Abstract
Hepatocellular carcinoma (HCC) is an aggressive liver cancer but clinically validated biomarkers that can predict natural history of malignant progression are lacking. The present study explored the proteome-wide patterns of HCC to identify biomarker signature that could distinguish cancerous and non-malignant liver tissues. A retrospective cohort of 80 HBV-associated HCC was included and both the tumor and adjacent non-tumor tissues were subjected to proteome-wide expression profiling by 2-DE method. The subjects were randomly divided into the training (n=55) and validation (n=25) subsets, and the data analyzed by classification-and-regression tree algorithm. Protein markers were characterized by MALDI-ToF/MS and confirmed by immunohistochemistry, western blotting and qPCR assays. Proteomic expression signature composed of six biomarkers (haptoglobin, cytochrome b5, progesterone receptor membrane component 1, heat shock 27 kDa protein 1, lysosomal proteinase cathepsin B, keratin I) was developed as a classifier model for predicting HCC. We further evaluated the model using both leave-one-out procedure and independent validation, and the overall sensitivity and specificity for HCC both are 92.5% respectively. Clinical correlation analysis revealed that these biomarkers were significantly associated with serum AFP, total protein levels and the Ishak’s score. The described model using biomarker signatures could accurately distinguish HCC from non-malignant tissues, which may also provide hints and guidance on how normal hepatocytes are transformed to malignant state during tumor progression.
Keywords: hepatocellular carcinoma, tumor markers, proteomics, diagnosis and prognosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common and aggressive malignancies, and is the third leading cause of cancer death worldwide.1 Chronic infection with hepatitis B or C virus (HBV or HCV) is the major etiologic factor that accounts for about 80% of all HCC cases. More than 50% of the HCC incidences are diagnosed in China where HBV vaccination program has just begun and hepatitis B is still prevalent, posing a serious health threat and burdening the already burdened medical care system. Surgical treatments including hepatectomy, liver transplantation, locoregional ablation and transarterial chemoembolization therapies are the common therapies for HCC.2 However, the procedures are costly and the clinical outcomes still remain dismal. The overall 5-year survival rate is less than 10%, and about one third of the HCC patients relapse within 1 year after curative surgery. The poor prognosis is mainly attributed to the late diagnosis and the high recurrence rate of the neoplasm.3
Effective management of HCC relies on accurate detection of the disease in a high-risk population (subjects with cirrhosis or HBV carriers); allowing clinicians to identify patients at the earliest curable stages when the tumor are usually resectable and to deliver effective interventions that contribute to the reduction in mortality and morbidity. Circulating α-fetoprotein (AFP) in serum has been widely used as a conventional biomarker for HCC surveillance.4 Elevated serum AFP level is also a useful prognostic indicator for poor survival rate and tumor recurrence in HCC patients.5 Nevertheless, serum AFP is not particularly efficient in detecting HCC at early stages, and its specificity is often shadowed by high false positive signals from patients with other liver-related diseases such as viral hepatitis and cirrhosis.6 In this regard, several groups have recently suggested several soluble tumor markers such as glypican-3,7 γ-glutamyltransferase II,8 des-γ-carboxypro thrombin,9 lens culinaris agglutinin-reactive AFP10 and α1-antitrypsin,11 that may improve the detection rate for early HCC. Nevertheless, their clinical utilities remain to be further determined in multicenter clinical studies.
In spite of our recent belief that a panel of biomarkers is required for early diagnosis of liver cancer, the precise set of such molecules that can be used to differentiate HCC from nonmalignant and normal liver tissues is not yet available. The technological advance in proteomics profiling promises to be an effective strategy for cancer biomarkers discovery.12–15 Differential proteome analysis of the matched tumor and non-tumor tissues also enable us to delineate the global changes in the expression patterns, shedding new insights into the molecular understanding of tumor progression and identifying novel therapeutic targets for early interventions.16
In this study, we analyzed the proteomic expression profiles by 2-dimensional gel electrophoresis (2-DE) of resected tumor and adjacent non-malignant tissue samples from 80 HCC patients. “Artificial intelligence” algorithm was then applied for analysis of the profiling data in order to generate a unique biomarker signature that could distinguish HCC with high accuracy from the non-malignant and normal liver samples. Clinical correlation of each biomarker with different clinicopathological features in HCC was also evaluated.
PATIENTS AND METHODS
Clinical Specimens
Tumor and adjacent non-malignant tissues were collected from 80 patients who were diagnosed with primary HCC and elected for hepatic surgery at Queen Mary Hospital (Pokfulam, Hong Kong) between 1998 and 2006. The demographic data and clinicopathological features of the patients were summarized in Table 1. In addition, 10 normal liver tissues obtained from residual donor grafts of healthy subjects were also included in this study as control. This study was vetted and approved by the Institutional Ethics Committee, and all the tissues were collected with informed consent from patients. Tissues were snap-frozen in liquid nitrogen after surgical resection and stored at −80°C until use. H&E staining was performed for histology examination, and samples showing tissue homogeneity (>90%) were used in this study.
Table 1.
Demographics and Clinicopathological Features of HCC Patients
| Characteristics | Training Set (n = 55) |
Validation Set (n = 25) |
P value |
|---|---|---|---|
| Gender (M:F) | 48:7 | 20:5 | 0.405 |
| Age | 53.4 ± 5.8 (Median: 54) | 57.0 ± 2.2 (Median: 59) | 0.228 |
| Hepatitis (HBV / HCV / negative) | 48 / 0 / 7 | 20 / 1 / 4 | 0.534 |
| Tumor Size (cm) | 8.79 ± 0.61 (Median: 8.00) | 7.42 ± 1.06 (Median: 7.00) | 0.277 |
| Tumor Stage (AJCC TNM) | |||
| I–II | 33 | 18 | 0.307 |
| III–IV | 22 | 7 | |
| Tumor Recurrence | 22 | 8 | 0.331 |
| Child’s Grade | |||
| A | 53 | 25 | |
| B | 2 | 0 | 0.340 |
| C | 0 | 0 |
Note: Both tumor and non-tumor specimens from 80 HCC patients were randomly assigned for the training and validation purposes. There was no apparent difference between the 2 sample sets.
2-DE Proteomics Profiling
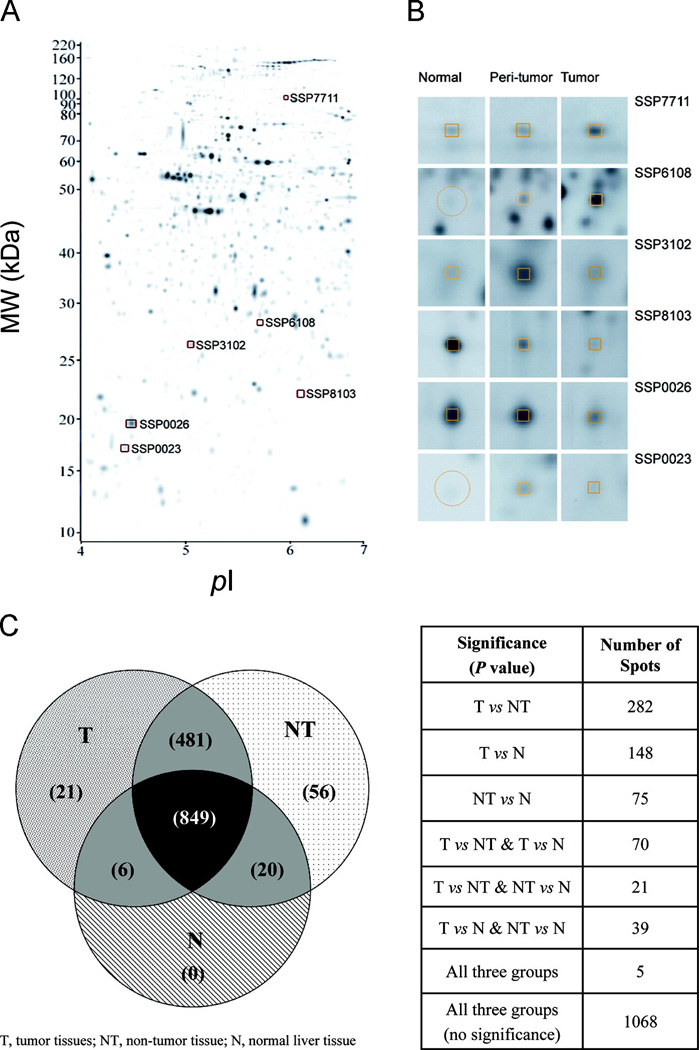
Proteins were extracted from 10 mg tissues using the ReadyPrep Sequential Extraction Kit (Bio-Rad, Hercules, CA) and the concentration determined by PlusOne 2-D Quant Kit (GE Healthcare, Uppsala, Sweden). Proteomic expression profiling of liver tissue (15 μg soluble lysates) was done by 2-DE gel electrophoresis using the IPGphor system and the Ettan™ DALTsix Electrophoresis Unit (GE Healthcare) as previously described.13,17,18 The silver-stained gel was scanned using a GS-800 Calibrated Densitometer (Bio-Rad) and a gray scale format of 2,700 × 2,200 pixels was used. As shown in Fig. 1, the digitized images were analyzed using the PDQuest software version 7.2 (Bio-Rad) for spot detection, matching, and normalization. Protein spot abundance was reported as relative value in ppm.
Fig. 1. Differential expression profiles of HCC proteome.
(A) Liver tissue lysates were separated by IEF in the range of pI 4–7, followed by second dimension (MW, 10–250 kDa) of PAGE and silver staining.
(B) Representative gel pictures of the six reference points from HCC, peritumor and normal liver tissues were shown.
(C) Venn diagram showing the numerical distribution of protein spots presents in tumor (T), non-tumor (NT) and normal (N) livers. The numbers of protein spots with significant changes (P<0.05) among the three different comparison groups (T vs NT, T vs N, and NT vs N) were tabulated. Statistical analysis was performed by one-way ANOVA and student-T-test (p < 0.05).
MALDI-ToF/ToF MS
Protein spots of interest were excised from the gel and subjected to destaining, washing, and trypsin digestion (MS grade, Promega, Madison, WI) at 37°C overnight. The digested peptides were recovered, reconstituted, purified by ZipTip® (Millipore, Bedford, MA) and then mixed with an equal volume of matrix (10 mg/ml α-cyano-4-hydroxycinnamic acid in 50% acetonitrile/0.1% TFA) for mass spectrometer analysis (4800 MALDI-TOF/TOF Analyzer, Applied Biosystems Inc., Foster City, CA).
Statistics and data-mining analysis
Sample size was estimated and calculated using Statmate program for Windows (GraphPad Software, Inc., San Diego, CA). After pilot experiments, a sample size of about 60 cases was used (90% power) to detect a statistically significant difference in intensity of protein spots between tumor and non-tumor samples. One-way ANOVA followed by Tukey or Dunnett’s T3 tests, Student’s t-test and Pearson Chi-Square Test were used to analyze spot intensities among normal, adjacent non-malignant and tumor samples. The discrimination performance was evaluated by plotting the receiver operator characteristic (ROC) curve and by calculating the area under the ROC curve (AUC). P <0.05 was considered as statistically significant. SPSS for Windows (version 13, SPSS, Inc., Chicago, IL) was used for statistical and correlation analysis in this study.
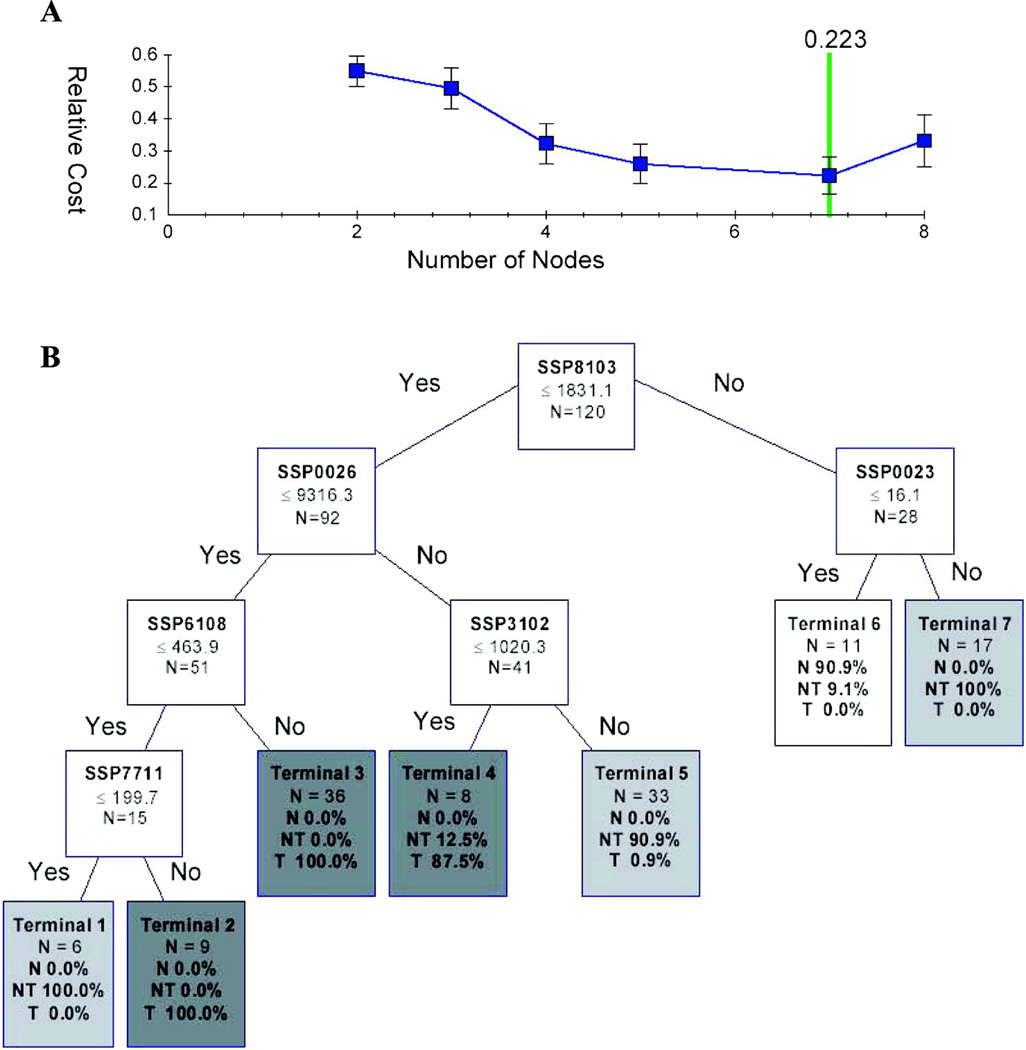
For data-mining analysis of the proteome datasets by classification and regression tree (CART) algorithm, the Biomarker Pattern Software (Ciphergen Biosystems, Inc., Fremont, CA) was employed to facilitate the decision-tree model building using the Gini impurity criterion for splitting and estimation using 10-fold cross-validation. The splitting decision in this case is based on the intensity of a spot or cluster at a defined cut-off value, with the following terminal nodes (tumor, non-tumor and normal) (Fig. 2). Before the data-mining analysis, the proteome datasets were randomly divided into (i) the “training” group (n = 120 cases; 55 tumor, 55 paired non-tumor and 10 normal) for model building, and (ii) the “blinded validation” group (n =50 cases; 25 tumors and 25 non-tumors) for evaluation of the diagnostic model accuracy on discriminating HCC cases.
Fig. 2. The optimal classification tree generated by CART.
(A) The cost value of decision trees with varying number of terminal nodes.
(B) The optimal decision tree is composed of 6 discriminative classifiers. The decision making process involves the evaluation of if-then rules of each node from top to bottom, which eventually reaches a terminal node with designated class outcome: tumor (T), non-tumor (NT) or normal (N).
Quantitative PCR
Total RNA was extracted from frozen liver tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), following manufacturer’s instructions. After DNase treatment, RNA was reverse-transcribed to cDNA using the TaqMan Reverse Transcription Reagents (Applied Biosystems), and the resulting products were used as templates for qPCR using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) and 200 nM gene-specific primers (Hp2: 5’- CAGAAAATGCAACAGCGAAA-3’; 5’-TAACCCACACGCCCTACTTC-3’; CYB5A: 5’- CGCCTTGATGTATCGCCTAT-3’, 5’-AAATTTGAGCGCAGAAAGGA-3’; PGRMC1: 5’- CCTCTGCATCTTCCTGCTCT-3’, 5’-CGTTGATGGCCATGAGTATG-3’; HSPB1: 5’- ACGAGCATGGCTACATCTCC-3’, 5’-CTTTACTTGGCGGCAGTCTC-3’; KRT1: 5’- AGGAGGTGGACGTGGTAGTG-3’, 5’-GAGGGCAGACAGGACCATAA-3’; CTSB: 5’- AGAATGGCACACCCTACTGG-3’, 5’-TGCATTTCTACCCCGATCTC -3’) in a final volume of 20μl. The cycling parameters were set as followings: 50°C for 2 min, 1 cycle; 95°C for 2 min, 1 cycle; 95°C for 15s and 60°C for 30s, 50 cycles. Calculation and confirmation of the qPCR were performed according to previous procedures.18,19
Immunohistochemistry
Tissue sections at 4-μm thick were used for immunohistochemical staining. After blocking with 2% BSA in PBS, the sections were incubated at 4°C overnight with the following primary antibodies (1:100 dilution) against haptoglobin (Rockland Immunochemicals, Gilbertsville, PA), cytochrome b5, heat shock protein 27, cathepsin B (Santa Cruz Biotechnology), and keratin I (Invitrogen). For negative controls, primary antibodies were substituted with the corresponding purified isotype-matched immunoglobulins (Invitrogen or Sigma). Horseradish peroxidaseconjugated secondary antibodies (1:700 in blocking solution) were used, and signals were developed using diaminobenzidine solution (Invitrogen) and counterstained with hematoxylin (Vector Laboratories, Burlingame, CA).20,21
Western blot analysis
Protein extracts (25μg) were separated on 10% polyacrylamide SDS gels and electroblotted on nitrocellulose membranes for western blotting according to previous procedures.22–24 After blocking with 5% non-fat dry milk in TBS-T (20 mM Tris, 137 mM NaCl, 0.1% Tween-20, pH 7.6), the membrane was incubated with the primary antibodies as described above at 4 °C overnight, followed by peroxidase-conjugated secondary antibody (Invitrogen). Mouse anti-human β-actin antibody (Santa Cruz Biotechnology, CA) at 1:1000 dilution was included in parallel as a loading reference control. After substrate development (ECL detection reagents, GE Healthcare), densitometry data were analyzed by Quantity One (Bio-Rad) for protein quantification of each marker normalized with β-actin.
RESULTS
Differential proteomic analysis of HCC tissues: tumor vs non-tumor
The 2-DE-based proteomic profiling approach was employed to analyze 80 pairs of tumor and the matched non-tumor liver tissues resected from HCC patients who received curative hepatic surgery. The samples were randomly divided into 2 groups, (i) for model construction in the training set and (ii) for blind validation in the testing set. There was no significant difference (P < 0.05) between the 2 sample sets with respect to demographic and clinicopathological data such as gender, age, hepatitis, tumor pathology, and AFP level, etc (Table 1). In addition, a panel of 10 normal liver tissues from donor residual grafts was also included as healthy subject controls for comparison.
Fig. 1A showed a representative HCC proteome by 2-DE and the coordinates of the six discriminative biomarkers (as described below: SSP8103, SSP0026, SSP0023, SSP6108, SSP3102, SSP7711). Differential expression patterns of the six selected biomarkers that were composed of the proteomic signature discriminating among the HCC, non-tumor and normal liver tissues were depicted in Fig. 1B. Overall, there were 849 proteins commonly shared. Distribution of all the protein spots differentially expressed among different tissue types was summarized in the Venn diagram (Fig.1C). There were 21 and 56 exclusively expressed in the tumor and non-tumor livers, respectively, whereas 148 (HCC vs normal) and 282 (HCC vs non-tumor) were significantly different in their protein levels by One-way ANOVA (P < 0.05).
Proteomic classification model for distinguishing HCC from non-malignant tissues
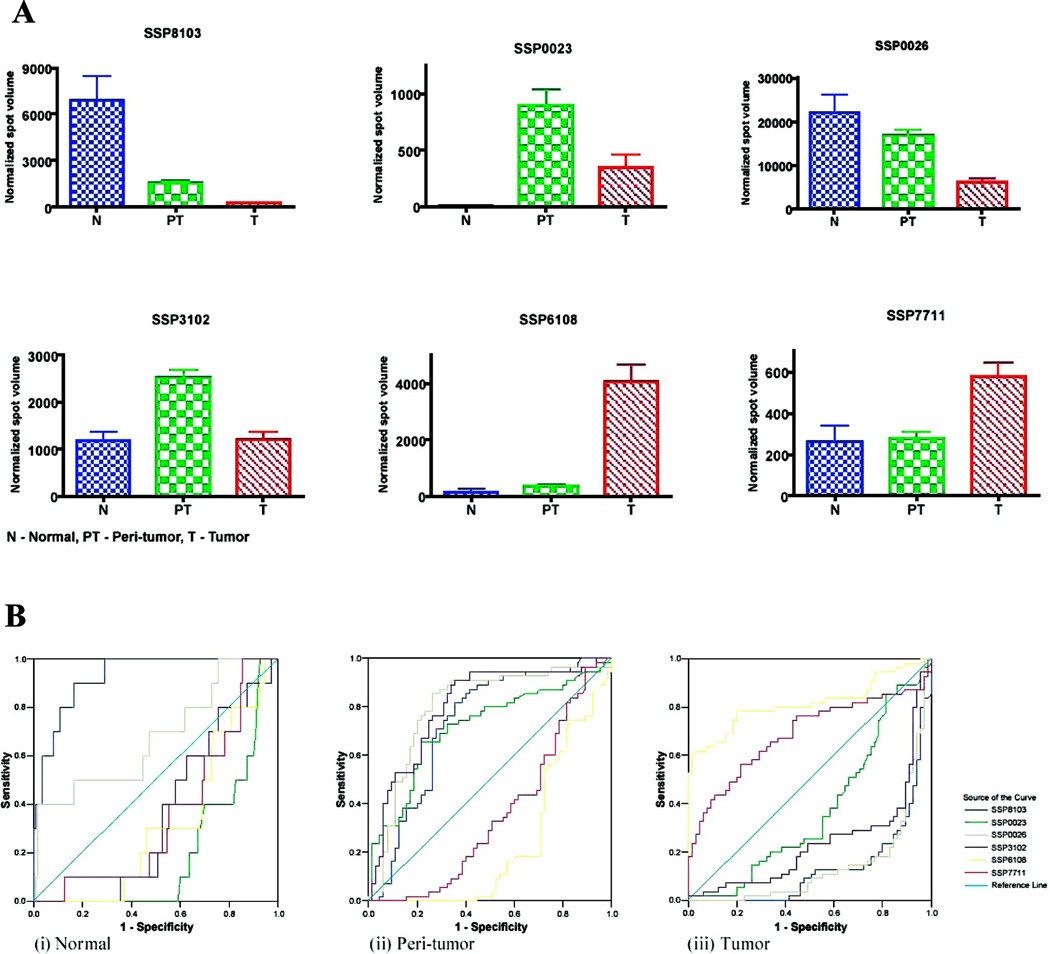
To facilitate building a proteomic classification model, the CART algorithm data-mining method was employed using the Biomarker Pattern Software to analyze all the normalized, matched protein spot intensities from each proteome profile (Total n = 120; 55 HCC, 55 non-malignant, 10 healthy controls) in the training set. Figure 2A showed the list of candidate decision-tree outputs that were generated, and the optimal tree model with minimal cross-validation cost at 0.223 was taken and illustrated in Figure 2B. The choice binary decision tree consisted of six distinct biomarker classifiers: SSP8103, SSP0026, SSP0023, SSP6108, SSP7711, and SSP3102 (Figure 3A), and all of these biomarkers showed significant discriminative performance when analyzed by the ROC curves to distinguish at least one phenotype among the normal, non-malignant and tumor tissues (Fig. 3B). The sensitivity and specificity of the six-biomarker proteomic signature model for the training and the validation sets were described in Table 2.
Fig. 3. Spot intensities and ROC curves of each six protein markers differentially expressed in HCC, non-tumor and normal liver tissues.
(A) Spot intensities on 2-DE gel of the six protein markers were graphically presented in respective histogram charts. Each bar represents a mean ± SD of the normalized spot intensity in ppm. Data were from the training set and the sample sizes were 55 (T), 55 (NT) and 10 (N). Statistical analysis was performed by student-T-test (p < 0.05) when compared T vs NT and T vs N.
(B) ROC curves of the 6 protein markers for their discriminative powers in the categories of normal livers (N), peri-tumor tissues (PT), and tumor tissues (T). For example, SSP8103 and SSP0026 showed preferential values for normal liver; SSP3102, SSP0023, SSP0026, SSP8103 for non-tumor, and SSP6108, SSP7711 for tumor, respectively. Data were from the training set.
Table 2.
Sensitivities and Specificities of Proteomics Expression Signature for Identification of HCC
| Sample Size (n) |
Sensitivity / Specificity |
Sample Classification (N) | |||
|---|---|---|---|---|---|
| Normal | Peri-Tumor | Tumor | |||
| Training Set | |||||
| Normal | 10 | 100% / 90.9% | 10 | 0 | 0 |
| Peri-tumor | 55 | 96.4% / 94.6% | 1 | 53 | 1 |
| Tumor | 55 | 94.5% / 98.1% | 0 | 3 | 52 |
| Blind validation Set | |||||
| Peri-tumor | 25 | 76.0% / 90.4% | 1 | 19 | 5 |
| Tumor | 25 | 88.0% / 81.5% | 0 | 3 | 22 |
| Overall | |||||
| Normal | 10 | 100% / 90.9% | 10 | 0 | 0 |
| Peri-tumor | 80 | 90.0% / 83% | 2 | 72 | 6 |
| Tumor | 80 | 92.5% / 92.5% | 0 | 6 | 74 |
Note: “Peri-tumor” is non-malignant liver tissue adjacent to the tumor; “Normal” is residual liver graft tissue.
Initially, the model was able to discriminate different sample types (tumor, non-tumor, normal liver) at high accuracy with 94.5% sensitivity and 98.1% specificity for HCC in the training set. We then further evaluated the model using both leave-one-out procedure and independent validation. In the blind validation test (samples n = 50), the biomarker signature remained at good performance with 88% sensitivity and 81.5% specificity respectively. Overall, based on the 170 samples that were tested, the performance of the 6-biomarker proteomic signature was satisfactorily with 92.5% at both the sensitivity and specificity to discriminate HCC from the non-malignant and normal liver tissues.
The Six-Biomarker Proteomic Signature
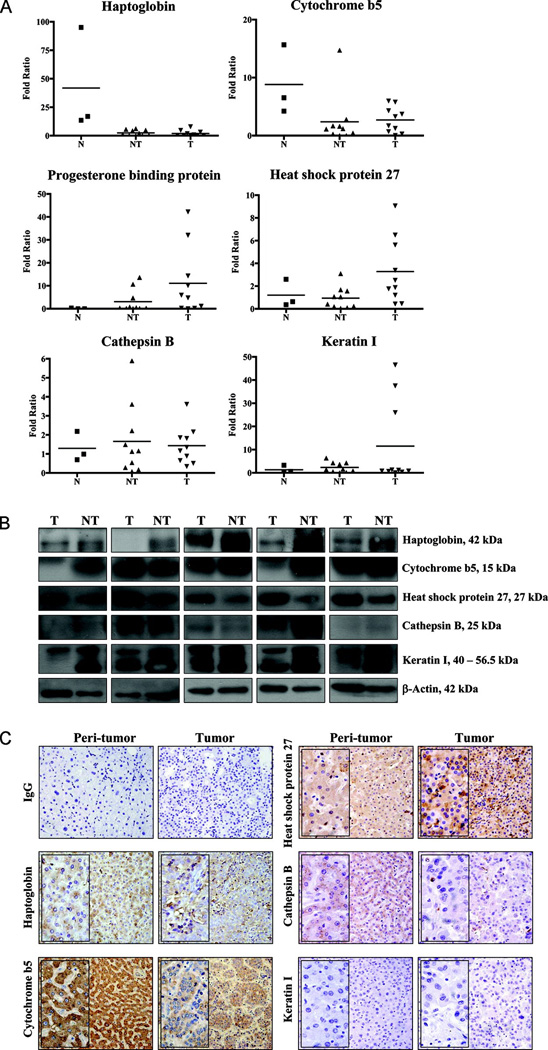
The six biomarker composition in the CART diagnostic model were subsequently identified by MALDI-ToF/ToF and unambiguously confirmed by tandem MS/MS, with at least 2 mass matched trypsin digested peptides in each protein spot, and each experiment was repeated at least twice. As presented in Table 3, the protein biomarker SSP8103 was identified as haptoglobin (Hp2), which possesses protease and hemoglobin binding activities; SSP0026 as cytochrome b5 (CYB5A), a major phase I xenobiotic metabolic enzyme subtype in liver parenchyma; SSP0023 as progesterone receptor membrane component I (or progesterone receptor) (PGRMC1; other name Hpr6.6), a novel class of steroid receptor expressed on the cytoplasmic membrane, which is linked to cellular survival and possibly cancer progression as well; SSP6108 as heat shock 27 kDa protein 1 (or HSP27) (HSPB1), one of the chaperones that is involved in protein folding and protection against cellular stress, which have been implicated in tumor development and progression in HCC and other malignancies; SSP7711 as keratin I (KRT1), a cytoskeleton component in normal cells, and SSP3102 as lysosomal proteinase cathepsin B (or cathepsin B) (CTSB) of the double-chain form, an active form of cysteine proteinase that was suggested to mediate cell death in tumors. Their relative mRNA expression values in the normal, adjacent non-tumor, and tumor tissues were evaluated by quantitative PCR (qPCR) (Fig. 4A). Except for the progesterone receptor with no commercial antibody available at present, western blot and immunohistochemistry were also performed for the other five protein biomarkers to examine the expression level and localization in the tumor and non-tumor tissues (Fig. 4B and 4C). In general, the qPCR data were comparable to the 2-DE differential expression profile pattern, except for the PGRMC1 gene that yielded higher mRNA level in the tumors (Fig. 4A). The results derived from western blot and immunohistochemistry were largely comparable with those derived from 2-DE experiments. Higher expressions of haptoglobin, cytochrome b5, cathepsin B, keratin I were found in adjacent non-tumor tissues, whereas high expression of heat shock protein 27 was associated with tumor tissues (Fig. 4B). Immunohistochemistry analysis showed strong expression of heat shock protein 27 in HCC while immunostaining of haptoglobin, cytochrome b5, and cathepsin B were stronger in the non-tumor tissues. By contrast, the immunoreactivities of keratin I appeared weak in both cases (Fig. 4C).
Table 3.
MS/MS Characterization of The Six Protein Markers in Proteomic Signature Model of HCC.
| Spot | Protein identity (Gene name) | MW (kDa) | pI | Mascot Score |
Matched Peaks |
Sequenced Peptides | Accession Numbers |
|---|---|---|---|---|---|---|---|
| SSP8103 | Haptoglobin (Hp2) | 41,743 | 6.23 | 173 | 1311.6 1439.6 895.5 |
TEGDGVYTLNDK TEGDGVYTLNNEK NPANPVQR |
1006264A |
| SSP0026 | Cytochrome b5 (CYB5A) | 15,330 | 4.88 | 76 | 1186.6 1134.59 1511.73 |
YYTLEEIQK STWLILHHK FLEEHPGGEEVLR |
P00167 |
| SSP0023 | Progesterone receptor membrane component 1 (PGRMC1) | 21,671 | 4.56 | 85 | 1644.8 1516.7 |
KFYGPEGPYGVFAGR FYGPEGPYGVFAGR |
NP_006658 |
| SSP6108 | Heat shock 27kDa protein 1 (HSPB1) | 22,782 | 5.98 | 73 | 961.4 1163.62 1104.53 |
GPSWDPFR LFDQAFGLPR QDEHGYISR |
NP_001531 |
| SSP3102 | Lysosomal proteinase cathepsin B (CTSB) | 23,077 | 5.19 | 164 | 1314.6 1634.70 1823.90 |
ICEPGYSPTYK HYGYNSYSVSNSEK GQDHCGIESEVVAGIPR |
AAA52125 |
| SSP7711 | Keratin I (KRT1) | 66,038 | 8.15 | 68 | 1179.60 1475.70 |
YEELQITAGR FLEQQNQVLQTK |
NP_006112 |
Note: Protein identity was based on at >2 sequenced peptides from at least 2 separate MS/MS analysis. Internal standards: des-Arg1 Bradykinin, Angiotensin I and Glu1-Fibrino-peptide B (Monoisotopic MH+ m/z 904.4681, 1296.6853, 1570.6774, respectively) were used for mass calibration. The tandem mass spectra were collected in product ion mode on the peptide of interest. Measured peptide and fragment ion masses were used to search the NCBI and SWISS-PROT databases for protein identifications using the Mascot (www.matrixscience.com), with the following settings: mass tolerance at 50 ppm, one missed cleavage allowed, at least two matching peptides and searches limited to Homo sapiens species.
Fig. 4. Relative gene and protein expressions of the six protein markers in human liver tissues.
(A) Real time PCR was performed using total RNA isolated from normal human livers (N) (n = 3), non-tumor (NT) (n = 10) and tumor (T) (n = 10) liver tissues from HCC patients to examine the gene abundance. The relative gene expressions of haptoglobin, cytochrome b5, progesterone binding protein, heat shock protein 27, cathepsin B, and cytokeratin I were presented by fold ratio difference. The mean value of each category was also shown accordingly.
(B) Western blot was performed to reveal the protein levels of target proteins in peri-tumor (NT) and corresponding tumor (T) tissues of HCC patients. 10 pairs of NT and T clinical specimens were included and 5 representative sets of western blots are shown herein.
(C) Immunohistochemistry was performed to reveal the localizations and expressions of the target proteins in peri-tumor and corresponding tumor tissues of HCC patients. The primary antibodies targeting haptoglobin, cytochrome b5, heat shock protein 27, cathepsin B, cytokeratin I, or the respective IgG (negative control) were used at 1:100 dilution to reveal the corresponding immunoreactivities. A commercial antibody against progesterone membrane receptor component 1 is not available and thus not included. Representative micrographs of each antibody and selected IgG isotype control were shown. Magnification, ×200 (insert ×400).
Diagnosis performance and clinical relevance of each individual biomarker
The diagnosis test performance of individual protein biomarkers in the proteomic signature model were calculated and summarized in Table 4. While HSP27 (SSP6108) appeared to be the key biomarker for distinguishing the tumor type, both the cytochrome b5 (SSP0026) and haptogloblin (SSP8103) were indicators of healthy state in the normal liver. To discriminate the non-malignant liver tissues, the indicative biomarkers were progesterone receptor (SSP0023) and cathepsin B (SSP3102) as well as cytochrome b5 and haptogloblin at lower cut-off thresholds. By contrast, keratin I (SSP7711) was insufficient per se as a diagnostic biomarker based on its expression level alone.
Table 4.
Individual Diagnostic Performance of Biomarkers to Discriminate Tumor, Non-tumor, and Normal Liver Tissues
| Classifiers in each tissue type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tumor | Non-Tumor | Normal | ||||||
| Test criteria | SSP6108 (HSP27) |
SSP7711 (keratin I) |
SSP0023 (progesterone receptor) |
SSP0026 (cytochrome b5) |
SSP3102 (cathepsin B) |
SSP8103 (haptoglobin) |
SSP0026 (cytochrome b5) |
SSP8103 (haptoglobin) |
| AUC | 0.820 | 0.697 | 0.720 | 0.783 | 0.801 | 0.724 | 0.733 | 0.950 |
| Cut-off point | 6.298 | 5.675 | 5.630 | 9.190 | 7.176 | 6.697 | 9.171 | 7.735 |
| Sensitivity | 0.782 | 0.764 | 0.667 | 0.778 | 0.852 | 0.736 | 0.800 | 0.900 |
| Specificity | 0.797 | 0.562 | 0.779 | 0.738 | 0.672 | 0.708 | 0.523 | 0.870 |
| False positive rate, FPR | 0.203 | 0.438 | 0.221 | 0.262 | 0.328 | 0.292 | 0.477 | 0.130 |
| False negative rate, FNR | 0.218 | 0.236 | 0.333 | 0.222 | 0.148 | 0.264 | 0.200 | 0.100 |
| Accuracy | 0.790 | 0.663 | 0.723 | 0.756 | 0.756 | 0.563 | 0.546 | 0.958 |
| Youden's Index | 0.579 | 0.326 | 0.446 | 0.516 | 0.524 | 0.444 | 0.323 | 0.770 |
| Positive likelihood ratio, LR+ | 3.852 | 1.744 | 3.018 | 2.970 | 2.598 | 2.521 | 1.677 | 6.923 |
| Negative likelihood ratio, LR− | 0.225 | 0.420 | 0.428 | 0.301 | 0.220 | 0.373 | 0.382 | 0.115 |
| Positive predictive value, PV+ | 0.768 | 0.600 | 0.706 | 0.712 | 0.852 | 0.672 | 0.132 | 0.281 |
| Negative predictive value, PV− | 0.810 | 0.735 | 0.735 | 0.800 | 0.846 | 0.459 | 0.966 | 0.990 |
Next we evaluated their potential clinical values by Pearson correlation analysis. Expression level of each protein biomarker in the panel was examined for their association with various clinicopathological features in HCC (Table 5). The results showed that cytochrome b5, progesterone receptor and keratin I were shown to have significant relevance with serum AFP (>400 ng/ml), total proteins levels and the Ishak’s score, respectively. Ishak’s score is a scoring system that takes architectural disturbance, fibrosis, and cirrhosis into account in assessing the liver status.25
Table 5.
Pearson Correlation of Protein Markers with Clinicopathological Data in HCC
| Protein Markers* | ||||||
|---|---|---|---|---|---|---|
| SSP0026 (cytochrome b5) |
SSP0023 (progesterone receptor) |
SSP7711 (keratin I) |
||||
| Characteristics | Coefficient | P value | Coefficient | P value | Coefficient | P value |
| Ishak’s Score** | −0.143 | 0.505 | −0.271 | 0.200 | −0.652 | 0.001 |
| Edmonson Grade | −0.117 | 0.405 | −0.081 | 0.565 | −0.247 | 0.080 |
| Total Bilirubin (umol/l) | 0.185 | 0.185 | 0.015 | 0.915 | 0.026 | 0.857 |
| Albumin (g/l) | −0.097 | 0.488 | 0.121 | 0.388 | −0.108 | 0.444 |
| SGOT (U/L) | 0.188 | 0.187 | −0.048 | 0.738 | 0.136 | 0.340 |
| SGPT (U/L) | 0.026 | 0.856 | 0.003 | 0.985 | 0.158 | 0.267 |
| AFP | −0.390 | 0.000 | −0.152 | 0.128 | −0.064 | 0.524 |
| HBsAg | −0.026 | 0.798 | 0.100 | 0.313 | −0.087 | 0.382 |
| Serum Total Protein | −0.000 | 0.998 | 0.223 | 0.024 | −0.052 | 0.604 |
SSP8103, SSP6108 and SSP3102 that showed no significant correlation (P <0.05, 2-tailed) were not presented.
Ishak score: 0, none fibrosis; 1–2, portal fibrotic expansion; 3–4, bridging fibrosis; 5–6, cirrhosis
DISCUSSION
To identify potential diagnostic biomarkers for HCC, we performed proteomics profiling analysis on matched tumor and non-tumor tissues resected from 80 HCC patients mostly associated with HBV together with 10 normal liver controls. First, the CART diagnostic model was developed by supervised algorithm analysis of 120 proteome profiles in the training set. Second, it was subjected to validation test with separate blind samples (25 pairs of T/NT tissues) and gave similar efficiency. As a whole, based on all the 170 samples (the largest cohort studied in this kind), the described CART model performed satisfactorily (overall sensitivity and specificity were 92.5%).
The decision-tree analysis also led to the identification of six highly distinctive protein biomarkers (as reflected by ROC in Figure 3 and AUC in Table 4) that correspond to tumor, non-malignant and normal liver tissues. By using the MALDI-ToF/ToF and tandem MS/MS analytical methods, the biomarkers were identified as (i) haptoglobin – a fragment isoform of 42 kDa, (ii) cytochrome b5, (iii) progesterone receptor– a fragment isoform of 21 kDa, (iv) HSP27, (v) cathepsin B, and (vi) keratin I, respectively. Dysregulated expression of these protein biomarkers was also reported to have implications in tumor development and progression in various malignancies (Table 3). Our present findings indicated that cytochrome b5 (CYB5A), progesterone receptor (PGRMC1) and keratin I (KRT1) respectively correlated significantly with the following clinical parameters in HCC: - serum AFP, total protein levels and the Ishak’s score, whilst the other three biomarkers did not reveal any significant association. Noteworthy from other studies, decreased expression of cytochrome b5 in HCC patients was associated with increased level of serum AFP,26,27 whereas progesterone receptor level was elevated in cirrhotic livers as well as in other cancerous tissues.28 Keratin I level was also found higher in HCC tissues, which seemed to link with the histopathological condition of liver tissue damages. This study supports further investigations into the molecular understanding and regulatory mechanisms of these biomarkers in hepatocarcinogenesis.
There is a trend of down regulation of metabolic biomarkers from healthy to diseased liver tissues to HCC. Haptoglobin (Hp2) functions as a heme-binding protein, together with transferrin. Previous studies have identified the Hp2 β and α2 chains as potential serum biomarkers of HBV-associated liver inflammation.29 In most chronic liver disease or HCC tissues, the haptoglobin level was found down-modulated as revealed by a similar proteomic approach.30 The present study also revealed lower mRNA level of Hp2 in tumor and non-tumor tissues as compared to the normal liver. Lower hemoglobin-binding capacity and increase in free iron-mediated oxidative stress may become a predisposing factor for hepato-carcinogenesis. Another example is cytochrome b5, which is one of the phase I catabolic enzymes of xenobiotics in normal liver. Down-regulation of cytochrome b5 was shown in HCV-related HCC in other studies.26,27 The inhibition of the detoxification enzymes in tumor may be related to the disruption of the membrane-bounded polysome attachment that is required for their synthesis in the endoplasmic reticulum. Thus, loss of haptoglobin and cytochrome b5 seems to be of general phenomenon in HCC that may indicate potential risk for tumor initiation or predisposition.
Tumors are complex biological systems, and tumorigenesis results from a progressive sequence of genetic alterations and post-translational protein modifications that promote malignant transformation of normal cells and neoplasm invasiveness. Progesterone receptor (PGRMC1), a novel class of membranous progesterone receptor, has been underscored in the cytochrome p450-dependent oxidative damage response pathway as demonstrated in breast cancer cells. Over-expression of progesterone receptor has been indicated in breast, cervix, colon and thyroid cancers.28 In this study, the observed molecular weight of progesterone receptor, 17 kDa, is smaller than the native progesterone receptor (Hpr6.6) of 22 kDa, probably due to post-translational modifications or enzymatic cleavage process in the tumor microenvironment. The PGRMC1 17 kDa–form was first described in HCC here and would require more detailed structure-function characterization once specific antibodies are available.
Specific members of stress response chaperones or heat shock proteins are frequently upregulated in tumor tissues of various malignancies.31 In this study, the HSP27 level was drastically elevated in tumor tissues. It has been suggested to inhibit apoptosis and promote cellular survival in tumor and regulate endothelial cell migration, the key mechanisms that are responsible for tumor proliferation and malignant progression.32 In liver, up-regulated expression of HSP27 and other HSP members such as HSP70, GRP75 and GRP78, may be closely related to the pro-survival mechanisms in HCC in response to unfavorable conditions (e.g. hypoxia and nutrient depletion) that were induced during cancer development.15,30
Upregulation of matrix proteinase enzymes and dysregulation of cytokeratins are often detected in the pre-malignant and lesion sites. Cathepsin B is a cysteine proteinase that is often over-expressed in colon, gastric, and lung cancers.33 Its function was suggested to help degrade fibronectin, and laminin and some types of collagen during extracellular matrix remodeling,34 a process essential for tumor migration and invasion. There was a positive correlation of cathepsin B with increased angiogenesis in colorectal cancer and patient ages. Inhibition of the proteinase was reported to decrease cell invasion, angiogenesis and metastasis in various cancers.35 In our study, its expression was higher in the adjacent non-tumor tissues. Keratin I is one of the cytoskeletal and microfibrillar keratins in normal cells, and is first found as one of the cytokeratins in amyloid deposits in amyloidosis. Our present findings also indicated its clinical correlation with the degree of liver damage as reflected by the Ishak’s score. Cytokeratin profiling in several cancers also indicated its diagnostic utility in squamous cell carcinoma and HCC,36 while other cytokeratin family members, such as cytokeratin 8, 10, and 18, were shown to be down-regulated in the context of HCC.26
Indeed, query might exist justifying the use of this panel of protein markers to diagnose liver cancer because we only show their expressions in clinical tissues, but not in serum. Although we have not demonstrated the circulation of these protein markers in patients’ sera, there are reports in the literature showing that some of these markers, such as cathepsin B and HSP27, are actually found in the bloodstream under certain clinical, or even HCC, conditions. The serum HSP27 level is elevated in HCC patients when compared to those with hepatitis B virus infection.37 A higher serum level of cathepsin B is observed in patients with HCC and cirrhosis than normal subjects.38 An elevated serum haptoglobin level can distinguish HCC patients from those patients with chronic liver diseases and hepatitis.37,39 However, no study reporting on the presence of the fragment isoform of haptoglobin that we identified in this study in blood circulation is yet performed. Besides, no report demonstrates the presence of cytochrome b5 in human serum. Based on these previous studies, it is believed that some of them might be secreted into the blood circulation in the normal liver condition or during liver tumorigenesis. It is of noted that some known serological markers of HCC nowadays, such as glypican-3, are first identified in liver tissues40 and later confirmed to be present in patients’ sera.41,42 For this purpose, large-scale and retrospective studies should be followed to illustrate their presence in blood circulation of normal subjects and patients. Results found in this paper form the foundation for further investigating the applicability of using this panel of markers for HCC diagnosis in future studies.
In conclusion, we have developed a proteomic signature model composed of six discriminative biomarkers that allow us to discriminate HCC from non-malignant and normal liver tissues at high accuracy. Some of these biomarkers are functionally related to pre-conditioning the stromal microenvironment or directly involved in the pro-survival and invasive mechanisms of HCC during tumor progression. HSP27 appears to be the key identifier for HCC whereas haptoglobin and cytochrome b5 are indicators for normal and healthy state of the liver.
Table 6.
The Biological Functions of the Protein Markers in the HCC Signature Model
| Protein markers | General Functions* | Implications in cancer |
References |
|---|---|---|---|
| Haptoglobin | binding of free plasma hemoglobin; iron homeostasis; hemoglobin clearance | breast, kidney, liver ovary | 43–45 |
| Cytochrome b5 | stimulation of cytochrome P450 reactions; cytochrome P450-related drug metabolism | liver | 46–48 |
| Progesterone receptor | distant homolog of cytochrome b5; having similar functions as cytochrome b5 | breast | 49–51 |
| Heat shock protein 27 | stress resistance; molecular chaperone; drug resistance; cytoprotective functions | breast, liver, tongue ovary | 52–54 |
| Cathepsin B | cysteine protease; protein catabolism; protein degradation | colon, lung, stomach | 55,56 |
| Keratin I | a type of intermediate filament; structural protein; scaffolding functions | liver | 57,58 |
This table describes the general functions of these protein markers and their implications in cancer malignancies. Readers should refer to the text regarding to the cancer- or liver-related functions of these protein markers
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the clinical supports and technical assistance provided by Wan-Ching Yu, Pauline Leung, Brian Lam and Stanley Lam of the Department of Surgery of The University of Hong Kong.
Financial Support: The work was supported by the Research Grants Council of Hong Kong (N_HKU 718/03), Innovation and Technology Fund (ITS/120/07) and Sun Chieh Yeh Research Foundation for Hepatobiliary and Pancreatic Surgery.
Abbreviations
- 2-DE
2-dimensional gel electrophoresis
- AFP
alpha fetoprotein
- CART
classification-and-regression tree
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- MALDI-ToF MS
matrix assisted laser desorption ionization-time of flight mass spectrometry
- ROC
receiver operating characteristics
- T/NT
tumor/non-tumor
Footnotes
Conflict of interest statement
None declared
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics 2002. CA Cancer J Clin. 2002;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST. Is primary resection and salvage transplantation for hepatocellular carcinoma a reasonable strategy? Ann Surg. 2004;240(5):925–928. doi: 10.1097/01.sla.0000143808.63039.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, Wong J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19(12):3037–3044. doi: 10.1200/JCO.2001.19.12.3037. [DOI] [PubMed] [Google Scholar]
- 4.Lok AS, Lai CL. alpha-Fetoprotein monitoring in Chinese patients with chronic hepatitis B virus infection: role in the early detection of hepatocellular carcinoma. Hepatology. 1989;9(1):110–115. doi: 10.1002/hep.1840090119. [DOI] [PubMed] [Google Scholar]
- 5.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnu L, Zoli M, Borzio F, Bernardi M, Trevisani F. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 6.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(5) Suppl 1:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Moriguchi H, Sato C. The values and limitations of glypican-3 as a novel tumor marker for hepatocellular carcinoma from clinical and economic viewpoints. Gastroenterology. 2004;127(2):679–680. doi: 10.1053/j.gastro.2004.03.082. [DOI] [PubMed] [Google Scholar]
- 8.Pompili M, Addolorato G, Pignataro G, Rossi C, Zuppi C, Covino M, Grieco A, Gasbarrini G, Rapaccini GL. Evaluation of the albumin-gamma-glutamyltransferase isoenzyme as a diagnostic marker of hepatocellular carcinoma-complicating liver cirrhosis. J Gastroenterol Hepatol. 2003;18(3):288–295. doi: 10.1046/j.1440-1746.2003.02962.x. [DOI] [PubMed] [Google Scholar]
- 9.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37(5):1114–1121. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 10.Khien VV, Mao HV, Chinh TT, Ha PT, Bang MH, Lac BV, Hop TV, Tuan NA, Don LV, Taketa K, Satomura S. Clinical evaluation of lentil lectin-reactive alpha-fetoprotein-L3 in histology-proven hepatocellular carcinoma. Int J Biol Markers. 2001;16(2):105–111. doi: 10.1177/172460080101600204. [DOI] [PubMed] [Google Scholar]
- 11.Lee HB, Yoo OJ, Ham JS, Lee MH. Serum alpha 1-antitrypsin in patients with hepatocellular carcinoma. Clin Chim Acta. 1992;206(3):225–230. doi: 10.1016/0009-8981(92)90092-5. [DOI] [PubMed] [Google Scholar]
- 12.Sun S, Lee NP, Poon RT, Fan ST, He QY, Lau GK, Luk JM. Oncoproteomics of hepatocellular carcinoma: from cancer markers' discovery to functional pathways. Liver Int. 2007;27(8):1021–1038. doi: 10.1111/j.1478-3231.2007.01533.x. [DOI] [PubMed] [Google Scholar]
- 13.Yi X, Luk JM, Lee NP, Peng J, Leng X, Guan XY, Lau GK, Beretta L, Fan ST. Association of mortalin (HSPA9) with liver cancer metastasis and prediction for early tumor recurrence. Mol Cell Proteomics. 2008;7(2):315–325. doi: 10.1074/mcp.M700116-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Luk JM, Lam BY, Lee NP, Ho DW, Sham PC, Chen L, Peng J, Leng X, Day PJ, Fan ST. Artificial neural networks and decision tree model analysis of liver cancer proteomes. Biochem Biophys Res Commun. 2007;361(1):68–73. doi: 10.1016/j.bbrc.2007.06.172. [DOI] [PubMed] [Google Scholar]
- 15.Luk JM, Lam CT, Siu AF, Lam BY, Ng IO, Hu MY, Che CM, Fan ST. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics. 2006;6(3):1049–1057. doi: 10.1002/pmic.200500306. [DOI] [PubMed] [Google Scholar]
- 16.Feng JT, Shang S, Beretta L. Proteomics for the early detection and treatment of hepatocellular carcinoma. Oncogene. 2006;25(27):3810–3817. doi: 10.1038/sj.onc.1209551. [DOI] [PubMed] [Google Scholar]
- 17.Luk JM, Su YC, Lam SC, Lee CK, Hu MY, He QY, Lau GK, Wong FW, Fan ST. Proteomic identification of Ku70/Ku80 autoantigen recognized by monoclonal antibody against hepatocellular carcinoma. Proteomics. 2005;5(7):1980–1986. doi: 10.1002/pmic.200401084. [DOI] [PubMed] [Google Scholar]
- 18.Lee NP, Leung KW, Cheung N, Lam BY, Xu MZ, Sham PC, Lau GK, Poon RT, Fan ST, Luk JM. Comparative proteomic analysis of mouse livers from embryo to adult reveals an association with progression of hepatocellular carcinoma. Proteomics. 2008;8(10):2136–2149. doi: 10.1002/pmic.200700590. [DOI] [PubMed] [Google Scholar]
- 19.Wang XQ, Luk JM, Leung PP, Wong BW, Stanbridge EJ, Fan ST. Alternative mRNA splicing of liver intestine-cadherin in hepatocellular carcinoma. Clin Cancer Res. 2005;11(2 Pt 1):483–489. [PubMed] [Google Scholar]
- 20.Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39–47. doi: 10.1016/j.jim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Lee NP, Tong MK, Leung PP, Chan VW, Leung S, Tam PC, Chan KW, Lee KF, Yeung WS, Luk JM. Kidney claudin-19: localization in distal tubules and collecting ducts and dysregulation in polycystic renal disease. FEBS Lett. 2006;580:923–931. doi: 10.1016/j.febslet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Wong WS, Luk JM. Signaling mechanisms of pertussis toxin-induced myelomonocytic cell adhesion: role of tyrosine phosphorylation. Biochem Biophys Res Commun. 1997;236(2):479–482. doi: 10.1006/bbrc.1997.6986. [DOI] [PubMed] [Google Scholar]
- 23.Lee NP, Leung KW, Wo JY, Tam PC, Yeung WS, Luk JM. Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis. 2006;11(7):1215–1229. doi: 10.1007/s10495-006-6981-2. [DOI] [PubMed] [Google Scholar]
- 24.Lee NP, Tsang S, Cheng RH, Luk JM. Increased solubility of integrin betaA domain using maltose-binding protein as a fusion tag. Protein Pept Lett. 2006;13(5):431–435. doi: 10.2174/092986606776819493. [DOI] [PubMed] [Google Scholar]
- 25.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 26.Blanc JF, Lalanne C, Plomion C, Schmitter JM, Bathany K, Gion JM, Bioulac-Sage P, Balabaud C, Bonneu M, Rosenbaum J. Proteomic analysis of differentially expressed proteins in hepatocellular carcinoma developed in patients with chronic viral hepatitis C. Proteomics. 2005;5(14):3778–3789. doi: 10.1002/pmic.200401194. [DOI] [PubMed] [Google Scholar]
- 27.Kim W, Oe Lim S, Kim JS, Ryu YH, Byeon JY, Kim HJ, Kim YI, Heo JS, Park YM, Jung G. Comparison of proteome between hepatitis B virus- and hepatitis C virus-associated hepatocellular carcinoma. Clin Cancer Res. 2003;9(15):5493–5500. [PubMed] [Google Scholar]
- 28.Crudden G, Loesel R, Craven RJ. Overexpression of the cytochrome p450 activator hpr6 (heme-1 domain protein/human progesterone receptor) in tumors. Tumour Biol. 2005;26(3):142–146. doi: 10.1159/000086485. [DOI] [PubMed] [Google Scholar]
- 29.He QY, Lau GK, Zhou Y, Yuen ST, Lin MC, Kung HF, Chiu JF. Serum biomarkers of hepatitis B virus infected liver inflammation: a proteomic study. Proteomics. 2003;3(5):666–674. doi: 10.1002/pmic.200300394. [DOI] [PubMed] [Google Scholar]
- 30.Lee IN, Chen CH, Sheu JC, Lee HS, Huang GT, Yu CY, Lu FJ, Chow LP. Identification of human hepatocellular carcinoma-related biomarkers by two-dimensional difference gel electrophoresis and mass spectrometry. J Proteome Res. 2005;4(6):2062–2069. doi: 10.1021/pr0502018. [DOI] [PubMed] [Google Scholar]
- 31.Beere HM. Stressed to death: regulation of apoptotic signaling pathways by the heat shock proteins. Sci STKE. 2001;2001(93):RE1. doi: 10.1126/stke.2001.93.re1. [DOI] [PubMed] [Google Scholar]
- 32.Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G. HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle. 2003;2(6):579–584. [PubMed] [Google Scholar]
- 33.Kruszewski WJ, Rzepko R, Wojtacki J, Skokowski J, Kopacz A, Jaskiewicz K, Drucis K. Overexpression of cathepsin B correlates with angiogenesis in colon adenocarcinoma. Neoplasma. 2004;51(1):38–43. [PubMed] [Google Scholar]
- 34.Creemers LB, Jansen ID, Docherty AJ, Reynolds JJ, Beertsen W, Everts V. Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biol. 1998;17(1):35–46. doi: 10.1016/s0945-053x(98)90123-8. [DOI] [PubMed] [Google Scholar]
- 35.Lakka SS, Gondi CS, Yanamandra N, Olivero WC, Dinh DH, Gujrati M, Rao JS. Inhibition of cathepsin B and MMP-9 gene expression in glioblastoma cell line via RNA interference reduces tumor cell invasion, tumor growth and angiogenesis. Oncogene. 2004;23(27):4681–4689. doi: 10.1038/sj.onc.1207616. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka S, Hirohashi K, Uenishi T, Yamamoto T, Hamba H, Kubo S, Tanaka H, Shuto T, Ogawa M, Kinoshita H. A mixed hepatocellular carcinoma and cholangiocarcinoma: dual expression of biliary-type cytokeratin and hepatocyte specific marker. Hepatogastroenterology. 2004;51(57):839–841. [PubMed] [Google Scholar]
- 37.Feng JT, Liu YK, Song HY, Dai Z, Qin LX, Almofti MR, Fang CY, Lu HJ, Yang PY, Tang ZY. Heat-shock protein 27: a potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics. 2005;5(17):4581–4588. doi: 10.1002/pmic.200401309. [DOI] [PubMed] [Google Scholar]
- 38.Leto G, Tumminello FM, Pizzolanti G, Montalto G, Soresi M, Gebbia N. Lysosomal cathepsins B and L and Stefin A blood levels in patients with hepatocellular carcinoma and/or liver cirrhosis: potential clinical implications. Oncology. 1997;54(1):79–83. doi: 10.1159/000227666. [DOI] [PubMed] [Google Scholar]
- 39.Ang IL, Poon TC, Lai PB, Chan AT, Ngai SM, Hui AY, Johnson PJ, Sung JJ. Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: a glycoproteomic approach. J Proteome Res. 2006;5(10):2691–2700. doi: 10.1021/pr060109r. [DOI] [PubMed] [Google Scholar]
- 40.Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Buchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48(4):558–564. doi: 10.1136/gut.48.4.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakatsura T, Kageshita T, Ito S, Wakamatsu K, Monji M, Ikuta Y, Senju S, Ono T, Nishimura Y. Identification of glypican-3 as a novel tumor marker for melanoma. Clin Cancer Res. 2004;10(19):6612–6621. doi: 10.1158/1078-0432.CCR-04-0348. [DOI] [PubMed] [Google Scholar]
- 42.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125(1):89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 43.Lim SK, Ferraro B, Moore K, Halliwell B. Role of haptoglobin in free hemoglobin metabolism. Redox Rep. 2001;6(4):219–227. doi: 10.1179/135100001101536364. [DOI] [PubMed] [Google Scholar]
- 44.Gordon S. Homeostasis: a scavenger receptor for haemoglobin. Curr Biol. 2001;11(10):R399–R401. doi: 10.1016/s0960-9822(01)00218-4. [DOI] [PubMed] [Google Scholar]
- 45.Madsen M, Graversen JH, Moestrup SK. Haptoglobin and CD163: captor and receptor gating hemoglobin to macrophage lysosomes. Redox Rep. 2001;6(6):386–388. doi: 10.1179/135100001101536490. [DOI] [PubMed] [Google Scholar]
- 46.Porter TD. The roles of cytochrome b5 in cytochrome P450 reactions. J Biochem Mol Toxicol. 2002;16(6):311–316. doi: 10.1002/jbt.10052. [DOI] [PubMed] [Google Scholar]
- 47.Schenkman JB, Jansson I. The many roles of cytochrome b5. Pharmacol Ther. 2003;97(2):139–152. doi: 10.1016/s0163-7258(02)00327-3. [DOI] [PubMed] [Google Scholar]
- 48.Lewis DF, Ito Y. Human cytochromes P450 in the metabolism of drugs: new molecular models of enzyme-substrate interactions. Expert Opin Drug Metab Toxicol. 2008;4(9):1181–1186. doi: 10.1517/17425255.4.9.1181. [DOI] [PubMed] [Google Scholar]
- 49.Mifsud W, Bateman A. Membrane-bound progesterone receptors contain a cytochrome b5-like ligand-binding domain. Genome Biol. 2002;3(12) doi: 10.1186/gb-2002-3-12-research0068. RESEARCH0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105(1–5):16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Losel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1--many tasks for a versatile protein. Steroids. 2008;73(9–10):929–934. doi: 10.1016/j.steroids.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Ciocca DR, Oesterreich S, Chamness GC, McGuire WL, Fuqua SA. Biological and clinical implications of heat shock protein 27,000 (Hsp27): a review. J Natl Cancer Inst. 1993;85(19):1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- 53.Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C. Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal. 2005;7(3–4):404–413. doi: 10.1089/ars.2005.7.404. [DOI] [PubMed] [Google Scholar]
- 54.Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C. Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med. 2008;12(3):743–761. doi: 10.1111/j.1582-4934.2008.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mort JS, Buttle DJ. Cathepsin B. Int J Biochem Cell Biol. 1997;29(5):715–720. doi: 10.1016/s1357-2725(96)00152-5. [DOI] [PubMed] [Google Scholar]
- 56.Moin K, Demchik L, Mai J, Duessing J, Peters C, Sloane BF. Observing proteases in living cells. Adv Exp Med Biol. 2000;477:391–401. doi: 10.1007/0-306-46826-3_40. [DOI] [PubMed] [Google Scholar]
- 57.Oriolo AS, Wald FA, Ramsauer VP, Salas PJ. Intermediate filaments: a role in epithelial polarity. Exp Cell Res. 2007;313(10):2255–2264. doi: 10.1016/j.yexcr.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magin TM, Vijayaraj P, Leube RE. Structural and regulatory functions of keratins. Exp Cell Res. 2007;313(10):2021–2032. doi: 10.1016/j.yexcr.2007.03.005. [DOI] [PubMed] [Google Scholar]