Abstract
Chronic Hepatitis B (HB) is the main risk factor for chronic liver disease (CLD) and hepatocellular carcinoma (HCC) in many low-resource countries, where diagnosis is constrained by lack of clinical, histopathological and biomarker resources. We have used proteomics to detect plasma biomarkers that outperform α-Fetoprotein (AFP), the most widely used biomarker for HCC diagnosis in low-resource contexts. Deep plasma proteome analysis was performed in HCC patients, patients with chronic liver disease (CLD) and in HB-carrier controls from Thailand (South-East Asia) and The Gambia (West-Africa). Mass spectrometry profiling identified Latent-Transforming Growth Factor β Binding-Protein 2 (LTBP2) and Osteopontin (OPN) as being significantly elevated in HCC versus CLD and controls. These two proteins were further analysed by ELISA in a total of 684 plasma samples, including 183 HCC, 274 CLD and 227 asymptomatic controls. When combined, LTBP2 and OPN showed an area under the receiver operating curve (ROC) of 0.85 in distinguishing HCC from CLD in subjects with α-Fetoprotein (AFP) < 20 ng/mL. In a prospective cohort of 115 CLD patients from Korea, increased plasma levels of LTBP2 and/or OPN were detected in plasma collected over 2 years prior to diagnosis in 21 subjects who developed HCC. Thus, the combination of LTBP2 and OPN outperformed AFP for diagnosis and prediction of HCC and may therefore improve biomarker-based detection of HBV-related HCC.
Keywords: Biomarkers, chronic liver disease, hepatocellular carcinoma, LTBP2, OPN
Introduction
Hepatocellular carcinoma (HCC) represents over 80% of primary liver cancer (PLC) cases and is the 3rd most frequent cause of cancer-related death worldwide, with considerable geographic variation in rates and aetiology 1. These variations are due to differences in the distribution of risk factors such as chronic infections with hepatitis B (HBV) virus or hepatitis C (HCV) virus, dietary exposure to the mycotoxin aflatoxin B1 (AFB1), alcohol consumption and metabolic syndromes associated with overweight/obesity. Most areas of high incidence are in low-resource countries, including sub-Saharan Africa, Egypt, central Asia, and large areas of south-eastern Asia in particular in China. In the Asia-Pacific region and in west Africa, PLC ranks as the most frequent cancer in males and the 3rd in females, with age standardized incidence rates (ASR, World standard) of 53 in males and 20 in females in The Gambia, and of 41 in males and 20 in females in Thailand 2, 3. In both countries, the main attributable risk factor is HBV, with however significant differences in HBV genotypes (B and/or C, in Thailand, E in The Gambia) and in levels of exposure to AFB1, which is considered as a minor, although non-negligible, risk factor in Thailand but represents a major contaminant of staple diets in The Gambia 4, 5. In addition, in Thailand, cholangiocarcinoma (CC), the second most common form of PLC, represents about 30% of the cases and up to 75% in the northern and eastern parts of the country 6.
In low-resource areas, most HCC are diagnosed on the basis of clinical signs and at advanced stages. Because of late presentation and absence of curative therapeutic options, histopathological analysis is rarely performed and diagnosis commonly relies on a triad consisting of clinical symptoms, suggestive ultrasonography and elevated levels of a plasma protein marker, α-Fetoprotein (AFP) 7. AFP levels above 400 ng/mL are considered as diagnostic for HCC; nevertheless, such high levels are detected only in a subset of patients8. In clinical practice, levels above 100 ng/mL are considered as highly suspicious, as well as lower levels if there is a continuous increase of expression over a short period of time 9. In subjects meeting these criteria, the National Comprehensive Cancer Network (NCCN) guidelines and the American Association for the Study of Liver Diseases (AASLD) recommend further assessment using imaging techniques10. However, AFP remains unsatisfactory for diagnosis and screening as many patients develop cancer without elevated AFP, and AFP levels above 100 ng/mL may be observed in some patients with non-cancer chronic liver diseases (CLD) 9-11. Other markers have been proposed as alternatives to AFP, including lens culinaris-reactive AFP (AFP-L3), des-gamma carboxyprothrombin (DCP) and glypican-3 (GPC3) 12. Thus far, most of these markers have not demonstrated better performance than AFP either alone or or when combined with ultrasonography 7. In a recent study, we have used deep-plasma proteomics to identify Osteopontin (OPN) as candidate new diagnosis biomarker for HCC, which may be applicable in areas of high incidence and HBV chronicity 13. OPN was shown to outperform AFP for distinguishing between patients with liver cirrhosis or HCC. OPN levels were also found to be elevated up to one year prior to HCC diagnosis in a cohort of chronically infected subjects from the US.
In the present study, we have used the same deep-plasma proteomics approach to investigate candidate markers in the plasma of HCC patients from Thailand and The Gambia, two regions of high prevalence of HB carriage and high incidence of HCC. The aim of this study was to identify new protein markers for improving the detection of HCC against CLD in an etiological context of high HB prevalence. In addition to the previously reported OPN, we report the identification of Latent-Transforming Growth Factor β Binding-Protein 2 (LTBP2) as a potential candidate for HCC detection and diagnosis 13. We used plasma samples series from case-control studies performed in The Gambia, in Thailand and in France, as well as from a pilot prospective cohort in Korea, to demonstrate that the combined use of LTBP2 and OPN shows specificity and sensitivity for HCC even in cases considered as negative for AFP (<20 ng/mL).
Materials and Methods
Study design and patient characteristics
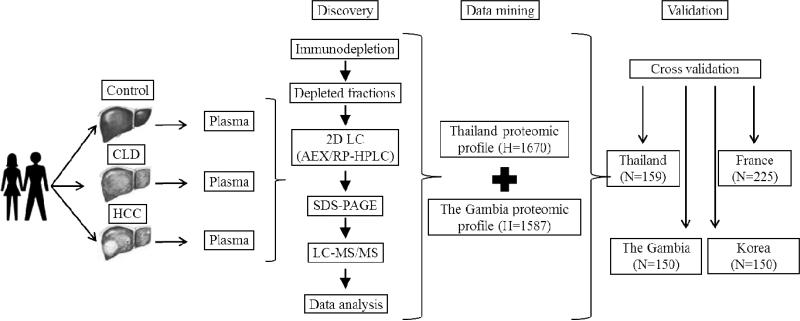
This study was performed in the framework of the International Liver Cancer Study (ILCS), an international initiative aimed at prevention, early diagnosis and control of liver cancer through the understanding of causes and mechanisms in different populations 14. The study included two steps, first a proteomics discovery pipeline and second, a translational validation approach (Figure 1). The proteomics discovery pipeline has been described previously 13.
Figure 1. Outline of study design, from protein discovery to biomarker validation.
The study included three steps, with first plasma proteomics, second data mining to identify candidate markers and third validation in several cohorts of patients. First, biospecimens (plasma) were collected in two structured hospital-based case-control studies in two regions of high incidence of HB carriage and of HCC, The Gambia and Thailand, using protocols optimized from plasma proteomics. These case-control studies included control individuals (no liver symptoms), individuals with chronic liver disease patients (CLD, chronic active hepatitis B or hepatitis C), and patients with confirmed diagnosis of HCC. A small number of plasma specimens of cases (HCC, n=10), CLD (n=10) and controls (n=10) from each region were extensively analysed using a deep-plasma proteomic approach. Next, data generated from The Gambia and from Thailand were mined to identify candidate biomarkers that were differentially expressed in cases, CLD and controls in both regions. Third, two of these candidate markers, OPN and LTBP2, were quantified in the entire collection of plasma from the two case-control studies and in two additional series, a case series from France (CLD and/or HCC) and a prospective study of HB carriers from Korea who subsequently developed HCC. H: number of identified proteins; N: number of plasma specimens tested.
For validation studies, plasma specimens collected in structured epidemiological studies were used. In Thailand, specimens were obtained from patients and hospital-based controls recruited at the Cancer Control Unit of the National Cancer Institute of Thailand, Bangkok (TLCS, Thailand Liver Cancer Study, Case-control 1). The study was conducted from April 2008 to December 2009. All cases of PLC were recruited and matched controls were obtained from outpatient clinics. Differential diagnosis of HCC versus CC was established by a combination of clinical examination, imaging using ultrasonography, computerized tomography (CT) or Magnetic Resonance Imaging (MRI), biochemistry (AFP and liver function enzymes testing) and histological confirmation on a small subset of patients from whom needle biopsies were available. In The Gambia (GLCS, Gambia Liver Cancer Study, Case-control 2), specimens were obtained in the course of a nationwide case-control study performed between 1997 and 2001 in three tertiary referral hospitals as described previously 15. Diagnosis of HCC was based on the classical triad of clinical examination, ultrasonography and AFP testing. The clinical characteristics of these patients have been extensively described 16, 17. Histological confirmation on needle biopsies was available for less than 15% of the cases 15. In France (FLCS, French Liver Cancer Study, Case-control 3), specimens were obtained from patients and controls recruited at Hopital Croix-Rousse in Lyon between September 2011 and May 2012. HCC was diagnosed according to AASLD guidelines and Barcelona Clinic Liver Cancer staging system (BCLC). In Korea a prospective cohort was assembled using specimens obtained from chronic hepatitis or cirrhosis patients and controls recruited in at the Kosin University Hospital in Busan between 1999 and 2001, with subsequent follow-up until 2006 (KLCS, Korean Liver Cancer Study, Cohort 1). Incident HCC was diagnosed according to AASLD guidelines. Overall, a total of 684 plasma samples including 183 HCC patients, 274 CLD patients (including chronic active HB (CHB) and HC (CHC) patients), and 227 controls without liver symptoms were selected from the four studies and used in the validation step. In TLCS (Thailand) and in GLDS (The Gambia), all CLD patients had CHB. In contrast, in FLDS (France), only CLD patients with CHC were included. In the KLDS prospective cohort, the CLD group included patients with CHB, CHC as well as patients with liver cirrhosis developing in a context of either HB or HC carriage. All steps of the study (proteomics discovery, data mining and validation) were approved by national ethics review boards in Thailand, The Gambia, France and Korea, and by the Institutional Review Board of the International Agency for Research on Cancer.
Biomarker discovery and validation
Plasma proteomics was performed at the Fred Hutchinson Cancer Research Center (FHCRC, Seattle, WS, USA) and was previously described by Shang et al. 13. Validation studies were focused on two markers that emerged from the proteomics discovery studies, OPN and LTBP2. Levels of OPN were measured using human Osteopontin Quantikine ELISA kit (R&D Systems, Inc., Lille, France, kit reference number: DOTS00) and AFP levels were measured using a standard protocol as described previously 13.
Levels of LTBP2 were measured with a commercial sandwich ELISA kit (USCN Life Science Inc., Wuhan, P. R. China; kit reference number: SEB630Hu) in 100 μl of diluted (1:10) plasma. Samples were added to the plates pre-coated with the LTBP2 monoclonal antibody MAB630Hu22and incubated for 2 h at 37°C. Next, the biotin-conjugated rabbit polyclonal antibody PAB630Hu01 was added to each well and the plate incubated for 1 h at 37°C. After three washes, 100 μl of avidin conjugated to horseradish peroxidase was added and the plate incubated for 30 min at 37°C. After five washes, 90μl of TMB solution was added and the plate was incubated for 15 min at 37°C in the dark followed by 50μl of stop solution (sulphuric acid solution). The absorbance was measured at 450 nm with a Multiskan* GO Microplate spectrophotometer (Thermo Fisher Scientific Inc, Saint Herblain, France). Levels of OPN were measured using a commercial ELISA kit (R&D Systems, Inc., Lille, France) as previously described in Shang et al.13. AFP levels were measured using standard protocols as described previously 13.
Statistical analysis
For each biomarker test, receiver operating characteristic (ROC) curves were plotted and area under the ROC curve (AUROC) was calculated as well as its 95% confidence intervals (CI) using PROC LOGISTIC program and %PLOTROC macro and comparison of area under the ROC curve was performed using the %ROC macro of the SAS statistical software package (SAS 9.2, SAS Institute Inc, North Carolina, USA). Optimal cut-offs were calculated based on the minimum distance to the top-left corner of the ROC curve and the maximum likelihood ratio with highest achievable sensitivity and sensitivity. The sensitivity and specificity and corresponding 95% CI were calculated for AFP, LTBP2, and OPN utilizing Usage Note 24170 (http://support.sas.com/kb/24/170.html). All analyses were conducted with and without stratification for hepatitis virus aetiology and for AFP levels. Multivariate analysis and Student's t-tests (p<0.01) were conducted using the STATA 11 (StataCorp LP, Texas, USA).
Results
Latent-Transforming Growth Factor β Binding-Protein 2 as potential HCC protein biomarker
To identify plasma protein markers for detection and diagnosis of HCC in low-resource settings, we used a biomarker discovery and validation pipeline based on deep plasma proteomics profiling followed by data mining and validation studies using ELISA tests (Figure 1). The proteomics approach included immunodepletion of major plasma proteins and protein separation by two-dimensional HPLC followed by SDS-PAGE and tandem mass spectrometry analysis as described previously 13. This analysis has led to the identification of OPN as a candidate biomarker, as previously described 13. Here, we have identified another potential biomarker, LTBP2. This biomarker was identified by mass spectrometry with high confidence, characterized by high peptide and protein probabilities (≥99% and ≥97%, respectively) with sequence coverage of 54% in plasma samples of CLD and HCC patients. To further evaluate LTBP2, we used plasma samples from patients with HCC or CLD with diverse aetiological backgrounds and natural history of progressive chronic liver disease. Case-control 1 (TLCS, Thailand) and 2 (GLDS, The Gambia), have recruited patients with mostly advanced forms of HCC, whereas the CLD group was constituted of patients with chronic active hepatitis B (CHB). Case-control 3 (FLDS, France), was constituted of patients with less advanced HCC; in this study the CLD group included patients with active chronic hepatitis C. Cohort 1 (Korea) was constituted of subjects with chronic hepatitis B who were prospectively followed-up for HCC. Moreover, the different case series also differed in the protocols used for plasma collection. Case-control studies 1 (TLCS, Thailand) and 3 (FLCS, France) were developed using a proteomics-optimized protocol for specimen collection (ILCS protocol, see Supporting Information) while Case-control 2 (GLCS, The Gambia) and Cohort 1 (KLCS, Korea) were part of previously described studies in which blood samples were collected and fractionated using standard protocols 15, 18, 19. In each case-control and cohort study, we compared LTBP2 with OPN and with levels of AFP to evaluate the performance of detection algorithms that combine several markers.
Levels and performance of LTBP2 in HCC patients in case-control 1 (Thailand)
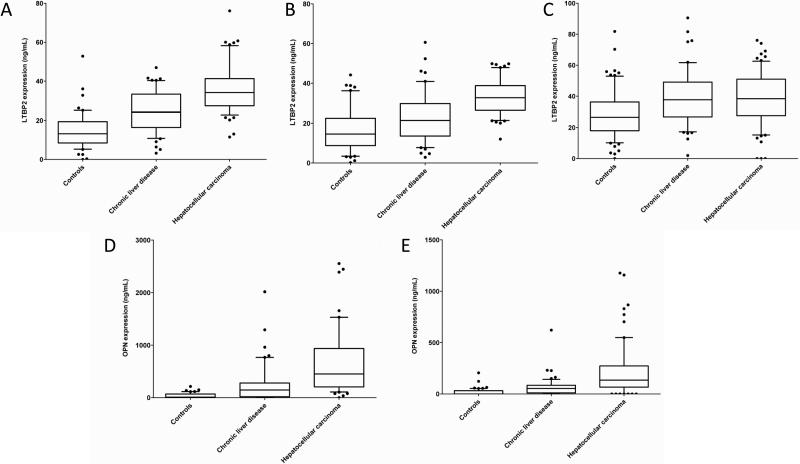
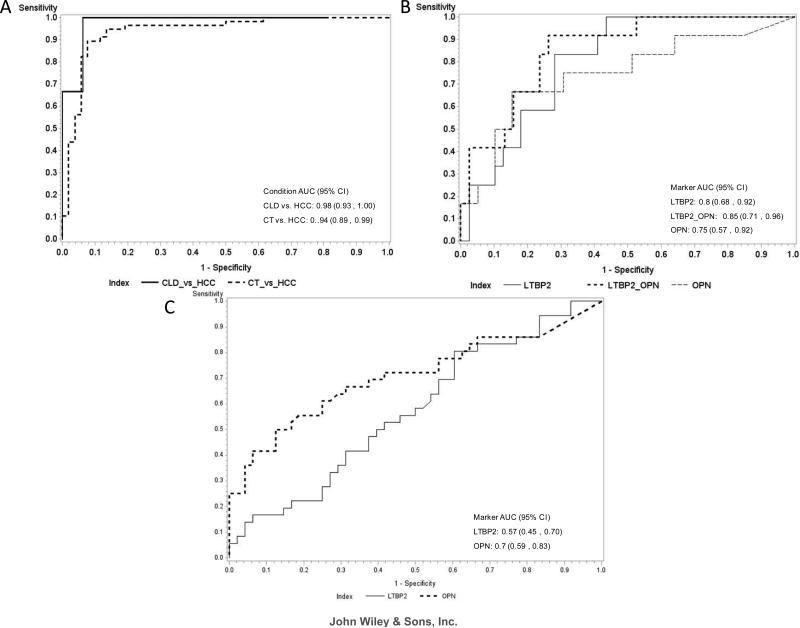
We analysed LTBP2 plasma levels in Case-control 1 (TLCS, Supporting Table S1), comprising a total of 159 plasma samples collected from 52 controls, 49 CLD patients and 58 HCC patients. Plasma levels of LTBP2 were measured blinded to clinical status. They were found to be significantly higher in HCC patients (mean = 35.96 ng/mL) than in CLD patients (mean = 24.21 ng/mL, p < 0.0001) and controls (mean = 14.67 ng/mL, p < 0.0001) (Figure 2 A). To analyse the performance of LTBP2 in discriminating between HCC, CLD and controls, AUROC analysis was performed at the level of 27 ng/mL LTBP2 as the cut-off value. The performance of LTBP2 in distinguishing HCC patients from controls was demonstrated by an AUROC of 0.94 (95% CI: 0.89 – 0.99) (Supporting Table S2). Next, to determine whether measuring LTBP2 may significantly improve HCC detection and diagnosis in patients with low serum levels of AFP, we analysed the performance of LTBP2 (at a cut-off of 27 ng/mL) in distinguishing HCC from CLD patients with AFP levels below 20 ng/mL. In this comparison, the AUROC of LTBP2 was 0.98 (95% CI: 0.93 – 1.00) (Figure 3 A, Table 1). Overall, when comparing LTBP2 cut-off of 27 ng/mL and AFP at a cut-off level of 20 ng/mL, LTBP2 had better performance than AFP alone, with a sensitivity of 100% (95 CI: 57% - 100%) and a specificity of 94% (95% CI: 70% - 99%) compared to a sensitivity of 85% (95% CI: 72% - 93%) and a specificity of 100% (95% CI: 79% - 100%) for AFP (Table 1). Moreover, measuring LTBP2 was particularly useful to distinguish between patients with HCC or with chronic liver disease under the AFP cut-off value of 20 ng/mL.
Figure 2. Levels of LTBP2 and OPN in the plasma of control, CLD and HCC patients.
Box and whisker plots are shown. (A-C): levels of LTBP2 in controls, chronic liver disease and hepatocellular carcinoma (HCC) in case-control study 1 (Thailand; A); case-control study 2 (The Gambia; B); (C) case-control study 3 (France; C); (D-E): levels of OPN in controls, chronic liver disease and HCC patients in case-control study 2 (The Gambia; D); case-control study 3 (France, E).
Figure 3. Receiver operating curves (ROC).
(A) LTBP2 in controls vs. HCC patients (CT vs. HCC) and chronic liver disease vs. HCC patients (CLD vs. HCC) at an AFP cut-off ≤ 20 ng/mL in case-control 1; (B) LTBP2, OPN and LTBP2 combined to OPN in chronic liver disease vs. HCC patients at an AFP cut-off ≤ 20 ng/mL in case-control 2; (C) LTBP2 and OPN in chronic liver disease vs. HCC patients at an AFP cut-off ≤ 20 ng/mL in case-control 3. The area under the ROC curve (AUROC) is shown with its 95% confidence intervals.
Table 1.
LTBP2, OPN and AFP performance when comparing various sample groups in the three case-control studies.
| Case-control study | Group 1 (Marker) | Group 2 (Marker) | AUROC | 95% CI | Cutoff (ng/mL) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| Thailand | CLD (AFP) | HCC (AFP) | 0.57 | 0.31-0.84 | 20 ng/mL | 85%; CI: 72%-93% | 100%; CI: 79%-100% |
| CLD (LTBP2 with AFP<20 ng/mL) | HCC (LTBP2 with AFP<20 ng/mL) | 0.98 | 0.93-1.00 | 27 ng/mL | 100%; CI: 57%-100% | 94%; CI: 70%-99% | |
| The Gambia | CLD (AFP) | HCC (AFP) | 0.59 | 0.39-0.79 | 20 ng/mL | 76%; CI: 61%-87% | 86%; CI: 72%-94% |
| CLD (LTBP2+OPN with AFP<20 ng/mL) | HCC (LTBP2+OPN with AFP<20 ng/mL) | 0.85 | 0.74-0.96 | 27&237 ng/mL | 92%; CI: 62%-99% | 70%; CI: 53%-83% | |
| France | CLD (AFP) | HCC (AFP) | 0.57 | 0.45-0.69 | 20 ng/mL | 49%; CI: 37%-62% | 86%; CI: 74%-94% |
| CLD (LTBP2 with AFP<20 ng/mL) | HCC (LTBP2 with AFP<20 ng/mL) | 0.57 | 0.45-0.70 | 27 ng/mL | 69%; CI: 54%-81% | 36%; CI: 20%-54% | |
| CLD (OPN with AFP<20 ng/mL) | HCC (OPN with AFP<20 ng/mL) | 0.70 | 0.59-0.83 | 91 ng/mL | 83%; CI: 69%-93% | 56%; CI: 38%-72% |
Sensitivity and specificity of LTBP2, OPN and AFP are assessed for corresponding cutoffs according to sample groups analysed. AUROC = area under the receiver operating curve, CLD = chronic liver disease patients, HCC = hepatocellular carcinoma patients.
Levels and performance of LTBP2 and OPN in HCC patients in case-control 2 (The Gambia)
We subsequently analysed LTBP2 and OPN plasma levels in a second, independent cohort performed in The Gambia between 1998 and 2001 (GLCS; case-control 2). We analysed a total of 150 plasma samples, collected from 50 controls, 50 CLD patients and 50 HCC patients (Supporting Table S3). Case-control 2 includes samples collected using a standard clinical protocol for blood collection and fractionation, therefore providing an “in-the-field” validation for both protein biomarkers. Firstly, we found that plasma levels of LTBP2 were significantly higher in HCC patients (mean = 33.31 ng/mL) than in CLD patients (mean = 23.13 ng/mL, p < 0.0001) and controls (mean = 16.72 ng/mL, p < 0.0001) (Figure 2 B). Plasma levels of OPN were also significantly higher in HCC patients (mean = 671.1 ng/mL) than in CLD patients (mean = 268.0 ng/mL, p < 0.0001) and controls (mean = 36.39 ng/mL, p < 0.0001) (Figure 2 D). Secondly, we analysed the performance of LTBP2 and OPN, independently and combined, in discriminating between HCC patients, patients with CLD and controls. Using a cut-off level of 27 ng/mL for LTBP2 and 237 ng/mL for OPN, both LTBP2 and OPN showed high performance in discriminating between HCC and controls, in particular when used in combination (LTBP2: AUROC 0.87, 95% CI: 0.79 – 0.94; OPN: AUROC 0.96, 95% CI: 0.93 – 1.00; LTBP2+OPN: AUROC 0.98, 95% CI: 0.97 – 1.00) (Supporting Table S2). When comparing HCC and CLD patients with AFP levels lower than 20 ng/mL, LTBP2 (cut-off: 27 ng/mL) and OPN (cut-off: 237 ng/mL), independently or combined, outperformed AFP (LTBP2: AUROC 0.80, 95% CI: 0.68 – 0.92; OPN: AUROC 0.75, 95% CI: 0.57 – 0.92; LTBP2+OPN: AUROC 0.85, 95% CI: 0.74 – 0.96; AFP: AUROC 0.59, 95% CI: 0.39 – 0.79) (Figure 3 B, Table 1).
Overall, when using as cut-off values <20 ng/mL for AFP, 27 ng/mL for LTBP2 and 237 ng/mL for OPN, the combination of LTBP2 and OPN showed better performance than AFP, with an increased sensitivity [LTBP2+OPN: sensitivity 92% (95% CI: 62% - 99%); AFP: sensitivity 76% (95% CI: 61% - 87%)].
Levels and performance of LTBP2 and OPN in HCC patients in case-control 3 (France)
To assess whether LTBP2 may also serve as marker in patients with “western-type” natural history of CLD and HCC aetiology, we extended our validation studies to a case-control study performed in France (case-control study 3; FLDS; cases and controls recruited in 2011 and 2012). In this series, HCV, alcohol and metabolic syndromes, were the most significant etiological risk factors for HCC and the pattern of CLD was dominated by cirrhosis. We analysed a total of 225 plasma samples, collected from 75 controls, 75 CLD patients and 75 HCC patients (diagnosed according to AASLD and BCLD guidelines; Supporting Table S4). First, we found that plasma levels of LTBP2 were significantly higher in HCC patients (mean = 50.46 ng/mL) compared to controls (mean = 34.69 ng/mL, p < 0.0001), as well as in CLD patients (mean = 53.63 ng/mL,) compared to controls (p < 0.0001) (Figure 2 C). Nevertheless, we did not find a significant difference in plasma levels between HCC and CLD patients. In contrast, plasma levels of OPN were significantly higher in HCC patients (mean = 253.24 ng/mL) compared to CLD patients (mean = 67.75 ng/mL, p < 0.0001) and controls (mean = 13.46 ng/mL, p < 0.0001) (Figure 2 E). Next, we analysed the performance of LTBP2 and OPN in distinguishing between HCC patients, patients with CLD and controls. Using a cut-off level of 27 ng/mL for LTBP2 and 91 ng/mL for OPN, we compared HCC and CLD patients with AFP levels <20 ng/mL. Whereas LTBP2 had a similar performance as AFP (Figure 3 C, Table 1), OPN greatly out performed AFP (OPN: AUROC 0.70, 95% CI: 0.59 – 0.83; AFP: AUROC 0.57, 95% CI: 0.45 – 0.69) with an increase in sensitivity [OPN: sensitivity 83% (95% CI: 69% - 93%); AFP: sensitivity 49% (95% CI: 37% - 62%)] (Figure 3 C, Table 1). Overall, OPN at a cut-off level of 91 ng/mL, but not LTBP2, appeared to be effective in distinguishing CLD from HCC in this case-control study from France.
Pre-diagnostic potential of combined LTBP2 and OPN levels in cohort 1 (Korea)
To evaluate whether a combined panel of protein biomarkers (AFP, LTBP2 and OPN) may assist in early detection/diagnosis of HCC, we investigated the levels of LTBP2 and OPN in an independent hepatitis prospective cohort developed in Korea between 1999 and 2001, with a follow up for HCC incident in 2006 (KLCS, cohort 1). We analysed a total of 150 plasma samples, collected from 50 controls, 50 patients with Chronic HB or HC, and 50 patients with liver cirrhosis (Supporting Table S5). Of these, 115 were considered at risk of developing HCC based on being CHB carriers, active hepatitis patients or cirrhotic patients. By the end of the follow up (median: 6 years), 21 patients (1 HBV carrier control, 3 hepatitis and 17 cirrhotic patients) had developed incident HCC . We found that plasma levels of LTBP2 were statistically significantly higher in hepatitis patients (mean = 29.2 ng/mL) or cirrhotic patients (mean = 22.17 ng/mL) than in controls (mean = 18.1 ng/mL, p < 0.0001). Plasma levels of OPN were also statistically significantly higher in hepatitis patients (mean = 56.43 ng/mL) or cirrhotic patients (mean = 184.22 ng/mL) than in controls (mean = 27.43 ng/mL, p < 0.0001).
Among the 21 subjects who developed HCC, 5 had AFP levels above 20 ng/mL and 19 had LTBP2 or OPN levels above 27 ng/mL or 91 ng/mL, respectively, at the time of recruitment. In patients who developed HCC and had AFP levels below 20 ng/mL (76% of cases), LTBP2 and OPN levels were shown to be above their respective cut-off values in 67% of the cases. Among these cases, 78% had plasma levels elevated up to 24 months before diagnosis while 58% could be detected with elevated plasma levels more than 24 months prior to diagnosis. The median time between inclusion into the study/blood sample collection and diagnosis of HCC was 29 months. Table 2 compares the sensitivity, specificity and positive/negative (PPV and NPV) predictive values for early detection of HCC using different combinations of markers. The PPV for LTBP2 and/or OPN positivity in subjects with AFP < 20 ng/mL was 70%, while the NPV was 98% (Table 2). Thus, increased levels of LTBP2 and/or OPN in the plasma of subjects with chronic liver disease may be suggestive of early, sub-clinical HCC.
Table 2.
Evaluation of positive and negative predictive values for AFP, LTBP2 and OPN when comparing the performance of the markers in cohort 1 (Korea) for the early detection/diagnosis of HCC.
| Parameter | AFP | 95% CI | LTBP2 or OPN | 95% CI | LTBP2 or OPN (for patients with AFP < 20 ng/mL) | 95% CI |
|---|---|---|---|---|---|---|
| Sensitivity | 24% | 8% – 47% | 90% | 68% – 98% | 95% | 75% – 99% |
| Specificity | 81% | 71% – 88% | 72% | 63% – 81% | 90% | 82% – 96% |
| PLR | 1.24 | 0.52 – 2.97 | 3.27 | 2.30 – 4.65 | 9.97 | 5.12 – 19.43 |
| NLR | 0.94 | 0.73 – 1.22 | 0.14 | 0.04 – 0.52 | 0.06 | 0.01 – 0.37 |
| PPV | 21% | 8% – 44% | 40% | 26% – 56% | 70% | 50% – 86% |
| NPV | 82% | 73% – 90% | 97% | 90% – 99% | 98% | 93% – 99% |
PLR = positive likelihood ration, NLR = negative likelihood ratio, PPV = positive predictive value, NPV = negative predictive value.
Discussion
In most low-resource regions, detection and diagnosis of HCC commonly relies on a triad consisting of clinical symptoms, ultrasonography findings, and elevated levels of a serum biomarker, AFP. In practice, the updated guidelines from the AASLD no longer recommends that AFP testing be part of the diagnostic evaluation 10. The panel of experts considered that imaging findings of classical enhancement to be more definitive in a diagnosis setting since the levels of serum AFP may be elevated in certain non-malignant conditions, or within normal limits in a substantial percentage of patients with HCC. However, in many low-resource regions with high HCC incidence, quality imaging procedures are not available and AFP is still used as part of the diagnostic panel. For example, the Asian Pacific Association for the Study of the Liver still recommends AFP usage at a cut-off of 200 ng/mL, a concentration at which this marker is highly specific for HCC but only moderately sensitive 11. Therefore, there is an urgent need for better markers to replace or complement AFP usage in particular for diagnosing HCC in patients with levels of AFP under the commonly recommended cut-off values.
The natural history of HCC and of its precursor liver disease is extremely variable according to populations, contexts of viral infections, and socio-economic conditions. About 80% of the cases of HCC occur in low-resource countries, mainly in a background of endemic HB carriage, and these cancers are almost invariably fatal because patients are presented at a very late stage, with no realistic therapeutic options, and often without a significant phase of precursor cirrhosis. This situation calls for widely applicable, cheap and effective markers for improving early diagnosis in large groups of subjects considered as at risk for HCC. In contrast, in western countries, HCC often develops as a sequel of protracted chronic liver diseases such as cirrhosis, in a context of chronic HC carriage and of liver damage caused by chronic alcoholism or by metabolic diseases. So far, most efforts at identifying biomarkers for early detection have been developed in patients with “western” HCC patterns. In this study, we have set to develop an approach in patients representing the low-resource contexts in which the majority of HCC occurs. We have used the deep-plasma proteomics discovery and validation previously described by Shang et al. 13 to detect and evaluate candidate protein markers in case-control studies from The Gambia (west Africa) and Thailand (south east Asia). The study by Shang et al. 13 has demonstrated the performance of OPN as a marker for HCC versus chronic liver disease in both “western” (US) and “low-resource” (west Africa) contexts. Here, we identify LTBP2 and further confirm OPN as candidate markers for detection and diagnosis of HBV-related HCC. Furthermore, using a case-control comparison nested in a prospective cohort of HB carriers from Korea, we show that the combined use of LTBP2 and OPN enables the detection of patients at high risk of developing HCC in particular when these patients have levels of AFP below 20 ng/ml. In contrast, LTBP2 was found ineffective in distinguishing between patients with liver cirrhosis and with HCC in a “western” case-control study developed in France, although levels in HCC patients was significantly elevated as compared to control subjects.
The performance of LTBP2 and OPN in “non-western” studies surpassed that of AFP in differentiating HCC patients from CLD patients, namely in patients with AFP levels below cut-off levels of 20 ng/mL. The best performance was obtained by combining LTBP2 and OPN as a complementary panel with AFP in HCC diagnosis in case-control 2 (The Gambia). The combination of the two markers increased the sensitivity in detecting HCC, but at the expense of lower specificity, which could be partially compensated by adding AFP in the panel of biomarkers. In contrast, the performance of LTBP2 was limited compared to AFP in case-control 3 (France). This study differs from the 3 other studies by several characteristics, including the facts that chronic HB is not a significant risk factor, and that the pattern of CLD in patients from France is dominated with cirrhosis. Thus, our observations suggest that elevated LTBP2 may be of particular significance for HBV-related HCC. Alternatively, LTBP2 may accumulate as the consequence of a form of damage to liver tissue that occurs with the occurrence of HCC in patients from The Gambia or Thailand (in whom precursor cirrhosis is infrequent), but also with liver cirrhosis in patients from France. Further studies are needed to address these hypotheses. Of note, in case-control study 3 (France) OPN outperformed AFP at a cut-off level (91 ng/mL) optimized for proteomics-based blood collection protocols, confirming previous results on a case-control study from the US 13.
Our results on the prospective cohort from Korea underlines the potential interest of OPN and LTBP2 as candidate markers for early detection of HCC. Overall, at least one of these markers was detected at baseline (recruitment in the cohort) in 17 of 21 patients who subsequently developed HCC. The low proportion of false positives (7%) and the important proportion of true negatives (71%) in at risk patients who did not develop HCC further supports the interest of these markers for early detection/diagnosis of HCC. Of note, the levels of LTBP2 or OPN were elevated in pre-diagnostic patients who showed AFP levels below 20 ng/mL.
The overall performance of the 3 markers, LTBP2, OPN and AFP in case-control 2 (The Gambia) is of particular interest since plasma samples for this study were not collected using an optimized protocol for plasma proteomics but were instead obtained using a standard clinical protocol for blood collection and fractionation. Furthermore, the specimens of this study were stored at −80°C for about 10 years prior to usage in the present study. Whilst these differences did not alter the performance of the markers, it is interesting to note that an OPN cut-off level of 237 ng/mL had to be used for case-control study 2, compared to 91 ng/mL in case-control studies 1 and 3 (as well as in a cohort from the US 13. This observation underlines that OPN may be less stable than AFP or LTBP2 and may be more dependent upon pre-analytical and sample storage conditions LTBP2 and OPN are components of the extracellular matrix (ECM). LTBP-2 is a protein with multiple heparin/heparan sulfate binding sites 20. Unlike other members of the LTBP family, LTBP2 does not bind transforming growth factor β (TGF-β) family members and does not use its RGD domain for interacting with integrins. It regulates the assembly of elastic fibres through binding to DANCE/fibulin-5 and its expression appears to be increased in intrinsically aged skin 21, 22. In human cancer, LTBP2 has been suggested to act as a suppressor in oesophageal squamous cell carcinoma and is up regulated in human pancreatic ductal adenocarcinoma 23-25. In a recent study that used proteomics to characterize cancer-associated fibroblasts in a mouse model of colorectal carcinogenesis, LTBP2 was identified as one of the components of a desmoplastic signature of 4 markers that significantly increased in tumour stroma, without significant expression in the cancer epithelial cells26. On the other hand, OPN is a well know ECM component that participates in wound repair, immune response, inflammation and cancer. OPN expression is increased during tumourigenesis and facilitates invasion and metastasis 27. Levels of OPN were reported to be increased in the plasma of patients with CLD as compared to healthy individuals 27. In HCC patients, high plasma levels of OPN were correlated with reduced liver function and late tumour stage as well as with high rate of postoperative recurrence 28. OPN has also been proposed as predictor of cirrhosis in patients with HBV infection and as potential early diagnosis marker for HCC 13, 27, 29-31.
We suggest that levels of OPN and LTBP2 may increase in the plasma as a consequence of the remodelling and degradation of ECM resulting from the expansion of transformed hepatocytes 32, 33. The release of these proteins might require the expression of specific ECM-degrading enzymes by tumour cells and may depend upon the specific structure of excess ECM accumulated during liver fibrosis that precedes liver cancer. Further studies are needed to understand the factors that regulate the activity of hepatic stellate cells (HSC) and the cross talks between HSC and transformed hepatocytes during the early steps of liver carcinogenesis, contributing to the release of high levels of specific ECM components in the plasma. It will also be important to evaluate whether plasma levels of OPN and LTBP2 are elevated in other cancer pathologies than HCC.
Our study provides evidence that measuring AFP, OPN and LTBP2 in plasma of subjects with suspicious liver symptoms may be effective for biomarker-based detection and diagnosis of HCC, opening new opportunities for improved diagnosis accuracy in clinical contexts where other diagnostic methods are difficult to implement. The main limitations of the study are the lack of AFP measurements in control groups, preventing us to compare the performance of AFP, LTBP2 and OPN in distinguishing HCC patients from subjects with no clinical liver symptoms. Larger validation studies, including prospective studies on groups of subjects with different risk factors and patterns of chronic liver disease, are needed, allowing for stratification of patients in relevant subgroups and detailed analyses according to tumour occurrence, size and clinical parameters. It will also be important to develop further studies to determine whether metastasis of other cancers to the liver may significantly increase plasma levels of OPN and LBPT2. However, in the context of regions of HBV-endemicity, adding LTBP2 and OPN detection to current AFP usage may hold promises for improved and earlier diagnosis of HCC by combining the high sensitivity of LTBP2 and OPN with the high specificity of AFP.
Supplementary Material
What's new:
Deep-plasma proteomics was used to identify candidate biomarkers for diagnosis of hepatocellular carcinoma (HCC), focusing on HBV-related HCC. Validation studies on a total of 684 samples showed that elevated levels of LTBP2 and/or OPN levels are highly specific and sensitive markers for distinguishing HCC from chronic liver disease, in particular in patients with low or undetectable levels of α-Fetoprotein. LTBP2 appears highly associated with HBV-related HCC, while OPN shows a robust and consistent association with HCC in both HBV and HCV contexts.
Acknowledgements
The authors acknowledge Dr. Behnoush Abedi for expert advice and in the interpretation of clinical implications of changes to liver histopathology during inflammation and cancer. The technical assistance of Mrs Adam Jeng for specimen collection and storage in The Gambia is acknowledged.
Grant Support: This work was supported by the European Union Collaborative Project Prolifica, 7th Framework Programme, FP7-AFRICA-2010, Health-F2-2011-265994 (Grant agreement no. 265994). ANdC was a recipient of a Postdoctoral Fellowship from the IARC, partially supported by the EC FP7 Marie Curie Actions – People – Co-funding of regional, national and international programmes (COFUND).
Abbreviations
- AASLD
the American Association for the Study of Liver Diseases
- AFP
α-Fetoprotein
- AFP-L3
lectin-bound AFP
- AUC
area under the ROC curve
- BCLC
Barcelona Clinic Liver Cancer staging system
- CC
cholangiocarcinoma
- CLD
chronic liver disease
- CT
computerized tomography
- CHB
chronic hepatitis B
- CHC
chronic hepatitis C
- CI
confidence interval
- DCP
des-gamma carboxyprothrombin
- GPC3
glypican-3
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HPLC
high-performance liquid chromatography
- ILCS
International Liver Cancer Study
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- LTBP2
latent-transforming growth factor β binding-protein 2
- OPN
osteopontin
- PLC
primary liver cancer
- ROC
receiver operating characteristic
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
- 1.Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infectious Disease Clinics of North America. 2010;24:899–919. doi: 10.1016/j.idc.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet] Vol. 2011. International Agency for Research on Cancer; Lyon, France: 2010. [Google Scholar]
- 3.Yuen MF, Hou JL, Chutaputti A. Asia Pacific Working Party P. Hepatocellular carcinoma in the Asia pacific region. Journal of Gastroenterology and Hepatology. 2009;24:346–53. doi: 10.1111/j.1440-1746.2009.05784.x. [DOI] [PubMed] [Google Scholar]
- 4.Srivatanakul P, Honjo S, Kittiwatanachot P, Jedpiyawongse A, Khuhaprema T, Miwa M. Hepatitis viruses and risk of cholangiocarcinoma in Northeast Thailand. Asian Pacific Journal of Cancer Prevention. 2010;11:985–8. [PubMed] [Google Scholar]
- 5.Szymanska K, Lesi OA, Kirk GD, Sam O, Taniere P, Scoazec JY, Mendy M, Friesen MD, Whittle H, Montesano R, Hainaut P. Ser-249 TP53 mutation in tumour and plasma DNA of hepatocellular carcinoma patients from a high incidence area in the Gambia, West Africa. International Journal of Cancer. 2004;110:374–9. doi: 10.1002/ijc.20103. [DOI] [PubMed] [Google Scholar]
- 6.Villar S, Le Roux-Goglin E, Gouas DA, Plymoth A, Ferro G, Boniol M, Lereau M, Bah E, Hall AJ, Wild CP, Mendy M, Norder H, et al. Seasonal variation in TP53 R249S-mutated serum DNA with aflatoxin exposure and hepatitis B virus infection. Environmental Health Perspectives. 2011;119:1635–40. doi: 10.1289/ehp.1103539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomaa AI, Khan SA, Leen ELS, Waked I, Taylor-Robinson SD. Diagnosis of hepatocellular carcinoma. World Journal of Gastroenterology. 2009;15:1301–14. doi: 10.3748/wjg.15.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, Rapaccini G, Del Poggio P, Di Nolfo MA, Benvegnu L, Zoli M, Borzio F, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: Both or neither? American Journal of Gastroenterology. 2006;101:524–32. doi: 10.1111/j.1572-0241.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- 9.Trevisani F, D'Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti HCV status. Journal of Hepatology. 2001;34:570–5. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 10.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omata M, Lesmana LA, Tateishi R, Chen P-J, Lin S-M, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RTP, Shiina S, Cheng AL, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatology International. 2010;4:439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanueva A, Minguez B, Forner A, Reig M, Llovet JM. Hepatocellular carcinoma: Novel molecular approaches for diagnosis, prognosis, and therapy. Annual Review of Medicineed. 2010;61:317–28. doi: 10.1146/annurev.med.080608.100623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, Hainaut P, Marrero JA, Beretta L. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–90. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plymoth A, Boffetta P, Chuang S-C, Hainaut P. International efforts to combat liver cancer: IARC and ILCA initiatives. Journal of Hepatology. 2008;49:675. [Google Scholar]
- 15.Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, Hall AJ, Whittle H, Montesano R. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211–9. doi: 10.1002/hep.20027. [DOI] [PubMed] [Google Scholar]
- 16.Kuniholm MH, Lesi OA, Mendy M, Akano AO, Sam O, Hall AJ, Whittle H, Bah E, Goederts JJ, Hainaut P, Kirk GD. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in The Gambia, West Africa. Environmental Health Perspectives. 2008;116:1553–7. doi: 10.1289/ehp.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umoh NJ, Lesi OA, Mendy M, Bah E, Akano A, Whittle H, Hainaut P, Kirk GD. Aetiological differences in demographical, clinical and pathological characteristics of hepatocellular carcinoma in The Gambia. Liver International. 2011;31:215–21. doi: 10.1111/j.1478-3231.2010.02418.x. [DOI] [PubMed] [Google Scholar]
- 18.Shin A, Cho ER, Kim J, Sung J, Park KW, Lim MK, Shin HR. Factors associated with awareness of infection status among chronic hepatitis B and C carriers in Korea. Cancer Epidemiol Biomarkers Prev. 2009;18:1894–8. doi: 10.1158/1055-9965.EPI-08-1228. [DOI] [PubMed] [Google Scholar]
- 19.Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, Noh HI, Pisani P, Park JG. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005;37:325–31. doi: 10.4143/crt.2005.37.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyytiainen M, Keski-Oja J. Latent TGF-beta binding protein LTBP-2 decreases fibroblast adhesion to fibronectin. Journal of Cell Biology. 2003;163:1363–74. doi: 10.1083/jcb.200309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biology. 2007;26:213–23. doi: 10.1016/j.matbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Vehvilainen P, Hyytiainen M, Keski-Oja J. Matrix association of Latent TGF-Beta Binding Protein 2 (LTBP-2) is dependent on Fibrillin-1. Journal of Cellular Physiology. 2009;221:586–93. doi: 10.1002/jcp.21888. [DOI] [PubMed] [Google Scholar]
- 23.Azmanov DN, Dimitrova S, Florez L, Cherninkova S, Draganov D, Morar B, Saat R, Juan M, Arostegui JI, Ganguly S, Soodyall H, Chakrabarti S, et al. LTBP2 and CYP1B1 mutations and associated ocular phenotypes in the Roma/Gypsy founder population. European Journal of Human Genetics. 2011;19:326–33. doi: 10.1038/ejhg.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan SHK, Ko JMY, Chan KW, Chan YP, Tao Q, Hyytiainen M, Keski-Oja J, Law S, Srivastava G, Tang J, Tsao SW, Chen H, et al. The ECM protein LTBP-2 is a suppressor of esophageal squamous cell carcinoma tumor formation but higher tumor expression associates with poor patient outcome. International Journal of Cancer. 2011;129:565–73. doi: 10.1002/ijc.25698. [DOI] [PubMed] [Google Scholar]
- 25.Turtoi A, Musmeci D, Wang Y, Dumont B, Somja J, Bevilacqua G, De Pauw E, Delvenne P, Castronovo V. Identification of novel accessible proteins bearing diagnostic and therapeutic potential in human pancreatic ductal adenocarcinoma. Journal of Proteome Research. 2011;10:4302–13. doi: 10.1021/pr200527z. [DOI] [PubMed] [Google Scholar]
- 26.Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, Pelaez-Garcia A, Pena C, Lopez-Lucendo M, Villar-Vazquez R, de Herreros AG, Bonilla F, Casal JI. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6006–19. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 27.Ramaiah SK, Rittling S. Pathophysiological role of osteopontin in hepatic inflammation, toxicity, and cancer. Toxicological Sciences. 2008;103:4–13. doi: 10.1093/toxsci/kfm246. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, Cho SY, Hong Y-J, Park HY, Lee M, Jung HH, Lee KH, et al. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. American Journal of Gastroenterology. 2006;101:2051–9. doi: 10.1111/j.1572-0241.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhang CH, Xu GL, Jia WD, Ge YS, Li JS, Ma JL, Ren WH. Prognostic significance of osteopontin in hepatocellular carcinoma: a meta-analysis. International Journal of Cancer. 2011 doi: 10.1002/ijc.26301. [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Dong L, Liu B, Wu G, Xu D, Chen J, Li K, Tong X, Dai J, Yao S, Wu M, Guo Y. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008;135:956–68. doi: 10.1053/j.gastro.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Xie H, Wang J, Han Y, Liu Z, Fan D. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. International Journal of Clinical Practice. 2008;62:1056–62. doi: 10.1111/j.1742-1241.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- 32.Lai KKY, Shang S, Lohia N, Booth GC, Masse DJ, Fausto N, Campbell JS, Beretta L. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and PTEN null mouse models. Plos Genetics. 2011:7. doi: 10.1371/journal.pgen.1002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez Gea V, Friedman SL, Abbas AKGSJHPM. Pathogenesis of liver fibrosis. Annual Review of Pathology: Mechanisms of Disease, Vol 6ed. 2011;6:425–56. doi: 10.1146/annurev-pathol-011110-130246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.