: 10.1016/s0028-3908(00)00020-4. [DOI] [PubMed] [Google Scholar]
- Minuzzi L, Nomikos GG, Wade MR, Jensen SB, Olsen AK, Cumming P. Interaction between LSD and dopamine D2/3 binding sites in pig brain. Synapse. 2005;56:198–204. doi: 10.1002/syn.20141. [DOI] [PubMed] [Google Scholar]
- Nedergaard S, Bolam JP, Greenfield SA. Facilitation of a dendritic calcium conductance by 5-hydroxytryptamine in the sub-stantia nigra. Nature. 1988;333:174–177. doi: 10.1038/333174a0. [DOI] [PubMed] [Google Scholar]
- Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Raap DK, Van de Kar LD. Selective serotonin reuptake inhibitors and neuroendocrine function. Life Sci. 1999;65:1217–1235. doi: 10.1016/s0024-3205(99)00169-1. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Gjedde A, Olsen AK, Jensen SB, Munk OL, Watanabe H, Cumming P. MDMA-evoked changes in [11C]raclopride and [11C]NMSP binding in living pig brain. Synapse. 2004;53:222–233. doi: 10.1002/syn.20053. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Fadayel G, Sullivan C, Taylor V. 5HT-2 receptors exert a state-dependent regulation of dopaminergic function: Studies with MDL 100,907 and the amphetamine analog, 3,4-methylenedioxymethamphetamine. Eur J Pharmacol. 1992;223:65–74. doi: 10.1016/0014-2999(92)90819-p. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Baumann P, Muller MJ, Annen O, Amey M, Hemmeter U, Hatzinger M, Chardon F, Holsboer-Trachsler E. Neuroendocrine effects of a 20-mg citalopram infusion in healthy males. A placebo-controlled evaluation of citalopram as 5-HT function probe. Neuropsychopharmacology. 1996;14:253–263. doi: 10.1016/0893-133X(95)00117-V. [DOI] [PubMed] [Google Scholar]
- Smith G, Dewey S, Schloesser R, Logan J, Brodie J, Simkowitz P, Vitkun S, Alexoff D, Martin T, Jenkins D, Shea C, Volkow N. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Smith G, Kramer E, Hermann C, Goldberg S, Ma Y, Dhawan V, Barnes A, Chaly T, Belakhleff A, Laghrissi-Thode F, Greenwald B, Eidelberg D, Pollock B. Serotonin modulation of cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry. 2002a;10:715–723. [PubMed] [Google Scholar]
- Smith G, Ma Y, Dhawan V, Gunduz H, Carbon M, Kirshner M, Larson J, Chaly T, Kramer E, Greenwald B, Kane J, Laghrissi-Thode F, Pollock B, Eidelberg D. Serotonin modulation of cerebral glucose metabolism measured with positron emission tomography (PET) in human subjects. Synapse. 2002b;45:105–112. doi: 10.1002/syn.10088. [DOI] [PubMed] [Google Scholar]
- Smith GS, Dewey SL, Brodie JD, Logan J, Vitkun SA, Simkowitz P, Schloesser R, Alexoff DA, Hurley A, Cooper T, Volkow ND. Serotonergic modulation of dopamine measured with [11C]raclopride and PET in normal human subjects. Am J Psychiatry. 1997;154:490–496. doi: 10.1176/ajp.154.4.490. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuoppamaki M, Nagren K, Bergman J, Eronen E, Syvalahti E, Hietala J. Serotonergic modulation of striatal D2 dopamine receptor number in humans measured with positron emission tomography. Psychopharmacology. 1996;126:277–280. doi: 10.1007/BF02247377. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624:188–198. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G-J, Dewey S, Schleyer D, MacGregor R, Logan J, Alexoff D, Shea C, Hitzemann R, Angrist B, Wolf A. Reproducibility of repeated measures of Carbon-11 raclopride binding in the human brain. J Nucl Med. 1993;34:609–613. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man–a PET study with [11C] raclopride. Neuropsychopharmacology. 1999;20:424–433. doi: 10.1016/S0893-133X(98)00108-0. [DOI] [PubMed] [Google Scholar]