Summary
Background
Idiopathic Parkinson’s disease can present with symptoms similar to those of multiple system atrophy or progressive supranuclear palsy. We aimed to assess whether metabolic brain imaging combined with spatial covariance analysis could accurately discriminate patients with parkinsonism who had different underlying disorders.
Methods
Between January, 1998, and December, 2006, patients from the New York area who had parkinsonian features but uncertain clinical diagnosis had fluorine-18-labelled-fluorodeoxyglucose-PET at The Feinstein Institute for Medical Research. We developed an automated image-based classification procedure to differentiate individual patients with idiopathic Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. For each patient, the likelihood of having each of the three diseases was calculated by use of multiple disease-related patterns with logistic regression and leave-one-out cross-validation. Each patient was classified according to criteria defined by receiver-operating-characteristic analysis. After imaging, patients were assessed by blinded movement disorders specialists for a mean of 2·6 years before a final clinical diagnosis was made. The accuracy of the initial image-based classification was assessed by comparison with the final clinical diagnosis.
Findings
167 patients were assessed. Image-based classification for idiopathic Parkinson’s disease had 84% sensitivity, 97% specificity, 98% positive predictive value (PPV), and 82% negative predictive value (NPV). Imaging classifications were also accurate for multiple system atrophy (85% sensitivity, 96% specificity, 97% PPV, and 83% NPV) and progressive supranuclear palsy (88% sensitivity, 94% specificity, 91% PPV, and 92% NPV).
Interpretation
Automated image-based classification has high specificity in distinguishing between parkinsonian disorders and could help in selecting treatment for early-stage patients and identifying participants for clinical trials.
Funding
National Institutes of Health and General Clinical Research Center at The Feinstein Institute for Medical Research.
Introduction
Many of the common late-onset neurodegenerative diseases have characteristic signs and symptoms. However, the main features of neurodegenerative disorders often overlap with those of other diseases, and early signs and symptoms can be difficult to diagnose. For example, the classic clinical features of idiopathic Parkinson’s disease that are associated with degeneration of the nigrostriatal pathway—tremor, rigidity, and bradykinesia—can also be present in patients with corticobasal ganglionic degeneration, diffuse Lewy body disease, essential tremor, drug-induced parkinsonism, or vascular parkinsonism, particularly in the early stages of disease. About 80% of patients misdiagnosed as having idiopathic Parkinson’s disease actually have multiple system atrophy or progressive supranuclear palsy—the two most common atypical parkinsonian syndromes.1,2
Accurate early diagnosis of parkinsonian disorders is important for several reasons. First, prognosis for different disorders differs greatly: for example, idiopathic Parkinson’s disease does not substantially shorten a patient’s lifespan, but patients with multiple system atrophy and progressive supranuclear palsy typically have a life expectancy of only several years after diagnosis. Second, some treatments have different outcomes: for example, deep brain stimulation is usually effective in patients with idiopathic Parkinson’s disease but not in patients with atypical parkinsonism.3,4 Third, clinical trials of potentially disease-modifying drugs5,6 might include misdiagnosed patients. In fact, up to 15% of patients diagnosed with early idiopathic Parkinson’s disease at the time of recruitment into trials were later found to have other parkinsonian syndromes.7
Because of the diagnostic challenges, better techniques are needed for differential diagnosis of parkinsonian diseases. Neuroimaging methods have not been sufficiently accurate in individual patients, especially in those with short symptom duration.8–12 Abnormal nigrostriatal innervation on dopaminergic imaging in patients with suspected idiopathic Parkinson’s disease helps to rule out essential tremor, drug-induced parkinsonism, vascular parkinsonism, and Alzheimer’s disease,9 but does not rule out progressive atypical parkinsonian syndromes (eg, multiple system atrophy and progressive supranuclear palsy).9,10
In the past 5 years, voxel-based spatial covariance mapping has been applied to fluorine-18-labelled fluorodeoxyglucose (FDG)-PET images to identify specific disease-related metabolic patterns in patients with neurodegenerative disorders.13,14 Using this technique, we have mapped and validated characteristic patterns of abnormality in patients with idiopathic Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy.15,16 In each of these diseases, quantitative indices of pattern expression can discriminate between patients with the disease and healthy individuals.15–17 However, several covariance patterns might be needed to discriminate between patients who have similar clinical features but have different underlying disorders.18
We developed a multiple-pattern imaging technique to calculate the probability of patients with parkinsonism but uncertain clinical diagnosis at the time of imaging having idiopathic Parkinson’s disease, multiple system atrophy, or progressive supranuclear palsy. For each patient, the initial image-based diagnosis was compared with the final clinical diagnosis.
Methods
Patients
Between January, 1998, and December, 2006, patients who were routinely assessed by movement disorders specialists in New York and who had been diagnosed with either non-specific parkinsonism or two or more possible diagnoses were referred to the functional neuroimaging laboratory at The Feinstein Institute for Medical Research (NY, USA) for FDG-PET. Inclusion criteria were clinical follow-up for at least 6 months after FDG-PET; a final clinical diagnosis of idiopathic Parkinson’s disease, multiple system atrophy, or progressive supranuclear palsy made by the movement disorders specialist on at least two consecutive visits; subsequent confirmation of the clinical diagnosis on chart review by a second blinded movement disorders specialist (KLP) using published clinical criteria;19 and no evidence of structural brain abnormalities (ie, mass lesions, white matter changes, or ischaemia) on MRI that could have caused the clinical findings.
The patients included in this study are typical of patients referred to tertiary movement-disorders centres because of diagnostic uncertainty.2 In our specialty clinics, the proportion of patients referred with parkinsonism who actually had an atypical parkinsonian syndrome was about 30%,20 which is higher than expected based solely on the population prevalence.21
Ethics permission for this study was obtained from the institutional review boards of the participating institutions. Each patient gave written informed consent after detailed explanation of the procedure.
Procedures
Patients fasted overnight for 12 h before FDG-PET. In patients receiving daily treatment with oral anti-parkinsonian medications, these drugs were withheld for at least 12 h before the scan. FDG-PET and network quantification were done as described previously.15,18 We developed a multiple-pattern analytical technique to compute the probability of a patient having idiopathic Parkinson’s disease, multiple system atrophy, or progressive supranuclear palsy from FDG-PET scans of patients with parkinsonism who had uncertain clinical diagnosis at the time of imaging. Scores that quantify expression of specific metabolic covariance patterns related to Parkinson’s disease,15 multiple system atrophy,16 and progressive supranuclear palsy16 were calculated for each patient. These scores were represented mathematically as the multiplication of each voxel value by the corresponding voxel weight in each disease-related covariance pattern. The values for all voxels were then summed over the whole brain volume.14,15 The resulting pattern scores represent the extent to which a patient expresses each of the three disease-related patterns. These scores were then Z-transformed with respect to a reference population (42 healthy volunteers, mean age 51·6 years [SD 14·6]) so that the normal mean was 0·0 (SD 1·0).
After FDG-PET, patients were assessed and given a clinical diagnosis by movement disorders specialists at each of the participating centres; specialists were unaware of the results of the image-based classification procedure. For each patient, we compared our initial image-based classification with the final clinical diagnosis.
In a subgroup of patients we did a separate imaging classification procedure on repeat scans to assess the reproducibility of the initial image-based diagnosis. In another subgroup of patients we compared our image-based classification with the diagnosis made at post-mortem examination at the Columbia University Medical Center Brain Bank. A neuropathologist (J-PV) used established criteria to diagnose patients on the basis of pathological assessment.22–24 Each post-mortem diagnosis was compared with the initial image-based classification and with the final clinical diagnosis.
Statistical analysis
Published clinicopathological data show a very high concordance (99% positive predictive value [PPV]) between final clinical diagnosis after at least 2 years of follow-up by a movement disorders specialist and the findings at post-mortem examination.2 Therefore, we used the final clinical diagnosis as the gold standard with which to compare the initial image-based classification in individual patients.
We developed a two-level algorithm to classify patients on the basis of their pattern scores. For first-level analysis, we grouped patients with clinically diagnosed multiple system atrophy and progressive supranuclear palsy into a single atypical parkinsonian syndrome group. Discrimination between the idiopathic Parkinson’s disease and atypical parkinsonian syndrome groups was examined with a logistic regression model25 with a dependent variable (idiopathic Parkinson’s disease or atypical parkinsonian syndrome) and three predictor variables (scores for the individual covariance patterns related to Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy). We selected the model with the best discrimination between the two groups, defined by the lowest Akaike’s information criteria value. Furthermore, we used leave-one-out cross-validation26 to obtain probabilities for idiopathic Parkinson’s disease and atypical parkinsonian syndrome in a given patient without bias from the patient’s own data. We did logistic regression of the pattern scores from other patients to compute the probability of each patient having idiopathic Parkinson’s disease or atypical parkinsonian syndrome. For each patient, the probabilities for idiopathic Parkinson’s disease and atypical parkinsonian syndrome sum to 1·0.
To establish the diagnostic criteria for the image-based classification of the patients, receiver-operating-characteristic (ROC) curves were plotted for the probability values for idiopathic Parkinson’s disease and atypical parkinsonian syndrome. The area under the curve was estimated for each curve with an SAS Macro ROC. The optimum cut-off probability values for classifying individual patients as having idiopathic Parkinson’s disease or atypical parkinsonian syndrome were calculated by identifying an inflection point on each ROC curve that corresponded to a combination of high specificity (>90%) and sensitivity (>80%). We chose a higher specificity than sensitivity because, in the clinical setting, FDG-PET imaging is used mainly as a confirmatory test rather than a screening test. Patients were classified as having idiopathic Parkinson’s disease or atypical parkinsonian syndrome if their probability value for one of these classifications was higher than the cut-off value. Patients were classified as having indeterminate parkinsonism if the probability of each classification was lower than the cut-off value for both the idiopathic Parkinson’s disease and the atypical parkinsonian syndrome classifications. On the basis of individual classification results, we calculated discriminative measures—sensitivity, specificity, PPV, and negative predictive value (NPV)—for patients clinically diagnosed with idiopathic Parkinson’s disease or atypical parkinsonian syndrome.
For second-level analysis, patients classified as having atypical parkinsonian syndrome were analysed to differentiate between multiple system atrophy and progressive supranuclear palsy. We used a logistic-regression model to differentiate between multiple system atrophy and non-multiple system atrophy (ie, progressive supranuclear palsy and idiopathic Parkinson’s disease). We used another logistic regression model to differentiate between progressive supranuclear palsy and non-progressive supranuclear palsy (ie, multiple system atrophy and idiopathic Parkinson’s disease). For patients with atypical parkinsonian syndrome, we computed the predicted probabilities of multiple system atrophy and progressive supranuclear palsy and then generated the ROC curves to identify optimum cut-off probabilities for each disorder using the method described above. As before, patients were classified as having multiple system atrophy or progressive supranuclear palsy if the probability value for that disease was higher than the cut-off value. Patients were classified as having indeterminate parkinsonism if the probability of each disease was lower than the corresponding cut-off values. We then calculated discriminative measures for patients clinically diagnosed with multiple system atrophy and for those with progressive supranuclear palsy.
For both analyses we calculated the discriminative measures for patients who had a short symptom duration (≤2 years) and for those who had a long symptom duration (>2 years). Patients with short symptom duration can be difficult to diagnose because many have not developed characteristic disease-specific clinical features. Because clinical diagnosis is usually more accurate after longer follow-up,2 we also calculated the discriminative measures in subgroups of patients with short (≤2 years) or long (>2 years) clinical follow-up.
Role of the funding source
The sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding author had full access to all the data and had final responsibility for the decision to submit the paper for publication.
Results
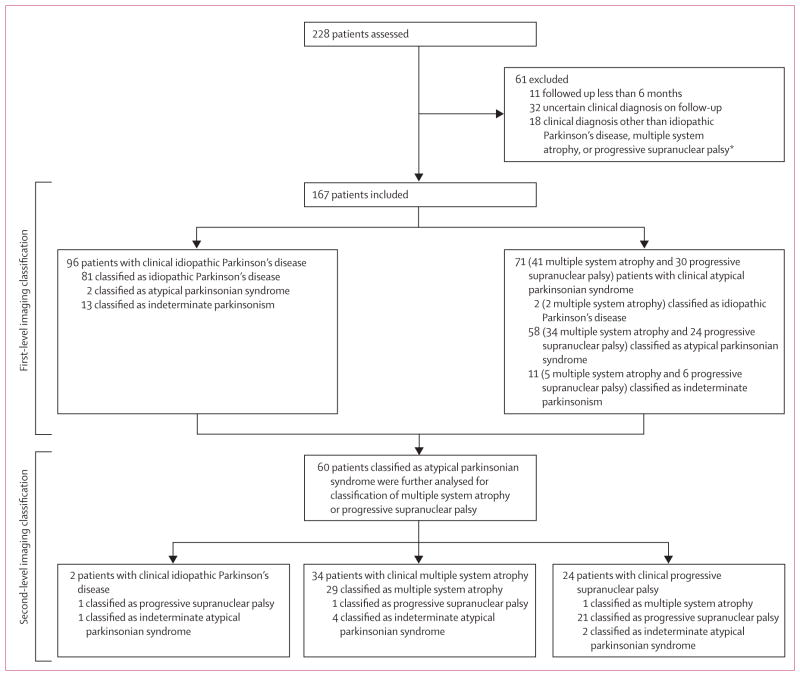
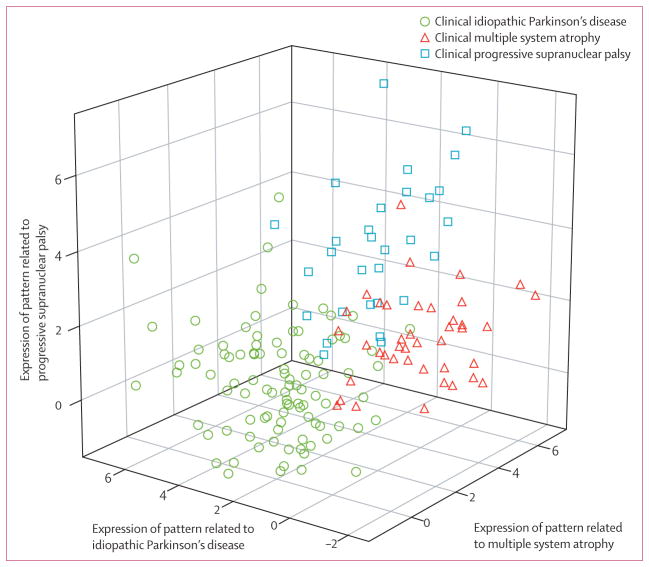
167 patients were included in the study (figure 1). The disease-specific pattern scores for each of the patients are shown in figure 2. 96 patients had a final clinical diagnosis of idiopathic Parkinson’s disease, 41 multiple system atrophy, and 30 progressive supranuclear palsy (table 1). The mean interval between FDG-PET and final clinical diagnosis was 2·6 years (SD 2·4). Clinical and imaging data from 97 of the 167 patients (66 with idiopathic Parkinson’s disease, 17 multiple system atrophy, and 14 progressive supranuclear palsy) have been published previously.18
Figure 1. Study protocol.
*This group included seven patients with corticobasal ganglionic degeneration, three with infectious parkinsonism or prion disease, three with vascular parkinsonism with MRI abnormalities, one with psychogenic disease, one with an autoimmune disorder, one with rapid-onset dystonia-parkinsonism, one with progressive lateral sclerosis with atypical parkinsonism, and one with multiple sclerosis and idiopathic Parkinson’s disease.
Figure 2.
3D plot of FDG-PET pattern expression
Table 1.
Demographic features of patient subgroups by duration of symptoms and clinical follow-up
| Symptom duration ≤2 years
|
Symptom duration >2 years
|
|||
|---|---|---|---|---|
| ≤2 years of clinical follow-up | >2 years of clinical follow-up | ≤2 years of clinical follow-up | >2 years of clinical follow-up | |
| Idiopathic Parkinson’s disease | ||||
|
| ||||
| Number of patients | 9 | 21 | 8 | 58 |
| Sex (male, female) | 5, 4 | 15, 6 | 5, 3 | 41, 17 |
| Age at FDG-PET (years) | 60·4 (11·2) | 54·7 (10·1) | 59·3 (7·5) | 57·7 (9·4) |
| Symptom duration at FDG-PET (years) | 1·4 (0·4) | 1·5 (0·5) | 6·1 (3·3) | 8·3 (3·9) |
| Hoehn and Yahr stage | 1·8 (0·7) | 1·6 (0·6) | 2·5 (0·4) | 2·7 (0·8) |
| UPDRS | 14·1 (8·8) | 15·8 (12·3) | 26·7 (9·3) | 29·9 (12·3) |
| Clinical follow-up (years) | 1·2 (0·4) | 5·5 (2·1) | 1·4 (0·4) | 6·1 (2·6) |
|
| ||||
| Multiple system atrophy | ||||
|
| ||||
| Number of patients | 5 | 6 | 11 | 19 |
| Sex (male, female) | 2, 3 | 3, 3 | 5, 6 | 8, 11 |
| Age at FDG-PET (years) | 59·3 (8·7) | 59·1 (6·5) | 63·8 (9·8) | 60·6 (9·0) |
| Symptom duration at FDG-PET (years) | 1·8 (0·4) | 1·7 (0·5) | 4·0 (1·8) | 5·1 (1·9) |
| Hoehn and Yahr stage | 2·3 (0·6) | 2·6 (1·1) | 3·3 (1·0) | 3·9 (1·1) |
| UPDRS | 28·7 (8·7) | 27·2 (15·8) | 36·1 (17·3) | 37·6 (11·7) |
| Clinical follow-up (years) | 1·0 (0·5) | 6·0 (3·9) | 1·0 (0·2) | 4·2 (1·6) |
|
| ||||
| Progressive supranuclear palsy | ||||
|
| ||||
| Number of patients | 8 | 6 | 9 | 7 |
| Sex (male, female) | 4, 4 | 3, 3 | 4, 5 | 3, 4 |
| Age at FDG-PET (years) | 66·2 (4·8) | 66·6 (9·8) | 70·1 (6·6) | 74·5 (6·1) |
| Symptom duration at FDG-PET (years) | 2·0 (0·0) | 1·5 (0·5) | 4·6 (1·0) | 3·6 (0·8) |
| Hoehn and Yahr stage | 3·0 (0·0) | 2·8 (0·5) | 3·3 (0·5) | 3·5 (1·0) |
| UPDRS | 24·1 (11·8) | 20·8 (10·2) | 23·3 (14·2) | 32·8 (23·4) |
| Clinical follow-up (years) | 1·1 (0·5) | 3·2 (0·7) | 1·1 (0·5) | 2·7 (0·9) |
Values are mean (SD). UPDRS=Unified Parkinson’s disease rating scale, part III.
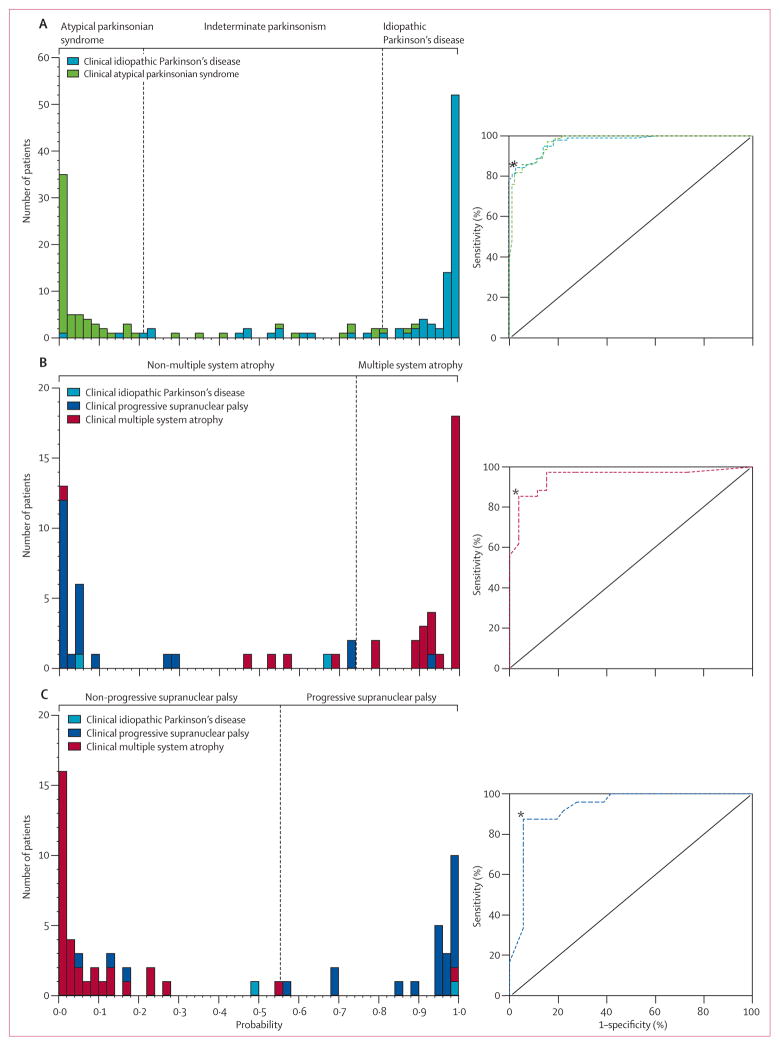
On first-level analysis, patients with idiopathic Parkinson’s disease were accurately differentiated from those with atypical parkinsonian syndrome (p<0·0001, logistic regression) by a discriminant function involving the expression of patterns related to all three diseases (idiopathic Parkinson’s disease p<0·0001, multiple system atrophy p<0·0001, progressive supranuclear palsy p=0·0393). To show the relation between sensitivity and specificity at different levels of disease probability, we plotted the ROC curves for idiopathic Parkinson’s disease and atypical parkinsonian syndrome for all patients (figure 3). The area under both curves was 0·97 (95% CI 0·95–0·99). The optimum cut-off probability for classifying idiopathic Parkinson’s disease was 0·81 and for atypical parkinsonian syndrome it was 0·79. Therefore, patients were classified as having idiopathic Parkinson’s disease if the probability value for idiopathic Parkinson’s disease was greater than 0·81, and atypical parkinsonian syndrome if the probability value for atypical parkinsonian syndrome was greater than 0·79. Patients whose probability values for idiopathic Parkinson’s disease and atypical parkinsonian syndrome were lower than their cut-off values were classified as having indeterminate parkinsonism. On the basis of individual results, imaging classification resulted in 84% sensitivity, 97% specificity, 98% PPV, and 82% NPV for patients clinically diagnosed with idiopathic Parkinson’s disease (table 2). For patients clinically diagnosed with atypical parkinsonian syndrome, image-based classification resulted in 82% sensitivity, 98% specificity, 97% PPV, and 88% NPV. 24 of the 167 patients (13 idiopathic Parkinson’s disease, five multiple system atrophy, and six progressive supranuclear palsy) were classified as having indeterminate parkinsonism (figure 1).
Figure 3. Predicted disease probability for differential diagnosis of parkinsonism.
Frequency distributions (left) for idiopathic Parkinson’s disease and atypical parkinsonian syndrome (A), multiple system atrophy (B), and progressive supranuclear palsy (C). The probability of atypical parkinsonian syndrome is the inverse of the probability of idiopathic Parkinson’s disease. ROC curves for each classification (right). *Inflection points on ROC curves chosen to identify the optimum cut-off probabilities for classification (vertical dashed lines).
Table 2.
Imaging classification relative to final clinical diagnosis: discriminative measures
| All patients | Symptom duration (years)
|
Symptom duration, clinical follow-up (years)
|
|||||
|---|---|---|---|---|---|---|---|
| ≤2 | >2 | ≤2, ≤2 | ≤2, >2 | >2, ≤2 | >2, >2 | ||
| Idiopathic Parkinson’s disease | |||||||
|
| |||||||
| Sensitivity | 84% (81/96) | 77% (23/30) | 88% (58/66) | 67% (6/9) | 81% (17/21) | 50% (4/8) | 93% (54/58) |
| Specificity | 97% (69/71) | 92% (23/25) | 100% (46/46) | 92% (12/13) | 92% (11/12) | 100% (20/20) | 100% (26/26) |
| Positive predictive value | 98% (81/83) | 92% (23/25) | 100% (58/58) | 86% (6/7) | 94% (17/18) | 100% (4/4) | 100% (54/54) |
| Negative predictive value | 82% (69/84) | 77% (23/30) | 85% (46/54) | 80% (12/15) | 73% (11/15) | 83% (20/24) | 87% (26/30) |
|
| |||||||
| Atypical parkinsonian syndrome | |||||||
|
| |||||||
| Sensitivity | 82% (58/71) | 72% (18/25) | 87% (40/46) | 62% (8/13) | 83% (10/12) | 80% (16/20) | 92% (24/26) |
| Specificity | 98% (94/96) | 97% (29/30) | 98% (65/66) | 89% (8/9) | 100% (21/21) | 88% (7/8) | 100% (58/58) |
| Positive predictive value | 97% (58/60) | 95% (18/19) | 98% (40/41) | 89% (8/9) | 100% (10/10) | 94% (16/17) | 100% (24/24) |
| Negative predictive value | 88% (94/107) | 81% (29/36) | 92% (65/71) | 62% (8/13) | 91% (21/23) | 64% (7/11) | 97% (58/60) |
|
| |||||||
| Multiple system atrophy | |||||||
|
| |||||||
| Sensitivity | 85% (29/34) | 88% (7/8) | 85% (22/26) | 67% (2/3) | 100% (5/5) | 88% (7/8) | 83% (15/18) |
| Specificity | 96% (25/26) | 91% (10/11) | 100% (15/15) | 83% (5/6) | 100% (5/5) | 100% (9/9) | 100% (6/6) |
| Positive predictive value | 97% (29/30) | 88% (7/8) | 100% (22/22) | 67% (2/3) | 100% (5/5) | 100% (7/7) | 100% (15/15) |
| Negative predictive value | 83% (25/30) | 91% (10/11) | 79% (15/19) | 83% (5/6) | 100% (5/5) | 90% (9/10) | 67% (6/9) |
|
| |||||||
| Progressive supranuclear palsy | |||||||
|
| |||||||
| Sensitivity | 88% (21/24) | 90% (9/10) | 86% (12/14) | 80% (4/5) | 100% (5/5) | 75% (6/8) | 100% (6/6) |
| Specificity | 94% (34/36) | 100% (9/9) | 93% (25/27) | 100% (4/4) | 100% (5/5) | 78% (7/9) | 100% (18/18) |
| Positive predictive value | 91% (21/23) | 100% (9/9) | 86% (12/14) | 100% (4/4) | 100% (5/5) | 75% (6/8) | 100% (6/6) |
| Negative predictive value | 92% (34/37) | 90% (9/10) | 93% (25/27) | 80% (4/5) | 100% (5/5) | 78% (7/9) | 100% (18/18) |
Data are % (calculation).
In 55 patients who had a short symptom duration, the PPV was 92% for idiopathic Parkinson’s disease and 95% for atypical parkinsonian syndrome. Of these patients, 33 were followed up for at least 2 years after imaging; in these patients the PPV for idiopathic Parkinson’s disease was 94% and that for atypical parkinsonian syndrome was 100% (table 2). Moreover, the PPVs were 100% for idiopathic Parkinson’s disease and atypical parkinsonian syndrome in 27 patients who had high expression (>1·0, corresponding to more than 1·0 SD above the normal mean) of one or more of the three disease-related patterns. By contrast, the PPV was 80% for idiopathic Parkinson’s disease in the six patients with low expression (≤1·0) of the three patterns.
60 patients (34 clinically diagnosed with multiple system atrophy, 24 progressive supranuclear palsy, and two idiopathic Parkinson’s disease) were classified as having atypical parkinsonian syndrome on first-level analysis. Patients with multiple system atrophy were accurately classified relative to those with non-multiple system atrophy (p<0·0001, logistic regression). The discriminant function involved expression of patterns related to multiple system atrophy (p=0·0183) and progressive supranuclear palsy (p=0·0013), but not idiopathic Parkinson’s disease (p=0·7296). The area under the ROC curve for multiple system atrophy was 0·95 (95% CI 0·89–1·00, figure 3). According to this curve, the optimum cut-off probability for multiple system atrophy was 0·74. Multiple-pattern imaging also accurately discriminated between patients with progressive supranuclear palsy and those with non-progressive supranuclear palsy (p<0·0001, logistic regression). The discriminant function involved expression of patterns related to progressive supranuclear palsy (p=0·0004) and multiple system atrophy (p=0·0157), but not idiopathic Parkinson’s disease (p=0·2722). The area under the ROC curve for progressive supranuclear palsy (figure 3) was 0·93 (95% CI 0·86–0·99). According to this curve, the optimum cut-off for progressive supranuclear palsy classification was 0·55. Therefore, each patient classified as having atypical parkinsonian syndrome was further classified as having multiple system atrophy if the probability of multiple system atrophy was greater than 0·74 and progressive supranuclear palsy if the probability of progressive supranuclear palsy was greater than 0·55. If a patient’s probability values for progressive supranuclear palsy and multiple system atrophy were both lower than the cut-off values then that patient was classified as having indeterminate atypical parkinsonian syndrome.
Imaging classification for patients clinically diagnosed with multiple system atrophy resulted in 85% sensitivity, 96% specificity, 97% PPV and 83% NPV (table 2). For patients with multiple system atrophy who had short symptom duration the PPV was 88% and in the five of these patients who also had long clinical follow-up the PPV was 100%. Image-based classification for patients with progressive supranuclear palsy resulted in 88% sensitivity, 94% specificity, 91% PPV, and 92% NPV. The PPV was 100% for patients with progressive supranuclear palsy who had a short symptom duration, five of whom had long clinical follow-up after imaging. Of the 60 patients classified as having atypical parkinsonian syndrome on first-level analysis, seven (one clinically diagnosed with idiopathic Parkinson’s disease, four multiple system atrophy, and two progressive supranuclear palsy) were subsequently classified as having indeterminate atypical parkinsonian syndrome on second-level analysis (figure 1).
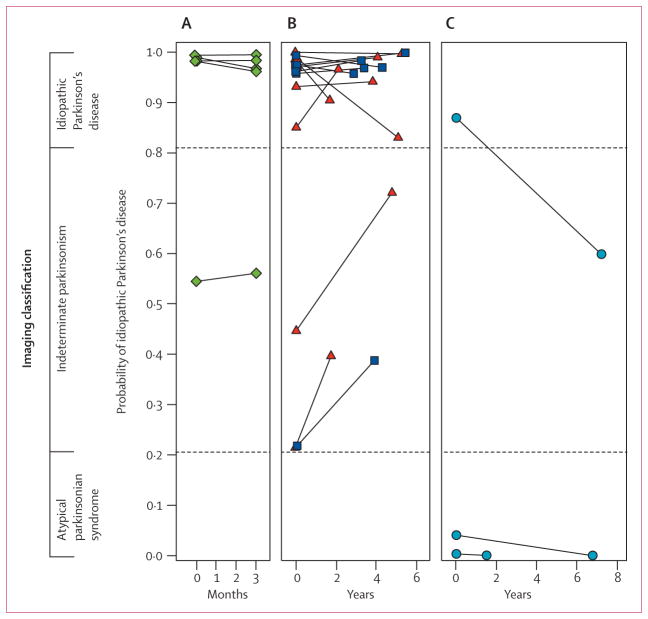
22 patients had repeat imaging. 19 were clinically diagnosed with idiopathic Parkinson’s disease and three with multiple system atrophy; the mean interval between scans was 3·1 years (SD 2·2). There was strong agreement between the imaging classifications for the two scans (weighted kappa 0·92, 95% CI 0·77–1·00, p<0·0001). In 21 of the patients, the same disease classification was made from both scans (figure 4). One patient who had a final clinical diagnosis of multiple system atrophy was initially classified as having idiopathic Parkinson’s disease but as having indeterminate parkinsonism on repeat imaging 7·2 years later.
Figure 4. Reliability of imaging classification on repeat testing.
Probabilities of idiopathic Parkinson’s disease and atypical parkinsonian syndrome computed from the initial and repeat scans of 22 patients. Values from the two scans from each patient are connected by solid lines. Significant agreement (p<0·0001) was found between the image-based classifications from the two scans for these patients. Probability of atypical parkinsonian syndrome is the inverse of that for idiopathic Parkinson’s disease. (A) Five patients clinically diagnosed with idiopathic Parkinson’s disease who were drug-naive at the time of the initial scan and who were rescanned after 3 months of oral carbidopa plus levodopa treatment. (B) 14 patients with clinical idiopathic Parkinson’s disease who were scanned twice in the off-state. Six patients (blue squares) were drug-naive at baseline and eight (red triangles) were receiving chronic oral treatment at the time of the first scan. All were receiving levodopa treatment chronically at the time of repeat scanning. (C) Three patients clinically diagnosed with multiple system atrophy who had repeat scanning.
Of the 22 patients, 11 with short symptom duration who were clinically diagnosed with idiopathic Parkinson’s disease were drug naive at the time of the first scan and were subsequently treated with chronic oral carbidopa plus levodopa. Five of these patients were rescanned in the on-state (1 h after their morning dose) 3 months after starting a daily treatment regimen (mean levodopa dose 360 mg per day, SD 82·16). We used these scans to assess whether the initiation of levodopa treatment changed the image-based classification. The other six drug-naive patients were rescanned in the off-state (12 h after their last drug dose; between scan interval 2·9–5·4 years). These scans were used to assess the ability to replicate the imaging classifications over a period of disease progression, without the potential confounding effect of levodopa treatment. The other 11 patients (eight clinically diagnosed with idiopathic Parkinson’s disease and three with multiple system atrophy) were on chronic oral dopaminergic medication at the time of both the initial and repeat scans (between-scan interval 1·5–7·2 years). In these patients, both scans were done in the off-state and were used to assess ability to replicate the imaging diagnoses in chronically treated patients. We investigated the agreement between classification results for the initial and repeat scans with the Cohen’s kappa test. The classification results remained consistent in the repeat imaging for the five drug-naive patients with idiopathic Parkinson’s disease who were subsequently treated with carbidopa plus levodopa for 3 months (figure 4, left), and for six patients who were rescanned after a longer period of chronic treatment. Thus, neither levodopa treatment nor disease progression altered the classification.
Nine patients (one clinically diagnosed as having idiopathic Parkinson’s disease, five multiple system atrophy, and three progressive supranuclear palsy) were examined after death. The mean interval from imaging to death was 3·6 years (SD 1·5). For six of these patients (one with idiopathic Parkinson’s disease, four multiple system atrophy, and one progressive supranuclear palsy) the diagnosis made at post-mortem investigation was the same as both the final clinical diagnosis and the initial imaging classification (figure 5). The other three patients were classified by imaging as having indeterminate parkinsonism, two of whom had a final clinical diagnosis of progressive supranuclear palsy, which was confirmed at autopsy. The third patient had a final clinical diagnosis of multiple system atrophy, but had evidence of corticobasal ganglionic degeneration at autopsy.
Figure 5. Disease-related metabolic patterns and post-mortem findings.
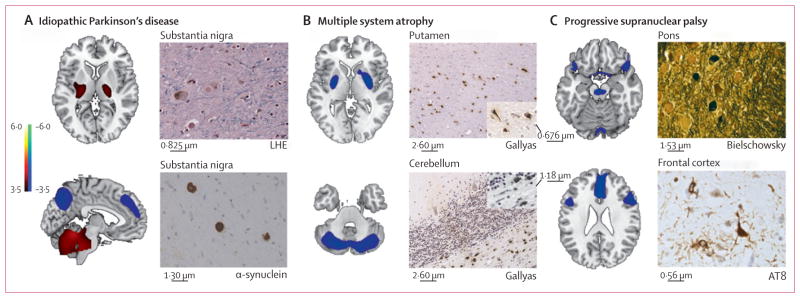
(A) The pattern related to idiopathic Parkinson’s disease (left)15 is characterised by increased (red areas) pallidothalamic and pontocerebellar metabolic activity associated with relative reductions (blue areas) in the premotor cortex, supplementary motor area, and parietal association regions. Neuropathological findings (right) from the substantia nigra pars compacta of a patient classified as having idiopathic Parkinson’s disease with a likelihood of 99% on the basis of fluorine-18-labelled-fluorodeoxyglucose (FDG)-PET 5·8 years before death. Diagnosis was confirmed at post-mortem examination, with the demonstration of Lewy-body containing neurons and severe cell loss in this region (LHE, 630X; top). Neuronal inclusions stained positively for α-synuclein (α-synuclein antibody, 400X; bottom). (B) The multiple system atrophy-related pattern (left)16 is characterised by bilateral metabolic reductions in putamen and cerebellar activity. Neuropathological findings (right) from a patient classified as having multiple system atrophy with a likelihood of 98% on the basis of FDG-PET 3 years before death. Autopsy revealed characteristic changes in abnormal hypometabolic pattern areas, with neuronal loss and gliosis in the putamen (top) and cerebellum (bottom). Both regions displayed glial cytoplasmic inclusions (Gallyas stain, 200X). Insets: putamen, 400X; cerebellum, 630X. (C) The progressive supranuclear palsy-related pattern (left)16 is characterised by metabolic reductions in the upper brainstem, medial frontal cortex, and medial thalamus. Neuropathological findings (right) from a patient classified as having progressive supranuclear palsy with a likelihood of 99% on the basis of FDG-PET 3·9 years before death. Post-mortem examination confirmed this diagnosis, with characteristic histopathological changes in abnormal hypometabolic pattern areas, in the pons (top) and frontal cortex (bottom). Argyrophilic globosum neuronal tangles were noted in the basis pontis (Bielschowsky stain 400X). A neuronal tangle with cytoplasmic inclusions and neuropil threads is displayed from the fifth cortical layer of the prefrontal region (AT8 stain, 630X). Tufted astrocytes (not shown) were present in this cortical region, the amygdala, globus pallidus, and claustrum. LHE=Luxol fast blue with haematoxylin and eosin.
Discussion
By use of three disease-specific, voxel based, FDG-PET metabolic patterns together, our automated image-based classification approach produced accurate differential diagnosis in patients with parkinsonism caused by different underlying neurodegenerative diseases. This method is not dependent on visual interpretation yet accurately distinguishes idiopathic Parkinson’s disease from multiple system atrophy and progressive supranuclear palsy, the two parkinsonian disorders most commonly misdiagnosed as idiopathic Parkinson’s disease at clinical diagnosis. By computing disease probabilities for idiopathic Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy, the accuracy of initial clinical diagnosis was enhanced.
Patients with a short symptom duration are ideal participants in clinical trials of potentially disease-modifying drugs. However, these patients are difficult for clinicians to diagnose because many have not yet developed disease-defining symptoms. We found that the PPV of an initial classification of idiopathic Parkinson’s disease was high (94%) in patients with short symptom duration who were subsequently clinically assessed for at least 2 years. On the basis of published clinicopathological data,2 we estimated the PPV of an initial diagnosis of idiopathic Parkinson’s disease to be about 75% relative to the final clinical diagnosis made by a movement disorders specialist after at least 2 years of follow-up. Therefore, by reducing the number of patients with atypical parkinsonian disorders misdiagnosed as idiopathic Parkinson’s disease, image-based classification improved diagnostic accuracy by about 20% in patients with suspected early idiopathic Parkinson’s disease. Thus, imaging classification was accurate several years before the final clinical diagnosis. This is especially relevant when considering treatment options for patients with medically refractory parkinsonism because invasive surgical approaches such as deep brain stimulation are generally ineffective for patients with atypical parkinsonian syndrome.3
The sensitivity of imaging classification was high in patients who had a short symptom duration and a clinical diagnosis of multiple system atrophy or progressive supranuclear palsy. In these patients the specificity was also high, as was the PPV. In the patients with short symptom duration who were clinically followed up for at least 2 years, all discriminative measures reached 100% for both diseases. This contrasts with the low sensitivity (<60%) currently associated with initial clinical diagnosis of early multiple system atrophy and progressive supranuclear palsy.27,28 In these diseases, diagnostic clinical features commonly appear several years after symptom onset.29 Although strict clinical criteria can improve the specificity (but not sensitivity) of diagnosing these parkinsonian diseases,2,27,28 this image-based classification approach can substantially improve the sensitivity of initial clinical assessments of patients with atypical parkinsonian syndromes who had a short symptom duration while maintaining high specificity. This will be of particular advantage when assessing potential participants in clinical trials of drugs to treat these progressive disorders.
Furthermore, we noted that the image-based classification technique improved diagnostic accuracy in patients who were followed up for at least 2 years, irrespective of symptom duration (table 2). Demographic features of these patients (age and Hoehn and Yahr stage at initial clinical assessment, and mean duration of clinical follow-up) were similar to those in previous studies, in which the final clinical diagnosis was substantiated as the gold standard owing to the high level of agreement (99% PPV) with the findings at autopsy.1,2 Indeed, in this group, PPV and specificity of all imaging classifications were above 90%, suggesting that discrepancies between the imaging classification and the final clinical diagnosis in patients with short follow-up (≤2 years) probably relate to clinical uncertainty rather than to inaccuracies in the image-based assessments.
Classifications based on repeat scans obtained in 22 patients were in strong agreement with the classifications from the initial scans. Also, the classification of drug-naive patients with idiopathic Parkinson’s disease remained unchanged after the initiation of dopaminergic therapy and was not altered by subsequent disease progression. Therefore, image-based classifications are highly reproducible in individual patients, irrespective of disease stage and treatment status.
For six of the nine patients who were examined after death, the pathological diagnosis matched that made both clinically and by the image-based classification. The other three patients examined post mortem were classified as having indeterminate parkinsonism on first-level analysis. Classification of patients in the indeterminate parkinsonism category as either having idiopathic Parkinson’s disease or atypical parkinsonian syndrome was not possible probably because characteristic metabolic signatures of disease had not developed sufficiently at the time of imaging. Given that pattern expression develops with disease progression,30,31 the change in disease likelihood in indeterminate cases is unsurprising.
Patients with indeterminate parkinsonism might have other atypical parkinsonian syndromes such as corticobasal ganglionic degeneration, as was the case in one patient who had post-mortem examination. In our sample, patients classified as having indeterminate parkinsonism had almost even odds of idiopathic Parkinson’s disease (54%; 13 out of 24, figure 1) or atypical parkinsonian syndrome (46%) at final clinical diagnosis. Therefore, these patients might not be appropriate participants in clinical trials of treatments for idiopathic Parkinson’s disease. The identification of such individuals before enrolment by image-based classification would thus be an advantage. Patients in the second-level indeterminate atypical parkinsonian syndrome category (12%), although not classified as having either multiple system atrophy or progressive supranuclear palsy, had already been identified as having atypical parkinsonian syndrome as opposed to idiopathic Parkinson’s disease, which is the most relevant distinction for clinical investigation and management.
Diagnostic accuracy for idiopathic Parkinson’s disease was lower in patients who had a short symptom duration and low (≤1 SD) expression of all three patterns. This subgroup might include patients with slowly progressing idiopathic Parkinson’s disease, atypical parkinsonism, or secondary non-degenerative causes of parkinsonism, including patients with psychogenic diseases.17 The image-based classification of these patients should be interpreted with caution, and extended clinical follow-up is necessary.
We recognise that the reported discriminative measures apply only to our sample of patients who received a final clinical diagnosis of idiopathic Parkinson’s disease, multiple system atrophy, or progressive supranuclear palsy after follow-up. This classification approach cannot be used to differentiate other forms of parkinsonism (eg, corticobasal ganglionic degeneration). However, once specific patterns for these other forms of parkinsonism are identified and validated, we will be able to differentiate these disorders by including the new patterns into the classification algorithm. Blinded, prospective imaging studies—ideally involving multiple centres, a larger validation group, repeat imaging, and more extensive post-mortem confirmation—are needed to establish the accuracy of this pattern-based categorisation procedure.
Acknowledgments
DE was funded by the NIH (grants R01 NS 35069 and P50 NS 38370). This work was supported by the general clinical research center at The Feinstein Institute for Medical Research (M01 RR018535). The authors thank Toni Fitzpatrick and Loreta Quartarolo for administrative assistance and Matthew Bussa for assistance with data management.
Footnotes
Conflicts of interest
DE is co-inventor of US patents 5 632 276 (filed Jan 27, 1995, granted May 27, 1997) and 5 873 823 (filed Sept 4, 1996, granted Feb 23, 1999) for the use of spatial patterns for the diagnosis of brain disease. DE has no financial conflicts of interest. All other authors have no conflicts of interest.
Contributors
DE and CCT designed the study. CCT, KLP, TE, and VD did the research. CCT and ML analysed the data. CCT, KLP, and DE wrote the Article. J-PV, ML, AF, SF, TE, SF, MG, and VD revised the manuscript. VD, AF, SF, MG, J-PV, and SF gave administrative, technical, and/or material support.
References
- 1.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 2001;57:S34–38. [PubMed] [Google Scholar]
- 2.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 3.Shih LC, Tarsy D. Deep brain stimulation for the treatment of atypical parkinsonism. Mov Disord. 2007;22:2149–55. doi: 10.1002/mds.21648. [DOI] [PubMed] [Google Scholar]
- 4.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA. 2002;287:1653–61. doi: 10.1001/jama.287.13.1653. [DOI] [PubMed] [Google Scholar]
- 6.Jankovic J, Rajput AH, McDermott MP, Perl DP. The evolution of diagnosis in early Parkinson disease. Arch Neurol. 2000;57:369–72. doi: 10.1001/archneur.57.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 8.Gaenslen A, Unmuth B, Godau J, et al. The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson’s disease: a prospective blinded study. Lancet Neurol. 2008;7:417–24. doi: 10.1016/S1474-4422(08)70067-X. [DOI] [PubMed] [Google Scholar]
- 9.Vlaar AM, de Nijs T, van Kroonenburgh MJ, et al. The predictive value of transcranial duplex sonography for the clinical diagnosis in undiagnosed parkinsonian syndromes: comparison with SPECT scans. BMC Neurol. 2008;8:42. doi: 10.1186/1471-2377-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seppi K, Scherfler C, Donnemiller E, et al. Topography of dopamine transporter availability in progressive supranuclear palsy: a voxelwise [123I]beta-CIT SPECT analysis. Arch Neurol. 2006;63:1154–60. doi: 10.1001/archneur.63.8.1154. [DOI] [PubMed] [Google Scholar]
- 11.Brooks DJ, Seppi K. Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Mov Disord. 2009;24:949–64. doi: 10.1002/mds.22413. [DOI] [PubMed] [Google Scholar]
- 12.Chung EJ, Lee WY, Yoon WT, Kim BJ, Lee GH. MIBG scintigraphy for differentiating Parkinson’s disease with autonomic dysfunction from parkinsonism-predominant multiple system atrophy. Mov Disord. 2009;24:1650–55. doi: 10.1002/mds.22649. [DOI] [PubMed] [Google Scholar]
- 13.Eckert T, Tang C, Eidelberg D. Assessment of the progression of Parkinson’s disease: a metabolic network approach. Lancet Neurol. 2007;6:926–32. doi: 10.1016/S1474-4422(07)70245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32:548–57. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: test–retest reproducibility. J Cereb Blood Flow Metab. 2007;27:597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckert T, Tang C, Ma Y, et al. Abnormal metabolic networks in atypical parkinsonism. Mov Disord. 2008;23:727–33. doi: 10.1002/mds.21933. [DOI] [PubMed] [Google Scholar]
- 17.Eckert T, Feigin A, Lewis DE, Dhawan V, Frucht S, Eidelberg D. Regional metabolic changes in parkinsonian patients with normal dopaminergic imaging. Mov Disord. 2007;22:167–73. doi: 10.1002/mds.21185. [DOI] [PubMed] [Google Scholar]
- 18.Spetsieris PG, Ma Y, Dhawan V, Eidelberg D. Differential diagnosis of parkinsonian syndromes using PCA-based functional imaging features. Neuroimage. 2009;45:1241–52. doi: 10.1016/j.neuroimage.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society scientific issues committee report: SIC task force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord. 2003;18:467–86. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 20.Fahn S, Jankovic J. Principles and practice of movement disorders. New York: Elsevier Health Sciences; 2007. Parkinsonism-plus syndromes and secondary parkinsonian disorders; pp. 233–84. [Google Scholar]
- 21.Schrag A, Ben-Shlomo Y, Quinn NP. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet. 1999;354:1771–75. doi: 10.1016/s0140-6736(99)04137-9. [DOI] [PubMed] [Google Scholar]
- 22.Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117:235–43. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 24.Litvan I, Hauw JJ, Bartko JJ, et al. Validity and reliability of the preliminary NINDS neuropathologic criteria for progressive supranuclear palsy and related disorders. J Neuropathol Exp Neurol. 1996;55:97–105. doi: 10.1097/00005072-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Allison PD. Logistic regression using the SAS system: theory and application. Cary: SAS Institute Inc; 1999. [Google Scholar]
- 26.Hastie T, Tibshirani R, Friedman J. Model assessment and selection—the elements of statistical learning: data mining, inference, and prediction. 2. New York: Springer; 2009. pp. 219–60. [Google Scholar]
- 27.Osaki Y, Wenning GK, Daniel SE, et al. Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology. 2002;59:1486–91. doi: 10.1212/01.wnl.0000028690.15001.00. [DOI] [PubMed] [Google Scholar]
- 28.Osaki Y, Ben-Shlomo Y, Lees AJ, et al. Accuracy of clinical diagnosis of progressive supranuclear palsy. Mov Disord. 2004;19:181–89. doi: 10.1002/mds.10680. [DOI] [PubMed] [Google Scholar]
- 29.Wenning GK, Ben-Shlomo Y, Hughes A, Daniel SE, Lees A, Quinn NP. What clinical features are most useful to distinguish definite multiple system atrophy from Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;68:434–40. doi: 10.1136/jnnp.68.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Tang C, Feigin A, et al. Changes in network activity with the progression of Parkinson’s disease. Brain. 2007;130:1834–46. doi: 10.1093/brain/awm086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poston K, Tang C, Eckert T, Ma Y, Frucht S, Eidelberg D. Longitudinal changes in regional metabolism and network activity in multiple system atrophy. Neurology. 2009;72:A67. [Google Scholar]